Abstract
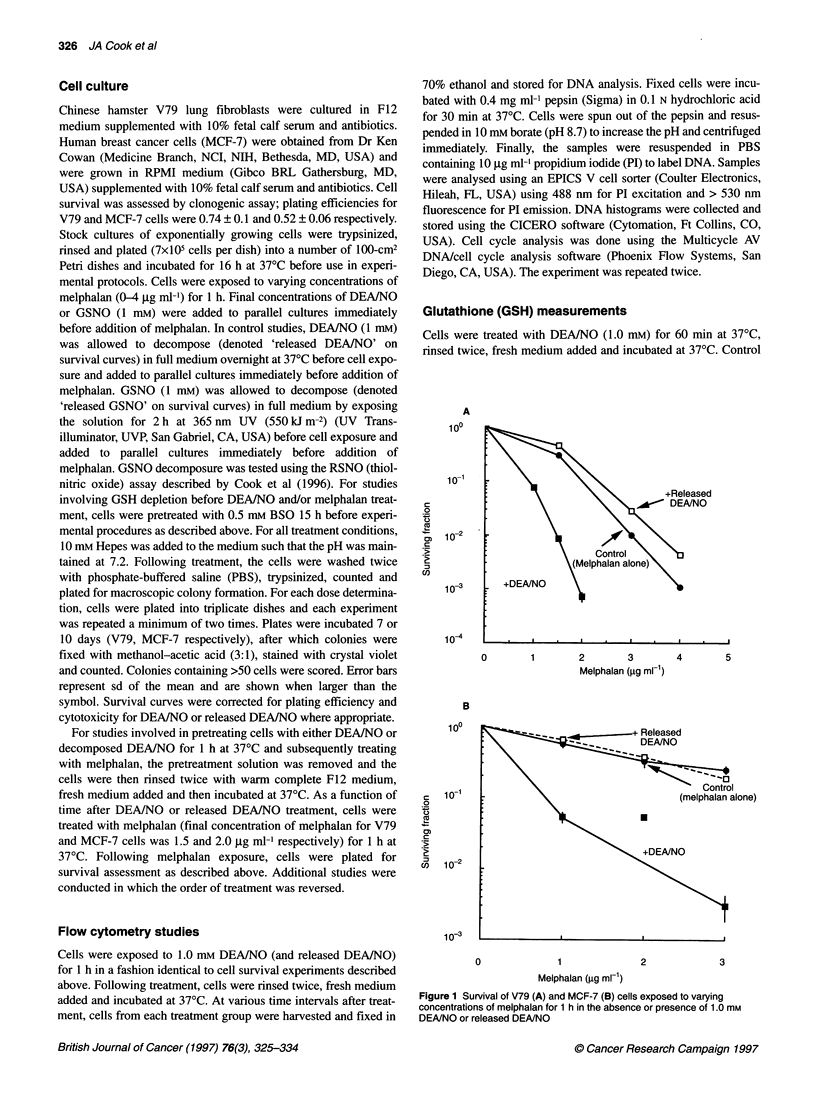
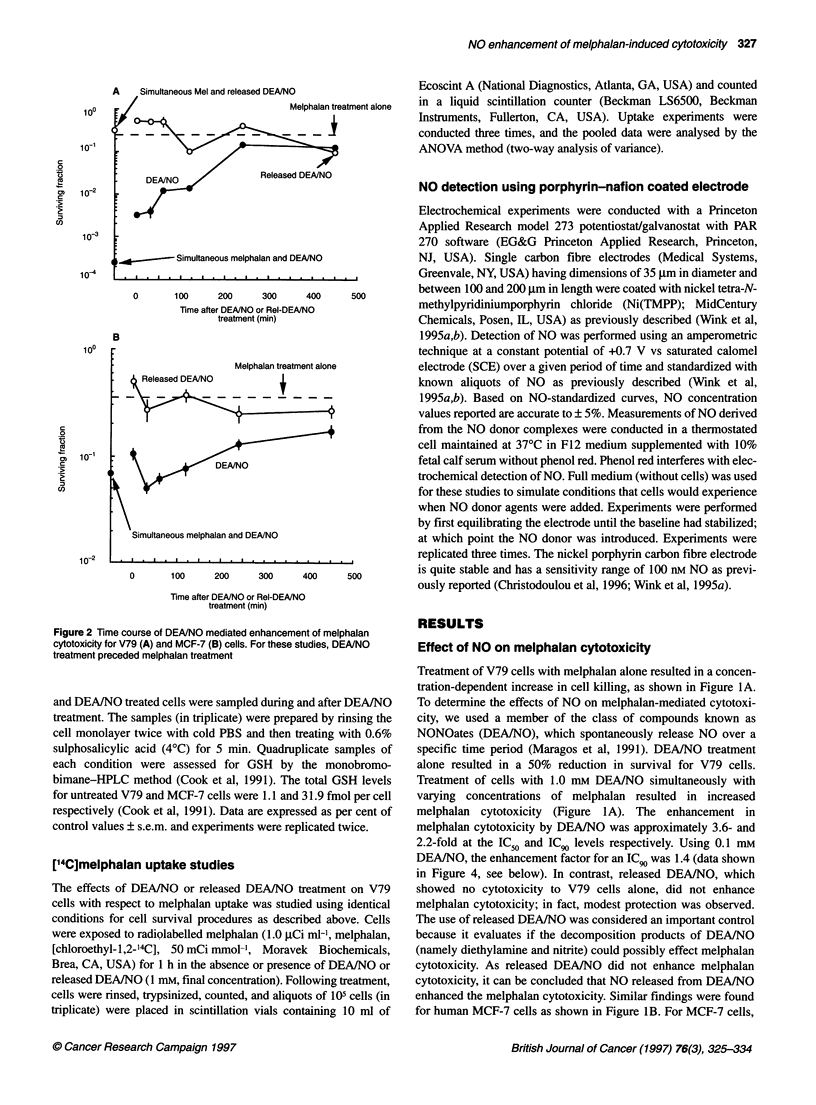
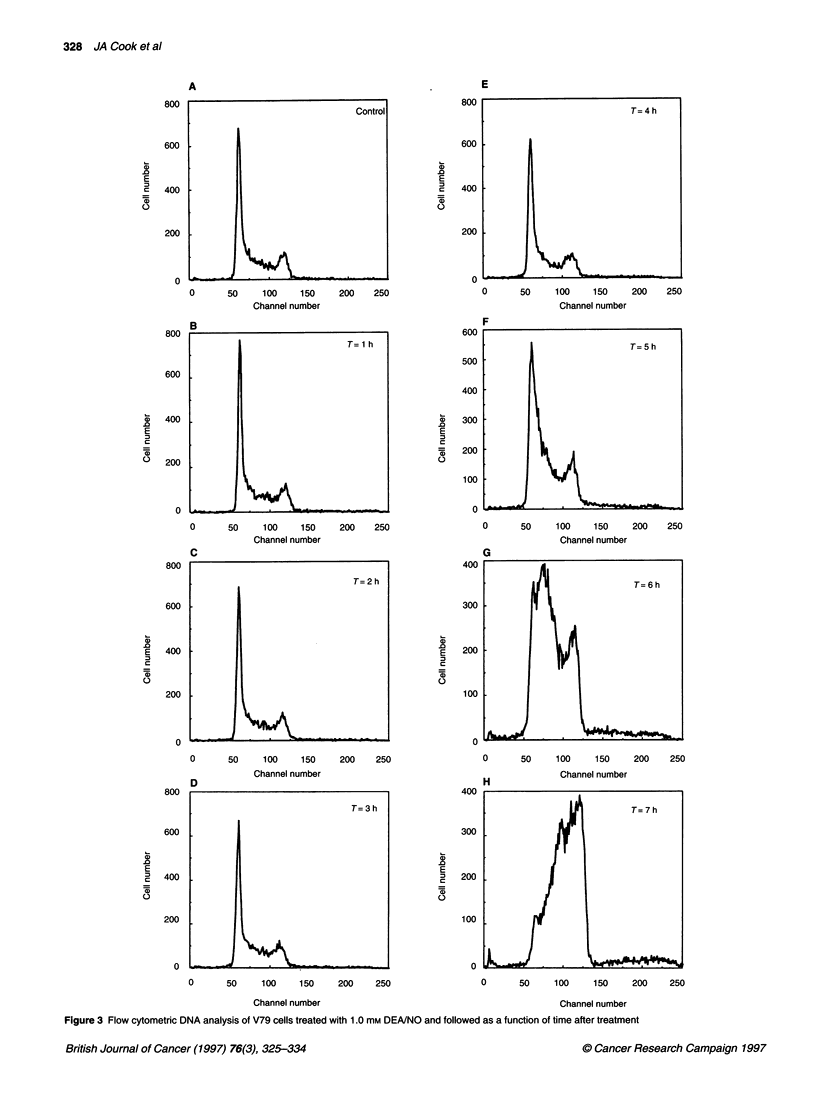
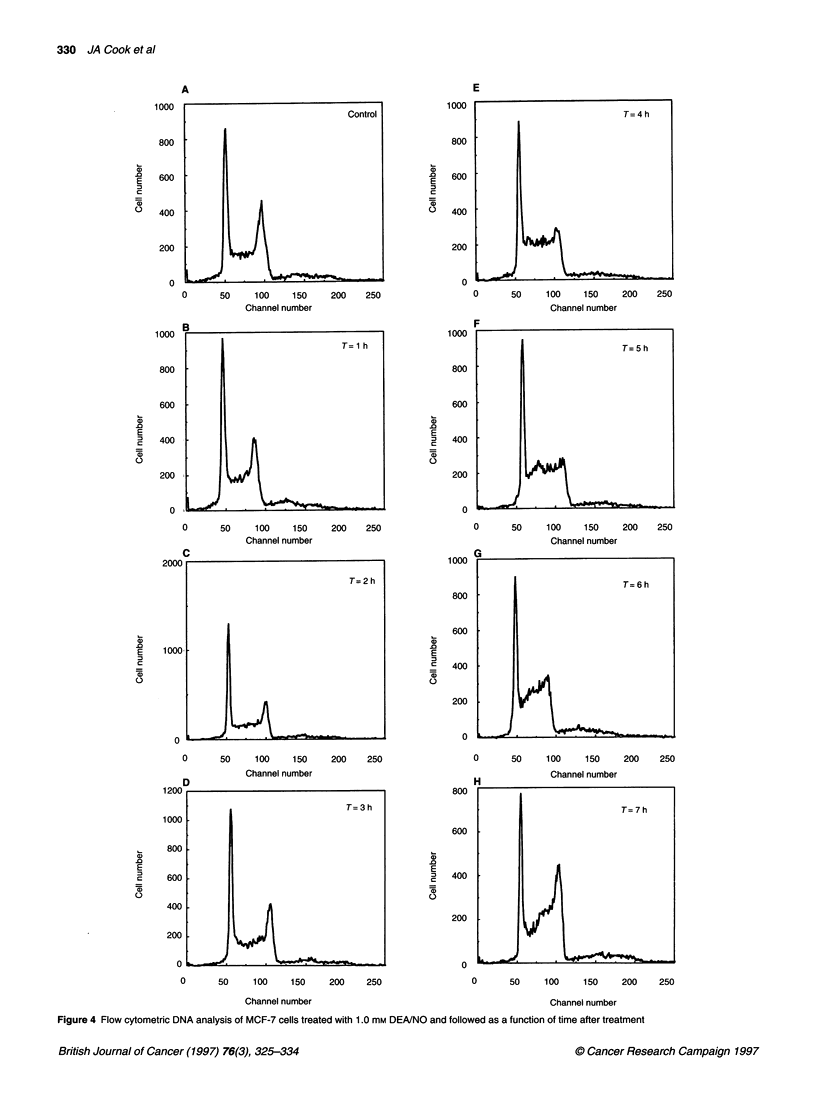
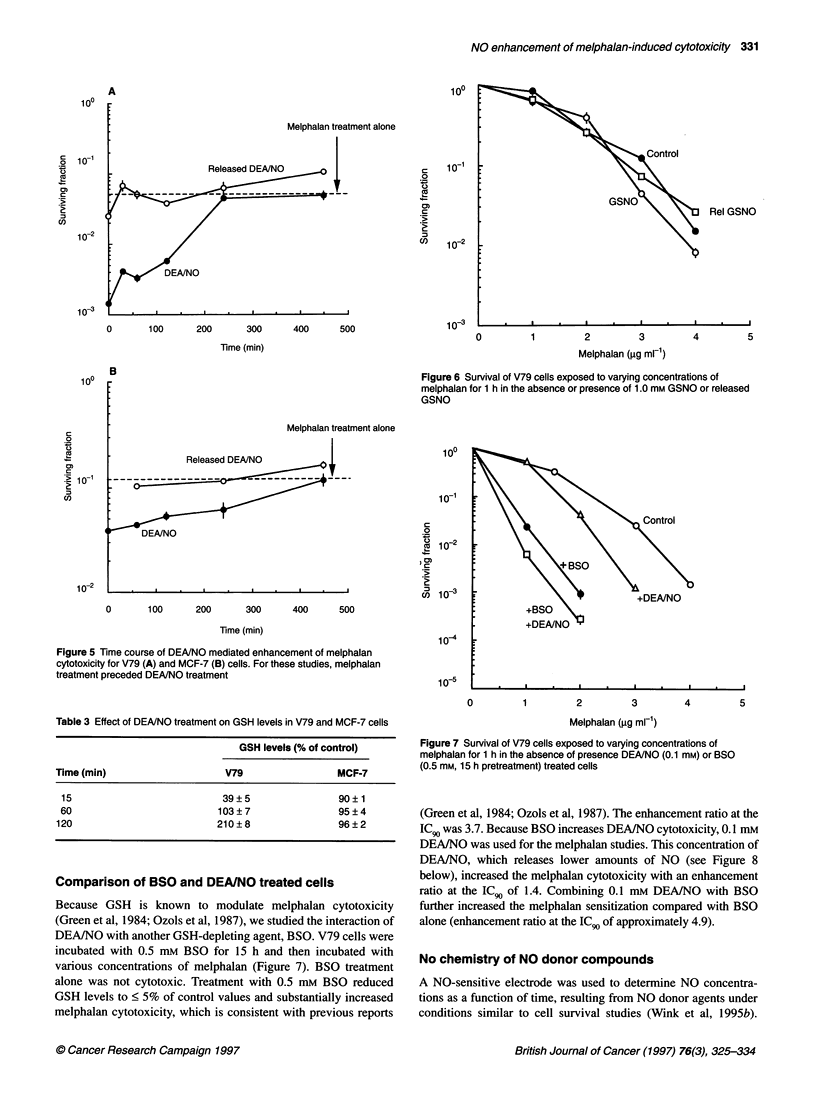
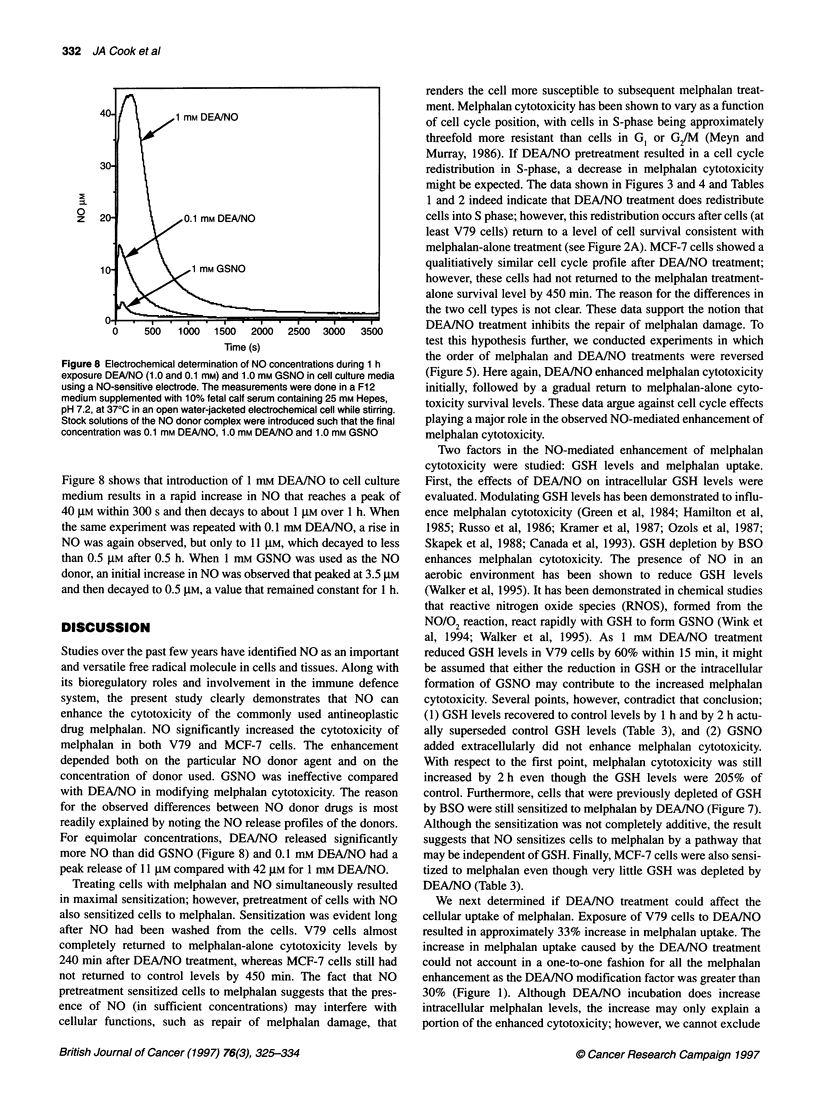
The effects of the diatomic radical, nitric oxide (NO), on melphalan-induced cytotoxicity in Chinese hamster V79 and human MCF-7 breast cancer cells were studied using clonogenic assays. NO delivered by the NO-releasing agent (C2H5)2N[N(O)NO]- Na+ (DEA/NO; 1 mM) resulted in enhancement of melphalan-mediated toxicity in Chinese hamster V79 lung fibroblasts and human breast cancer (MCF-7) cells by 3.6- and 4.3-fold, respectively, at the IC50 level. Nitrite/nitrate and diethylamine, the ultimate end products of DEA/NO decomposition, had little effect on melphalan cytotoxicity, which suggests that NO was responsible for the sensitization. Whereas maximal sensitization of melphalan cytotoxicity by DEA/NO was observed for simultaneous exposure of DEA/NO and melphalan, cells pretreated with DEA/NO were sensitized to melphalan for several hours after NO exposure. Reversing the order of treatment also resulted in a time-dependent enhancement in melphalan cytotoxicity. To explore possible mechanisms of NO enhancement of melphalan cytotoxicity, the effects of DEA/NO on three factors that might influence melphalan toxicity were examined, namely NO-mediated cell cycle perturbations, intracellular glutathione (GSH) levels and melphalan uptake. NO pretreatment resulted in a delayed entry into S phase and a G2/M block for both V79 