Abstract
The process by which the intracellular parasite Toxoplasma gondii exits its host cell is central to its propagation and pathogenesis. Experimental induction of motility in intracellular parasites results in parasite egress, leading to the hypothesis that egress depends on the parasite's actin-dependent motility. Using a novel assay to monitor egress without experimental induction, we have established that inhibiting parasite motility does not block this process, although treatment with actin-disrupting drugs does delay egress. However, using an irreversible actin inhibitor, we show that this delay is due to the disruption of host cell actin alone, apparently resulting from the consequent loss of membrane tension. Accordingly, by manipulating osmotic pressure, we show that parasite egress is delayed by releasing membrane tension and promoted by increasing it. Therefore, without artificial induction, egress does not depend on parasite motility and can proceed by mechanical rupture of the host membrane.
The protozoan parasite Toxoplasma gondii is capable of infecting virtually any nucleated cell from a wide range of mammalian and avian species (11, 23). T. gondii is one of the most widespread and successful protozoan parasites among warm-blooded animals and causes a common infection in humans; it has become one of the main opportunistic pathogens for AIDS patients (27). As an obligately intracellular parasite, T. gondii must successfully enter a cell, replicate, and then exit by a process known as egress. Parasite egress results in the death of the host cell and is directly and indirectly (by the ensuing inflammatory response) responsible for major tissue damage (3). Despite the importance of egress in the survival of T. gondii and the pathology of a T. gondii infection, relatively little is known about this process.
Most studies of egress have taken advantage of the fact that T. gondii can be rapidly induced to exit its host cells through permeabilization of the host cell with detergents or bacterial toxins (2, 30) or by exposing cells and parasites to calcium ionophores (13) or dithiothreitol (40). The induced egress resulting from host cell permeabilization seems to be specifically due to the consequent loss of K+ from the host cell (30). This was demonstrated by the lack of egress when host cells were permeabilized in a buffer with a high [K+], which prevents a decrease in intracellular [K+] (30). Furthermore, the ability of host cell K+ efflux to induce egress is confirmed by the fact that treatment of infected cells with the K+ ionophore nigericin effectively causes the parasite to exit (18). Interestingly, the loss of host cell [K+] results in a rise in cytoplasmic [Ca2+] within the parasite, as measured by using the calcium indicator dye Fura-PE3(AM) for extracellular parasites whose medium was switched from a high-[K+] to a low-[K+] medium (30). How the decrease in extraparasitic [K+] is transduced to release of intraparasitic Ca2+ stores is not fully clear, but the process appears to involve the activation of a parasite phospholipase C (PLC), since the PLC inhibitor U73122 blocks permeabilization-induced egress (30). The correlation between the induction of calcium fluxes and egress is underscored by the fact that, as mentioned above, altering Ca2+ levels in the parasite and host cell directly through the use of calcium ionophores also results in rapid egress, a phenomenon known as ionophore-induced egress (IIE) (2, 13).
Both the reduction of extraparasitic [K+] and calcium fluxes within the parasite are known to activate the parasite's actin-dependent motility machinery. For instance, buffers containing K+ levels that mimic the high concentrations normally found within host cells block the motility of extracellular parasites (15, 24). This effect is reversed when [K+] is reduced to normal extracellular concentrations (15, 24). Similarly, intraparasitic calcium fluxes activate and regulate motility-related events such as secretion of adhesion molecules and cytoskeletal rearrangements (26, 44). Therefore, it is likely that the loss of K+ from the host cell and calcium ionophore treatment both induce egress by activating the motility machinery of the parasite. Indeed, motility is required for induced egress, as evidenced by the fact that egress cannot be induced by any method if the parasites are pretreated with the actin inhibitor cytochalasin D (2, 18, 30), which is a potent inhibitor of parasite motility (10, 39).
The requirement for motility and calcium fluxes during induced egress has led to the hypothesis that in some aspects egress mimics invasion (21). Time lapse video microscopy of parasites leaving their host cell upon IIE shows that instead of rupturing the host cell during egress, the parasites appear to penetrate the vacuolar membrane and come out of the host cell at discrete sites, constricting their bodies through the plasma membrane as they do during invasion (3). Interestingly, it has been shown that a parasite protein, RON4, that localizes to the constriction ring during invasion is specifically seen around the constriction of the parasite during calcium ionophore-induced egress (1). Nonetheless, these events have been reported only for induced egress, so their significance in egress that is not experimentally induced is not known.
