Abstract
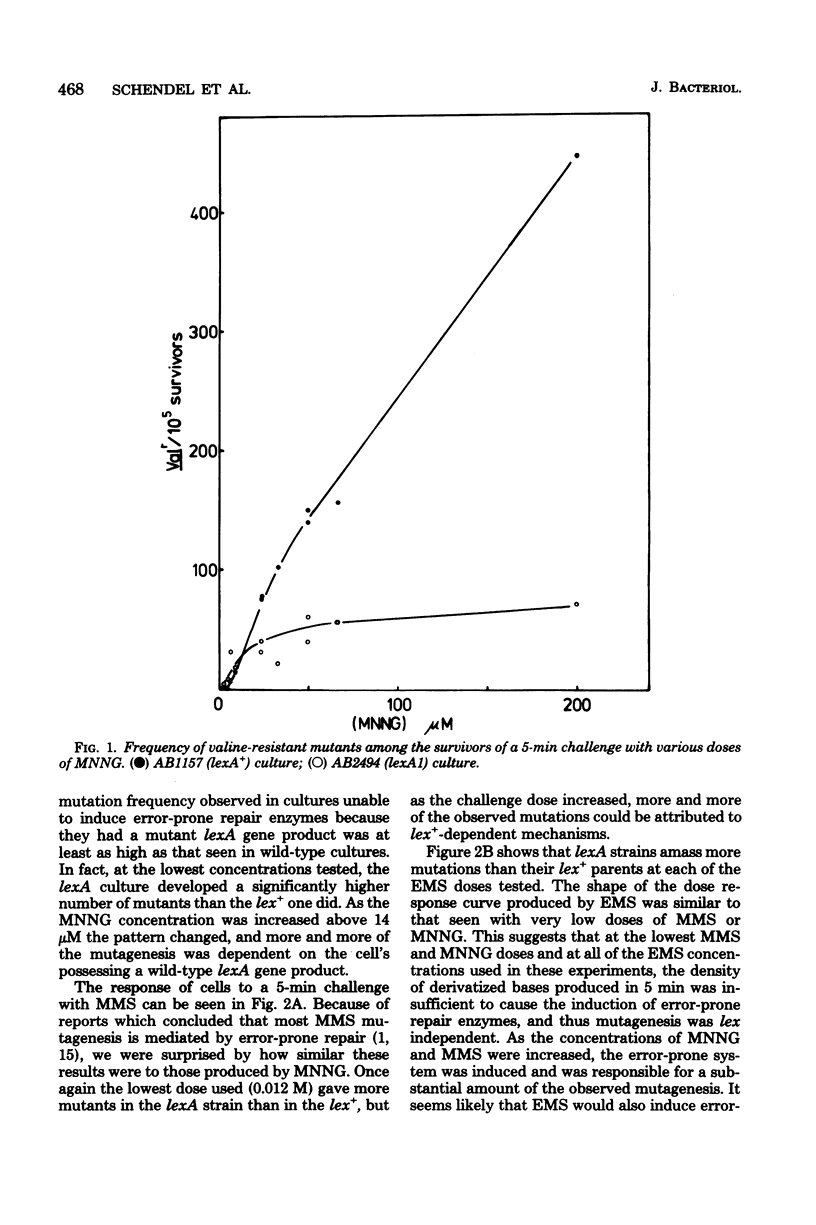
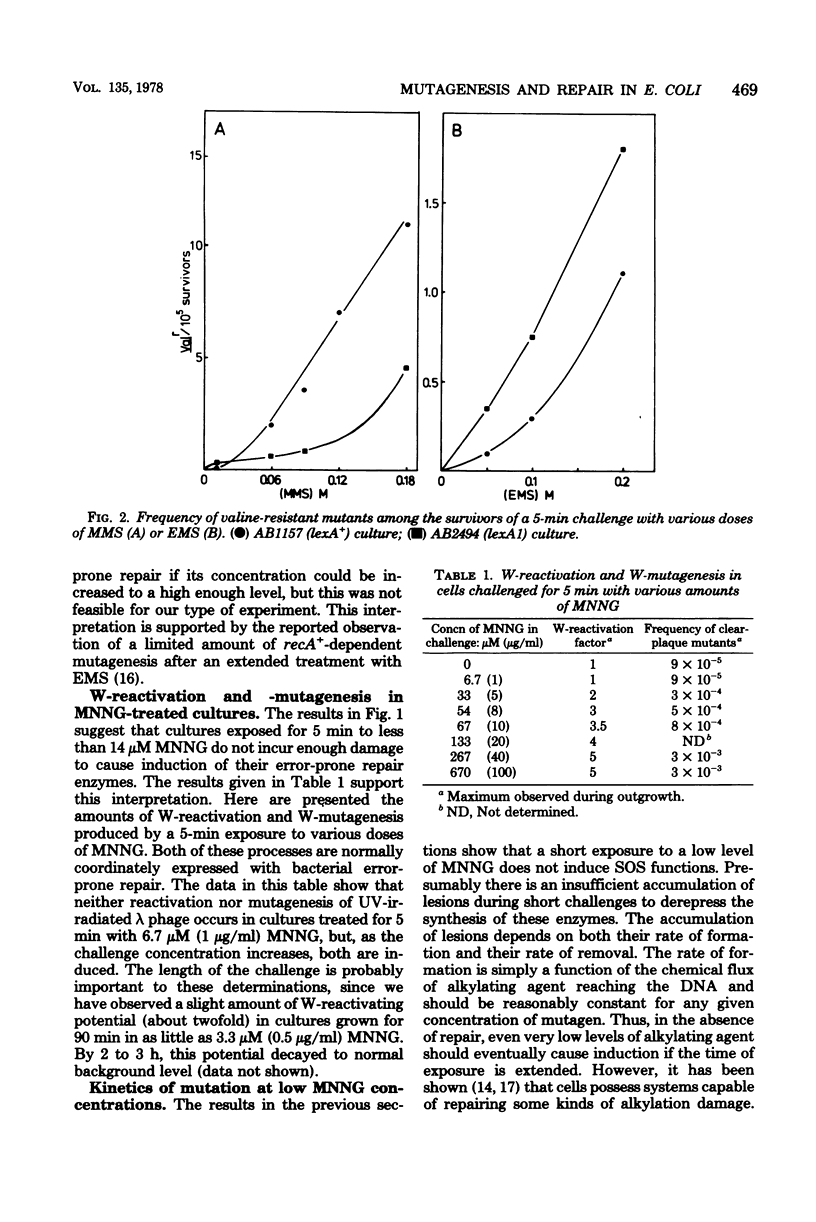
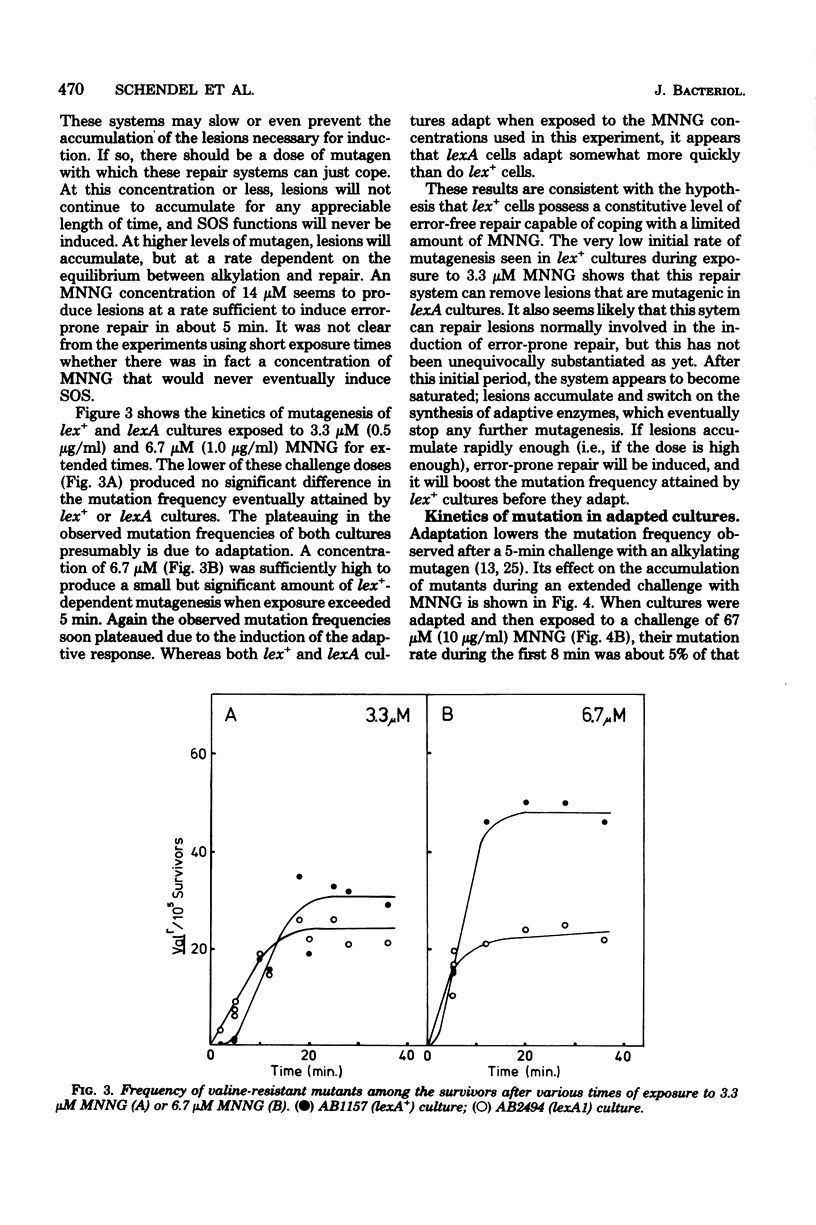
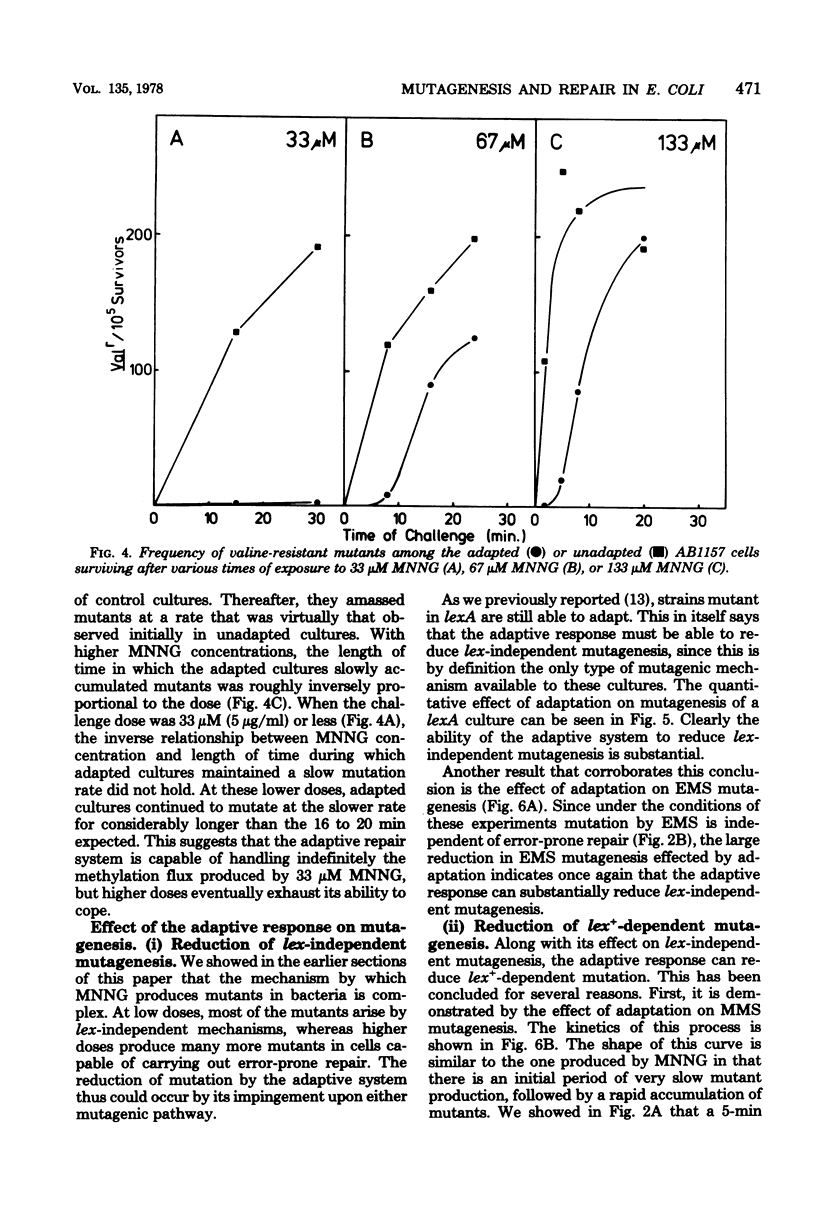
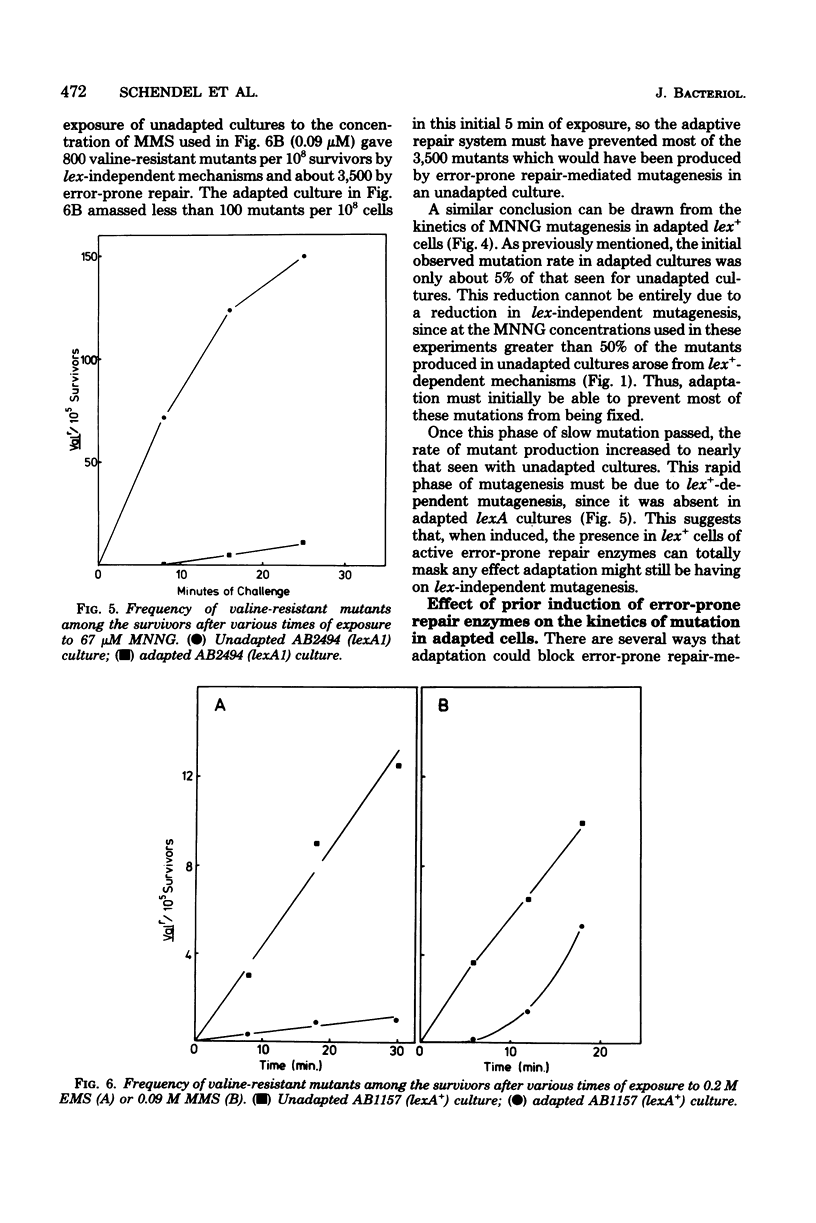
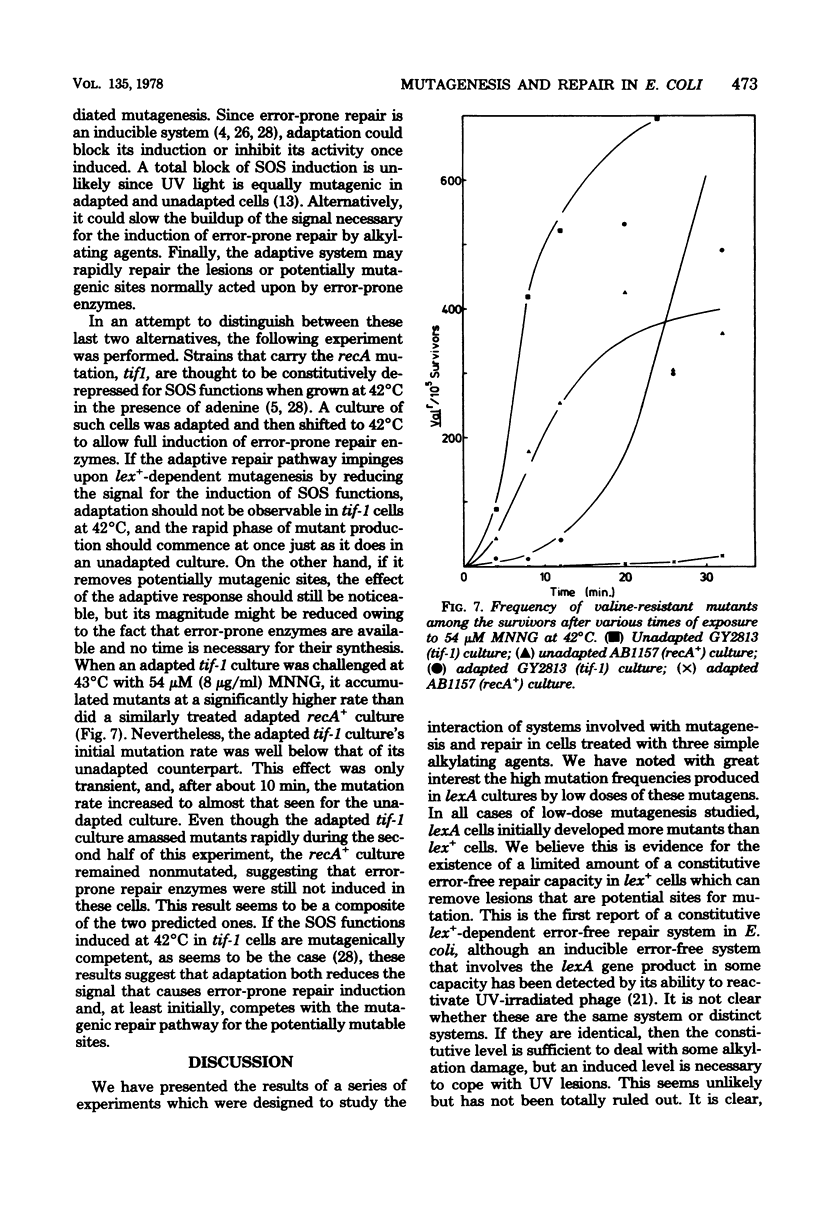
Mutagenesis by simple alkylating agents is thought to occur by either a lexA+-dependent process called error-prone repair or a lex-independent process often attributed to mispairing during replication. We show here that error-prone repair is responsible for the majority of mutants formed after a large dose of alkylating agent, but it is unlikely that it contributes significantly to mutagenesis during exposure to low concentrations of these chemicals. The mutagenicity of these low doses of alkylating agent is reduced by a repair system constitutively present in lexA+ cells but absent in lexA mutants. This system reduces mutagenesis until a second error-free system, called the adaptive responses, can be induced [P. Jeggo, M. Defais, L. Samson, and P. Schendel, Mol. Gen. Genet, 157:1-9, 1977; L. Samson and J. Cairns, Nature (London) 267:281-283, 