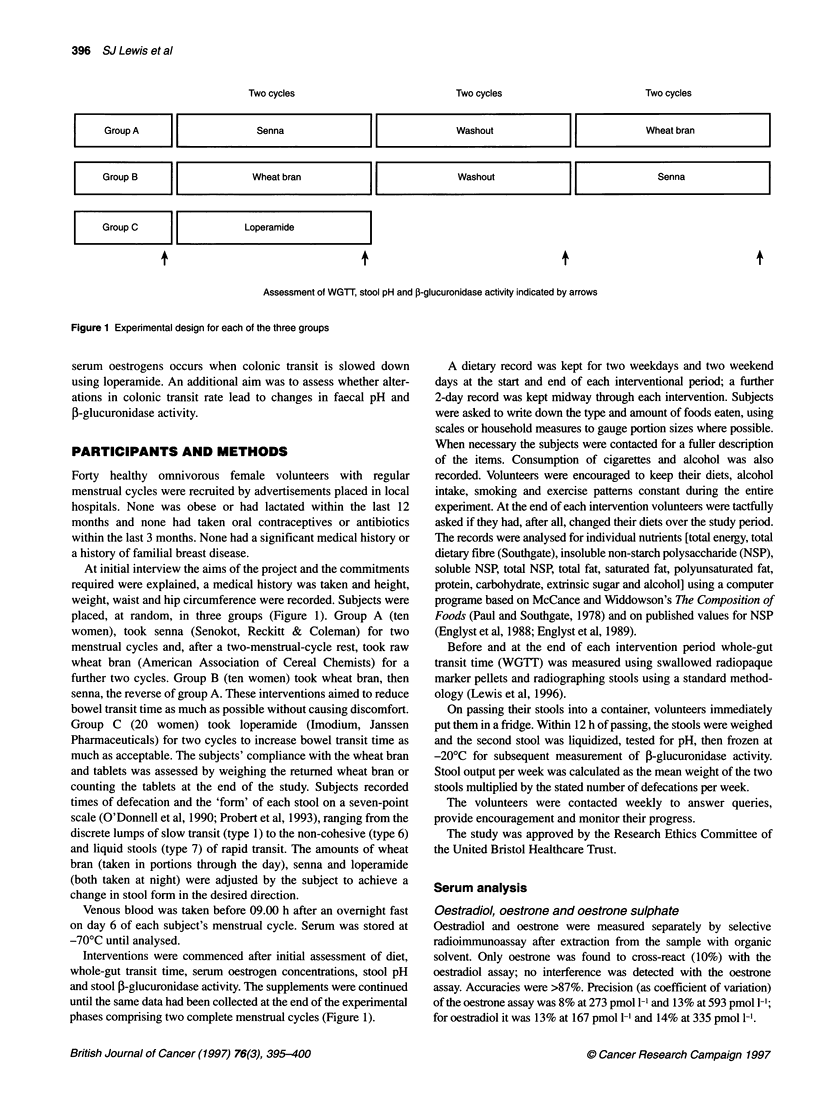
Abstract
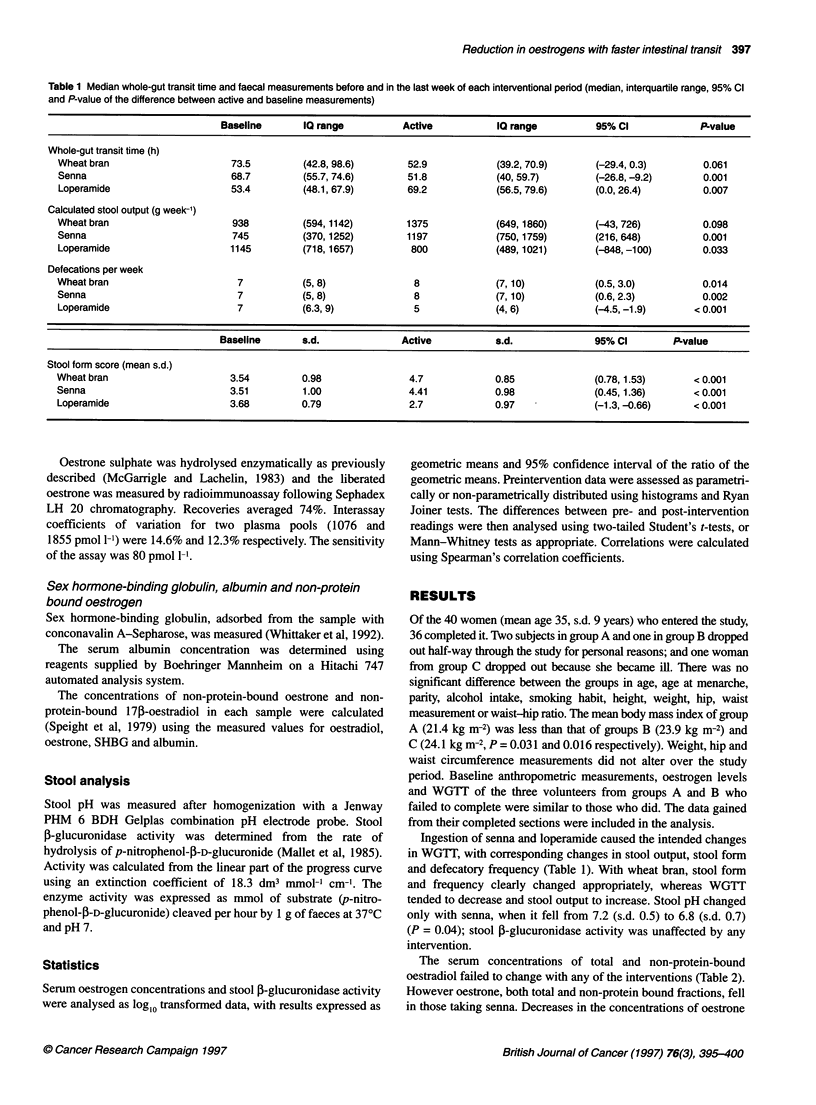
Increased fibre intake has been shown to reduce serum oestrogen concentrations. We hypothesized that fibre exerts this effect by decreasing the time available for reabsorption of oestrogens in the colon. We tested this in volunteers by measuring changes in serum oestrogen levels in response to manipulation of intestinal transit times with senna and loperamide, then comparing the results with changes caused by wheat bran. Forty healthy premenopausal volunteers were placed at random into one of three groups. The first group took senna for two menstrual cycles then, after a washout period, took wheat bran, again for two menstrual cycles. The second group did the reverse. The third group took loperamide for two menstrual cycles. At the beginning and end of each intervention a 4-day dietary record was kept and whole-gut transit time was measured; stools were taken for measurement of pH and beta-glucuronidase activity and blood for measurement of oestrone and oestradiol and their non-protein-bound fractions and of oestrone sulphate. Senna and loperamide caused the intended alterations in intestinal transit, whereas on wheat bran supplements there was a trend towards faster transit. Serum oestrone sulphate fell with wheat bran (mean intake 19.8 g day(-1)) and with senna; total- and non-protein-bound oestrone fell with senna. No significant changes in serum oestrogens were seen with loperamide. No significant changes were seen in faecal beta-glucuronidase activity. Stool pH changed only with senna, in which case it fell. In conclusion, speeding up intestinal transit can lower serum oestrogen concentrations.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adlercreutz H., Martin F., Lehtinen T., Tikkanen M. J., Pulkkinen M. O. Effect of ampicillin administration on plasma conjugated and unconjugated estrogen and progesterone levels in pregnancy. Am J Obstet Gynecol. 1977 Jun 1;128(3):266–271. doi: 10.1016/0002-9378(77)90620-2. [DOI] [PubMed] [Google Scholar]
- Anderson J. W., Bridges S. R., Tietyen J., Gustafson N. J. Dietary fiber content of a simulated American diet and selected research diets. Am J Clin Nutr. 1989 Feb;49(2):352–357. doi: 10.1093/ajcn/49.2.352. [DOI] [PubMed] [Google Scholar]
