Abstract
Aspergillus nidulans gapA1, a mutation leading to compact, fluffy colonies and delayed polarity establishment, maps to a gene encoding a Ras GTPase-activating protein. Domain organization and phylogenetic analyses strongly indicate that GapA regulates one or more “true” Ras proteins. A gapAΔ strain is viable. gapA colonies are more compact than gapA1 colonies and show reduced conidiation. gapAΔ strains have abnormal conidiophores, characterized by the absence of one of the two layers of sterigmata seen in the wild type. gapA transcript levels are very low in conidia but increase during germination and reach their maximum at a time coincident with germ tube emergence. Elevated levels persist in hyphae. In germinating conidiospores, gapAΔ disrupts the normal coupling of isotropic growth, polarity establishment, and mitosis, resulting in a highly heterogeneous cell population, including malformed germlings and a class of giant cells with no germ tubes and a multitude of nuclei. Unlike wild-type conidia, gapAΔ conidia germinate without a carbon source. Giant multinucleated spores and carbon source-independent germination have been reported in strains carrying a rasA dominant active allele, indicating that GapA downregulates RasA. gapAΔ cells show a polarity maintenance defect characterized by apical swelling and subapical branching. The strongly polarized wild-type F-actin distribution is lost in gapAΔ cells. As GapA-green fluorescent protein shows cortical localization with strong predominance at the hyphal tips, we propose that GapA-mediated downregulation of Ras signaling at the plasma membrane of these tips is involved in the polarization of the actin cytoskeleton that is required for hyphal growth and, possibly, for asexual morphogenesis.
Guanine nucleotide binding protein (G-protein)-mediated signaling is one fundamental mechanism by which eukaryotic cells adapt to changing environments and extracellular signals. Monomeric, typically 21-kDa members of the Ras (rat sarcoma) superfamily (for reviews, see references 5 and 76) represent a major class of G-proteins that includes members of the Arf-Sar, Rho, Rab, Ran, and Ras families. Like the structurally related α subunits of heterotrimeric G-proteins, Ras superfamily members alternate between GTP- and GDP-bound states corresponding to different conformations. The GTP-bound conformation represents the active state, leading to productive interactions with downstream effector proteins, whereas the GDP-bound conformation represents the inactive state. Guanine nucleotide exchange factors (GEFs) bind GDP-bound G-proteins and catalyze replacement of GDP by GTP to activate the molecular switch. Conversely, as G-protein signaling terminates when bound GTP is hydrolyzed to GDP and Pi, the rate of GTP hydrolysis determines the lifetime of the activated state. Most G-proteins themselves have the ability to hydrolyze bound GTP, but their intrinsically slow GTPase activity is stimulated by GTPase-activating proteins (GAPs), which critically regulate G-protein function (68).
Potentially oncogenic mammalian Ras proteins are the founding members of the “true” Ras protein subfamily (47). Mammalian “true” Ras proteins have been intensively studied because its deregulation results in malignant cell transformation. However, “true” Ras proteins are also present in the proteomes of fungi, where they have been shown to play important roles in cell growth and morphogenesis. In Saccharomyces cerevisiae haploid cells, “true” Ras proteins respond to nutritional signals and couple cell growth to nutrient availability. While Saccharomyces cerevisiae Ras2p is involved in glucose sensing by signaling through the cyclic AMP-protein kinase A pathway, it is well established that yeast Ras proteins have morphogenetic functions, mediated by the Rho family member Cdc42 (reviewed in reference 43), which plays a key role in cell polarization through its regulation of the actin cytoskeleton organization. In diploid cells, Ras2p signals through a mitogen-activated protein kinase (MAPK) module to promote pseudohyphal growth under conditions of nutrient starvation. Signaling to the MAPK module is mediated by Cdc42p via its effector kinase, Ste20p (49). In agreement with this Ras morphogenetic role, Ho and Bretscher demonstrated the involvement of Ras in regulating the actin cytoskeleton by showing that ras2Δ results in loss of Cdc42p and F-actin polarity at 37°C (32). In turn, actin cytoskeleton dynamics and the Ras pathway are mechanistically linked via the actin regulatory factor Srv2p/cyclase-associated protein (24). In Schizosaccharomyces pombe, Ras1 mediates the pheromone-dependent mating response by signaling through a MAPK pathway and independently controls the elongated shape of fission yeast through Cdc42 because the fission yeast Cdc42 exchange factor Scd1 is a Ras1 effector (10). ras1Δ mutants and those specifically deficient in the Ras1/Scd1 pathway are abnormally round (23).
In view of the morphogenetic role that “true” Ras proteins play in these ascomycete yeasts, the involvement of Ras in filamentous, polarized fungal growth and development came as no surprise. NC-ras2 regulates hyphal growth in Neurospora crassa (37). In Ustilago maydis, expression of dominant active Ras2 or its activating GEF Sql2 promotes filamentation (50). Dominant active Ras2 promotes Magnaporthe grisea appressorium formation (58). Dominant active Candida albicans Ras1 promotes filamentation and is required for the morphogenetic switch (and thus, for pathogenicity) (41, 65). Cryptococcus neoformans Ras1, which additionally activates the pheromone response pathway, is required for filamentation and for growth at 37°C (and thus, for pathogenicity of this basidiomycete) (2).
However, our understanding of the mechanisms by which Ras regulates morphogenesis in “true” filamentous fungi is relatively limited. Filamentous fungi usually contain a pair of “true” Ras paralogues. Most filamentous ascomycetes and basidiomycetes undergo morphological switches and developmental processes involving relatively complex specialized reproductive structures, and Ras paralogues may show partially overlapping contributions to these processes. Moreover, the role of Ras proteins is not restricted to the regulation of morphogenesis. In N. crassa, the characterization of the Ras1bd dominant allele has revealed the involvement of ras1 in circadian regulation of regulated conidial formation (4). Last, polarized filamentous growth involves specialized apical growth regulatory factors that are absent from yeasts (26). One example is the presence in “true” filamentous fungi of Rac homologues. Rac, like their related monomeric G-proteins Cdc42 and Rho, is a key regulator of the actin cytoskeleton in mammalian cells (36), acting downstream of Ras (71). Filamentous fungal Rac proteins reportedly regulate polarized hyphal growth (11, 45). Penicillium marneffei CflACdc42 and CflBRac act downstream of Ras to coordinately regulate polarization in hyphae (7), and in the dimorphic yeast Yarrowia lipolytica, Rac is required for hyphal growth, but not for pseudohyphal or yeast growth (34). Notably, in C. neoformans, Cdc42 seems to mediate the function of Ras in polarity establishment and maintenance in yeast cells, while Rac1 is required for Ras-dependent filamentation, which illustrates how different morphogenetic processes are regulated by Ras acting through different pathways (51).
One additional level of complexity stems from the fact that different thresholds of Ras activity may be required at different developmental/morphogenetic processes within the same organism. In their pioneer study of “true” Ras function in filamentous fungi, Som and Kolaparti (74) demonstrated that high RasA activity is required for Aspergillus nidulans conidiospore germination but prevents the morphogenetic switch from isotropic to polarized growth. Their data additionally indicate that levels of active RasA critically contribute to the fate of aerial hyphae, such that high levels of RasA determine vegetative hyphal growth, whereas lower levels promote conidiophore development (74). Thus, understanding the role of Ras and the relative contributions of downstream Cdc42, Rac, and perhaps other Rho GTPases to filamentous fungal polarity and development clearly awaits further genetic and biochemical analyses.
Recent work in S. pombe emphasized another major aspect of Ras regulation by showing that not only the lifetime of the GTP-bound state (determined by the GAPs [see above]) but also the site at which Ras is activated determines the actual signaling. Spatially restricted Ras signaling from endomembranes determines cell morphology through Scd1-Cdc42 effectors, whereas Ras signaling at the plasma membrane through the Byr2 MAPK cascade determines mating (53).
Here we identify an A. nidulans gene involved in polarity establishment and maintenance which encodes a Ras-GAP that contributes to the normal organization of the polarized actin cytoskeleton in hyphae. Remarkably, this Ras-GAP localizes to the plasma membrane but strongly predominates in the apexes, strongly suggesting that spatially restricted downregulation of Ras at the hyphal tip is an important determinant of hyphal growth.
MATERIALS AND METHODS
Aspergillus nidulans and growth media.
Aspergillus nidulans strains are shown in Table 1. Gene symbols are defined at the http://www.gla.ac.uk/ibls/molgen/aspergillus/loci.html website. Standard complete and minimal media for A. nidulans were used (14). In minimal medium, the sole nitrogen and carbon sources were 5 mM ammonium l-(+)-tartrate and 1% (wt/vol) glucose, respectively, unless indicated otherwise.
TABLE 1.
Aspergillus nidulans strains used in this work
| Strain | Relevant genotype | Purpose |
|---|---|---|
| CS2498 | pabaA1 | Wild-type control |
| CS1717 | uapA::amdS niiA4 biA1 | Mutagenized to obtain gapA1 |
| LG10 | gapA1 prnB117 pabaA1 | Genetic mapping of gapA1 |
| FGSC A622 | pantoC3 cnxH3 sC12 | Genetic mapping of gapA1 |
| LG12 | gapA1 pantoC3 pabaA1 yA2 | Genetic mapping of gapA1 |
| LG13 | galE9 sC12 | Genetic mapping of gapA1 |
| LH11 | gapA1 uapAΔ pyrG89 azgA4 pabaA1 pyroA4 | Cloning of gapA |
| LH29 | pabaA1 pantoB100 pyrG89 | Recipient strain for deleting gapA |
| LH102 | gapAΔ::pyrG pabaA1 yA2 | gapAΔ strain for immunofluorescence |
| MAD1425 | pyrG89 pyroA4 argB2 nkuAΔ::argB+ | Recipient strain for gapA-GFP |
| MAD1768 | gapA::gfp::pyrG pyroA4 nkuAΔ::argB+ | Strain with gapA::gfp gene replaced |
Conidiation.