1977]. The adaptive response is capable of dealing with a much larger amount of alkylation damage than the constitutive system and, when induced, appears to be able to reduce mutagenesis by both decreasing the number of sites available for mutagenesis and delaying the induction of error-prone repair enzymes. Finally, we discuss a model of chemically induced mutagenesis based on these findings which maintains that the observed mutation frequency is dependent on a "race" between these two error-free systems and the two mutagenic pathways.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bridges B. A., Mottershead R. P., Green M. H., Gray W. J. Mutagenicity of dichlorvos and methyl methanesulphonate for Escherichia coli WP2 and some derivatives deficient in DNA repair. Mutat Res. 1973 Sep;19(3):295–303. doi: 10.1016/0027-5107(73)90229-7. [DOI] [PubMed] [Google Scholar]
- Castellazzi M., George J., Buttin G. Prophage induction and cell division in E. coli. I. Further characterization of the thermosensitive mutation tif-1 whose expression mimics the effect of UV irradiation. Mol Gen Genet. 1972;119(2):139–152. doi: 10.1007/BF00269133. [DOI] [PubMed] [Google Scholar]
- Defais M., Caillet-Fauquet P., Fox M. S., Radman M. Induction kinetics of mutagenic DNA repair activity in E. coli following ultraviolet irradiation. Mol Gen Genet. 1976 Oct 18;148(2):125–130. doi: 10.1007/BF00268375. [DOI] [PubMed] [Google Scholar]
- Defais M., Fauquet P., Radman M., Errera M. Ultraviolet reactivation and ultraviolet mutagenesis of lambda in different genetic systems. Virology. 1971 Feb;43(2):495–503. doi: 10.1016/0042-6822(71)90321-7. [DOI] [PubMed] [Google Scholar]
- George J., Castellazzi M., Buttin G. Prophage induction and cell division in E. coli. III. Mutations sfiA and sfiB restore division in tif and lon strains and permit the expression of mutator properties of tif. Mol Gen Genet. 1975 Oct 22;140(4):309–332. [PubMed] [Google Scholar]
- Green M. H., Muriel W. J., Bridges B. A. Use of a simplified fluctuation test to detect low levels of mutagens. Mutat Res. 1976 Feb;38(1):33–42. doi: 10.1016/0165-1161(76)90077-7. [DOI] [PubMed] [Google Scholar]
