Abstract
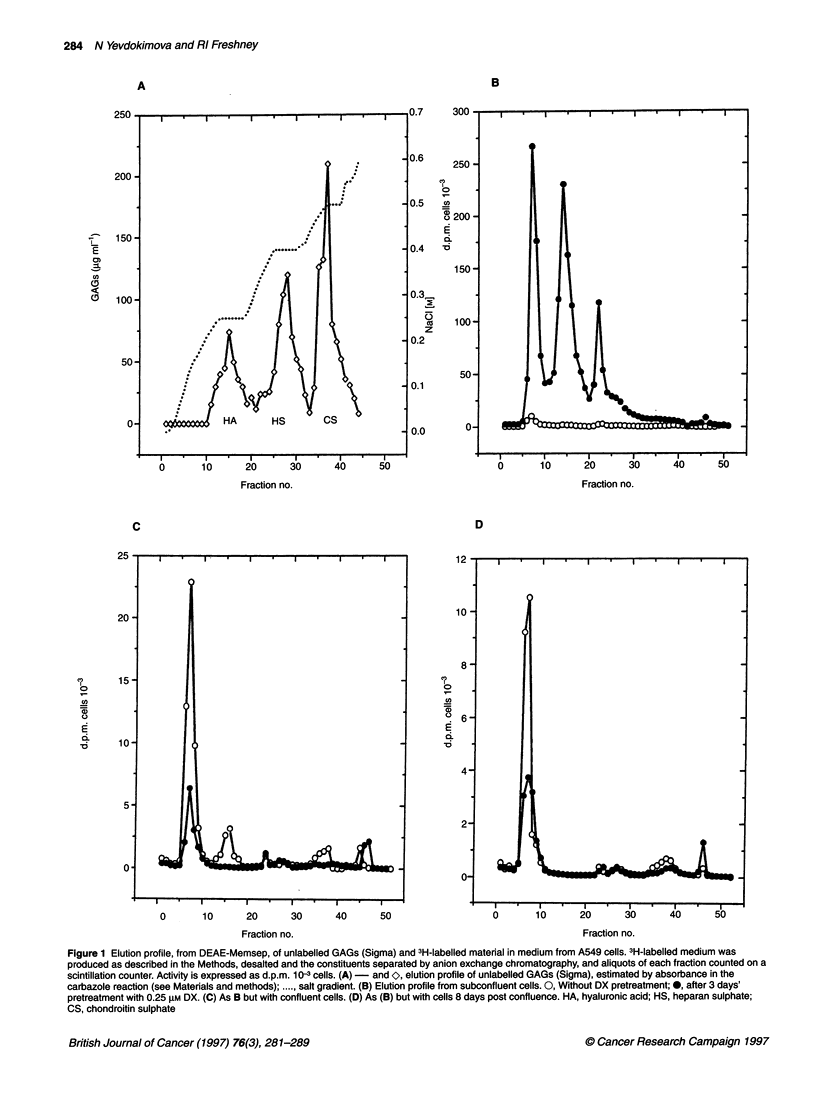
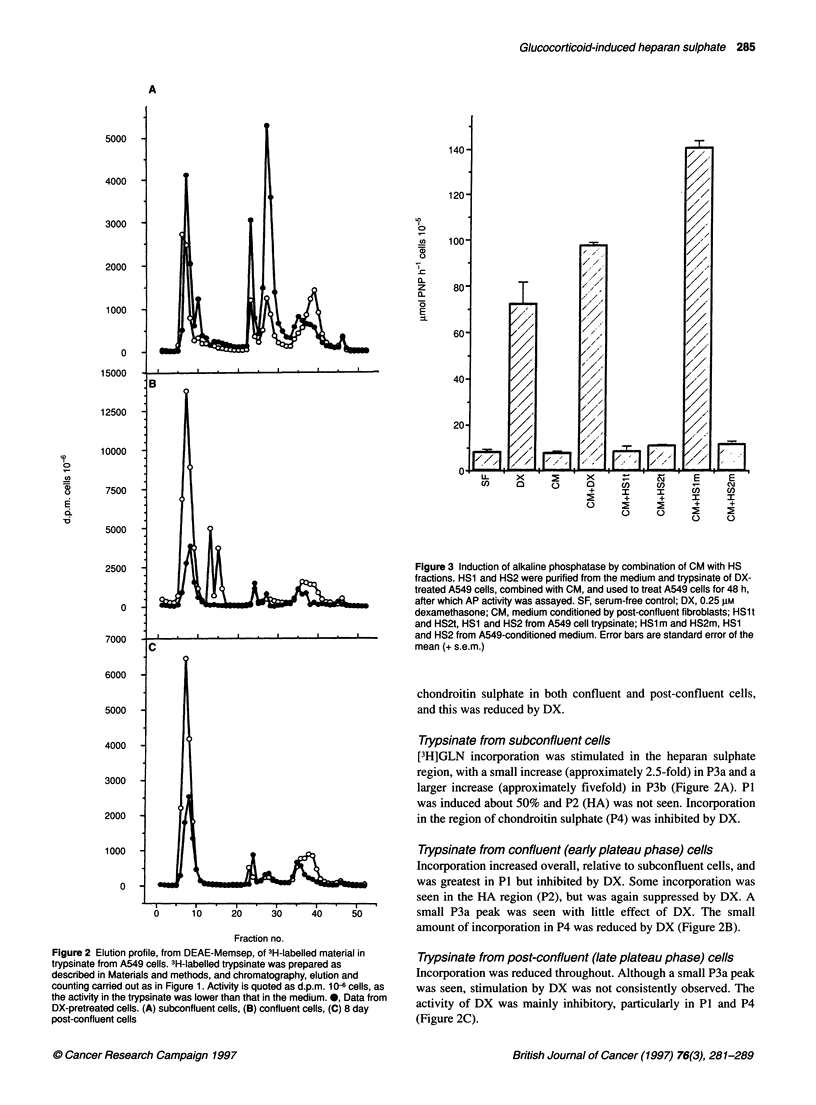
Alkaline phosphatase, a marker of differentiation in the human alveolar adenocarcinoma cell line A549, is inducible by conditioned medium from lung fibroblasts and by cytokines including oncostatin M and interleukin 6, but only in the presence of a glucocorticoid, dexamethasone. Dexamethasone was shown to induce incorporation of [3H]glucosamine into three fractions of medium and cell trypsinate from subconfluent A549 cells, eluting from DEAE ion-exchange chromatography. The first peak did not correspond to any of the unlabelled glycosaminoglycans and was not characterized further. Induction was seen in two other peaks, corresponding to hyaluronic acid and heparan sulphate. Of these, heparan sulphate, eluting as one well-defined peak (referred to as HS1) and another of lower activity and less well defined (HS2), was selected as the most likely to interact with growth factors and cytokines and was isolated from the eluate, concentrated and desalted, and used in alkaline phosphatase induction experiments in place of dexamethasone. HS1 isolated from the medium (HS1m) of subconfluent A549 cells was shown to replace dexamethasone in induction experiments with fibroblast-conditioned medium, oncostatin M and interleukin 6. HS1 from the cell trypsinate and HS2 from the medium and trypsinate were inactive. As the activity of HS1m could be abolished by heparinase and heparitinase but not by chondroitinase ABC, it was concluded that HS1m was a fraction of heparan sulphate involved in the regulation of paracrine growth factor activity in lung fibroblast-conditioned medium, and in the regulation of other growth factors with potential roles in the paracrine control of cell differentiation.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bruce A. G., Linsley P. S., Rose T. M. Oncostatin M. Prog Growth Factor Res. 1992;4(2):157–170. doi: 10.1016/0955-2235(92)90029-h. [DOI] [PubMed] [Google Scholar]
- Edelson J. D., Shannon J. M., Mason R. J. Alkaline phosphatase: a marker of alveolar type II cell differentiation. Am Rev Respir Dis. 1988 Nov;138(5):1268–1275. doi: 10.1164/ajrccm/138.5.1268. [DOI] [PubMed] [Google Scholar]
- Fernig D. G., Gallagher J. T. Fibroblast growth factors and their receptors: an information network controlling tissue growth, morphogenesis and repair. Prog Growth Factor Res. 1994;5(4):353–377. doi: 10.1016/0955-2235(94)00007-8. [DOI] [PubMed] [Google Scholar]