To measure the levels of conidiation, spores were inoculated onto minimal medium and incubated at 37°C for 8 days. Three 9.6-cm2 circles were imprinted onto the plate surface, and conidia were scraped from these areas, resuspended in 10 ml of 0.1% Tween, and counted in triplicate. The number of spores per ml was divided by 9.6 cm2 to give a value of spores per cm2, and mean values and standard errors were calculated.
Genetic methods.
Standard genetic methods were used throughout. The strains used to map gapA1 are shown in Table 1. Mutagenesis of A. nidulans strain CS1717 with N-methyl-N′nitro-N-nitrosoguanidine (13) resulted in 95% kill. Mutagenized conidiospores were plated onto medium containing uric acid (0.1 mg/ml) as the sole nitrogen source and grown at 25°C for 5 days. As strain CS1717 carries a null uapA mutation (15), utilization of uric acid depends on the UapC transporter, which is not active at 25°C (16). We attempted to obtain a mutant where the UapC transporter was active at 25°C. One colony growing above the background of the mycelia not utilizing uric acid was identified and isolated. On further testing, the isolated colony (carrying the mutation that we further characterized as gapA1) is, due to its compact morphology, an apparent and nonspecific suppressor of every mutation tested, which results in leaky, straggly growth on a given nitrogen source.
DNA and RNA manipulations.
Oligonucleotides used in this study are listed in Table S1 in the supplemental material. Total RNA was isolated from resting conidiospores, germlings, or mycelia of A. nidulans as described previously (44), treated with glyoxal, and separated on a 1% agarose gel. A PCR-amplified fragment of gapA (see Table S1 in the supplemental material) was used as a probe in Northern blots. The 3′ terminus of the gapA transcript was determined using the 5′/3′-RACE kit (Roche Diagnostic, Indianapolis, IN) using the specific oligonucleotide 3RACEgap.
Cloning the A. nidulans gapA gene.
A. nidulans LH11 was transformed with the gene library constructed in the autonomously replicating plasmid Prg3-AMA1-Not1 (54), obtained from A. Apostolaki. This plasmid contains the N. crassa pyr4 selective marker complementing the A. nidulans pyrG89 mutation. Transformed protoplasts were plated on selective (minus pyrimidine) minimal medium and incubated at 37°C for 3 days. Transformants with wild-type morphology were selected. From one of these, a plasmid able to complement the morphological phenotype caused by gapA1 was isolated (T1pl11). Primers pRG3F and pRG3R, were used to amplify the boundaries of the insert. These sequences were used to identify by BLAST analysis the cognate contig in the A. nidulans genomic database. New primers (F2, R2, F3, and R3; see Table S1 in the supplemental material) were designed and used to extend the sequence, and by reiterating this procedure, the whole insert of plasmid T1pl11 was obtained. The putative open reading frames of AN4998 and AN4997 were amplified from genomic DNA with primer pairs pRG3F/fGap and pSec/pRG3R, respectively; the complete insert was amplified with primers pRG3F/pRG3R. The three PCR products were cloned in pGEM-T Easy (Promega). Strain LH11 was cotransformed with a mix of each of the newly constructed plasmids and the integrative plasmid pPyr4, which contains the pyr4 gene of N. crassa, to determine which of the two genes complements the gapA1 phenotype.
Deletion of gapA.
The gapAΔ strain was constructed by transforming a pyrG89 pabA1 pantoB100 strain (LH29 [Table 1]) with a gene disruption cassette built by double-joint PCR (80), using Long Expand polymerase (Roche). The upstream (3,011-bp) and downstream (2,914-bp) flanking regions of gapA gene were amplified from genomic DNA using oligonucleotides DEPC1 and DEPC2 and oligonucleotides DEPC5 and DEPC6, respectively (see Table S1 in the supplemental material). The Aspergillus fumigatus pyrG gene, used to replace the complete gapA coding region, was amplified using oligonucleotides DEPC3 and DEPC4 (see Table S1 in the supplemental material). The PCR fusion product was amplified with the nested primer pair DEPC7 and DEPC8, purified with the Qiagen PCR purification kit and used in transformation. Protoplasts were plated on selective (minus pyrimidine) minimal medium and incubated at 37°C for 3 days. Nine transformants that showed the compact morphology typical of gapA1 strains were purified and analyzed by Southern blotting. In all of them, the gapA gene had been replaced by pyrG. Strains with a single integration event were selected for further analysis.
Construction of a gapA::gfp fusion strain.
To generate the gapA::gfp transformation cassette, we used a three-way, PCR-based protocol for C-terminal tagging of proteins with green fluorescent protein (GFP) (79). Primers are listed in Table S1 in the supplemental material. The GFP-pyrG cassette was amplified by PCR, using primers GapGFP3 and GapGFP4 and a plasmid containing (Gly-Ala)5-GFP plus A. fumigatus pyrG (kindly provided by S. Osmani) as a template. The second fragment, corresponding to the upstream targeting region (the 3′ end in the gapA coding region), was PCR amplified using primers GapGFP1 and GapGFP2 with genomic DNA as a template. The third amplified fragment contains the 3′ untranslated region and was amplified using primers GapGFP5 and GapGFP6. The fusion product was amplified with primers GapGFP1 and GapGFP6 and used to transform A. nidulans strain MAD1425 (see Table 1), kindly provided by B. Oakley.
Microscopic techniques.
For staining, coverslips with adhered germlings were incubated for 5 min at room temperature in a solution containing 60 ng/ml 4′,6-diamidino-2-phenylindole (DAPI) from Sigma (diluted in 50% glycerol) or 10 μg/ml Calcofluor (fluorescent brightener 28; Sigma). The coverslips were then rinsed in distilled water and mounted. When indicated, phosphate-buffered saline with 4% (vol/vol) paraformaldehyde was used to fix cells prior to staining. All experiments were repeated at least three times with essentially identical results. Immunofluorescence detection of actin was done essentially as described previously (62), using anti-actin C4 monoclonal (ICN Biomedicals Inc.) (1/500) and Alexa Fluor 568-labeled goat anti-mouse immunoglobulin G (Molecular Probes) (1/1,000) as primary and secondary antibodies, respectively. Germlings of strains expressing GapA-GFP protein were cultured on the surface of glass coverslips immersed in 2.5 ml of appropriately supplemented “watch” minimal medium (WMM) (61) containing 1% glucose (wt/vol) as the sole carbon source. Fluorescence was detected with an ORCA-ER digital camera (Hamamatsu) coupled to a Nikon E-600 microscope equipped with 60× and 100× objectives. G2-A, B-2A, and UV-2A Nikon filters were used for red, green, and blue fluorescence, respectively. The subcellular distribution of fluorescence was photographed with an ORCA-ER digital camera (Hamamatsu) driven by Metamorph (Molecular Dynamics Inc.). Conidiophore imaging was carried out essentially as described previously (56).
Phylogenetic analysis.
A multiple-sequence alignment including residues corresponding to H-Ras positions 1 through 164 in the 29 protein sequences included in the tree was constructed using Clustal W. Pairwise amino acid sequence comparisons were computed using a pairwise-distance (p-distance) model. The phylogenetic tree was constructed with Mega 4.0 (http://www.megasoftware.net) using the neighbor-joining method. All positions containing alignment gaps and missing data were eliminated only in pairwise sequence comparisons (pairwise deletion option). There were a total of 224 positions in the final data set. Bootstrap values represent data from 1,000 replicates.
RESULTS
Isolation and genetic characterization of the gapA1 mutation.
In a mutagenic screen designed to obtain mutations altered in the folding or in the expression at the plasma membrane of the UapC purine permease (see Materials and Methods), we serendipitously isolated a mutation leading to compact colony morphology, which results in nonspecific apparent suppression of mutations that lead to straggly, diffuse growth on a number of nitrogen sources (data not shown). This mutation was denoted gapA1 (see below for gene designation). The growth rate of gapA1 colonies was considerably reduced compared to the wild type. Radial colony rates that we determined on complete medium at 37°C were 0.38 ± 0.007 mm/h and 0.65 ± 0.007 mm/h in gapA1 and gapA+ strains, respectively (Fig. 1A). Notably, microscopic observation of strains carrying the gapA1 mutation showed that a proportion of germinating conidia do not give rise to a germ tube but continue growing isotropically while nuclei undergo mitosis, yielding giant, multinucleate spherical cells (see below), suggesting that polarity establishment is affected by the mutation. gapA1 segregates 1:1 in crosses, is recessive in diploids, and was mapped by mitotic haploidization to chromosome III. Meiotic crosses established linkage with cnxH (29 cM), pantoC (25 cM), and galE (8 cM) in the left arm of chromosome III. Three point crosses established that gapA is centromere distal relative to galE, in agreement with its position in contig 1.84 of chromosome III (see below).
FIG. 1.
Genetic and molecular characterization of gapA. (A) Wild-type, gapA1, and gapAΔ strains inoculated on Aspergillus complete medium and incubated for 5 days at 37°C. (B) Genetic and physical mapping of gapA. Genetic distance (in centimorgans) and physical distance (in kilobases) are shown above and below the schematically depicted chromosome III (Chr III), respectively. gapA is AN4998, galE is AN4957, and cnxH is AN4841 in the A. nidulans genomic database. pantoC has not been annotated. The pantoC-cnxH distance was independently determined to be 18 cM (3). The circle represents the centromere, and the filled bar on the left indicates the telomere. (C) Domain structure of GapA and the truncated gapA1 product. The RasGAP (hatched box) and RasGAP_C (gray box) domains correspond to SM00323 and PF03836, respectively. Deletion of four bases in the gapA1 allele (from positions 1718 to 1721 of the nucleotide sequence) results in a GapA protein truncated after residue 536 (indicated by an arrow), containing a 35-residue C-terminal peptide translated in one incorrect reading frame (filled box in the diagram and lowercase letters for amino acid single-letter codes). The asterisk denotes a stop codon. aa, amino acids.