- Grossman L., Braun A., Feldberg R., Mahler I. Enzymatic repair of DNA. Annu Rev Biochem. 1975;44:19–43. doi: 10.1146/annurev.bi.44.070175.000315. [DOI] [PubMed] [Google Scholar]
- Hince T. A., Neale S. Physiological modification of alkylating-agent induced mutagenesis. I. Effect of growth rate and repair capacity on nitrosomethylurea-induced mutation of Escharichia coli. Mutat Res. 1977 Feb 1;46(1):1–10. doi: 10.1016/0165-1161(77)90105-4. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P. DNA repair and genetic recombination: studies on mutants of Escherichia coli defective in these processes. Radiat Res. 1966;(Suppl):156+–156+. [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P., Theriot L. Three loci in Escherichia coli K-12 that control the excision of pyrimidine dimers and certain other mutagen products from DNA. Genetics. 1966 Jun;53(6):1119–1136. doi: 10.1093/genetics/53.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa-Ryo H., Kondo S. Indirect mutagenesis in phage lambda by ultraviolet preirradiation of host bacteria. J Mol Biol. 1975 Sep 5;97(1):77–92. doi: 10.1016/s0022-2836(75)80023-4. [DOI] [PubMed] [Google Scholar]
- Ishii Y., Kondo S. Comparative analysis of deletion and base-change mutabilities of Escherichia coli B strains differing in DNA repair capacity (wild-type, uvrA-, polA-, recA-) by various mutagens. Mutat Res. 1975 Jan;27(1):27–44. doi: 10.1016/0027-5107(75)90271-7. [DOI] [PubMed] [Google Scholar]
- Jeggo P., Defais T. M., Samson L., Schendel P. An adaptive response of E. coli to low levels of alkylating agent: comparison with previously characterised DNA repair pathways. Mol Gen Genet. 1977 Nov 29;157(1):1–9. doi: 10.1007/BF00268680. [DOI] [PubMed] [Google Scholar]
- Kirtikar D. M., Goldthwait D. A. The enzymatic release of O6-methylguanine and 3-methyladenine from DNA reacted with the carcinogen N-methyl-N-nitrosourea. Proc Natl Acad Sci U S A. 1974 May;71(5):2022–2026. doi: 10.1073/pnas.71.5.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S., Ichikawa H., Iwo K., Kato T. Base-change mutagenesis and prophage induction in strains of Escherichia coli with different DNA repair capacities. Genetics. 1970 Oct;66(2):187–217. doi: 10.1093/genetics/66.