Abstract
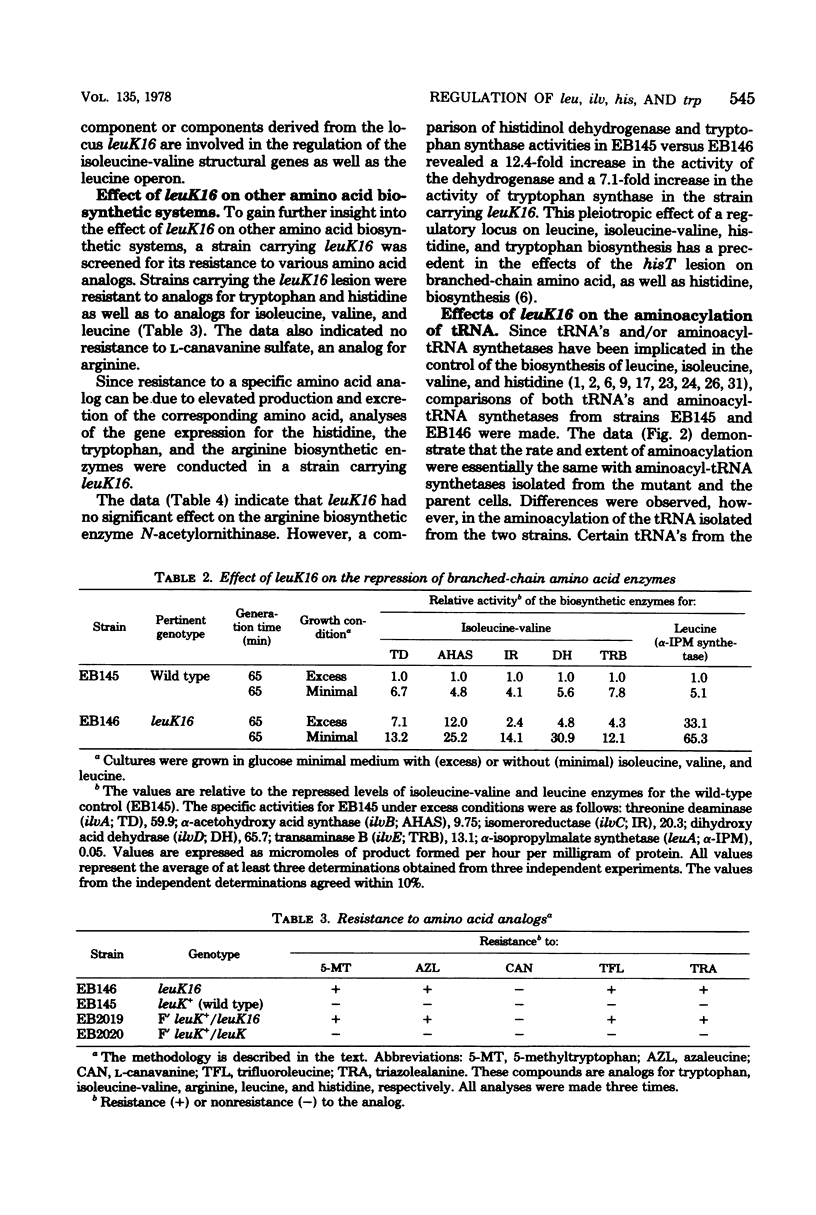
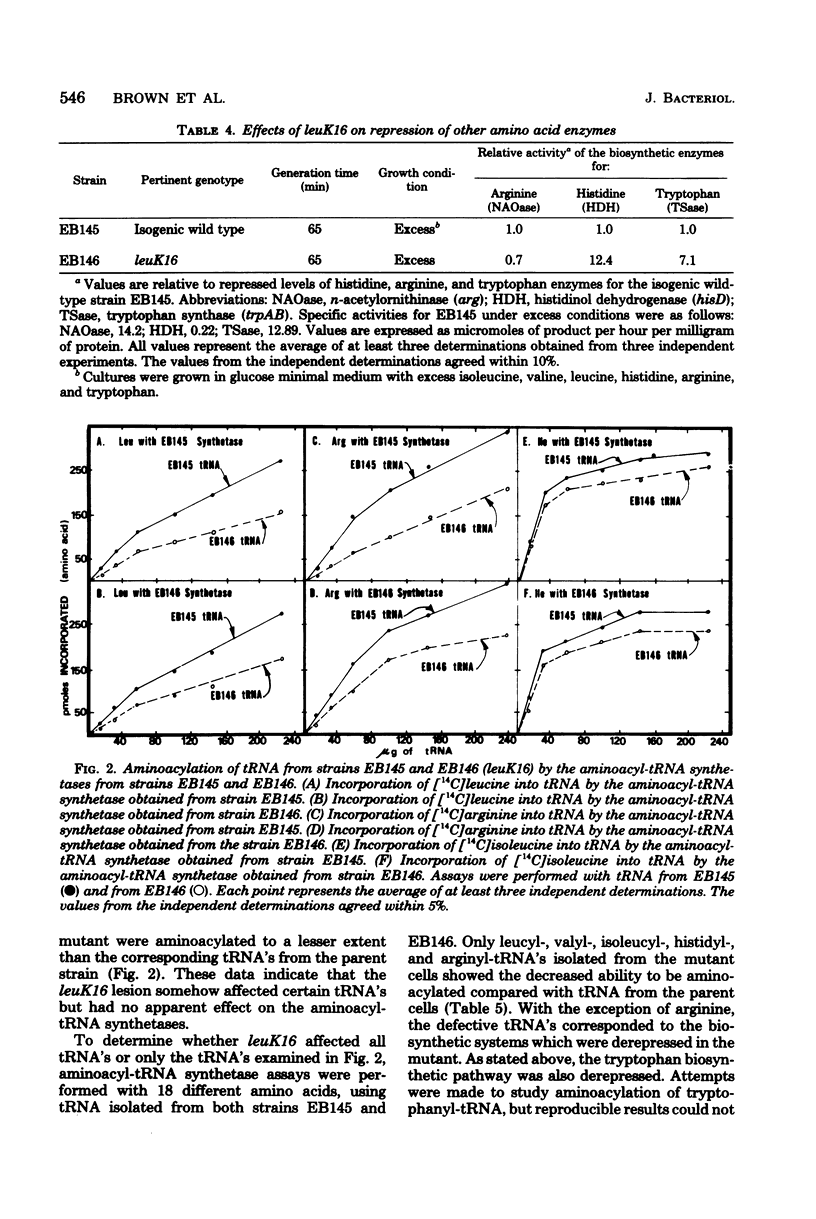
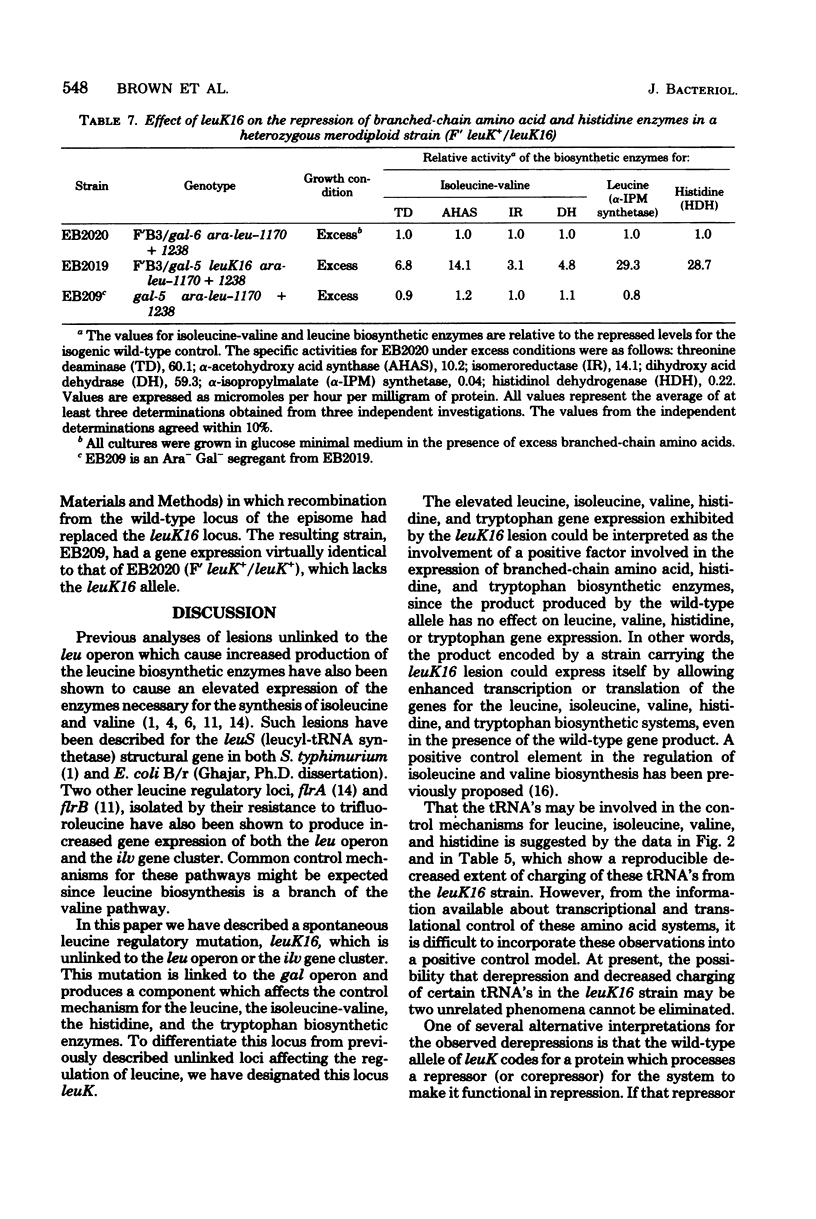
A locus (leuK) affecting regulation of the leucine operon was selected by isolating a spontaneous Ara+ derivative of an Escherichia coli B/r strain carrying an ara-leu fusion in which the arabinose operon is under leucine control. Genetic analyses by P1 transduction demonstrated that the lesion is located to the right of the galactose operon. Regulation of the biosynthetic enzymes for leucine, isoleucine-valine, histidine, and tryptophan was altered in a strain carrying leuK16. High-level gene expression in the heterozygous merodiploid strain F' leuK+/leuK16) demonstrated the dominance of the mutant allele to the wild-type allele. No apparent effect was observed in the mutant on N-acetylornithinase, a biosynthetic enzyme in the arginine pathway, nor on any of the 18 aminoacyl-tRNA synthetases examined. However, compared with that of the parent strain, the extent of the charging of leucyl-, isoleucyl-, valyl-, histidyl-, and arginyl-tRNA was decreased in the mutant.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander R. R., Calvo J. M., Freundlich M. Mutants of Salmonella typhimurium with an altered leucyl-transfer ribonucleic acid synthetase. J Bacteriol. 1971 Apr;106(1):213–220. doi: 10.1128/jb.106.1.213-220.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner M., Ames B. N. Histidine regulation in Salmonella typhimurium. IX. Histidine transfer ribonucleic acid of the regulatory mutants. J Biol Chem. 1972 Feb 25;247(4):1080–1088. [PubMed] [Google Scholar]