Molecular characterization of gapA.
The phenotype of the gapA1 mutation suggested that its main defect was in the establishment and/or maintenance of polarity (see below). The gapA gene was cloned by complementation of the morphological phenotype of a pyrG89 gapA1 strain with a genomic library constructed in the replicative plasmid pRG3-AMA1-Not1 (see Materials and Methods). Four clones among the 20,000 pyrimidine-independent transformants screened showed wild-type colony morphology at 37°C. Plasmids recovered from these transformants complemented the gapA1 compact colony growth phenotype and showed overlapping restriction patterns (data not shown). Nucleotide sequencing of one such plasmid revealed that the insert corresponds to A. nidulans supercontig 1.84 that, like gapA1, maps to the left arm of chromosome III according to the Aspergillus Comparative Database (http://www.broad.mit.edu/annotation/fungi/aspergillus/). The contig 1.84 region contained in the rescued plasmid comprises nucleotides 4311570 through 437570 and includes autocalled genes AN4998 and AN4997, respectively, encoding a GTPase-activating protein and an A. nidulans homologue of S. cerevisiae SEC14. Subcloning and transformation experiments showed that the AN4998 sequence, but not the AN4997 sequence, is able to complement the gapA1 mutation.
gapA1 maps ∼118 kb centromere distal to galE (autocalled gene AN4957), which lies within contig 1.84, and ∼481 kb centromere distal from cnxH. These physical distances correspond to ∼15 kb per cM, which is larger than the values of 2 to 9 kb per cM that have been reported for right arm chromosome III markers which do not lie in the proximity of the centromere (18). Physical linkage to galE represents preliminary evidence that reversion of the compact colony phenotype did not result from extragenic multicopy suppression.
Domain organization of GapA.
The 780-residue GapA polypeptide contains N-terminal RasGAP (PF00616) and C-terminal RasGAP_C (PF03836) PFAM domains characterizing regulatory proteins that accelerate the intrinsically slow GTPase activity of Ras protein family members (Fig. 1C). GapA shares its domain organization with Schizophyllum commune Gap1, Schizosaccharomyces pombe Sar1, and Dictyostelium discoideum DdRasGAP1p, which indeed represent its closest relatives (64%, 43%, and 29% amino acid sequence identity, respectively) among functionally characterized GAP proteins (42, 70, 78). The structural bases of human H-Ras GTPase activity stimulation by a cognate GAP are well understood (69). The presence in GapA of the four conserved motifs that characterize catalytically active Ras GAPs (Fig. 2), including the “Arg finger” residue which is critical to promote GTP hydrolysis by H-Ras (69), strongly suggests that the physiological role of GapA is stimulating the GTPase activity of Ras protein(s).
FIG. 2.
Multiple-sequence alignment including representative fungal and metazoan GAP proteins. The four amino acid sequence blocks characteristic of Ras GAPs (see Fig. 3 in reference 69) are boxed. The fully conserved “Arg finger” residue that plays a critical role in catalysis is indicated by an arrow. Numbers on the right indicate amino acid residue numbering. Abbreviations and GenBank accession numbers (in parentheses) are as follows: A. nidulans GapA (AAO38800); Sp_GAP1, S. pombe Gap1 (NP_595370); Sc IRA1 and Sc IRA2, S. cerevisiae Ira1p (P18963) and Ira2p (CAA99093), respectively; dmGAP1, Drosophila melanogaster GAP1 (M86655); rrGAP1m, Rattus rattus GAP1m (BAA06398); ceGAP1, Caenorhabditis elegans GAP1 (NP_509594); HsNF1, Homo sapiens NF1 (P21359); Hs p120GAP, H. sapiens p120 GAP (P20936). Gaps introduced to maximize alignment are indicated by dashes.
gapA1 is a partial loss-of-function mutation truncating the protein upstream of the conserved RasGAP_C domain.
To confirm that we had cloned the gapA gene and not a multicopy suppressor of the gapA1 phenotype, we sequenced the gapA1 allele from three different genetic backgrounds obtained by meiotic crossing. In each case, we found a frameshifting deletion of nucleotides 1718 through 1721 in AN4998, demonstrating that the cloned gene is not an extragenic suppressor. This frameshift mutation truncates GapA after residue 536, adding a 36-residue out-of-frame amino acid sequence at the C terminus of the mutant protein (Fig. 1C). This mutation does not affect transcript stability as determined by Northern blotting (see below). As the truncating mutation removes the entire RasGAP_C domain, these data suggest that this domain plays a functionally important role in GapA function (we note that we cannot rule out the possibility that the out-of-frame C-terminal tail leads to protein instability).
A complete gapA deletion results in a more extreme phenotype than the gapA1 deletion does.
To address whether gapA1 represents a complete loss-of-function mutation, we constructed a null gapA allele after substituting its complete coding region by the A. fumigatus pyrG gene, using a transforming linear DNA fragment assembled by double-joint PCR as described in Materials and Methods. Nine pyrG+ strains showing the characteristic gapA1 compact colony phenotype were isolated. All of them carried the expected gene replacement event, as demonstrated by Southern blot analysis (data not shown). One was selected for further study. The cognate mutation will be referred to as gapAΔ. Both gapA1 and gapAΔ strains show fluffy growth on some media, noticeably on those containing ammonium as the sole nitrogen source, but the compact colony phenotype is markedly more conspicuous in gapAΔ strains than in gapA1 strains, in agreement with their relative colony growth rates (0.32 ± 0.006 mm/h in gapAΔ compared to 0.38 ± 0.007 mm/h in gapA1; this gapAΔ growth rate is less than half that of the wild type [see above]). Fluffy growth denotes an abnormally high number of aerial nonconidiating hyphae. However, this phenotype differs from that previously described for fluffy mutations (1) in that it is not invasive and the aerial nonconidiating hyphae are confined to a compact colony (not shown). Unlike the boundaries of gapA+ and gapA1 colonies, those of gapAΔ strains are irregular. gapAΔ additionally results in a ∼50% reduction in conidiospore production compared to isogenic gapA+ and gapA1 strains (7.4 × 107 ± 0.1.1 × 107 in the gapAΔ strain compared to 1.5 × 108 ± 0.22 × 108 conidiospores per cm2 in the wild type and in the gapA1 mutant). We concluded that gapA1 is a partial loss-of-function mutation, and therefore, all subsequent phenotypic analyses were carried out with gapAΔ strains.
Bioinformatic analysis of A. nidulans Ras and Ras GAPs.
In view of the fact that gapA encodes a Ras GAP, we reexamined the Ras content of A. nidulans in the context of the complete genome sequence, using TBLASTN searches to identify putative Ras family members. We confirmed the presence of two “true” Ras proteins corresponding to the previously described A. nidulans/A. fumigatus RasA and RasB (21, 22, 74) and additionally identified two as-yet-unreported proteins with significant similarity to Ras. To determine their closest fungal relatives, we carried out a phylogeny analysis, using Ras-related Rheb orthologues as an outgroup to construct the phylogenetic tree shown in Fig. 3, as Rheb proteins are proteins relatively distant to “true” Ras and the A. fumigatus Rheb orthologue has been characterized (55). RasA and RasB cluster with “true” Ras proteins, including human H-Ras (Fig. 3, branches 1 and 2). One of the two as-yet-undescribed “Ras-like” proteins is clearly grouped (branch 3) with S. cerevisiae Rsr1p (Bud1p), which is involved in bud site selection (39, 66) and apparently corresponds to the A. nidulans orthologue of N. crassa Krev-1, a Ras-like protein that does not appear to play a role in hyphal growth (35). The second protein (the AN3434 product [branch 4]) is more distantly related to RasA/B and, notably, appears to be specific of aspergilli (data not shown).
FIG. 3.
Phylogenetic analysis of Ras proteins in filamentous fungi and yeasts. “True” Ras and “Ras-like” proteins are included in clades 1 through 4 (numbers within black circles). Ras-related Rheb proteins (clade 5) were used as an outgroup. Mega 4.0 software was used for constructing the phylogenetic tree (details in Materials and Methods). The numbers beside the nodes are bootstrap values corresponding to 1,000 replicates. Hypothetical proteins are denoted by Entrez database accession numbers. In those cases where the gene/protein has been described, protein designations have been given (for all A. nidulans proteins, the systematic gene number is also included). Entrez database accession numbers for the proteins are as follows: H. sapiens p21 H-ras, P01112; S. cerevisiae Ras1, P01119; S. cerevisiae Ras2, P01120; S. cerevisiae Rhb1, P25378; S. cerevisiae Rsr1, P13856; N. crassa Ras1, CAA37612; N. crassa Ras-2, BAA03708; N. crassa NCU01444, XP_955966; N. crassa Krev-1, BAA32410; U. maydis Ras1, AAO19640; U. maydis Ras2, AAO19639; U. maydis UM02047, XP_758194; U. maydis UM05654, EAK86057; S. pombe Ras1, P08647; S. pombe Rhb1, NP_595194; Ashbya gossypii AAS51658, AAS51658; A. gossypii Rsr1p, AAS53835; M. grisea Ras2, XP_369310; M. grisea MGG06914, XP370417; M. grisea Ras1, XP_ 364654; M. grisea MGG02727, XP_366651; A. nidulans RasA, XP_657786 (AN0182); A. nidulans RasB, XP_663436 (AN5832); A. nidulans AN3434, XP_661038; A. nidulans AN4685, XP_662289; A. nidulans RhbA, XP_682137 (AN8868); P. marneffei RasA, AAO64439; C. neoformans Ras1, AAD55937; and C. neoformans Ras2, AAG10598. Sequence similarities were scored using the Blosum62 matrix. H. sapiens, Homo sapiens.
