Abstract
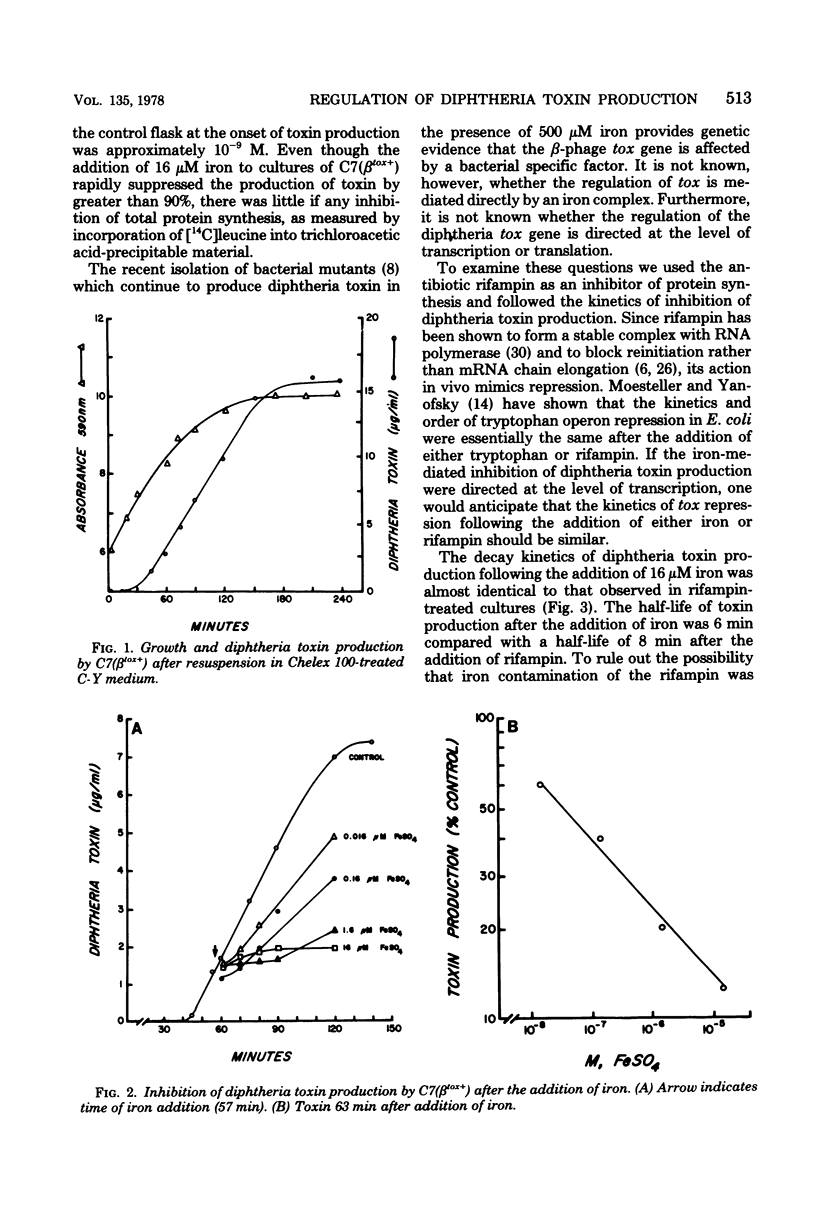
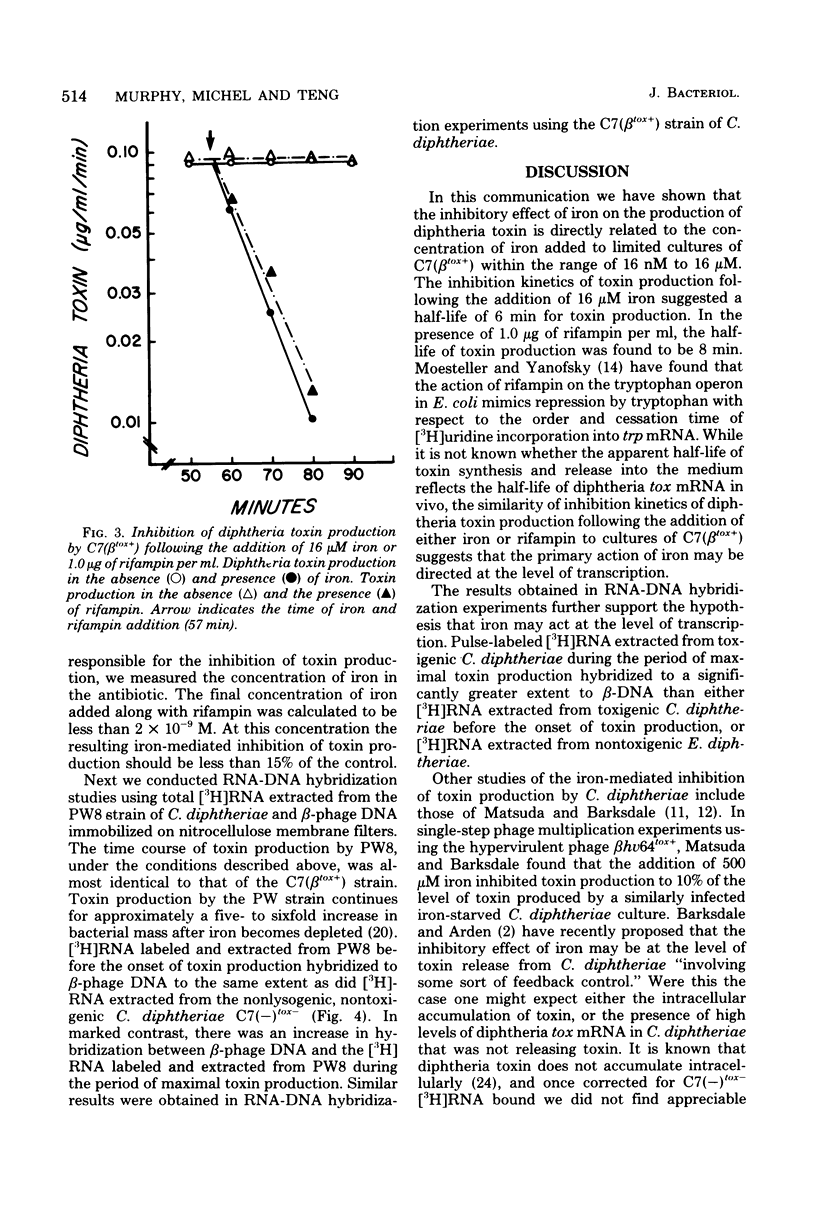
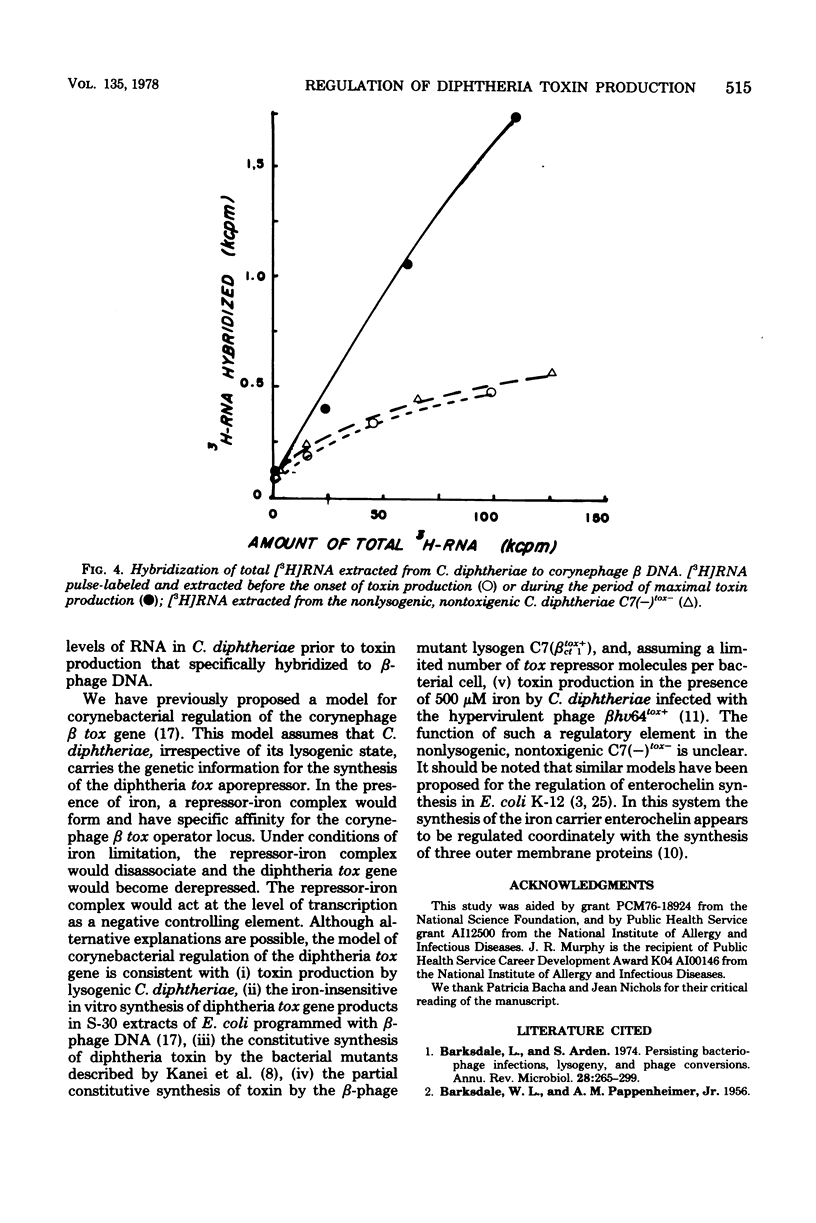
It has been known for several decades that iron inhibits the production of diphtheria toxin by Corynebacterium diphtheriae by preventing expression at maximal levels. We examined the inhibition kinetics of toxin production after the addition of either iron or rifampin to iron-limited cultures of C7 (betatox+). Iron-mediated inhibition of toxin production was found to be linear within the range of 16 nM to 16 micron. The inhibition kinetics following the addition of iron or rifampin was almost identical. [3H]RNA extracted from iron-limited toxigenic C. diphtheriae was found to hybridize to a greater extent to corynephage beta DNA than either [3H]RNA extracted from toxigenic C. diphtheriae before the onset of toxin production or [3H]RNA extracted from nonlysogenic, nontoxigenic C. diphtheriae.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barksdale L., Arden S. B. Persisting bacteriophage infections, lysogeny, and phage conversions. Annu Rev Microbiol. 1974;28(0):265–299. doi: 10.1146/annurev.mi.28.100174.001405. [DOI] [PubMed] [Google Scholar]
- Bryce G. F., Brot N. Iron transport in Escherichia coli and its relation to the repression of 2,3-dihydroxy-N-benzoyl-L-serine synthetase. Arch Biochem Biophys. 1971 Feb;142(2):399–406. doi: 10.1016/0003-9861(71)90503-0. [DOI] [PubMed] [Google Scholar]
