Abstract
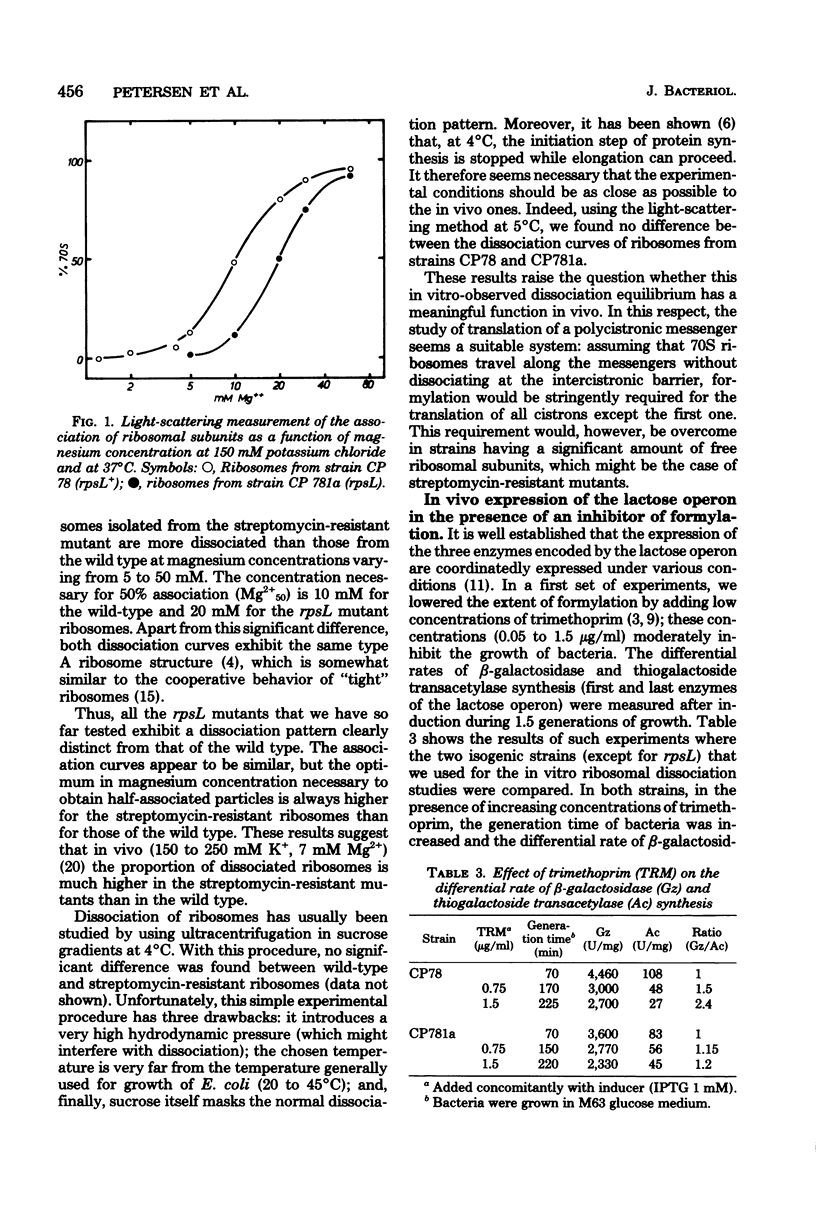
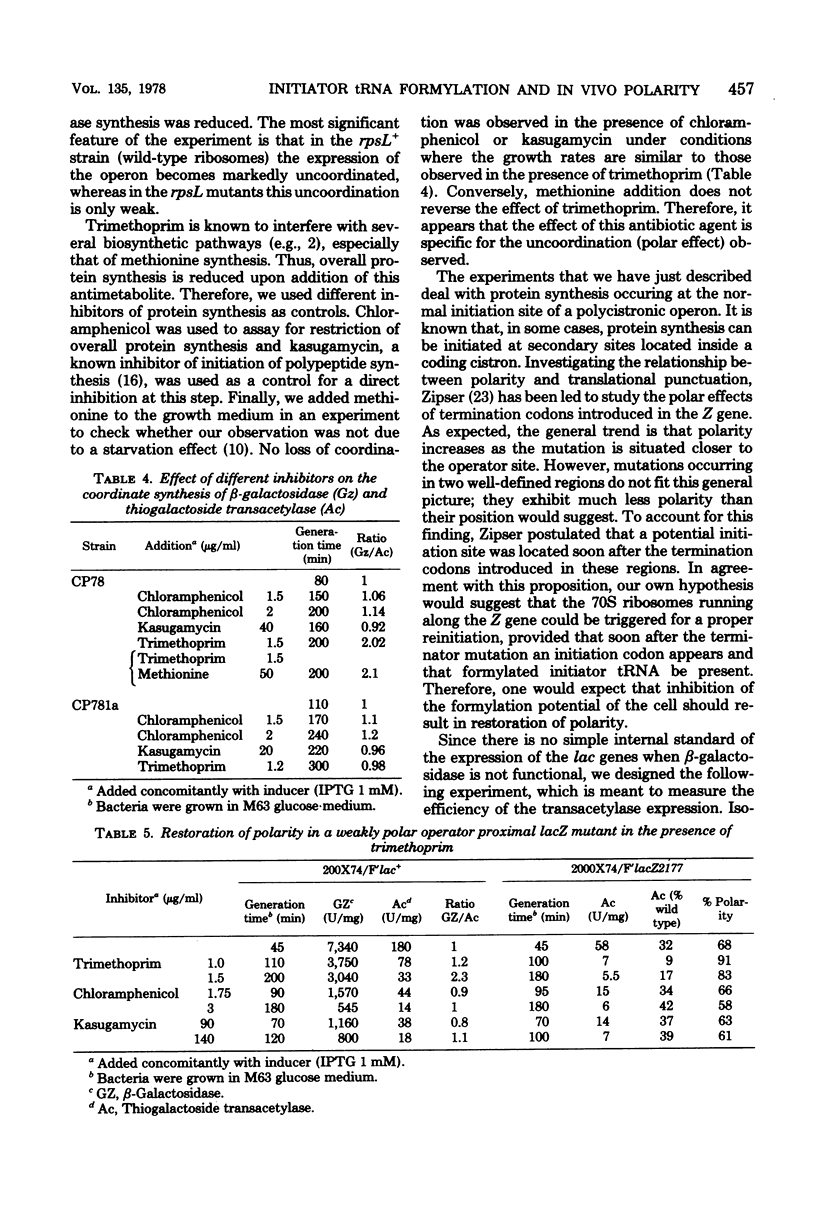
Eucaryotic and procaryotic organisms differ in two aspects of their translation machinery: polycistronic messengers are expressed as a sequence of individual proteins only in procaryotes, and the initiation of protein synthesis proceeds with an initiator tRNA which is found to be modified (formylated) in procaryotes and not in eucaryotes. In the present study, we show that formylation is required in vivo for the coordinate expression of the Escherichia coli lactose operon. Our experiments are consistent with a translation mechanism using dissociated ribosomes at the 5' end of the mRNA in a reaction that is only weakly dependent on formylation at this initiation step; the ribosomes then travel along the messenger and can reinitiate after the intracistronic barrier without dissociation. This latter initiation step is strongly dependent on the level of formylation: a low level of the formyl group, obtained by the antifolic agent trimethoprim, induces a strong polarity in the expression of the lactose operon. There exist mutant strains in which this polarity is much less apparent than in the wild type. We show here that such is the case of rpsL mutants. Ribosomes mutated in the S12 protein (rpsL) are found to be much more easily dissociated than the wild type. This might explain why the expression of the lactose operon on rpsL strains remains coordinated when the intracellular level of formylation is decreased.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berberich M. A., Kovach J. S., Goldberger R. F. Chain initiation in a polycistronic message: sequential versus simultaneous derepression of the enzymes for histidine biosynthesis in Salmonella typhimurium. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1857–1864. doi: 10.1073/pnas.57.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danchin A. Does formylation of initiator tRNA act as a regulatory signal in E. coli? FEBS Lett. 1973 Aug 15;34(2):327–332. doi: 10.1016/0014-5793(73)80823-3. [DOI] [PubMed] [Google Scholar]