- Calvo J. M., Freundlich M., Umbarger H. E. Regulation of branched-chain amino acid biosynthesis in Salmonella typhimurium: isolation of regulatory mutants. J Bacteriol. 1969 Mar;97(3):1272–1282. doi: 10.1128/jb.97.3.1272-1282.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo J. M., Morgolin P., Umbarger H. E. Operator constitutive mutations in the leucine operon of Salmonella typhimurium. Genetics. 1969 Apr;61(4):777–787. doi: 10.1093/genetics/61.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese R., Landsberg R., Haar R. A., Umbarger H. E., Ames B. N. Pleiotropy of hisT mutants blocked in pseudouridine synthesis in tRNA: leucine and isoleucine-valine operons. Proc Natl Acad Sci U S A. 1974 May;71(5):1857–1861. doi: 10.1073/pnas.71.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle W. F., Yanofsky C. Mutants of Escherichia coli with an altered tryptophanyl-transfer ribonucleic acid synthetase. J Bacteriol. 1968 Apr;95(4):1283–1294. doi: 10.1128/jb.95.4.1283-1294.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan D. E., Wechsler J. A. An assay for transaminase B enzyme activity in Escherichia coli K-12. Anal Biochem. 1973 Jan;51(1):67–79. doi: 10.1016/0003-2697(73)90453-3. [DOI] [PubMed] [Google Scholar]
- EIDLIC L., NEIDHARDT F. C. ROLE OF VALYL-SRNA SYNTHETASE IN ENZYME REPRESSION. Proc Natl Acad Sci U S A. 1965 Mar;53:539–543. doi: 10.1073/pnas.53.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg D., Mikulka T. W., Jones J., Calvo J. M. flrB, a regulatory locus controlling branched-chain amino acid biosynthesis in Salmonella typhimurium. J Bacteriol. 1974 Jun;118(3):942–951. doi: 10.1128/jb.118.3.942-951.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS J., ENGLESBERG E. Determination of the order of mutational sites governing L-arabinose utilization in Escherichia coli B/r bv transduction with phage Plbt. Virology. 1959 Nov;9:314–331. doi: 10.1016/0042-6822(59)90125-4. [DOI] [PubMed] [Google Scholar]