- Gill D. M., Uchida T., Singer R. A. Expression of diphtheria toxin genes carried by integrated and nonintegrated phage beta. Virology. 1972 Dec;50(3):664–668. doi: 10.1016/0042-6822(72)90420-5. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Hartmann G., Honikel K. O., Knüsel F., Nüesch J. The specific inhibition of the DNA-directed RNA synthesis by rifamycin. Biochim Biophys Acta. 1967;145(3):843–844. doi: 10.1016/0005-2787(67)90147-5. [DOI] [PubMed] [Google Scholar]
- Holmes R. K., Barksdale L. Genetic analysis of tox+ and tox- bacteriophages of Corynebacterium diphtheriae. J Virol. 1969 Jun;3(6):586–598. doi: 10.1128/jvi.3.6.586-598.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanei C., Uchida T., Yoneda M. Isolation from corynebacterium diphtheriae C7(beta) of bacterial mutants that produce toxin in medium with excess iron. Infect Immun. 1977 Oct;18(1):203–209. doi: 10.1128/iai.18.1.203-209.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M., Barksdale L. Phage-directed synthesis of diphtherial toxin in non-toxinogenic Corynebacterium diphtheriae. Nature. 1966 May 28;210(5039):911–913. doi: 10.1038/210911a0. [DOI] [PubMed] [Google Scholar]