Induced egress allows easy manipulation of the timing and synchronization of parasite egress: within minutes of host cell permeabilization or addition of a Ca2+ ionophore, virtually all parasites within a population will emerge (2, 13, 30). Consequently, induced egress has become an important model for how egress may occur under natural conditions (21). Nonetheless, from these studies it can only be concluded that K+ loss from the host cell, induction of calcium fluxes, and activation of motility are all sufficient to cause egress. Whether any of these events are required in order to initiate egress is not known. Determination of what occurs and what is required during egress has been made difficult by the fact that egress from cells in culture is highly variable in timing, typically occurring anywhere from 30 to 48 h postinvasion, and that it is asynchronous between parasites in different host cells (28; M. D. Lavine and G. Arrizabalaga, unpublished data). Thus, it is difficult to monitor egress in vitro, and as a consequence our understanding of this process is limited.
Here we use an assay to monitor the egress of T. gondii in vitro without experimental induction, and we find that, in contrast to induced egress, parasite motility is not required for noninduced egress. Instead, egress seems to proceed by a mechanism in which internal pressure generated by the parasite causes stretching and ultimate rupture of the host cell membrane.
MATERIALS AND METHODS
Parasite and host cell maintenance and reagents.
Strain RH parasites expressing green fluorescent protein (GFP) (18) were maintained by passage through human foreskin fibroblasts (HFFs) at 37°C under 5% CO2. Normal culture medium was Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum, 2 mM l-glutamine, and 100 U penicillin-100 μg streptomycin per ml.
Extracellular buffer (ECB) was 141.8 mM NaCl, 5.8 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 5.6 mM glucose, and 25 mM HEPES (pH 7.2). Intracellular buffer (ICB) was 5.8 mM NaCl, 141.8 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 5.6 mM glucose, and 25 mM HEPES (pH 7.2). BAPTA-AM (Sigma), U73122 (Sigma), cytochalasin D (MP Biomedical), mycalolide B (Calbiochem), and A23187 (Sigma) were dissolved in dimethyl sulfoxide as 50 mM, 5 mM, 50 mM, 1 mM, and 1 mM stock solutions, respectively. Lysophosphocholine 18:0 (1-lauroyl-2-hydroxy-sn-glycero-3-phosphocholine) (LPC) (Avanti Polar Lipids) was dissolved at a final concentration of 25 mM in AIM-V plus Albumax medium (Gibco) at 37°C immediately prior to its use. Stachyose (Sigma) was dissolved directly in normal culture medium at 20 mM, 40 mM, and 60 mM concentrations. Hypotonic stress medium was 75% normal growth medium and 25% H2O, 90% normal growth medium and 10% H2O, or 95% normal growth medium and 5% H2O. The osmolality of these solutions was measured using a Vapro 5520 vapor pressure osmometer (Wescor Inc.). To test the integrity of the host membrane during experiments, carboxy fluorescein succinimidyl ester (CFSE; Molecular Probes) was dissolved in dimethyl sulfoxide as a 5 mM stock and used to stain HFFs at a 10 μM final concentration according to the manufacturer's instructions.
Quantitation of egress.
A total of 1 × 105 parasites (representing a multiplicity of infection of approximately 0.8) were allowed to invade confluent cultures of HFFs in 24-well tissue culture plates for 1 h (unless otherwise indicated). At the indicated time point postinvasion, normal tissue culture medium was replaced with either normal medium plus the indicated drug, modified medium (i.e., hypotonic, hypertonic, ECB, or ICB), or normal medium plus the drug solvent only as a control. At this time, six positions per well were randomly marked using a felt-tip pen. These positions were photographed at ×400 using a Zeiss Axiovert CFL microscope. The RH-GFP parasites were readily identifiable by fluorescence microscopy. At the indicated time(s) postinvasion, the same positions were photographed again. The percentage of egress was determined by comparing the original photograph with photographs from later time points at the same position and determining how many of the original vacuoles had lysed (i.e., were no longer present as intact vacuoles in the later photographs).
Mycalolide B treatment.
For experiments in which both parasites and cells were exposed transiently to mycalolide B, we treated infected cells with 3 μM mycalolide B for 1 h starting at 30 h postinvasion. Excess drug was eliminated by washing cells five times with normal medium. Normal medium was then added to all wells, photographic images were taken of six distinct areas, and cultures were allowed to continue development. Egress was then monitored at the times indicated as described above.
To test the effect of treating host cells only with mycalolide B, HFFs were exposed to 3 μM mycalolide B for 10 min in DMEM. We then removed the drug and washed the monolayer five times with normal medium. Parasites were then allowed to invade for 1 h. Digital images of six areas were taken at 30 h postinvasion, and egress was monitored at the times indicated as described above.