- Kline E. L., Brown C. S., Coleman W. G., Jr, Umbarger H. E. Regulation of isoleucine-valine biosynthesis in an ilvDAC deletion strain of Escherichia coli K-12. Biochem Biophys Res Commun. 1974 Apr 23;57(4):1144–1151. doi: 10.1016/0006-291x(74)90816-x. [DOI] [PubMed] [Google Scholar]
- Kline E. L. New amino acid regulatory locus having unusual properties in heterozygous merodiploids. J Bacteriol. 1972 Jun;110(3):1127–1134. doi: 10.1128/jb.110.3.1127-1134.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levinthal M., Williams L. S., Umbarger H. E. Role of threonine deaminase in the regulation of isoleucine and valine biosynthesis. Nat New Biol. 1973 Nov 21;246(151):65–68. doi: 10.1038/newbio246065a0. [DOI] [PubMed] [Google Scholar]
- Lewis J. A., Ames B. N. Histidine regulation in Salmonella typhimurium. XI. The percentage of transfer RNA His charged in vivo and its relation to the repression of the histidine operon. J Mol Biol. 1972 Apr 28;66(1):131–142. doi: 10.1016/s0022-2836(72)80011-1. [DOI] [PubMed] [Google Scholar]
- Low B. Formation of merodiploids in matings with a class of Rec- recipient strains of Escherichia coli K12. Proc Natl Acad Sci U S A. 1968 May;60(1):160–167. doi: 10.1073/pnas.60.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse D. E., Morse A. N. Dual-control of the tryptophan operon is mediated by both tryptophanyl-tRNA synthetase and the repressor. J Mol Biol. 1976 May 15;103(2):209–226. doi: 10.1016/0022-2836(76)90310-7. [DOI] [PubMed] [Google Scholar]
