Abstract
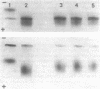
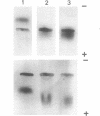
A mutant (PDs-) of Chlamydomonas reinhardi has been isolated which produces an altered neutral phosphatase. The wild-type (PDs+) and mutant (PDs-) phosphatases markedly differed in their thermosensitivities and electrophoretic mobilities. The heterozygous PDs-/PDs+ diploids produced only the wild-type electrophoretic form of the phosphatase. Mixing extracts of PDs- with extracts of various other strains in vitro resulted in the rapid transformation of the PDs- enzymic form into an enzymic variety, the properties (heat sensitivity, electrophoretic mobility) of which were similar to those of the wild-type neutral phosphatase. The results are discussed in relation to the idea that the PDs mutation is located not in the structural gene but rather in a modifying gene acting at the post-translational level.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Khan N. A., Eaton N. R. Genetic control of maltase formation in yeast. I. Strains producing high and low basal levels of enzyme. Mol Gen Genet. 1971;112(4):317–322. doi: 10.1007/BF00334433. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lalley P. A., Shows T. B. Lysosomal Acid phosphatase deficiency: liver specific variant in the mouse. Genetics. 1977 Oct;87(2):305–317. doi: 10.1093/genetics/87.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loppes R., Braipson J., Matagne R. F., Sassen A., Ledoux L. Regulation of the neutral phosphatase in Chlamydomonas reinhardi: an immunogenetic study of wild-type and mutant strains. Biochem Genet. 1977 Dec;15(11-12):1147–1157. doi: 10.1007/BF00484505. [DOI] [PubMed] [Google Scholar]
- Loppes R. Genes involved in the regulation of the neutral phosphatase in Chlamydomonas reinhardi. Mol Gen Genet. 1976 Nov 17;148(3):315–321. doi: 10.1007/BF00332906. [DOI] [PubMed] [Google Scholar]
- Loppes R., Matagne R. F. Acid phosphatase mutants in Chlamydomonas: isolation and characterization by biochemical, electrophoretic and genetic analysis. Genetics. 1973 Dec;75(4):593–604. doi: 10.1093/genetics/75.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loppes R. Release of enzymes by normal and wall-free cells of Chlamydomonas. J Bacteriol. 1976 Oct;128(1):114–116. doi: 10.1128/jb.128.1.114-116.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matagne R. F., Loppes R., Deltour R. Phosphatase of Chlamydomonas reinhardi: biochemical and cytochemical approach with specific mutants. J Bacteriol. 1976 May;126(2):937–950. doi: 10.1128/jb.126.2.937-950.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matagne R. F., Loppes R. Isolation and study of mutants lacking a derepressible phosphatase in Chlamydomonas reinhardi. Genetics. 1975 Jun;80(2):239–250. doi: 10.1093/genetics/80.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingle M., Halvorson H. O. Biochemical and genetic characterization of -glucosidase mutants in Saccharomyces lactis. J Bacteriol. 1972 Apr;110(1):196–201. doi: 10.1128/jb.110.1.196-201.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh-e A., Kakimoto S. Genes coding for the structure of the acid phosphatases in Saccharomyces cerevisiae. Mol Gen Genet. 1975 Dec 30;143(1):65–70. doi: 10.1007/BF00269421. [DOI] [PubMed] [Google Scholar]
- Yu S. A., Garrett K., Sussman A. S. Genetic control of multiple forms of trehalase in Neurospora crassa. Genetics. 1971 Aug;68(4):473–481. doi: 10.1093/genetics/68.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]