Quantitation of invasion.
Parasites exiting from control and mycalolide B-treated cultures were recovered by aspiration at 72 h postinvasion and resuspended in normal culture medium. The efficiency of invasion was determined by allowing 2 × 106 parasites to invade confluent HFFs for 60 min on coverslips and then counting the number of parasites that entered cells. Since at this time point intracellular and extracellular parasites are indistinguishable, we identified those parasites outside host cells by staining with antibodies against a parasite surface antigen without permeabilization of HFFs, leaving all intracellular parasites unstained. In summary, following fixation with 4% formaldehyde, external parasites were labeled with an antibody generated in rabbits against the parasite SAG1 protein (a gift from J. Boothroyd) without permeabilization of the cells. These external parasites were visualized with an anti-rabbit immunoglobulin G secondary antibody with a red fluorescent tag (Alexa Fluor 594; Molecular Probes). Since all parasites expressed GFP, external parasites were colabeled red and green, while intracellular parasites were labeled green only. In this manner we determined the number of intracellular parasites in 20 randomly chosen fields of view for each coverslip by using a Zeiss Axiovert 40 CFL microscope (magnification, ×400). The data were expressed as a ratio of the total number of intracellular parasites that had been recovered from mycalolide B-treated cells to the number of intracellular parasites that had been recovered from untreated cultures.
Quantitation of IIE.
To monitor the effect on IIE of the exposure of both parasites and HFFs to mycalolide B, 1 × 105 parasites were allowed to invade confluent HFFs for 1 h in a 24-well plate. Wells were then washed twice in phosphate-buffered saline and were refilled with normal culture medium plus 3 μM mycalolide B or DMEM solvent (control) for 10 min. Cells were then washed three times, for 5 min each time, in normal culture medium. Cell culture wells were marked and photographed 30 h postinvasion, as in noninduced-egress assays. The parasites were then incubated at 37°C in serum-free DMEM containing 1 μM A23187 calcium ionophore for 10 min. At this point, the same positions on the wells were photographed again, and percent egress was determined as for noninduced egress. In order to assay the effect on IIE of the exposure of HFFs alone to mycalolide B, confluent HFFs in 24-well plates were incubated in normal culture medium plus 3 μM mycalolide B or DMEM solvent (control) for 10 min. Cells were washed three times, for 5 min each time, in normal culture medium, and then 1 × 105 parasites per well were allowed to invade for 1 h. Cell culture wells were then marked, parasites were exposed to ionophore, and percent egress was determined as described above.
Quantitation of the parasite division rate.
To monitor the effect of exposure of both parasites and HFFs to mycalolide B on parasite development, 1 × 105 parasites were allowed to invade confluent HFFs for 1 h in a 24-well plate. Wells were then washed twice in phosphate-buffered saline and were refilled with normal culture medium plus 3 μM mycalolide B or DMEM solvent (control) for 10 min. Cells were then washed three times, for 5 min each time, in normal culture medium. At 24 h after parasite invasion, the cells were fixed in 4% formaldehyde. The number of parasites per vacuole for a minimum of 100 randomly chosen vacuoles was then counted for each treatment by using a Nikon Eclipse 2000-5 microscope at ×1,000 magnification. In order to assay the effect of exposure of HFFs alone to mycalolide B on parasite development, confluent HFFs in 24-well plates were incubated in normal culture medium plus 3 μM mycalolide B or DMEM solvent (control) for 10 min. Cells were washed three times, for 5 min each time, in normal culture medium, and then 1 × 105 parasites per well were allowed to invade for 1 h. Parasites were then allowed to develop for 24 h, and cells were fixed and vacuoles assayed as described above.
RESULTS
Inhibitors of motility and induced egress do not block egress.