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacoste L., Lacaille M., Brakier-Gingras L. The role of misrepair processes in the isolation of new types of streptomycin-resistant mutants in Escherichia coli. Mol Gen Genet. 1977 Dec 9;157(3):313–318. doi: 10.1007/BF00268668. [DOI] [PubMed] [Google Scholar]
- Lawley P. D., Orr D. J. Specific excision of methylation products from DNA of Escherichia coli treated with N-methyl-N'-nitro-N-nitrosoguanidine. Chem Biol Interact. 1970 Aug;2(2):154–157. doi: 10.1016/0009-2797(70)90047-5. [DOI] [PubMed] [Google Scholar]
- Loveless A. Possible relevance of O-6 alkylation of deoxyguanosine to the mutagenicity and carcinogenicity of nitrosamines and nitrosamides. Nature. 1969 Jul 12;223(5202):206–207. doi: 10.1038/223206a0. [DOI] [PubMed] [Google Scholar]
- Miura A., Tomizawa J. I. Studies on radiation-sensitive mutants of E. coli. 3. Participation of the rec system in induction of mutation by ultraviolet irradiation. Mol Gen Genet. 1968;103(1):1–10. doi: 10.1007/BF00271151. [DOI] [PubMed] [Google Scholar]
- Mount D. W., Kosel C. K., Walker A. Inducible, error-free DNA Repair in tsl recA mutants of E. coli. Mol Gen Genet. 1976 Jul 5;146(1):37–41. doi: 10.1007/BF00267980. [DOI] [PubMed] [Google Scholar]
- Mount D. W., Low K. B., Edmiston S. J. Dominant mutations (lex) in Escherichia coli K-12 which affect radiation sensitivity and frequency of ultraviolet lght-induced mutations. J Bacteriol. 1972 Nov;112(2):886–893. doi: 10.1128/jb.112.2.886-893.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson L., Cairns J. A new pathway for DNA repair in Escherichia coli. Nature. 1977 May 19;267(5608):281–283. doi: 10.1038/267281a0. [DOI] [PubMed] [Google Scholar]
- Sedgwick S. G. Inducible error-prone repair in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2753–2757. doi: 10.1073/pnas.72.7.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Thermal enhancement of ultraviolet mutability in a tif-1 uvrA derivative of Escherichia coli B-r: evidence that ultraviolet mutagenesis depends upon an inducible function. Proc Natl Acad Sci U S A. 1974 May;71(5):1930–1934. doi: 10.1073/pnas.71.5.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