and MCF-7 cells; however, cell cycle redistribution for V79 cells occurred after the cells returned to a level of cell survival, consistent with treatment with melphalan alone. After 15 min exposure of V79 cells to DEA/NO (1 mM), GSH levels were reduced to 40% of control values; however, GSH levels recovered fully after 1 h and were elevated 2 h after DEA/NO incubation. In contrast, DEA/NO (1 mM) incubation did not reduce GSH levels significantly in MCF-7 cells (approximately 10%). Melphalan uptake was increased by 33% after DEA/NO exposure in V79 cells. From these results enhancement of melphalan cytotoxicity mediated by NO appears to be complex and may involve several pathways, including possibly alteration of the repair of melphalan-induced lesions. Our observations may give insights for improving tumour kill with melphalan using either exogenous or possibly endogenous sources of NO.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano K., Chee C. B., Gaston B., Lilly C. M., Gerard C., Drazen J. M., Stamler J. S. Constitutive and inducible nitric oxide synthase gene expression, regulation, and activity in human lung epithelial cells. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):10089–10093. doi: 10.1073/pnas.91.21.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canada A., Herman L., Kidd K., Robertson C., Trump D. Glutathione depletion increases the cytotoxicity of melphalan to PC-3, an androgen-insensitive prostate cancer cell line. Cancer Chemother Pharmacol. 1993;32(1):73–77. doi: 10.1007/BF00685880. [DOI] [PubMed] [Google Scholar]