- Giard D. J., Aaronson S. A., Todaro G. J., Arnstein P., Kersey J. H., Dosik H., Parks W. P. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973 Nov;51(5):1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- Han Z. C., Bellucci S., Shen Z. X., Maffrand J. P., Pascal M., Petitou M., Lormeau J., Caen J. P. Glycosaminoglycans enhance megakaryocytopoiesis by modifying the activities of hematopoietic growth regulators. J Cell Physiol. 1996 Jul;168(1):97–104. doi: 10.1002/(SICI)1097-4652(199607)168:1<97::AID-JCP12>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Hawgood S., Clements J. A. Pulmonary surfactant and its apoproteins. J Clin Invest. 1990 Jul;86(1):1–6. doi: 10.1172/JCI114670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jandik K. A., Gu K., Linhardt R. J. Action pattern of polysaccharide lyases on glycosaminoglycans. Glycobiology. 1994 Jun;4(3):289–296. doi: 10.1093/glycob/4.3.289. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M., Baird A. A dual receptor system is required for basic fibroblast growth factor activity. Cell. 1991 Oct 18;67(2):229–231. doi: 10.1016/0092-8674(91)90173-v. [DOI] [PubMed] [Google Scholar]
- Kohlhepp E. A., Condon M. E., Hamburger A. W. Recombinant human interferon alpha enhancement of retinoic-acid-induced differentiation of HL-60 cells. Exp Hematol. 1987 May;15(4):414–418. [PubMed] [Google Scholar]
- Lieber M., Smith B., Szakal A., Nelson-Rees W., Todaro G. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int J Cancer. 1976 Jan 15;17(1):62–70. doi: 10.1002/ijc.2910170110. [DOI] [PubMed] [Google Scholar]
- Lotem J., Sachs L. Regulation of bcl-2, bcl-XL and bax in the control of apoptosis by hematopoietic cytokines and dexamethasone. Cell Growth Differ. 1995 Jun;6(6):647–653. [PubMed] [Google Scholar]
- López-Casillas F., Wrana J. L., Massagué J. Betaglycan presents ligand to the TGF beta signaling receptor. Cell. 1993 Jul 2;73(7):1435–1444. doi: 10.1016/0092-8674(93)90368-z. [DOI] [PubMed] [Google Scholar]
- Mackie A. E., Freshney R. I., Akturk F., Hunt G. Glucocorticoids and the cell surface of human glioma cells: relationship to cytostasis. Br J Cancer Suppl. 1988 Dec;9:101–107. [PMC free article] [PubMed] [Google Scholar]
- McCaffrey T. A., Falcone D. J., Vicente D., Du B., Consigli S., Borth W. Protection of transforming growth factor-beta 1 activity by heparin and fucoidan. J Cell Physiol. 1994 Apr;159(1):51–59. doi: 10.1002/jcp.1041590108. [DOI] [PubMed] [Google Scholar]
- McCormick C., Freshney R. I., Speirs V. Activity of interferon alpha, interleukin 6 and insulin in the regulation of differentiation in A549 alveolar carcinoma cells. Br J Cancer. 1995 Feb;71(2):232–239. doi: 10.1038/bjc.1995.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piepkorn M., Lo C., Plowman G. Amphiregulin-dependent proliferation of cultured human keratinocytes: autocrine growth, the effects of exogenous recombinant cytokine, and apparent requirement for heparin-like glycosaminoglycans. J Cell Physiol. 1994 Apr;159(1):114–120. doi: 10.1002/jcp.1041590115. [DOI] [PubMed] [Google Scholar]
