Abstract
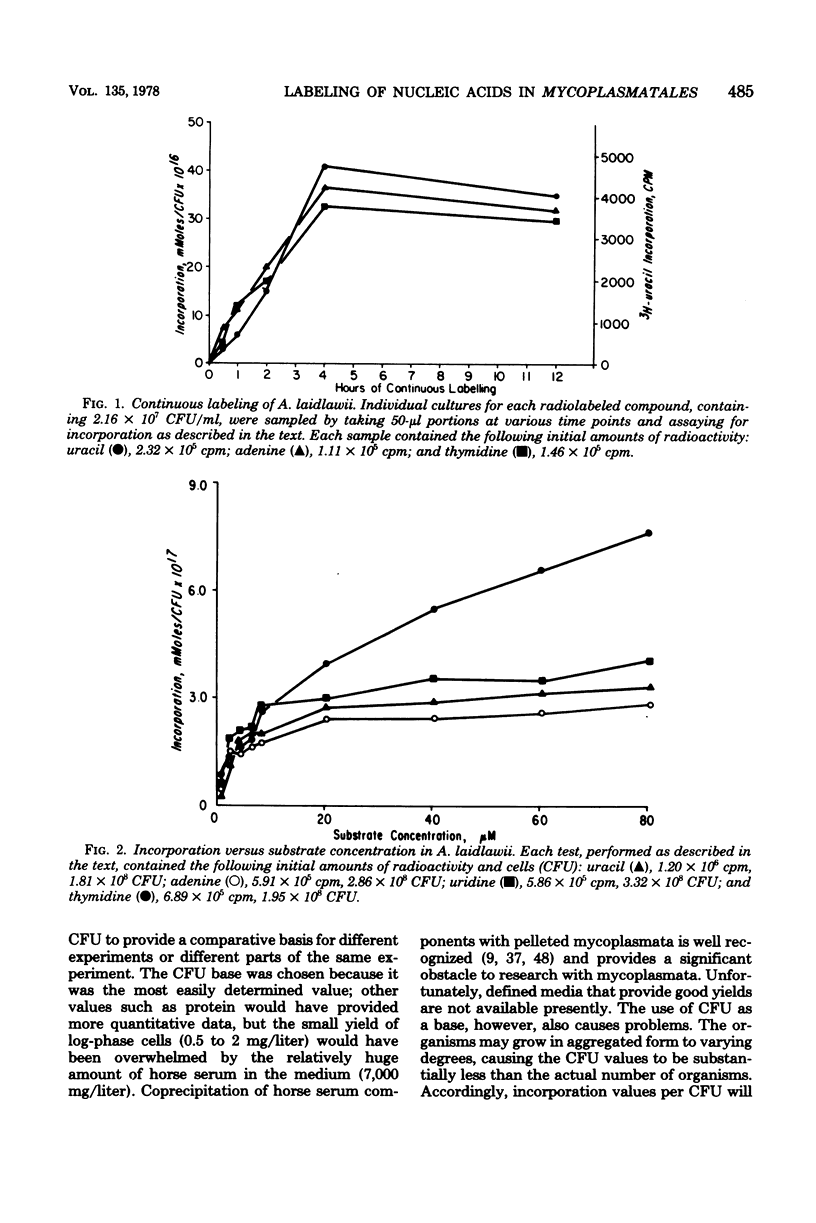
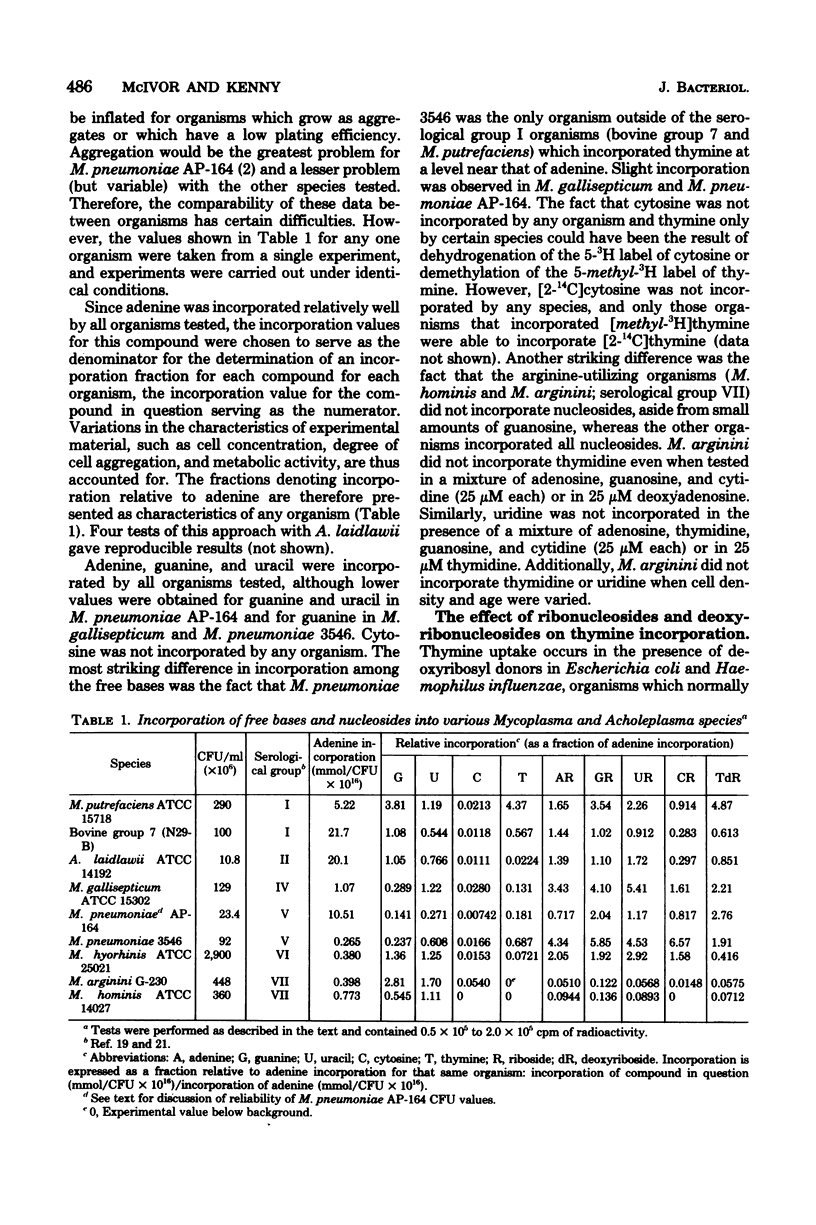
Eight species representative of the serological diversity of the Mycoplasmatales were tested for their ability to incorporate radiolabeled nucleic acid precursors into acid-insoluble material. Cultures in complex growth medium were centrifuged and resuspended in minimal essential medium (Eagle). For Acholeplasma laidlawii, labeling occurred mainly during the first 4 h of incubation, with substrate saturation at 20 micron. All organisms tested incorporated uracil, adenine, and guanine; none incorporated cytosine. Thymine was incorporated only by bovine group 7, Mycoplasma putrefaciens, and Mycoplasma pneumoniae (strain 3546), but deoxynucleosides enhanced thymine incorporation in A. laidlawii, Mycoplasma gallisepticum, M. pneumoniae (strain AP-164), and Mycoplasma hyorhinis. Nucleoside incorporation (adenosine, guanosine, uridine, cytidine, and thymidine) was not observed for the arginine-utilizing species, Mycoplasma hominis and Mycoplasma arginini, whereas all other organisms tested incorporated nucleosides. The incorporation pattern provides additional metabolic evidence to support the biochemical and antigenic diversity of these organisms. The recognition of differences in incorporation of nucleic acid precursors is important not only to the specific labeling of these organisms, but also to the study of metabolism and transport.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boatman E. S., Kenny G. E. Morphology and ultrastructure of Mycoplasma pneumoniae spherules. J Bacteriol. 1971 Jun;106(3):1005–1015. doi: 10.1128/jb.106.3.1005-1015.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmody J. M., Herriott R. M. Thymine and thymidine uptake by Haemophilus influenzae and the labeling of deoxyribonucleic acid. J Bacteriol. 1970 Feb;101(2):525–530. doi: 10.1128/jb.101.2.525-530.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- ENNY G. E., GRAYSTON J. T. EATON PLEUROPNEUMONIA-LIKE ORGANISM (MYCOPLASMA PNEUMONIAE) COMPLEMENT-FIXING ANTIGEN: EXTRACTION WITH ORGANIC SOLVENTS. J Immunol. 1965 Jul;95:19–25. [PubMed] [Google Scholar]