- Christodoulou D., Kudo S., Cook J. A., Krishna M. C., Miles A., Grisham M. B., Murugesan M., Ford P. C., Wink D. A. Electrochemical methods for detection of nitric oxide. Methods Enzymol. 1996;268:69–83. doi: 10.1016/s0076-6879(96)68010-0. [DOI] [PubMed] [Google Scholar]
- Cook J. A., Iype S. N., Mitchell J. B. Differential specificity of monochlorobimane for isozymes of human and rodent glutathione S-transferases. Cancer Res. 1991 Mar 15;51(6):1606–1612. [PubMed] [Google Scholar]
- Cook J. A., Kim S. Y., Teague D., Krishna M. C., Pacelli R., Mitchell J. B., Vodovotz Y., Nims R. W., Christodoulou D., Miles A. M. Convenient colorimetric and fluorometric assays for S-nitrosothiols. Anal Biochem. 1996 Jul 1;238(2):150–158. doi: 10.1006/abio.1996.0268. [DOI] [PubMed] [Google Scholar]
- Fast D. J., Lynch R. C., Leu R. W. Nitric oxide production by tumor targets in response to TNF: paradoxical correlation with susceptibility to TNF-mediated cytotoxicity without direct involvement in the cytotoxic mechanism. J Leukoc Biol. 1992 Sep;52(3):255–261. doi: 10.1002/jlb.52.3.255. [DOI] [PubMed] [Google Scholar]
- Feinstein D. L., Galea E., Roberts S., Berquist H., Wang H., Reis D. J. Induction of nitric oxide synthase in rat C6 glioma cells. J Neurochem. 1994 Jan;62(1):315–321. doi: 10.1046/j.1471-4159.1994.62010315.x. [DOI] [PubMed] [Google Scholar]
- Green J. A., Vistica D. T., Young R. C., Hamilton T. C., Rogan A. M., Ozols R. F. Potentiation of melphalan cytotoxicity in human ovarian cancer cell lines by glutathione depletion. Cancer Res. 1984 Nov;44(11):5427–5431. [PubMed] [Google Scholar]