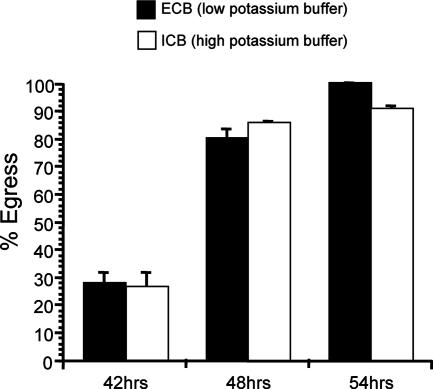
While it is clear that K+ efflux from the cell is sufficient to induce egress (18, 30), it is not clear whether it is necessary for egress. To answer this question, we developed an assay that allows us to follow the course of egress over time under different conditions. By marking exact positions on tissue culture plates and taking digital images of the same area at different time points, we can monitor the fate of individual vacuoles over time and determine the timing of egress in live cultures. Utilizing this method, we explored the effect of preventing a decrease in host cell [K+] by maintaining infected HFFs in a buffer that mimicked the high intracellular [K+] normally maintained by cells (ICB). Incubation of infected cells in ICB is known to block permeabilization-induced egress (30) and nigericin-induced egress (18). At 30 h postinvasion, we switched the cell culture medium to either ICB or a low-[K+] buffer (ECB) that allows K+ efflux, and we marked and photographed the tissue culture plates. We then photographed the same vacuoles at 42 h, 48 h, and 54 h postinvasion (12 h, 18 h, and 24 h post-buffer switch). Interestingly, we observed the same timing of egress regardless of whether the cells were maintained in ECB, which allows K+ efflux, or ICB, which does not (Fig. 1). Uninfected HFFs maintained in ICB or ECB were never lysed over the course of the experiment, indicating that lysis of host cells was not due to the action of the buffer alone but resulted from parasites exiting the cells. In addition, to verify that after 12 h in the high-[K+] ICB, K+ efflux and motility were still blocked, we tested whether egress could be induced by addition of 0.005% saponin for 10 min at 37°C, a treatment known to induce egress in a K+ efflux-dependent manner. While intracellular parasites incubated in the low-[K+] ECB showed 100% egress after saponin treatment, no egress was detected for those that had been incubated in the high-[K+] ICB for 12 h (data not shown). This result confirms that, as expected, a high extracellular [K+] blocks induced egress and that even after long incubation in the buffer, neither the cells nor the parasites can compensate for the lack of a K+ gradient, and thus, K+ efflux and motility are still blocked. Together our results suggest that while a drop in host cell cytoplasmic [K+] is sufficient to cause egress, it is not necessary for egress.
FIG. 1.
Effect of high extracellular [K+] on egress. At 30 h postinvasion, the culture medium was switched to either ECB, which allows K+ efflux, or ICB, which blocks K+ efflux. Percent egress is the percentage of lysed vacuoles at 42 h, 48 h, or 54 h postinvasion relative to the level at 30 h postinvasion. Data are means from three independent experiments. Error bars, standard deviations.
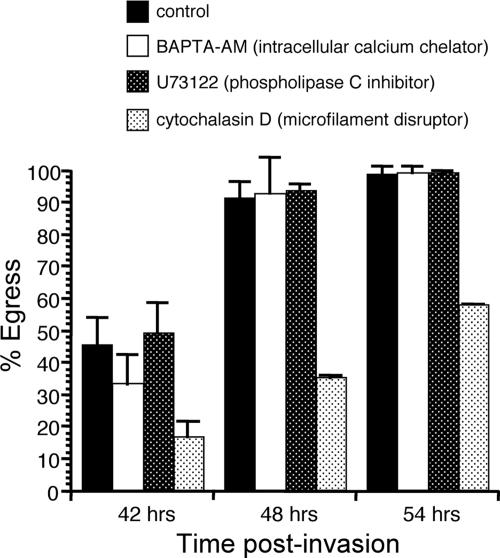
In experiments in which infected cells are incubated in ICB, K+ is maintained at a concentration that is known to be inhibitory to parasite motility (14, 24). Given that under these conditions parasites can still undergo egress, our results suggest that either the parasites do not need to become motile in order to exit their host cells or the initiation of motility during egress can occur regardless of the external [K+]. To investigate whether motility is required for egress, we tested the effects of the PLC inhibitor U73122, the intracellular Ca2+ chelator BAPTA-AM, and the actin inhibitor cytochalasin D on egress. All of these chemicals have been shown to inhibit the motility of extracellular parasites (10, 26, 30), as well as induced egress (2, 18, 30), although the target for U73122 is unclear, since it does not block the activity of a recombinant T. gondii PLC (TgPI-PLC) (17). Our overall strategy was to allow parasites to invade and replicate for 30 h, at which time point we captured images of specific vacuoles and added the particular inhibitor that was being tested. To assess the effect of the inhibitor on egress, we monitored the same vacuoles at 42, 48, and 54 h postinfection. Utilizing this assay, we observed that U73122 and BAPTA-AM did not block or delay egress relative to that of controls at any of the three time points (Fig. 2). Neither U73122 nor BAPTA-AM caused lysis of HFFs when parasites were not present, indicating that host cell lysis was a consequence of parasitism and not of direct drug action.