- Armstrong B. K., Brown J. B., Clarke H. T., Crooke D. K., Hähnel R., Masarei J. R., Ratajczak T. Diet and reproductive hormones: a study of vegetarian and nonvegetarian postmenopausal women. J Natl Cancer Inst. 1981 Oct;67(4):761–767. [PubMed] [Google Scholar]
- Armstrong B., Doll R. Environmental factors and cancer incidence and mortality in different countries, with special reference to dietary practices. Int J Cancer. 1975 Apr 15;15(4):617–631. doi: 10.1002/ijc.2910150411. [DOI] [PubMed] [Google Scholar]
- Arts C. J., Govers C. A., van den Berg H., Wolters M. G., van Leeuwen P., Thijssen J. H. In vitro binding of estrogens by dietary fiber and the in vivo apparent digestibility tested in pigs. J Steroid Biochem Mol Biol. 1991 May;38(5):621–628. doi: 10.1016/0960-0760(91)90321-u. [DOI] [PubMed] [Google Scholar]
- Buell P. Changing incidence of breast cancer in Japanese-American women. J Natl Cancer Inst. 1973 Nov;51(5):1479–1483. doi: 10.1093/jnci/51.5.1479. [DOI] [PubMed] [Google Scholar]
- Burkitt D. P., Walker A. R., Painter N. S. Effect of dietary fibre on stools and the transit-times, and its role in the causation of disease. Lancet. 1972 Dec 30;2(7792):1408–1412. doi: 10.1016/s0140-6736(72)92974-1. [DOI] [PubMed] [Google Scholar]
- Cummings J. H., Bingham S. A., Heaton K. W., Eastwood M. A. Fecal weight, colon cancer risk, and dietary intake of nonstarch polysaccharides (dietary fiber) Gastroenterology. 1992 Dec;103(6):1783–1789. doi: 10.1016/0016-5085(92)91435-7. [DOI] [PubMed] [Google Scholar]
- Drasar B. S., Irving D. Environmental factors and cancer of the colon and breast. Br J Cancer. 1973 Feb;27(2):167–172. doi: 10.1038/bjc.1973.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood M. A., Kirkpatrick J. R., Mitchell W. D., Bone A., Hamilton T. Effects of dietary supplements of wheat bran and cellulose on faeces and bowel function. Br Med J. 1973 Nov 17;4(5889):392–394. doi: 10.1136/bmj.4.5889.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmett P. M., Symes C. L., Heaton K. W. Dietary intake and sources of non-starch polysaccharide in English men and women. Eur J Clin Nutr. 1993 Jan;47(1):20–30. [PubMed] [Google Scholar]
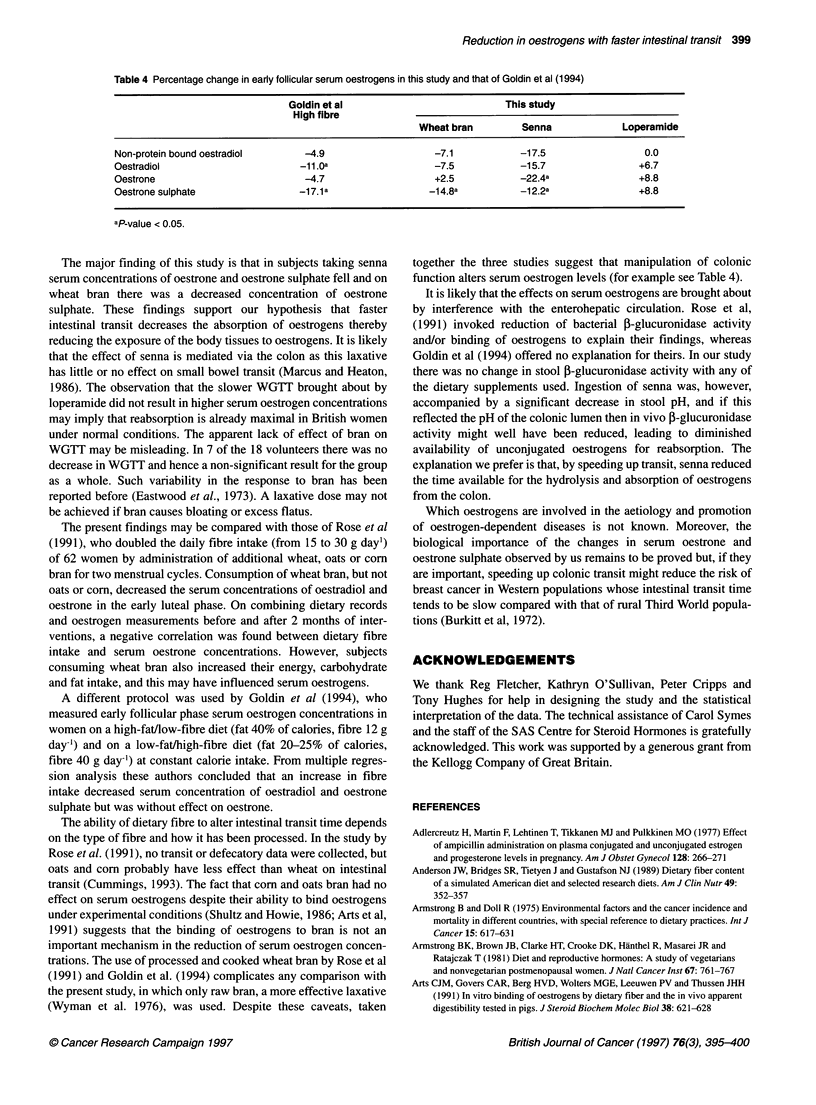