- Fenske J. D., Kenny G. E. Role of arginine deiminase in growth of Mycoplasma hominis. J Bacteriol. 1976 Apr;126(1):501–510. doi: 10.1128/jb.126.1.501-510.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAKALA M. T., HOLLAND J. F., HOROSZEWICZ J. S. Change in pyrimidine deoxyribonucleoside metabolism in cell culture caused by Mycoplasma (PPLO) contamination. Biochem Biophys Res Commun. 1963 Jun 20;11:466–471. doi: 10.1016/0006-291x(63)90094-9. [DOI] [PubMed] [Google Scholar]
- Hartzman R. J., Bach M. L., Bach F. H., Thurman G. B., Sell K. W. Precipitation of radioactively labeled samples: a semi-automatic multiple-sample processor. Cell Immunol. 1972 Jun;4(2):182–186. doi: 10.1016/0008-8749(72)90018-4. [DOI] [PubMed] [Google Scholar]
- Hatanaka M., Del Giudice R., Long C. Adenine formation from adenosine by mycoplasmas: adenosine phosphorylase activity. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1401–1405. doi: 10.1073/pnas.72.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K. F., Leer J. C., Nygaard P. Thymine utilization in Escherichia coli K12 on the role of deoxyribose 1-phosphate and thymidine phosphorylase. Eur J Biochem. 1973 Dec 17;40(2):345–354. doi: 10.1111/j.1432-1033.1973.tb03203.x. [DOI] [PubMed] [Google Scholar]
- Kenny G. E. Heat-lability and organic solvent-solubility of mycoplasma antigens. Ann N Y Acad Sci. 1967 Jul 28;143(1):676–681. doi: 10.1111/j.1749-6632.1967.tb27713.x. [DOI] [PubMed] [Google Scholar]
- Kenny G. E. Serological comparison of ten glycolytic Mycoplasma species. J Bacteriol. 1969 Jun;98(3):1044–1055. doi: 10.1128/jb.98.3.1044-1055.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu Y., Tanaka K. A showdomycin-resistant mutant of Escherichia coli K-12 with altered nucleoside transport character. Biochim Biophys Acta. 1972 Nov 2;288(2):390–403. doi: 10.1016/0005-2736(72)90260-x. [DOI] [PubMed] [Google Scholar]
- Levine E. M. Mycoplasma contamination of animal cell cultures: a simple, rapid detection method. Exp Cell Res. 1972 Sep;74(1):99–109. doi: 10.1016/0014-4827(72)90484-3. [DOI] [PubMed] [Google Scholar]
- Long C. W., DelGiudice R., Gardella R. S., Hatanaka M. Uracil phosphoribosyl transferase activity of mycoplasma and infected cell cultures. In Vitro. 1977 Jul;13(7):429–433. doi: 10.1007/BF02615103. [DOI] [PubMed] [Google Scholar]