- Hamilton T. C., Winker M. A., Louie K. G., Batist G., Behrens B. C., Tsuruo T., Grotzinger K. R., McKoy W. M., Young R. C., Ozols R. F. Augmentation of adriamycin, melphalan, and cisplatin cytotoxicity in drug-resistant and -sensitive human ovarian carcinoma cell lines by buthionine sulfoximine mediated glutathione depletion. Biochem Pharmacol. 1985 Jul 15;34(14):2583–2586. doi: 10.1016/0006-2952(85)90551-9. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Vavrin Z., Taintor R. R. L-arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J Immunol. 1987 Jan 15;138(2):550–565. [PubMed] [Google Scholar]
- Kramer R. A., Greene K., Ahmad S., Vistica D. T. Chemosensitization of L-phenylalanine mustard by the thiol-modulating agent buthionine sulfoximine. Cancer Res. 1987 Mar 15;47(6):1593–1597. [PubMed] [Google Scholar]
- Kröncke K. D., Fehsel K., Schmidt T., Zenke F. T., Dasting I., Wesener J. R., Bettermann H., Breunig K. D., Kolb-Bachofen V. Nitric oxide destroys zinc-sulfur clusters inducing zinc release from metallothionein and inhibition of the zinc finger-type yeast transcription activator LAC9. Biochem Biophys Res Commun. 1994 Apr 29;200(2):1105–1110. doi: 10.1006/bbrc.1994.1564. [DOI] [PubMed] [Google Scholar]
- Laval F., Wink D. A. Inhibition by nitric oxide of the repair protein, O6-methylguanine-DNA-methyltransferase. Carcinogenesis. 1994 Mar;15(3):443–447. doi: 10.1093/carcin/15.3.443. [DOI] [PubMed] [Google Scholar]