FIG. 2.
Effects of motility inhibitors on natural egress. At 30 h postinvasion, the normal tissue culture medium was switched to normal medium containing either 25 μM BAPTA-AM, 5 μΜ U73122, 10 μM cytochalasin D, or a solvent control. Percent egress was calculated as for other egress assays. Data are means from three independent experiments. Error bars, standard deviations.
Interestingly, disruption of actin polymerization by cytochalasin D did, however, have an effect on egress. By 42 h postinvasion (12 h post-drug treatment), cultures treated with 10 μM cytochalasin D showed only 16% egress while control cells showed 45% egress (Fig. 2). By 48 h postinvasion, cytochalasin D-treated cells showed 35% egress compared to 91% egress for control cells. By 54 h, egress levels were at 57% for cytochalasin D-exposed cells and 98% for controls. Just as with the other inhibitors used, lysis of the cells was due to parasite egress, since uninfected cells were not lysed when treated with cytochalasin D. The fact that cytochalasin D-treated cultures showed an increase in the percentage of vacuoles undergoing egress over time indicates that microfilament disruption delays, but does not completely block, egress. This result is in contrast to the effect of cytochalasin D on motility (10, 39) and on induced egress (2, 18), which is complete inhibition.
In order to verify that BAPTA-AM, U73122, and cytochalasin D remained active over the course of the experiment, at 42 h postinvasion (12 h after addition of the drugs) we added saponin to a final concentration of 0.005% and then monitored egress after 10 min of exposure at 37°C. All three treatments inhibited induced egress relative to that of controls (data not shown), indicating that the drugs were still active and could interfere with permeabilization-induced egress, as has been reported previously (2, 30). The fact that strong inhibitors of induced egress do not block noninduced egress indicates that these two phenomena have different requirements.
Mycalolide B permanently blocks parasite motility but not egress.
Although BAPTA-AM, U73122, and cytochalasin D disrupt motility in extracellular parasites (10, 26, 30), the experiments described above, showing that none of these inhibitors stops noninduced egress, do not unequivocally demonstrate that motility is not required for this process. This is because it is possible that parasites were somehow partially or fully protected from the actions of these inhibitors by virtue of the parasite's intracellular location within the parasitophorous vacuole, accounting for their ability to egress. To fully address this question, we would have to test the motility state of the parasites that undergo egress despite treatment with the inhibitors. However, given that these inhibitors are reversible, they are kept in the medium for the duration of the assay, and thus parasites are exposed to them in the ECB upon egress. Therefore, parasites would become paralyzed once outside the host cell, making it difficult to test whether the parasites were immotile during egress.
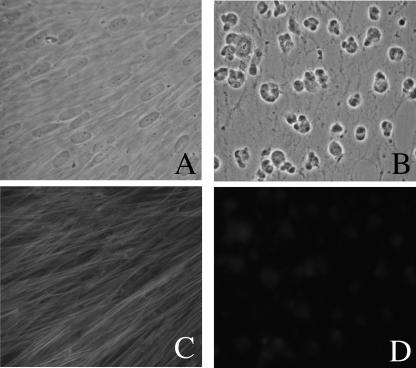
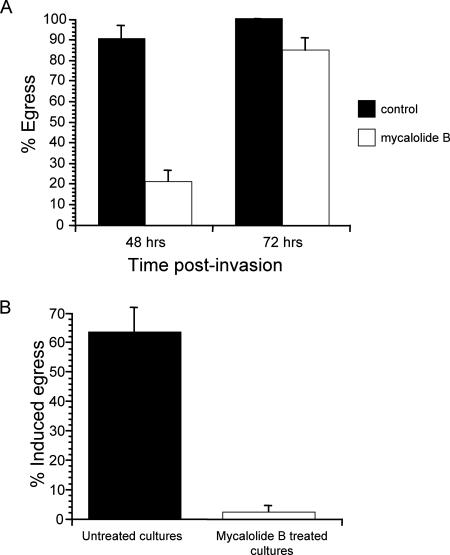
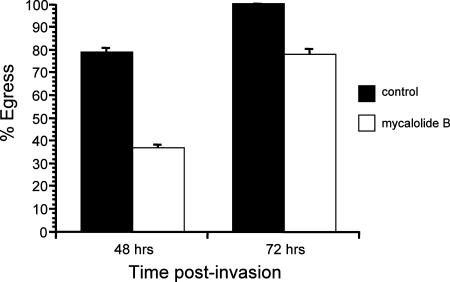
To overcome the limitation of the inhibitors described above, we have taken advantage of the actin depolymerizer mycalolide B, whose effect, unlike that of cytochalasin D, is irreversible, apparently due to covalent modification of actin (22, 35-37, 43). In fact, HFFs exposed to 3 μM mycalolide B for as little as 5 min still show complete disruption of their actin cytoskeletons 48 h later (Fig. 3). When exposed to 3 μM mycalolide B for 1 h at 30 h postinvasion, intracellular parasites can still exit their host cell at later time points (Fig. 4A). Furthermore, similar to what was observed after cytochalasin D treatment, transient exposure to mycalolide B results in a delay in egress (Fig. 4A). By 48 h postinvasion (18 h after the 1-h drug exposure), only 20% of mycalolide B-treated vacuoles had undergone egress, compared to 88% of controls. By 72 h, 81% of mycalolide B-treated vacuoles and 100% of control vacuoles had lysed.