- McGee Z. A., Rogul M., Wittler R. G. Molecular genetic studies of relationships among mycoplasma, L-forms and bacteria. Ann N Y Acad Sci. 1967 Jul 28;143(1):21–30. doi: 10.1111/j.1749-6632.1967.tb27639.x. [DOI] [PubMed] [Google Scholar]
- Mitchell A., Finch L. R. Pathways of nucleotide biosynthesis in Mycoplasma mycoides subsp. mycoides. J Bacteriol. 1977 Jun;130(3):1047–1054. doi: 10.1128/jb.130.3.1047-1054.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munch-Petersen A., Mygind B. Nucleoside transport systems in Escherichia coli K12: specificity and regulation. J Cell Physiol. 1976 Dec;89(4):551–559. doi: 10.1002/jcp.1040890410. [DOI] [PubMed] [Google Scholar]
- Nardone R. M., Todd J., Gonzalez P., Gaffney E. V. Nucleoside incorporation into strain L cells: inhibition by pleuropneumonia-like organisms. Science. 1965 Sep 3;149(3688):1100–1101. doi: 10.1126/science.149.3688.1100. [DOI] [PubMed] [Google Scholar]
- Parry T. E., Blackmore J. A. Serum 'uracil plus uridine' levels in normal subjects and their possible significance. J Clin Pathol. 1974 Oct;27(10):789–793. doi: 10.1136/jcp.27.10.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden K. W. A rapid and simple method for the detection of mycoplasma and other intracellular contaminants. Experientia. 1975 Sep 15;31(9):1111–1112. doi: 10.1007/BF02326990. [DOI] [PubMed] [Google Scholar]
- Perez A. G., Kim J. H., Gelbard A. S., Djordjevic B. Altered incorporation of nucleic acid precursors by mycoplasma-infected mammalian cells in culture. Exp Cell Res. 1972 Feb;70(2):301–310. doi: 10.1016/0014-4827(72)90140-1. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Richey D. P. Transport of nucleosides, nucleic acid bases, choline and glucose by animal cells in culture. Biochim Biophys Acta. 1974 Dec 16;344(3-4):263–305. doi: 10.1016/0304-4157(74)90010-0. [DOI] [PubMed] [Google Scholar]
- Quinlan D. C., Liss A., Maniloff J. Eagle's basal medium as a defined medium for Mycoplasma studies. Microbios. 1972 Sep-Oct;6(22):179–185. [PubMed] [Google Scholar]
- RAZIN S., KNIGHT B. C. A partially defined medium for the growth of Mycoplasma. J Gen Microbiol. 1960 Apr;22:492–503. doi: 10.1099/00221287-22-2-492. [DOI] [PubMed] [Google Scholar]