- Lepoivre M., Boudbid H., Petit J. F. Antiproliferative activity of gamma-interferon combined with lipopolysaccharide on murine adenocarcinoma: dependence on an L-arginine metabolism with production of nitrite and citrulline. Cancer Res. 1989 Apr 15;49(8):1970–1976. [PubMed] [Google Scholar]
- Liebmann J., DeLuca A. M., Coffin D., Keefer L. K., Venzon D., Wink D. A., Mitchell J. B. In vivo radiation protection by nitric oxide modulation. Cancer Res. 1994 Jul 1;54(13):3365–3368. [PubMed] [Google Scholar]
- Maragos C. M., Morley D., Wink D. A., Dunams T. M., Saavedra J. E., Hoffman A., Bove A. A., Isaac L., Hrabie J. A., Keefer L. K. Complexes of .NO with nucleophiles as agents for the controlled biological release of nitric oxide. Vasorelaxant effects. J Med Chem. 1991 Nov;34(11):3242–3247. doi: 10.1021/jm00115a013. [DOI] [PubMed] [Google Scholar]
- Misra R. R., Hochadel J. F., Smith G. T., Cook J. C., Waalkes M. P., Wink D. A. Evidence that nitric oxide enhances cadmium toxicity by displacing the metal from metallothionein. Chem Res Toxicol. 1996 Jan-Feb;9(1):326–332. doi: 10.1021/tx950109y. [DOI] [PubMed] [Google Scholar]
- Mitchell J. B., Wink D. A., DeGraff W., Gamson J., Keefer L. K., Krishna M. C. Hypoxic mammalian cell radiosensitization by nitric oxide. Cancer Res. 1993 Dec 15;53(24):5845–5848. [PubMed] [Google Scholar]
- O'Dwyer P. J., Hamilton T. C., LaCreta F. P., Gallo J. M., Kilpatrick D., Halbherr T., Brennan J., Bookman M. A., Hoffman J., Young R. C. Phase I trial of buthionine sulfoximine in combination with melphalan in patients with cancer. J Clin Oncol. 1996 Jan;14(1):249–256. doi: 10.1200/JCO.1996.14.1.249. [DOI] [PubMed] [Google Scholar]