- Debey P., Hui Bon Hoa G., Douzou P., Godefroy-Colburn T., Graffe M., Grunberg-Manago M. Ribosomal subunit interaction as studied by light scattering. Evidence of different classes of ribosome preparations. Biochemistry. 1975 Apr 22;14(8):1553–1559. doi: 10.1021/bi00679a001. [DOI] [PubMed] [Google Scholar]
- Dondon J., Godefroy-Colburn T., Graffe M., Grunberg-Manago M. IF-3 requirements for initiation complex formation with synthetic messengers in E. coli system. FEBS Lett. 1974 Sep 1;45(1):82–87. doi: 10.1016/0014-5793(74)80816-1. [DOI] [PubMed] [Google Scholar]
- Friedman H., Lu P., Rich A. Temperature control of initiation of protein synthesis in Escherichia coli. J Mol Biol. 1971 Oct 14;61(1):105–121. doi: 10.1016/0022-2836(71)90209-9. [DOI] [PubMed] [Google Scholar]
- Grunberg-Manago M., Dessen P., Pantaloni D., Godefroy-Colburn T., Wolfe A. D., Dondon J. Light-scattering studies showing the effect of initiation factors on the reversible dissociation of Escherichia coli ribosomes. J Mol Biol. 1975 May 25;94(3):461–478. doi: 10.1016/0022-2836(75)90215-6. [DOI] [PubMed] [Google Scholar]
- Grunberg-Manago M., Gros F. Initiation mechanisms of protein syntehesis. Prog Nucleic Acid Res Mol Biol. 1977;20:209–284. doi: 10.1016/s0079-6603(08)60474-2. [DOI] [PubMed] [Google Scholar]
- Harvey R. J. Growth and initiation of protein synthesis in Escherichia coli in the presence of trimethoprim. J Bacteriol. 1973 Apr;114(1):309–322. doi: 10.1128/jb.114.1.309-322.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemura T., Dahlberg J. E. Small ribonucleic acids of Escherichia coli. II. Noncoordinate accumulation during stringent control. J Biol Chem. 1973 Jul 25;248(14):5033–5041. [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- Leive L., Kollin V. Synthesis, utilization and degradation of lactose operon mRNA in Escherichia coli. J Mol Biol. 1967 Mar 14;24(2):247–259. doi: 10.1016/0022-2836(67)90330-0. [DOI] [PubMed] [Google Scholar]