- Goldin B. R., Adlercreutz H., Gorbach S. L., Warram J. H., Dwyer J. T., Swenson L., Woods M. N. Estrogen excretion patterns and plasma levels in vegetarian and omnivorous women. N Engl J Med. 1982 Dec 16;307(25):1542–1547. doi: 10.1056/NEJM198212163072502. [DOI] [PubMed] [Google Scholar]
- Goldin B. R., Gorbach S. L. The relationship between diet and rat fecal bacterial enzymes implicated in colon cancer. J Natl Cancer Inst. 1976 Aug;57(2):371–375. doi: 10.1093/jnci/57.2.371. [DOI] [PubMed] [Google Scholar]
- Goldin B. R., Woods M. N., Spiegelman D. L., Longcope C., Morrill-LaBrode A., Dwyer J. T., Gualtieri L. J., Hertzmark E., Gorbach S. L. The effect of dietary fat and fiber on serum estrogen concentrations in premenopausal women under controlled dietary conditions. Cancer. 1994 Aug 1;74(3 Suppl):1125–1131. doi: 10.1002/1097-0142(19940801)74:3+<1125::aid-cncr2820741521>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Gorbach S. L., Goldin B. R. Diet and the excretion and enterohepatic cycling of estrogens. Prev Med. 1987 Jul;16(4):525–531. doi: 10.1016/0091-7435(87)90067-3. [DOI] [PubMed] [Google Scholar]
- Howe G. R., Hirohata T., Hislop T. G., Iscovich J. M., Yuan J. M., Katsouyanni K., Lubin F., Marubini E., Modan B., Rohan T. Dietary factors and risk of breast cancer: combined analysis of 12 case-control studies. J Natl Cancer Inst. 1990 Apr 4;82(7):561–569. doi: 10.1093/jnci/82.7.561. [DOI] [PubMed] [Google Scholar]
- Katsouyanni K., Trichopoulos D., Boyle P., Xirouchaki E., Trichopoulou A., Lisseos B., Vasilaros S., MacMahon B. Diet and breast cancer: a case-control study in Greece. Int J Cancer. 1986 Dec 15;38(6):815–820. doi: 10.1002/ijc.2910380606. [DOI] [PubMed] [Google Scholar]
- Kim D. H., Kang H. J., Kim S. W., Kobashi K. pH-inducible beta-glucosidase and beta-glucuronidase of intestinal bacteria. Chem Pharm Bull (Tokyo) 1992 Jun;40(6):1667–1669. doi: 10.1248/cpb.40.1667. [DOI] [PubMed] [Google Scholar]
- Lea A. J. Dietary factors associated with death-rates from certain neoplasms in man. Lancet. 1966 Aug 6;2(7458):332–333. doi: 10.1016/s0140-6736(66)92615-8. [DOI] [PubMed] [Google Scholar]
- Lewis S., Bolton C., Heaton K. Lack of influence of intestinal transit on oxidative status in premenopausal women. Eur J Clin Nutr. 1996 Aug;50(8):565–568. [PubMed] [Google Scholar]