- Matsuda M., Barksdale L. System for the investigation of the bacteriophage-directed synthesis of diphtherial toxin. J Bacteriol. 1967 Feb;93(2):722–730. doi: 10.1128/jb.93.2.722-730.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh M. A., Earhart C. F. Coordinate regulation by iron of the synthesis of phenolate compounds and three outer membrane proteins in Escherichia coli. J Bacteriol. 1977 Jul;131(1):331–339. doi: 10.1128/jb.131.1.331-339.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosteller R. D., Yanofsky C. Transcription of the tryptophan operon in Escherichia coli: rifampicin as an inhibitor of initiation. J Mol Biol. 1970 Mar;48(3):525–531. doi: 10.1016/0022-2836(70)90064-1. [DOI] [PubMed] [Google Scholar]
- Murphy J. R., Bacha P., Teng M. Determination of Corynebacterium diphtheriae toxigenicity by a colorimetric tissue culture assay. J Clin Microbiol. 1978 Jan;7(1):91–96. doi: 10.1128/jcm.7.1.91-96.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. R., Pappenheimer A. M., Jr, de Borms S. T. Synthesis of diphtheria tox-gene products in Escherichia coli extracts. Proc Natl Acad Sci U S A. 1974 Jan;71(1):11–15. doi: 10.1073/pnas.71.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. R., Skiver J., McBride G. Isolation and partial characterization of a corynebacteriophage beta, tox operator constitutive-like mutant lysogen of Corynebacterium diphtheriae. J Virol. 1976 Apr;18(1):235–244. doi: 10.1128/jvi.18.1.235-244.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr Diphtheria toxin. Annu Rev Biochem. 