As reported previously (70), we detected only three Ras GAP-like proteins in A. nidulans. Domain organization and phylogenetic analysis clearly separate the GapA/Gap1/Sar1 fungal subfamily from the AN9463 and AN3735 products representing the two other Ras GAPs in the A. nidulans proteome (70). AN3735 contains lipid-binding C2 and RasGAP domains and is the almost certain homologue of Bud2p, the S. cerevisiae GAP for Rsr1p. Thus, in all likelihood, AN3735 is the GAP regulating the sole A. nidulans Rsr1p homologue. AN9463 contains calponin homology (CH) and IQ calmodulin binding domains, in addition to RasGAP and RasC-ter domains, making this protein a clear relative of human IQGAP1 and S. pombe Rng2, a potential effector of the Rho family of GTPases and a component of the actomyosin ring involved in cytokinesis (17). IQGAP proteins are Ras GTPase-related proteins without actual Ras GTPase activity that function as Cdc42 effectors (8, 31).
The third A. nidulans Ras GAP, GapA, is clearly the orthologue of S. pombe Sar1p (78). Sar1p is the GAP for Ras1p (31), the single fission yeast “true” Ras, whose closest homologue in A. nidulans is RasA. Thus, although we have not made any attempt to determine the specificity of GapA, it is very likely that this protein is a negative regulator of RasA and, possibly, of RasB, as GAPs show little specificity with regard to “true” Ras proteins (5).
GapA is involved in conidiophore development.
One predicted consequence of gapAΔ is Ras hyperactivation. In A. nidulans, a threshold of RasA activity is required for the initiation of conidiophore development. This activity must subsequently decrease for progression of aerial hyphae through the developmental pathway (74). In A. fumigatus, where RasA and RasB play different but overlapping developmental roles, dominant active RasA expression reduces conidiation and leads to malformed conidiophores, while expression of a dominant negative rasB allele promotes ectopic conidiophore development (21). These reports led us to examine the morphology of conidiophores, which are indeed abnormal (Fig. 4A and B). In the wild type, primary sterigmata, named metullae, bud directly from the conidiophore vesicle (Fig. 4A). These metullae give rise to a layer of secondary sterigmata, named phialides, from which conidia are originated (Fig. 4A). These two layers of sterigmata seen in the wild type are clearly absent in the mutant, where a single layer of abnormal sterigmata from which conidia bud directly was clearly observed (Fig. 4C). In some cases, it seems that cells that morphologically resemble conidia would appear to arise directly from the vesicle without the formation of metullae or phialides (Fig. 4C). However, we clearly observed in the mutant examples of abnormal, spherical sterigmata whose diameter was similar or slightly larger than that of conidiospores (Fig. 4D, mutant conidiospore showing several abnormal, spherical sterigmata; an ellipsoid sterigmata arising from the same vesicle is also indicated). The presence of these class of spherical sterigmata precluded us from reaching a firm conclusion on the possible absence of sterigmata in some mutant spore chains.
FIG. 4.
Deletion of GapA affects conidiophore development. (A) Wild-type conidiophore. v, vesicle; m, metullae; p, phialides; c, conidia. (B) Mutant conidiophore. (C) Mutant conidiospore where conidia arise from primary sterigmata (white arrowheads). A normal primary sterigmata is not discernible in the spore chain indicated with a white star. c, conidia. (D) Mutant conidiospore showing several abnormally round-shaped metullae (white stars) and an ellipsoid one (white arrow). Bars, 10 μm.
Despite the developmental effects of the gapAΔ mutation, we observed no difference in conidiospore viability between the wild type and the mutant. In the wild type, a single nucleus migrates into each bud during budding of primary sterigmata from the vesicle, and thus, daughter phialides and conidiospores are always uninucleate. However, about 6% of the gapAΔ conidiospores have two nuclei and spores with three nuclei were occasionally seen. We found no significant difference in conidiospore diameter between the wild type and the mutant when the whole population of gapAΔ conidiospores was considered (wild type, 3.5 ± 0.2 μm; gapAΔ, 3.5 ± 0.5 μm). However, gapAΔ binucleate spores were clearly larger (4.6 ± 0.6 μm in diameter; n = 15).
gapA transcript levels reach their maximum during polarity establishment.
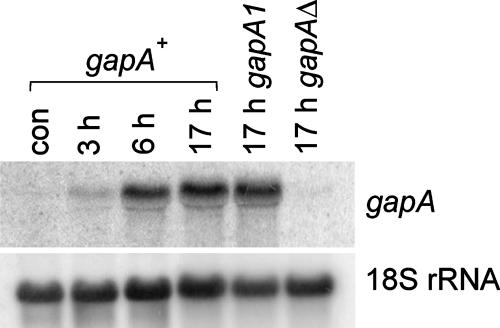
A. nidulans conidiospores contain a single nucleus arrested in G1. During the initial stages of germination, conidiospores undergo a period of isotropic growth and reenter the nuclear division cycle. In response to poorly understood signals, germinating conidiospores establish a polarity axis and switch from isotropic to polarized growth, which leads to the emergence of a germ tube (26, 28, 48, 77). Forced, high-level expression of mutant hyperactive RasAG17V in germinating conidiospores leads to large, swollen multinucleated cells that do not proceed any further (20, 74), indicating that high Ras activity impairs polarity establishment. Thus, we monitored gapA transcription in germinating conidiospores, using a synthetic minimal medium containing 10 mM ammonia and 1% glucose as the sole N and C sources, respectively. Under these conditions, germ tube emergence roughly coincides with the onset of the first mitosis (data not shown) and has essentially taken place in all germinating conidiospores after 7 h at 37°C (see also below). Figure 5 shows that gapA transcript levels, which are very low in conidiospores, increase during the isotropic growth phase (overlapping the 3-h time point) to reach their maximum at the 6-h time point (roughly corresponding to polarity establishment/germ tube emergence). Transcript levels remained essentially unchanged and unaffected by the gapA1 mutation after 17 h of incubation (Fig. 5), when germlings had already given rise to hyphae. These data, in conjunction with previous work (74), are consistent with involvement of GapA in polarity establishment and suggested that GapA might play an additional role in hyphal cells.
FIG. 5.
Transcript analysis of gapA mRNA during conidial germination. Transcript levels were determined by Northern blot analysis using an specific gapA probe and 18S rRNA as loading control. The experiment was carried out at 37°C. con, control.
Isotropic growth is uncoupled from spore polarization in a gapAΔ strain.
After a 7-h incubation under the above conditions, all wild-type germinating conidiospores (n = 130) had undergone the morphogenetic switch. These germlings contained two or four nuclei (Fig. 6A and E, wild-type strain). The even number of nuclei is in agreement with the report that the first three nuclear divisions occur simultaneously (29). In stark contrast, two classes were seen in the mutant population at the same time point. Nearly half of gapAΔ conidiospores (43%; n = 130) had germinated (“swelled”) but had not given rise to a germ tube and contained a variable number of nuclei, ranging from one to eight (Fig. 6D) (note that approximately 1/10 of these mutant swelled conidiospores have not undergone the first nuclear division). gapAΔ spores in the second class (57%) had led to a germ tube but showed delayed polarity establishment with apparent uncoupling of isotropic growth from polarity establishment and from nuclear division (Fig. 6E, gapAΔ strain). Delayed polarity establishment after an abnormally long isotropic growth was evident because the size of the mutant swelled conidiospores (6.5 ± 1.5 μm) was significantly larger than in the wild type (4.5 ± 0.3 μm), as determined using two-sample t and Wilcoxon-Mann-Whitney tests (P values of 6.1 × 10−21 and 1 × 10−12, respectively). In contrast, the mutant germ tubes were noticeably shorter (Fig. 6A and B). Finally, some mutant conidiospores did not give rise to a germ tube even after a 16-h incubation at 37°C, thus forming giant multinucleated spores resembling those described by Som and Kolaparthi (74). The diameter of these giant spores in some cases approaches 15 μm (Fig. 6C), and these cells may contain as many as 11 nuclei. Uncoupling of polarity establishment and nuclear division/migration was indicated by the broad distribution in the number of nuclei that we found in gapAΔ germlings, with a significant proportion of them (37%) having eight nuclei, many of which had not yet migrated into the germ tube (Fig. 6B and E). This was in striking contrast to the predominating single class in the wild type, containing two or four nuclei evenly spaced along the complete length of the germling (Fig. 6A and E).
FIG. 6.
Delayed polarity establishment and uncoupling of nuclear division from the morphogenetic switch. Germlings of the wild-type and gapAΔ strains were cultured in minimal medium for 7.5 h at 37°C, fixed, and stained with DAPI. (A) Wild-type germlings. The merged image of Nomarski (left) and DAPI (center) channel images is shown on the right. (B) gapAΔ strain (channels as in panel A). (C) An example of giant multinucleated spores seen with the gapAΔ strain. Bars, 10 μm. (D) Germinating gapAΔ conidiospores that had not undergone the morphogenetic switch (i.e., “isotropically growing cells” without a noticeable germ tube) at the time of the analysis were classified according to their number of nuclei. While this population involves 43% of the germinated gapAΔ spores, essentially all wild-type conidiospores have given rise to a germ tube at the sampled time point. (E) As in panel D, but including only germinated conidiospores that had given rise to a germ tube (“polarized cells”). Note the highly regular pattern observed in wild-type germlings (see panel A) and the relatively increased number of nuclei in the mutant (representing “isotropic” and “polarized” cells, denoted as total germinated conidia). gapA1 strains showed a similar phenotype.