- Post M., Floros J., Smith B. T. Inhibition of lung maturation by monoclonal antibodies against fibroblast-pneumonocyte factor. Nature. 1984 Mar 15;308(5956):284–286. doi: 10.1038/308284a0. [DOI] [PubMed] [Google Scholar]
- Post M., Smith B. T. Effect of fibroblast-pneumonocyte factor on the synthesis of surfactant phospholipids in type II cells from fetal rat lung. Biochim Biophys Acta. 1984 Apr 18;793(2):297–299. doi: 10.1016/0005-2760(84)90332-1. [DOI] [PubMed] [Google Scholar]
- Rose T. M., Bruce A. G. Oncostatin M is a member of a cytokine family that includes leukemia-inhibitory factor, granulocyte colony-stimulating factor, and interleukin 6. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8641–8645. doi: 10.1073/pnas.88.19.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Yamaguchi Y., Hildebrand A., Border W. A. Extracellular matrix/growth factor interactions. Cold Spring Harb Symp Quant Biol. 1992;57:309–315. doi: 10.1101/sqb.1992.057.01.035. [DOI] [PubMed] [Google Scholar]
- Skinner S. J., Post M., Torday J. S., Stiles A. D., Smith B. T. Characterization of proteoglycans synthesized by fetal rat lung type II pneumonocytes in vitro and the effects of cortisol. Exp Lung Res. 1987;12(3):253–264. doi: 10.3109/01902148709064304. [DOI] [PubMed] [Google Scholar]
- Speirs V., Ray K. P., Freshney R. I. Paracrine control of differentiation in the alveolar carcinoma, A549, by human foetal lung fibroblasts. Br J Cancer. 1991 Oct;64(4):693–699. doi: 10.1038/bjc.1991.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadnyk A. W. Cytokine production by epithelial cells. FASEB J. 1994 Oct;8(13):1041–1047. doi: 10.1096/fasebj.8.13.7926369. [DOI] [PubMed] [Google Scholar]
- Strain A. J., McGuinness G., Rubin J. S., Aaronson S. A. Keratinocyte growth factor and fibroblast growth factor action on DNA synthesis in rat and human hepatocytes: modulation by heparin. Exp Cell Res. 1994 Feb;210(2):253–259. doi: 10.1006/excr.1994.1037. [DOI] [PubMed] [Google Scholar]
- Walker A., Turnbull J. E., Gallagher J. T. Specific heparan sulfate saccharides mediate the activity of basic fibroblast growth factor. J Biol Chem. 1994 Jan 14;269(2):931–935. [PubMed] [Google Scholar]
- de Wynter E., Allen T., Coutinho L., Flavell D., Flavell S. U., Dexter T. M. Localisation of granulocyte macrophage colony-stimulating factor in human long-term bone marrow cultures. Biological and immunocytochemical characterisation. J Cell Sci. 1993 Nov;106(Pt 3):761–769. doi: 10.1242/jcs.106.3.761. [DOI] [PubMed] [Google Scholar]