- Ozols R. F., Louie K. G., Plowman J., Behrens B. C., Fine R. L., Dykes D., Hamilton T. C. Enhanced melphalan cytotoxicity in human ovarian cancer in vitro and in tumor-bearing nude mice by buthionine sulfoximine depletion of glutathione. Biochem Pharmacol. 1987 Jan 1;36(1):147–153. doi: 10.1016/0006-2952(87)90392-3. [DOI] [PubMed] [Google Scholar]
- Robbins R. A., Barnes P. J., Springall D. R., Warren J. B., Kwon O. J., Buttery L. D., Wilson A. J., Geller D. A., Polak J. M. Expression of inducible nitric oxide in human lung epithelial cells. Biochem Biophys Res Commun. 1994 Aug 30;203(1):209–218. doi: 10.1006/bbrc.1994.2169. [DOI] [PubMed] [Google Scholar]
- Russo A., Tochner Z., Phillips T., Carmichael J., DeGraff W., Friedman N., Fisher J., Mitchell J. B. In vivo modulation of glutathione by buthionine sulfoximine: effect on marrow response to melphalan. Int J Radiat Oncol Biol Phys. 1986 Jul;12(7):1187–1189. doi: 10.1016/0360-3016(86)90255-5. [DOI] [PubMed] [Google Scholar]
- Schwarz M. A., Lazo J. S., Yalowich J. C., Allen W. P., Whitmore M., Bergonia H. A., Tzeng E., Billiar T. R., Robbins P. D., Lancaster J. R., Jr Metallothionein protects against the cytotoxic and DNA-damaging effects of nitric oxide. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4452–4456. doi: 10.1073/pnas.92.10.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skapek S. X., Colvin O. M., Griffith O. W., Elion G. B., Bigner D. D., Friedman H. S. Enhanced melphalan cytotoxicity following buthionine sulfoximine-mediated glutathione depletion in a human medulloblastoma xenograft in athymic mice. Cancer Res. 1988 May 15;48(10):2764–2767. [PubMed] [Google Scholar]