The absence of gapA allows spore germination in the absence of carbon source.
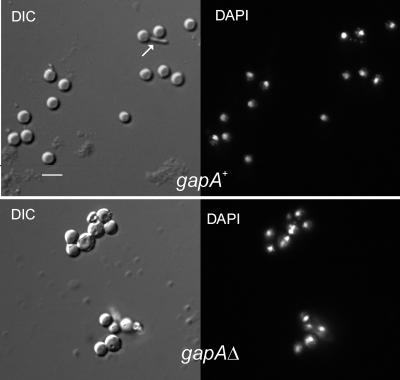
In S. cerevisiae, Ras is involved in carbon source sensing and couples cell growth to nutrient availability (for a review, see reference 67). Osherov and May (54) demonstrated that RasAG17V induces conidial germination in the absence of a carbon source. In their experiments, mutant conidia underwent swelling and arrested growth before the morphogenetic switch. We repeated these experiments using our gapAΔ allele. Both wild-type and gapAΔ conidiospores germinated in the presence of 10 mM glucose. However, while gapAΔ conidiospores underwent swelling in the absence of a carbon source, no isotropic growth was detected for the wild-type conidiospores after a 14-h incubation at 37°C under the same conditions (average diameters of gapAΔ and gapA+ conidiospores were 4.9 ± 0.9 μm and 3.5 ± 0.3 μm, respectively [Fig. 7]). In addition to conidiospore germination, gapAΔ activates the nuclear division cycle in the absence of glucose, as 30% of the swelled gapAΔ conidiospores contained two or four nuclei (Fig. 7; note that only 6% of mutant conidiospores were binucleated before incubation [see above]). These data closely resemble those obtained with RasAG17Vconidia (54) and thus strongly support our contention that RasA is a GapA substrate. We additionally observed that 5% of wild-type spores that do not swell in the absence of glucose gave rise to an abnormally narrow, abortive germ-tube primordium (Fig. 7). This observation appears to suggest that isotropic growth might not be an absolute requirement for polarity establishment/selection of a polarity axis.
FIG. 7.
Mutant gapAΔ conidiospores germinate in the absence of glucose. Conidia were fixed and stained with DAPI after 14 h of incubation at 37°C in minimal medium without a carbon source, a condition which prevents germination in the wild type (gapA+) but not in the gapAΔ mutant (gapAΔ). Nomarski and DAPI-stained images are shown. While the wild-type spores do not germinate in the absence of a carbon source, mutant spores undergo isotropic growth and activate nuclear division. We noticed that a minor proportion of wild-type spores gave rise to an abnormally slender and abortive germ tube (indicated by a white arrow in the Nomarski image). DIC, differential interference contrast. Bar, 5 μm.
GapA is required for polarity maintenance and signals to the actin cytoskeleton.
The most conspicuous phenotype of gapAΔ strains is a marked defect in polarity maintenance. Sixty-two percent of the mutant germlings (n = 117) showed morphological abnormalities (Fig. 8C to F), including an abnormal high frequency of apical branching, randomly localized increases in hyphal diameter, and loss of polarity which in some cases led to “budding yeast-like” germlings that resemble a class of abnormal cells seen in A. nidulans swoF mutants affected in conidiospore swelling (Fig. 8D) (73).
FIG. 8.
The actin cytoskeleton phenotype resulting from the gapAΔ mutation. (A) Nomarski image of a wild-type germling. (B) Example of a gapAΔ germling showing abnormal apical swelling. (C) Abnormal germling showing two germ tubes, one of which shows apical branching. (D) Abnormal, budding yeast-like germling. (E and F) Calcofluor staining of gapAΔ germlings showing the positions of the septa and abnormally swelled regions (white arrowheads in panel E), often associated with apical branching. (G and H) The wild-type actin cytoskeleton, as observed by indirect immunofluorescence using a mouse anti-actin monoclonal antibody. In panel G, the positions of two actin cables are indicated with black arrows. Actin patches predominate in the apical regions (black stars), where they lead to a much stronger signal than that of actin cables. (Note that these apical pixels become saturated when images are contrasted to observe actin cables). (I) The strong actin signal at the tip and the marked polarization of actin patches seen in the wild type are lost in mutant germlings. The black arrow indicates an actin cable. (J) An example of apical actin depolarization in a germling with an abnormally swelled tip. (K) Activation of a subapical branch leads to loss of actin polarization at the main tip. (L) Predominance of actin patches at the tip of an abnormal subapical branch; note that the main tip is clearly swelled and shows little actin polarization. Bars, 5 μm.
Germinating A. nidulans conidia typically give rise to a second germ tube after the primary polarity axis has been established. The normal pattern of germ tube emergence is dependent on the actin cytoskeleton (27). In most cases (95% for the wild type in our conditions; n = 150), the second germ tube emerges opposite or nearly opposite (∼180°) the first. In a minor proportion of wild-type germlings (5%), the second germ tube emerges at ∼90° relative to the first (quaterpolar pattern [27]). In marked contrast, 23% of the gapAΔ germlings (n = 150) showed a quaterpolar or nearly quaterpolar pattern (Fig. 8C). We additionally noticed that a proportion of gapAΔ germlings showed apical swelling, contrasting both with the wild type and with other relatively normal mutant germlings (Fig. 8A, B, and I). Both apical branching and isotropic swelling of the tip are indicative of defective organization of the actin cytoskeleton (46, 64, 75).
Ras involvement in the regulation of polarity and the actin cytoskeleton in fungi has been reported. In S. cerevisiae, Ras proteins function in the regulation of actin polarity (32) and Ras2p signals function through the key actin cytoskeleton regulator Cdc42p to induce diploid filamentous growth (49), which resembles hyphal growth in that it requires a highly polarized actin cytoskeleton (9). Thus, we examined the actin cytoskeleton of A. nidulans gapAΔ germlings using indirect immunofluorescence (Fig. 8G to L). In wild-type cells, this procedure reveals faintly labeled actin cables and strongly labeled, highly polarized actin patches (29) (L. Araujo, M. A. Peñalva, and E. Espeso, unpublished data) (Fig. 8G and H). In “normal” gapAΔ germlings, actin cables and patches were still visible (Fig. 8I and J). However, a marked loss of actin patch polarization was clearly seen in these germlings (Fig. 8I and J; compare to wild-type controls in panels G and H). To provide a semiquantitative estimation of actin polarization, we measured the relative fluorescence of a region containing the hyphal tip compared to an equivalent region at the base of the germ tube in 30 germlings from different experiments. We obtained values of 4.13 ± 1.6 and 1.8 ± 0.4 for wild-type and gapAΔ germlings, respectively. We conclude that an abnormal polarization of the actin cytoskeleton, predictably resulting from an abnormally high Ras activity, underlies the polarity maintenance defects shown by mutant germlings.
Branching was markedly more frequent in the gapAΔ mutant than in the wild type (4.8% of the mutant germlings showed branching compared to 0.7% in the wild type [n = 700]). In malformed gapAΔ germlings, abnormal apical branching associated with tip swelling was frequently observed (Fig. 8C and L). Branching in the mutant involves a complete loss of actin polarization in the tip of the main polarity axis and strong polarization of actin in the new branch tip (Fig. 8K and L), strongly suggesting that, in the gapAΔ mutant, activation of polarized growth in the branch occurs at the expense of the inactivation of the main polarity axis.
GapA-GFP localizes to the plasma membrane and is polarized in hyphae.
As the polarity establishment and maintenance roles of GapA in swelled conidiospores and hyphal tips, respectively, appear to involve regulation of the cortical actin cytoskeleton, we used a GapA-GFP fusion to determine the subcellular localization of GapA, using the gene replacement procedure reported by Yang et al. (79) to express the fusion protein at physiological levels. GapA-GFP localizes to the cytosol and to the cell periphery in swelled conidiospores, germlings, and hyphae (Fig. 9A to C). The cortical distribution showed some polarization in germlings (Fig. 9B) but was markedly polarized in hyphae (Fig. 9C), in agreement with the requirement of GapA in polarity maintenance described above. GapA-GFP additionally localized to septa (Fig. 9D). These and the above data strongly indicate that GapA is required to downregulate Ras activity at or near the plasma membrane and that this downregulation predominates at the cortical regions of hyphal tips and at septa, where the actin cytoskeleton plays major roles.
FIG. 9.
Subcellular localization of GapA-GFP. (A) Subcellular localization of GapA-GFP in young germlings, shortly after germ tube emergence. Bar, 10 μm. (B) Subcellular localization of GapA-GFP in longer germlings. Bar, 10 μm. (C) Marked polarization at the hyphal tip (GFP-stained and Nomarski images shown). Bar, 5 μm. (D) GapA-GFP localization to septa. GFP and Calcofluor images are shown. Bars, 5 μm.
DISCUSSION
This work shows that an A. nidulans Ras GAP plays an important role in polarity establishment and maintenance and is required for conidiophore development. Polarized A nidulans growth is crucially dependent on the actin and microtubule cytoskeletons (30, 33, 38, 64, 75). The actin cytoskeleton is highly polarized (29) (Araujo, Peñalva, and Espeso, unpublished). The absence of GapA results in actin cytoskeleton disorganization and depolarization defects.