- Lubin F., Wax Y., Modan B. Role of fat, animal protein, and dietary fiber in breast cancer etiology: a case-control study. J Natl Cancer Inst. 1986 Sep;77(3):605–612. doi: 10.1093/jnci/77.3.605. [DOI] [PubMed] [Google Scholar]
- Mallett A. K., Rowland I. R., Bearne C. A. Modification of rat caecal microbial biotransformation activities by dietary saccharin. Toxicology. 1985 Aug;36(2-3):253–262. doi: 10.1016/0300-483x(85)90058-7. [DOI] [PubMed] [Google Scholar]
- Marcus S. N., Heaton K. W. Intestinal transit, deoxycholic acid and the cholesterol saturation of bile--three inter-related factors. Gut. 1986 May;27(5):550–558. doi: 10.1136/gut.27.5.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin F., Peltonen J., Laatikainen T., Pulkkinen M., Adlercreutz H. Excretion of progesterone metabolites and estriol in faeces from pregnant women during ampicillin administration. J Steroid Biochem. 1975 Sep;6(9):1339–1346. doi: 10.1016/0022-4731(75)90363-5. [DOI] [PubMed] [Google Scholar]
- McGarrigle H. H., Lachelin G. C. Oestrone, oestradiol and oestriol glucosiduronates and sulphates in human puerperal plasma and milk. J Steroid Biochem. 1983 May;18(5):607–611. doi: 10.1016/0022-4731(83)90139-5. [DOI] [PubMed] [Google Scholar]
- Miller A. B. Role of nutrition in the etiology of breast cancer. Cancer. 1977 Jun;39(6 Suppl):2704–2708. doi: 10.1002/1097-0142(197706)39:6<2704::aid-cncr2820390657>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- O'Donnell L. J., Virjee J., Heaton K. W. Detection of pseudodiarrhoea by simple clinical assessment of intestinal transit rate. BMJ. 1990 Feb 17;300(6722):439–440. doi: 10.1136/bmj.300.6722.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probert C. S., Emmett P. M., Heaton K. W. Some determinants of whole-gut transit time: a population-based study. QJM. 1995 May;88(5):311–315. [PubMed] [Google Scholar]
- Reddy B., Engle A., Katsifis S., Simi B., Bartram H. P., Perrino P., Mahan C. Biochemical epidemiology of colon cancer: effect of types of dietary fiber on fecal mutagens, acid, and neutral sterols in healthy subjects. Cancer Res. 1989 Aug 15;49(16):4629–4635. [PubMed] [Google Scholar]
- Rose D. P., Goldman M., Connolly J. M., Strong L. E. High-fiber diet reduces serum estrogen concentrations in premenopausal women. Am J Clin Nutr. 1991 Sep;54(3):520–525. doi: 10.1093/ajcn/54.3.520. [DOI] [PubMed] [Google Scholar]