1977;46:69–94. doi: 10.1146/annurev.bi.46.070177.000441. [DOI] [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr, Uchida T., Harper A. A. An immunological study of the diphtheria toxin molecule. Immunochemistry. 1972 Sep;9(9):891–906. doi: 10.1016/0019-2791(72)90163-2. [DOI] [PubMed] [Google Scholar]
- RAYNAUD M., TURPIN A., MANGALO R., BIZZINI B. Croissance et toxinogenèse. Ann Inst Pasteur (Paris) 1954 Dec;87(6):599–616. [PubMed] [Google Scholar]
- Sippel A., Hartmann G. Mode of action of rafamycin on the RNA polymerase reaction. Biochim Biophys Acta. 1968 Mar 18;157(1):218–219. doi: 10.1016/0005-2787(68)90286-4. [DOI] [PubMed] [Google Scholar]
- Uchida T., Gill D. M., Pappenheimer A. M., Jr Mutation in the structural gene for diphtheria toxin carried by temperate phage . Nat New Biol. 1971 Sep 1;233(35):8–11. doi: 10.1038/newbio233008a0. [DOI] [PubMed] [Google Scholar]
- Uchida T., Pappenheimer A. M., Jr, Greany R. Diphtheria toxin and related proteins. I. Isolation and properties of mutant proteins serologically related to diphtheria toxin. J Biol Chem. 1973 Jun 10;248(11):3838–3844. [PubMed] [Google Scholar]
- Wehrli W., Knüsel F., Schmid K., Staehelin M. Interaction of rifamycin with bacterial RNA polymerase. Proc Natl Acad Sci U S A. 1968 Oct;61(2):667–673. doi: 10.1073/pnas.61.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]