In relatively young germlings, these defects correlate with an abnormally high frequency of apical branching in the proximity of an abnormally swelled tip where the actin cytoskeleton organization has been fully lost, strongly indicating that apical branching occurs as a result of loss of actin organization in the main growth axis tip. In longer hyphae, GapA deficiency results in a marked loss of F-actin polarization. In contrast, the gapA deletion has no obvious effect in microtubule organization as assessed with wild-type and mutant strains expressing a β-tubulin-GFP fusion (data not shown).
Predominance of tip swelling/apical branching in relatively young germlings suggests that accurate regulation of the actin cytoskeleton plays a more prominent role in these cells than in hyphae. Notably, Horio and Oakley have reported significant differences in growth rate and mitotic behavior of cytoplasmic microtubules between these two types of cells (33). Microtubules are not required for polarity establishment and germ tube emergence (52) but appear to be involved in focusing actin at the tips of growing hyphae (33). Thus, it is likely that apical actin organization is more sensitive to the absence of GapA function in germlings than in hyphae, where the contribution of other factors like microtubules reaching the hyphal tip might to some extent compensate for the consequences of the GapA deficiency, somehow preventing the complete disorganization of apical actin.
Bioinformatic analyses strongly indicate that GapA is a “true” Ras-specific GAP, and thus, gapAΔ would be expected to increase the activated lifetime of its Ras substrate(s), resulting in abnormally persistent signaling. Aspergilli contain two Ras genes, rasA and rasB, encoding the only “true” Ras proteins in the A. nidulans genome and the major candidates to mediate the physiological role of GapA. A. nidulans rasB has not yet been characterized. However, the findings that A. nidulans rasA is essential (74) whereas A. fumigatus rasB is not (22) and that, in A. fumigatus, rasA is expressed at relatively high levels whereas expression of rasB is very low (21) would suggest that RasA plays a more important physiological role.
Several arguments strongly suggest that GapA plays its physiological roles by regulating at least RasA. (i) gapAΔ promotes vegetative growth of aerial hyphae and reduces the production of conidiospores, resembling the consequences of expressing A. nidulans dominant active RasA at moderate levels (74). (ii) In A. fumigatus, expression of dominant active RasA at physiological levels leads to reduced growth and conidiation, promotes the formation of aerial hyphae, and results in malformed conidiophores, affecting the morphology of sterigmata. These phenotypes are strikingly similar to those of A. nidulans gapAΔ. (iii) In P. marneffei, RasA regulates polarized hyphal growth (7). (iv) During A. nidulans germination, morphogenesis is coordinated with nuclear division (25). One phenotypic consequence of gapAΔ is the uncoupling of germ tube emergence from nuclear division, such that mutant germlings contain, on average, more nuclei than the wild type. A similar phenotype has been observed in A. fumigatus after expression of a dominant active RasA protein at physiological levels (21). (v) We show that gapAΔ conidia are able to germinate in the absence of a carbon source. According to Osherov and May (54), this is a distinctive phenotype of A. nidulans conidia expressing high levels of dominant active RasA. Notably, this phenotype is also shown by A. fumigatus conidia expressing physiological levels of dominant active RasA (21). (vi) Finally, in both A. nidulans (20, 54, 74) and A. fumigatus (21), the formation of giant multinucleated spores resulting from delayed polarity establishment that we observed in gapAΔ conidia is a typical phenotype of conidia expressing dominant active RasA. However, despite the above evidence that GapA acts, at least in part, by regulating Ras activity, we cannot exclude the possibility that GapA has Ras-independent functions, like those played by the mammalian p120 Ras GAP in the regulation of cell motility (40).
GapA-GFP shows cytosolic and cortical localization, at or very near the plasma membrane. However, while GapA does not appear to be polarized in very young germlings, it becomes polarized in longer germlings and polarization is very conspicuous in hyphal cells, in agreement with its involvement in polarity maintenance and with the depolarization of the actin cytoskeleton seen in gapAΔ strains. GapA has no evident membrane-targeting domains, suggesting that it may be recruited to its cortical localization indirectly, perhaps by its cognate Ras. Ras is known to be targeted to the endoplasmic reticulum, Golgi bodies, and plasma membranes trough posttranslational modification (12), but the subcellular localization of A. nidulans RasA and RasB remains to be investigated.
A. nidulans GapA localization to a crescent at the hyphal tip and to septa resembles the localization of the single A. nidulans formin and tropomyosin proteins, respectively, denoted SepA and TmpA (60, 72). In S. cerevisiae, null alleles of RAS2 and TPM1 (encoding tropomyosin) are synthetically lethal, which strongly suggests that these genes are involved in a common cellular pathway.
A well understood example illustrating how a Ras family member can act directly upstream of the key actin cytoskeleton regulator Cdc42 takes place during S. cerevisiae vegetative growth, where Cdc42p recruitment/activation to the nascent bud site is directed by the bud site selection Ras-like G-protein Rsr1p/Bud1p (a Rap1A subfamily member and thus, not a “true” Ras). Cdc42p and its GEF (Cdc24p) are effectors of Rsr1-GTP (39; for reviews, see references 59 and 63). Recruitment of Cdc42p to sites of polarized growth underlies symmetry breaking/polarity establishment (81), as Cdc42p subsequently recruits the Bni1p formin to these sites to locally nucleate actin cables mediating targeted delivery of secretory vesicles (19).
In mammalian cells, “true” Ras proteins are upstream regulators of Rho family proteins, regulating the activation states of RhoA, Rac1, and Cdc42. “True” Ras effectors acting in fungal morphogenesis have been thoroughly investigated both in the yeasts S. cerevisiae (for a review, see reference 59) and S. pombe (53). It is well established that S. cerevisiae Ras2p signals through Cdc42 to promote pseudohyphal growth (43), which involves a marked polarization of the actin cytoskeleton (9). In S. cerevisiae yeast cells, Ras2p is involved in cell cycle-dependent actin polarization and in the localization of Cdc42p to the bud tips (32). Thus, Cdc42 would be a likely candidate to mediate the effects of gapAΔ on actin organization. However, unlike yeasts, filamentous fungi contain Rac homologues, and these have been shown to act in combination with Cdc42 to regulate different aspects of cell morphology in Penicillium marneffei and C. neoformans (see the introduction; also see references 6 and 51). Therefore, we anticipate that the elucidation of the specific Ras effector(s) acting at each morphogenetic or developmental decision will require intensive research combining genetic, biochemical, and (as illustrated here) subcellular localization studies.
Involvement of a Ras GAP in fungal morphogenesis was first reported for the Schizophyllum commune protein Gap1 (70). Deletion of the cognate gene mainly affects the sexual cycle, and thus, the dikaryon between two Δgap1 strains is unable to yield fertile basidiospores. However, the pleiotropic phenotype of the mutant strongly suggests that Gap1 is also involved in polarity maintenance.
Spatial regulation of Ras during fungal morphogenesis has been reported for Ras1 of S. pombe, where the Efc25 GEF regulates Cdc42-dependent Ras signaling from endomembranes to determine elongated cell morphology, whereas the Ste6 GEF regulates MAPK-dependent Ras signaling from the plasma membrane to control mating (53, 57). The subcellular localization of GapA in growing hyphae strongly suggests that downregulation of Ras signaling in a plasma membrane domain located at the hyphal tip plays an important role in polarized hyphal growth. Such local downregulation of Ras by GAP would provide a second way by which spatially restricted Ras signaling might be achieved.
ADDENDUM IN PROOF
A recent paper (A. Virag, M. P. Lee, H. Si, and S. D. Harris, Mol. Microbiol. 66:1569-1596, 2007) addresses the role of Cdc42 and Rac proteins in the regulation of A. nidulans hyphal morphogenesis.
Supplementary Material
Acknowledgments
We thank Ricardo Ehrlich, Alberto Rosa, and Ana Ramón for support and laboratory facilities in Oruguay; Elena Reoyo for technical assistance; Eduardo Espeso and Herb Arst for critical reading of the manuscript; Javier Valdez-Taubas and Christine Drevet for helpful discussions; Lidia Araujo and Olga Rodríguez-Galán for help with actin immunofluorescence detection; Bernard Labedan and Jorge Graneri for help with phylogeny and statistical analyses, respectively; Stephen Osmani and Berl Oakley for plasmids and strains; and one anonymous referee for helpful suggestions.
This work was supported by the DGCYT (Spain) through grant BIO2006-0556 to M.A.P., the International Foundation for Science (Stockholm, Sweden) through a grant to L.G. (Uruguay), and the French CNRS, the Université Paris-Sud, and the Institut Universitaire de France through grants to C.S. Cooperation between the Université Paris-Sud and the Universidad de la República was supported by ECOS-Sud project 00B01 to L.G. and C.S. L.H. was partially supported by the Direction des Rélations Internacionales (Université Paris-Sud) and by the Agencia Española de Cooperación Internacional (Spain).