- Martin J., Webster R. E. The in vitro translation of a terminating signal by a single Escherichia coli ribosome. The fate of the subunits. J Biol Chem. 1975 Oct 25;250(20):8132–8139. [PubMed] [Google Scholar]
- Okuyama A., Machiyama N., Kinoshita T., Tanaka N. Inhibition by kasugamycin of initiation complex formation on 30S ribosomes. Biochem Biophys Res Commun. 1971 Apr 2;43(1):196–199. doi: 10.1016/s0006-291x(71)80106-7. [DOI] [PubMed] [Google Scholar]
- Petersen H. U., Danchin A., Grunberg-Manago M. Toward an understanding of the formylation of initiator tRNA methionine in prokaryotic protein synthesis. I. In vitro studies of the 30S and 70S ribosomal-tRNA complex. Biochemistry. 1976 Apr 6;15(7):1357–1362. doi: 10.1021/bi00652a001. [DOI] [PubMed] [Google Scholar]
- Petersen H. U., Danchin A., Grunberg-Manago M. Toward an understanding of the formylation of initiator tRNA methionine in prokaryotic protein synthesis. II. A two-state model for the 70S ribosome. Biochemistry. 1976 Apr 6;15(7):1362–1369. doi: 10.1021/bi00652a002. [DOI] [PubMed] [Google Scholar]
- Rhoads D. B., Waters F. B., Epstein W. Cation transport in Escherichia coli. VIII. Potassium transport mutants. J Gen Physiol. 1976 Mar;67(3):325–341. doi: 10.1085/jgp.67.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R. E., Zinder N. D. Fate of the message-ribosome complex upon translation of termination signals. J Mol Biol. 1969 Jun 28;42(3):425–439. doi: 10.1016/0022-2836(69)90234-4. [DOI] [PubMed] [Google Scholar]
- Zalkin H., Yanofsky C., Squires C. L. Regulated in vitro synthesis of Escherichia coli tryptophan operon messenger ribonucleic acid and enzymes. J Biol Chem. 1974 Jan 25;249(2):465–475. [PubMed] [Google Scholar]
- Zipser D., Zabell S., Rothman J., Grodzicker T., Wenk M. Fine structure of the gradient of polarity in the z gene of the lac operon of Escherichia coli. J Mol Biol. 1970 Apr 14;49(1):251–254. doi: 10.1016/0022-2836(70)90392-x. [DOI] [PubMed] [Google Scholar]