- Stuehr D. J., Nathan C. F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989 May 1;169(5):1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen L. L., Lawton F. G., Knowles R. G., Beesley J. E., Riveros-Moreno V., Moncada S. Nitric oxide synthase activity in human gynecological cancer. Cancer Res. 1994 Mar 1;54(5):1352–1354. [PubMed] [Google Scholar]
- Walker M. W., Kinter M. T., Roberts R. J., Spitz D. R. Nitric oxide-induced cytotoxicity: involvement of cellular resistance to oxidative stress and the role of glutathione in protection. Pediatr Res. 1995 Jan;37(1):41–49. doi: 10.1203/00006450-199501000-00010. [DOI] [PubMed] [Google Scholar]
- Wink D. A., Cook J. A., Krishna M. C., Hanbauer I., DeGraff W., Gamson J., Mitchell J. B. Nitric oxide protects against alkyl peroxide-mediated cytotoxicity: further insights into the role nitric oxide plays in oxidative stress. Arch Biochem Biophys. 1995 Jun 1;319(2):402–407. doi: 10.1006/abbi.1995.1310. [DOI] [PubMed] [Google Scholar]
- Wink D. A., Hanbauer I., Krishna M. C., DeGraff W., Gamson J., Mitchell J. B. Nitric oxide protects against cellular damage and cytotoxicity from reactive oxygen species. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):9813–9817. doi: 10.1073/pnas.90.21.9813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink D. A., Laval J. The Fpg protein, a DNA repair enzyme, is inhibited by the biomediator nitric oxide in vitro and in vivo. Carcinogenesis. 1994 Oct;15(10):2125–2129. doi: 10.1093/carcin/15.10.2125. [DOI] [PubMed] [Google Scholar]