- Mosteller R. D., Yanofsky C. Evidence that tryptophanyl transfer ribonucleic acid is not the corepressor of the tryptophan operon of Escherichia coli. J Bacteriol. 1971 Jan;105(1):268–275. doi: 10.1128/jb.105.1.268-275.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J. R., Ames B. N. Histidine regulatory mutants in Salmonella typhimurium II. Histidine regulatory mutants having altered histidyl-tRNA synthetase. J Mol Biol. 1966 Dec 28;22(2):325–333. doi: 10.1016/0022-2836(66)90135-5. [DOI] [PubMed] [Google Scholar]
- SCHLESINGER S., MAGASANIK B. EFFECT OF ALPHA-METHYLHISTIDINE ON THE CONTROL OF HISTIDINE SYNTHESIS. J Mol Biol. 1964 Sep;9:670–682. doi: 10.1016/s0022-2836(64)80174-1. [DOI] [PubMed] [Google Scholar]
- Sheppard D. E., Englesberg E. Further evidence for positive control of the L-arabinose system by gene araC. J Mol Biol. 1967 May 14;25(3):443–454. doi: 10.1016/0022-2836(67)90197-0. [DOI] [PubMed] [Google Scholar]
- Silbert D. F., Fink G. R., Ames B. N. Histidine regulatory mutants in Salmonella typhimurium 3. A class of regulatory mutants deficient in tRNA for histidine. J Mol Biol. 1966 Dec 28;22(2):335–347. doi: 10.1016/0022-2836(66)90136-7. [DOI] [PubMed] [Google Scholar]
- Szentirmai A., Szentirmai M., Umbarger H. E. Isoleucine and valine metabolism of Escherichia coli. XV. Biochemical properties of mutants resistant to thiaisoleucine. J Bacteriol. 1968 May;95(5):1672–1679. doi: 10.1128/jb.95.5.1672-1679.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Yang H. L., Kessler D. P. Genetic analysis of the leucine region in Escherichia coli B-r: gene-enzyme assignments. J Bacteriol. 1974 Jan;117(1):63–72. doi: 10.1128/jb.117.1.63-72.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