Footnotes
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Adams, T. H., J. K. Wieser, and J. H. Yu. 1998. Asexual sporulation in Aspergillus nidulans. Microbiol. Mol. Biol. Rev. 6235-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alspaugh, J. A., L. M. Cavallo, J. R. Perfect, and J. Heitman. 2000. RAS1 regulates filamentation, mating and growth at high temperature of Cryptococcus neoformans. Mol. Microbiol. 36352-365. [DOI] [PubMed] [Google Scholar]
- 3.Arst, H. N., Jr. 1978. GABA transaminase provides an alternative route of beta-alanine synthesis in Aspergillus nidulans. Mol. Gen. Genet. 16323-27. [DOI] [PubMed] [Google Scholar]
- 4.Belden, W. J., L. F. Larrondo, A. C. Froehlich, M. Shi, C. H. Chen, J. J. Loros, and J. C. Dunlap. 2007. The band mutation in Neurospora crassa is a dominant allele of ras-1 implicating RAS signaling in circadian output. Genes Dev. 211494-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boguski, M. S., and F. McCormick. 1993. Proteins regulating Ras and its relatives. Nature 366643-654. [DOI] [PubMed] [Google Scholar]
- 6.Boyce, K. J., M. J. Hynes, and A. Andrianopoulos. 2003. Control of morphogenesis and actin localization by the Penicillium marneffei RAC homolog. J. Cell Sci. 1161249-1260. [DOI] [PubMed] [Google Scholar]
- 7.Boyce, K. J., M. J. Hynes, and A. Andrianopoulos. 2005. The Ras and Rho GTPases genetically interact to co-ordinately regulate cell polarity during development in Penicillium marneffei. Mol. Microbiol. 551487-1501. [DOI] [PubMed] [Google Scholar]
- 8.Brill, S., S. Li, C. W. Lyman, D. M. Church, J. J. Wasmuth, L. Weissbach, A. Bernards, and A. J. Snijders. 1996. The Ras GTPase-activating-protein-related human protein IQGAP2 harbors a potential actin binding domain and interacts with calmodulin and Rho family GTPases. Mol. Cell. Biol. 164869-4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cali, B. M., T. C. Doyle, D. Botstein, and G. R. Fink. 1998. Multiple functions for actin during filamentous growth of Saccharomyces cerevisiae. Mol. Biol. Cell 91873-1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang, E. C., M. Barr, Y. Wang, V. Jung, H. P. Xu, and M. H. Wigler. 1994. Cooperative interaction of S. pombe proteins required for mating and morphogenesis. Cell 79131-141. [DOI] [PubMed] [Google Scholar]
- 11.Chen, C., and M. B. Dickman. 2004. Dominant active Rac and dominant negative Rac revert the dominant active Ras phenotype in Colletotrichum trifolii by distinct signalling pathways. Mol. Microbiol. 511493-1507. [DOI] [PubMed] [Google Scholar]
- 12.Choy, E., V. K. Chiu, J. Silletti, M. Feoktistov, T. Morimoto, D. Michaelson, I. E. Ivanov, and M. R. Philips. 1999. Endomembrane trafficking of ras: the CAAX motif targets proteins to the ER and Golgi. Cell 9869-80. [DOI] [PubMed] [Google Scholar]
- 13.Clutterbuck, A. J., and U. Sinha. 1966. N-methyl-N′-nitro-N-nitrosoguanidine (NTG) as a mutagen for Aspergillus nidulans. Aspergillus Newsl. 7 12-13. [Google Scholar]
- 14.Cove, D. J. 1966. The induction and repression of nitrate reductase in the fungus Aspergillus nidulans. Biochim. Biophys. Acta 11351-56. [DOI] [PubMed] [Google Scholar]
- 15.Diallinas, G., and C. Scazzocchio. 1989. A gene coding for the uric acid-xanthine permease of Aspergillus nidulans: inactivational cloning, characterization and sequence of a cis-acting mutation. Genetics 122341-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diallinas, G., J. Valdez, V. Sophianopoulou, A. Rosa, and C. Scazzocchio. 1998. Chimeric purine transporters of Aspergillus nidulans define a domain critical for function and specificity conserved in bacterial, plant and metazoan homologues. EMBO J. 173827-3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eng, K., N. I. Naqvi, K. C. Wong, and M. K. Balasubramanian. 1998. Rng2p, a protein required for cytokinesis in fission yeast, is a component of the actomyosin ring and the spindle pole body. Curr. Biol. 8611-621. [DOI] [PubMed] [Google Scholar]
- 18.Espeso, E. A., L. Cobeño, and H. N. Arst, Jr. 2005. Discrepancies between recombination frequencies and physical distances in Aspergillus nidulans: implications for gene identification. Genetics 171835-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evangelista, M., D. Pruyne, D. C. Amberg, C. Boone, and A. Bretscher. 2002. Formins direct Arp2/3-independent actin filament assembly to polarize cell growth in yeast. Nat. Cell Biol. 4260-269. [DOI] [PubMed] [Google Scholar]
- 20.Fillinger, S., M. K. Chaveroche, K. Shimizu, N. Keller, and C. d'Enfert. 2002. cAMP and ras signalling independently control spore germination in the filamentous fungus Aspergillus nidulans. Mol. Microbiol. 441001-1016. [DOI] [PubMed] [Google Scholar]
- 21.Fortwendel, J. R., J. C. Panepinto, A. E. Seitz, D. S. Askew, and J. C. Rhodes. 2004. Aspergillus fumigatus rasA and rasB regulate the timing and morphology of asexual development. Fungal Genet. Biol. 41129-139. [DOI] [PubMed] [Google Scholar]
- 22.Fortwendel, J. R., W. Zhao, R. Bhabhra, S. Park, D. S. Perlin, D. S. Askew, and J. C. Rhodes. 2005. A fungus-specific ras homolog contributes to the hyphal growth and virulence of Aspergillus fumigatus. Eukaryot. Cell 41982-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukui, Y., T. Kozasa, Y. Kaziro, T. Takeda, and M. Yamamoto. 1986. Role of a ras homolog in the life cycle of Schizosaccharomyces pombe. Cell 44329-336. [DOI] [PubMed] [Google Scholar]
- 24.Gourlay, C. W., and K. R. Ayscough. 2006. Actin-induced hyperactivation of the Ras signaling pathway leads to apoptosis in Saccharomyces cerevisiae. Mol. Cell. Biol. 266487-6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris, S. D. 1999. Morphogenesis is coordinated with nuclear division in germinating Aspergillus nidulans conidiospores. Microbiology 1452747-2756. [DOI] [PubMed] [Google Scholar]
- 26.Harris, S. D. 2006. Cell polarity in filamentous fungi: shaping the mold. Int. Rev. Cytol. 25141-77. [DOI] [PubMed] [Google Scholar]
- 27.Harris, S. D., A. F. Hofmann, H. W. Tedford, and M. P. Lee. 1999. Identification and characterization of genes required for hyphal morphogenesis in the filamentous fungus Aspergillus nidulans. Genetics 1511015-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris, S. D., and M. Momany. 2004. Polarity in filamentous fungi: moving beyond the yeast paradigm. Fungal Genet. Biol. 41391-400. [DOI] [PubMed] [Google Scholar]
- 29.Harris, S. D., J. L. Morrell, and J. E. Hamer. 1994. Identification and characterization of Aspergillus nidulans mutants defective in cytokinesis. Genetics 136517-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris, S. D., N. D. Read, R. W. Roberson, B. Shaw, S. Seiler, M. Plamann, and M. Momany. 2005. Polarisome meets Spitzenkörper: microscopy, genetics, and genomics converge. Eukaryot. Cell 4225-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hart, M. J., M. G. Callow, B. Souza, and P. Polakis. 1996. IQGAP1, a calmodulin-binding protein with a rasGAP-related domain, is a potential effector for cdc42Hs. EMBO J. 152997-3005. [PMC free article] [PubMed] [Google Scholar]
- 32.Ho, J., and A. Bretscher. 2001. Ras regulates the polarity of the yeast actin cytoskeleton through the stress response pathway. Mol. Biol. Cell 121541-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horio, T., and B. R. Oakley. 2005. The role of microtubules in rapid hyphal tip growth of Aspergillus nidulans. Mol. Biol. Cell 16918-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hurtado, C. A., J. M. Beckerich, C. Gaillardin, and R. A. Rachubinski. 2000. A rac homolog is required for induction of hyphal growth in the dimorphic yeast Yarrowia lipolytica. J. Bacteriol. 1822376-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ito, S., Y. Matsui, A. Toh-e, T. Harashima, and H. Inoue. 1997. Isolation and characterization of the krev-1 gene, a novel member of ras superfamily in Neurospora crassa: involvement in sexual cycle progression. Mol. Gen. Genet. 255429-437. [DOI] [PubMed] [Google Scholar]
- 36.Jaffe, A. B., and A. Hall. 2005. Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 21247-269. [DOI] [PubMed] [Google Scholar]
- 37.Kana-uchi, A., C. T. Yamashiro, S. Tanabe, and T. Murayama. 1997. A ras homologue of Neurospora crassa regulates morphology. Mol. Gen. Genet. 254427-432. [DOI] [PubMed] [Google Scholar]
- 38.Konzack, S., P. E. Rischitor, C. Enke, and R. Fischer. 2005. The role of the kinesin motor KipA in microtubule organization and polarized growth of Aspergillus nidulans. Mol. Biol. Cell 16497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kozminski, K. G., L. Beven, E. Angerman, A. H. Tong, C. Boone, and H. O. Park. 2003. Interaction between a Ras and a Rho GTPase couples selection of a growth site to the development of cell polarity in yeast. Mol. Biol. Cell 144958-4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kulkarni, S. V., G. Gish, P. van der Geer, M. Henkemeyer, and T. Pawson. 2000. Role of p120 Ras-GAP in directed cell movement. J. Cell Biol. 149457-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leberer, E., D. Harcus, D. Dignard, L. Johnson, S. Ushinsky, D. Y. Thomas, and K. Schroppel. 2001. Ras links cellular morphogenesis to virulence by regulation of the MAP kinase and cAMP signalling pathways in the pathogenic fungus Candida albicans. Mol. Microbiol. 42673-687. [DOI] [PubMed] [Google Scholar]