- Shultz T. D., Howie B. J. In vitro binding of steroid hormones by natural and purified fibers. Nutr Cancer. 1986;8(2):141–147. doi: 10.1080/01635588609513887. [DOI] [PubMed] [Google Scholar]
- Shultz T. D., Leklem J. E. Nutrient intake and hormonal status of premenopausal vegetarian Seventh-day Adventists and premenopausal nonvegetarians. Nutr Cancer. 1983;4(4):247–259. doi: 10.1080/01635588209513765. [DOI] [PubMed] [Google Scholar]
- Southgate D. A. The definition and analysis of dietary fibre. Nutr Rev. 1977 Mar;35(3):31–37. doi: 10.1111/j.1753-4887.1977.tb06534.x. [DOI] [PubMed] [Google Scholar]
- Speight A. C., Hancock K. W., Oakey R. E. Non-protein-bound oestrogens in plasma and urinary excretion of unconjugated oestrogens in men. Clin Endocrinol (Oxf) 1979 Apr;10(4):329–341. doi: 10.1111/j.1365-2265.1979.tb02088.x. [DOI] [PubMed] [Google Scholar]
- Staszewski J., Haenszel W. Cancer mortality among the Polish-born in the United States. J Natl Cancer Inst. 1965 Aug;35(2):291–297. [PubMed] [Google Scholar]
- Whittaker J. A., Cawood M. L., Oakey R. E. A method for the determination of sex hormone binding globulin using Concanavalin A-sepharose. Ann Clin Biochem. 1992 Mar;29(Pt 2):168–171. doi: 10.1177/000456329202900208. [DOI] [PubMed] [Google Scholar]
- Willman K., Pulkkinen M. O. Reduced maternal plasma and urinary estriol during ampicillin treatment. Am J Obstet Gynecol. 1971 Mar 15;109(6):893–896. doi: 10.1016/0002-9378(71)90803-9. [DOI] [PubMed] [Google Scholar]
- Wyman J. B., Heaton K. W., Manning A. P., Wicks A. C. The effect on intestinal transit and the feces of raw and cooked bran in different doses. Am J Clin Nutr. 1976 Dec;29(12):1474–1479. doi: 10.1093/ajcn/29.12.1474. [DOI] [PubMed] [Google Scholar]
- Wyman J. B., Heaton K. W., Manning A. P., Wicks A. C. Variability of colonic function in healthy subjects. Gut. 1978 Feb;19(2):146–150. doi: 10.1136/gut.19.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaridze D., Lifanova Y., Maximovitch D., Day N. E., Duffy S. W. Diet, alcohol consumption and reproductive factors in a case-control study of breast cancer in Moscow. Int J Cancer. 1991 Jun 19;48(4):493–501. doi: 10.1002/ijc.2910480404. [DOI] [PubMed] [Google Scholar]