- Schneider E. L., Stanbridge E. J., Epstein C. J. Incorporation of 3H-uridine and 3H-uracil into RNA: a simple technique for the detection of mycoplasma contamination of cultured cells. Exp Cell Res. 1974 Mar 15;84(1):311–318. doi: 10.1016/0014-4827(74)90411-x. [DOI] [PubMed] [Google Scholar]
- Sin I. L., Finch L. R. Adenine phosphoribosyltransferase in Mycoplasma mycoides and Escherichia coli. J Bacteriol. 1972 Oct;112(1):439–444. doi: 10.1128/jb.112.1.439-444.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. W., Hanawalt P. C. Macromolecular synthesis and thymineless death in Mycoplasma laidlawii B. J Bacteriol. 1968 Dec;96(6):2066–2076. doi: 10.1128/jb.96.6.2066-2076.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanbridge E. J., Hayflick L., Perkins F. T. Modification of amino-acid concentrations induced by mycoplasmas in cell culture medium. Nat New Biol. 1971 Aug 25;232(34):242–244. doi: 10.1038/newbio232242a0. [DOI] [PubMed] [Google Scholar]
- Stanbridge E. J., Tischfield J. A., Schneider E. L. Appearance of hypoxanthine guanine phosphoribosyltransferase activity as a consequence of mycoplasma contamination. Nature. 1975 Jul 24;256(5515):329–331. doi: 10.1038/256329a0. [DOI] [PubMed] [Google Scholar]
- Stock D. A., Gentry G. A. Thymidine metabolism in Mycoplasma hominis. J Gen Microbiol. 1971 Jan;65(1):105–107. doi: 10.1099/00221287-65-1-105. [DOI] [PubMed] [Google Scholar]
- Studzinski G. P., Gierthy J. F., Cholon J. J. An autoradiographic screening test for mycoplasmal contamination of mammalian cell cultures. In Vitro. 1973 May-Jun;8(6):466–472. doi: 10.1007/BF02615948. [DOI] [PubMed] [Google Scholar]
- TOURTELLOTTE M. E., MOROWITZ H. J., KASIMER P. DEFINED MEDIUM FOR MYCOPLASMA LAIDLAWII. J Bacteriol. 1964 Jul;88:11–15. doi: 10.1128/jb.88.1.11-15.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully J. G., Barile M. F., Edward D. G., Theodore T. S., Erno H. Characterization of some caprine mycoplasmas, with proposals for new species, mycoplasma capricolum and mycoplasma putrefaciens. J Gen Microbiol. 1974 Nov;85(1):102–120. doi: 10.1099/00221287-85-1-102. [DOI] [PubMed] [Google Scholar]
- Yaguzhinskaya O. E. Detection of serum proteins in the electrophoretic patterns of total proteins of mycoplasma cells. J Hyg (Lond) 1976 Oct;77(2):189–198. doi: 10.1017/s002217240002461x. [DOI] [PMC free article] [PubMed] [Google Scholar]