- 42.Lee, S., R. Escalante, and R. A. Firtel. 1997. A Ras GAP is essential for cytokinesis and spatial patterning in Dictyostelium. Development 124983-996. [DOI] [PubMed] [Google Scholar]
- 43.Lengeler, K. B., R. C. Davidson, C. D'souza, T. Harashima, W.-C. Shen, P. Wang, X. Pan, M. Waugh, and J. Heitman. 2000. Signal transduction cascades regulating fungal development and virulence. Microbiol. Mol. Biol. Rev. 64746-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lockington, R., C. Scazzocchio, D. Sequeval, M. Mathieu, and B. Felenbok. 1987. Regulation of alcR, the positive regulatory gene of the ethanol utilization regulon of Aspergillus nidulans. Mol. Microbiol. 1275-281. [DOI] [PubMed] [Google Scholar]
- 45.Mahlert, M., L. Leveleki, A. Hlubek, B. Sandrock, and M. Bolker. 2006. Rac1 and Cdc42 regulate hyphal growth and cytokinesis in the dimorphic fungus Ustilago maydis. Mol. Microbiol. 59567-578. [DOI] [PubMed] [Google Scholar]
- 46.Malavazi, I., C. P. Semighini, M. R. Kress, S. D. Harris, and G. H. Goldman. 2006. Regulation of hyphal morphogenesis and the DNA damage response by the Aspergillus nidulans ATM homolog AtmA. Genetics 17399-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malumbres, M., and M. Barbacid. 2003. RAS oncogenes: the first 30 years. Nat. Rev. Cancer 3459-465. [DOI] [PubMed] [Google Scholar]
- 48.Momany, M. 2002. Polarity in filamentous fungi: establishment, maintenance and new axes. Curr. Opin. Microbiol. 5580-585. [DOI] [PubMed] [Google Scholar]
- 49.Mosch, H. U., R. L. Roberts, and G. R. Fink. 1996. Ras2 signals via the Cdc42/Ste20/mitogen-activated protein kinase module to induce filamentous growth in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 935352-5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Müller, P., J. D. Katzenberger, G. Loubradou, and R. Kahmann. 2003. Guanyl nucleotide exchange factor Sql2 and Ras2 regulate filamentous growth in Ustilago maydis. Eukaryot. Cell 2609-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nichols, C. B., Z. H. Perfect, and J. A. Alspaugh. 2007. A Ras1-Cdc24 signal transduction pathway mediates thermotolerance in the fungal pathogen Cryptococcus neoformans. Mol. Microbiol. 631118-1130. [DOI] [PubMed] [Google Scholar]
- 52.Oakley, B. R., and N. R. Morris. 1980. Nuclear movement is beta-tubulin-dependent in Aspergillus nidulans. Cell 19255-262. [DOI] [PubMed] [Google Scholar]
- 53.Onken, B., H. Wiener, M. R. Philips, and E. C. Chang. 2006. Compartmentalized signaling of Ras in fission yeast. Proc. Natl. Acad. Sci. USA 1039045-9050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Osherov, N., and G. May. 2000. Conidial germination in Aspergillus nidulans requires RAS signaling and protein synthesis. Genetics 155647-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Panepinto, J. C., B. G. Oliver, J. R. Fortwendel, D. L. Smith, D. S. Askew, and J. C. Rhodes. 2003. Deletion of the Aspergillus fumigatus gene encoding the Ras-related protein RhbA reduces virulence in a model of invasive pulmonary aspergillosis. Infect. Immun. 712819-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pantazopoulou, A., N. D. Lemuh, D. G. Hatzinikolaou, C. Drevet, G. Cecchetto, C. Scazzocchio, and G. Diallinas. 2007. Differential physiological and developmental expression of the UapA and AzgA purine transporters in Aspergillus nidulans. Fungal Genet. Biol. 44627-640. [DOI] [PubMed] [Google Scholar]
- 57.Papadaki, P., V. Pizon, B. Onken, and E. C. Chang. 2002. Two Ras pathways in fission yeast are differentially regulated by two Ras guanine nucleotide exchange factors. Mol. Cell. Biol. 224598-4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park, G., C. Xue, X. Zhao, Y. Kim, M. Orbach, and J. R. Xu. 2006. Multiple upstream signals converge on the adaptor protein Mst50 in Magnaporthe grisea. Plant Cell 182822-2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park, H. O., and E. Bi. 2007. Central roles of small GTPases in the development of cell polarity in yeast and beyond. Microbiol. Mol. Biol. Rev. 7148-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pearson, C. L., K. Xu, K. E. Sharpless, and S. D. Harris. 2004. MesA, a novel fungal protein required for the stabilization of polarity axes in Aspergillus nidulans. Mol. Biol. Cell 153658-3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peñalva, M. A. 2005. Tracing the endocytic pathway of Aspergillus nidulans with FM4-64. Fungal Genet. Biol. 42963-975. [DOI] [PubMed] [Google Scholar]
- 62.Prigozhina, N. L., R. A. Walker, C. E. Oakley, and B. R. Oakley. 2001. Gamma-tubulin and the C-terminal motor domain kinesin-like protein, KLPA, function in the establishment of spindle bipolarity in Aspergillus nidulans. Mol. Biol. Cell 123161-3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pruyne, D., A. Legesse-Miller, L. Gao, Y. Dong, and A. Bretscher. 2004. Mechanisms of polarized growth and organelle segregation in yeast. Annu. Rev. Cell Dev. Biol. 20559-591. [DOI] [PubMed] [Google Scholar]
- 64.Riquelme, M., R. Fischer, and S. Bartnicki-Garcia. 2003. Apical growth and mitosis are independent processes in Aspergillus nidulans. Protoplasma 222211-215. [DOI] [PubMed] [Google Scholar]
- 65.Rocha, C. R., K. Schroppel, D. Harcus, A. Marcil, D. Dignard, B. N. Taylor, D. Y. Thomas, M. Whiteway, and E. Leberer. 2001. Signaling through adenylyl cyclase is essential for hyphal growth and virulence in the pathogenic fungus Candida albicans. Mol. Biol. Cell 123631-3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ruggieri, R., A. Bender, Y. Matsui, S. Powers, Y. Takai, J. R. Pringle, and K. Matsumoto. 1992. RSR1, a ras-like gene homologous to Krev-1 (smg21A/rap1A): role in the development of cell polarity and interactions with the Ras pathway in Saccharomyces cerevisiae. Mol. Cell. Biol. 12758-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Santangelo, G. M. 2006. Glucose signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 70253-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scheffzek, K., and M. R. Ahmadian. 2005. GTPase activating proteins: structural and functional insights 18 years after discovery. Cell. Mol. Life Sci. 623014-3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scheffzek, K., M. R. Ahmadian, W. Kabsch, L. Wiesmuller, A. Lautwein, F. Schmitz, and A. Wittinghofer. 1997. The Ras-RasGAP complex: structural basis for GTPase activation and its loss in oncogenic Ras mutants. Science 277333-338. [DOI] [PubMed] [Google Scholar]
- 70.Schubert, D., M. Raudaskoski, N. Knabe, and E. Kothe. 2006. Ras GTPase-activating protein Gap1 of the homobasidiomycete Schizophyllum commune regulates hyphal growth orientation and sexual development. Eukaryot. Cell 5683-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Scita, G., P. Tenca, E. Frittoli, A. Tocchetti, M. Innocenti, G. Giardina, and P. P. Di Fiore. 2000. Signaling from Ras to Rac and beyond: not just a matter of GEFs. EMBO J. 192393-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sharpless, K. E., and S. D. Harris. 2002. Functional characterization and localization of the Aspergillus nidulans formin SEPA. Mol. Biol. Cell 13469-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shaw, B. D., C. Momany, and M. Momany. 2002. Aspergillus nidulans swoF encodes an N-myristoyl transferase. Eukaryot. Cell 1241-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Som, T., and V. S. R. Kolaparthi. 1994. Developmental decisions in Aspergillus nidulans are modulated by Ras activity. Mol. Cell. Biol. 145333-5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Torralba, S., M. Raudaskoski, A. M. Pedregosa, and F. Laborda. 1998. Effect of cytochalasin A on apical growth, actin cytoskeleton organization and enzyme secretion in Aspergillus nidulans. Microbiology 14445-53. [DOI] [PubMed] [Google Scholar]
- 76.Valencia, A., P. Chardin, A. Wittinghofer, and C. Sander. 1991. The ras protein family: evolutionary tree and role of conserved amino acids. Biochemistry 304637-4648. [DOI] [PubMed] [Google Scholar]
- 77.Virag, A., and S. D. Harris. 2006. The Spitzenkorper: a molecular perspective. Mycol. Res. 1104-13. [DOI] [PubMed] [Google Scholar]
- 78.Wang, Y., M. Boguski, M. Riggs, L. Rodgers, and M. Wigler. 1991. sar1, a gene from Schizosaccharomyces pombe encoding a protein that regulates ras1. Cell Regul. 2453-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang, L., L. Ukil, A. Osmani, F. Nahm, J. Davies, C. P. De Souza, X. Dou, A. Pérez-Balaguer, and S. A. Osmani. 2004. Rapid production of gene replacement constructs and generation of a green fluorescent protein-tagged centromeric marker in Aspergillus nidulans. Eukaryot. Cell 31359-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yu, J. H., Z. Hamari, K. H. Han, J. A. Seo, Y. Reyes-Domínguez, and C. Scazzocchio. 2004. Double-joint PCR: a PCR-based molecular tool for gene manipulations in filamentous fungi. Fungal Genet. Biol. 41973-981. [DOI] [PubMed] [Google Scholar]
- 81.Ziman, M., D. Preuss, J. Mulholland, J. M. O'Brien, D. Botstein, and D. I. Johnson. 1993. Subcellular localization of Cdc42p, a Saccharomyces cerevisiae GTP-binding protein involved in the control of cell polarity. Mol. Biol. Cell 41307-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.