FIG. 3.
Effect of mycalolide B on HFFs. HFFs were exposed for 5 min either to solvent alone in medium (control) or to 3 μM mycalolide B. They were then washed, and 48 h later, they were photographed. (A) Phase micrograph of control HFFs. (B) Phase micrograph of HFFs exposed to mycalolide B. (C) Phalloidin staining of cells in panel A, showing microfilaments. (D) Phalloidin staining of cells in panel B, showing continued disruption of microfilaments.
FIG. 4.
Effects of treating HFFs and parasites with mycalolide B on noninduced and induced egress. (A) Noninduced egress was monitored at 48 h and 72 h postinvasion, after exposure of infected cells to either 3 μM mycalolide B or a solvent control for 1 h starting at 30 h postinvasion. (B) Infected cells were exposed to 1 μM A23187 for 10 min, 30 h after being exposed to either 3 μM mycalolide B or DMEM solvent (control) for 10 min. Percent egress indicates the percentage of lysed vacuoles after ionophore exposure relative to the preexposure level. In both panels, data are means from three independent experiments.
In the experiment described above, parasites were exposed to mycalolide B only transiently while inside the host cell, which allows us to test whether the parasites were immotile while exiting. At 72 h, we recovered all parasites that had exited either from the untreated cultures or from the cultures that had been transiently exposed to mycalolide B 40 h prior, and we assessed whether the parasites retained their motility by testing their invasion efficiency. This assay consisted of incubating equal numbers of parasites that had exited from control or mycalolide B-treated cultures with host cells for 1 h. We then determined the number of intracellular parasites in an equal number of fields of view for both parasite samples and expressed our results as the ratio of the number of invading mycalolide B-exposed parasites to the number of invading control parasites. Since no mycalolide B-exposed parasites ever were observed to have invaded a host cell, our ratio was zero (the mean number of invading parasites per 20 fields of view for the control cells, from three independent replicates, was 118). This result confirms that parasites lose their motility when exposed intracellularly to mycalolide B and that this paralysis persists days after drug exposure. Furthermore, since these parasites were capable of exiting their host cells (Fig. 4A), our data demonstrate that egress can proceed without functional motility.
To further verify the loss of motility for parasites transiently exposed to mycalolide B within host cells, we tested them for their ability to respond to egress induction by the calcium ionophore A23187 (Fig. 4B). As mentioned above, IIE depends on parasite motility, and therefore if transient intracellular exposure to mycalolide B irreversibly disrupts motility, this treatment is expected to inhibit IIE. For this test we allowed parasites to invade host HFFs for 1 h and then exposed them to 3 μM mycalolide B for 10 min. Parasites were allowed to develop for 30 h after drug treatment and were then exposed to 1 μM A23187. After 10 min of ionophore exposure, only 2% of the mycalolide B-exposed vacuoles had undergone egress, compared to 63% of control vacuoles. This result further demonstrates that mycalolide B irreversibly disrupts parasite motility and confirms that experimentally induced egress has different requirements than noninduced egress (Fig. 4A and B).
Delay in egress upon actin disruption is due to effects on the host cell cytoskeleton.
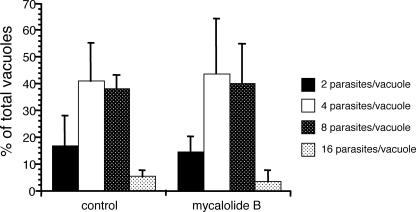
Despite the evidence that parasites can exit host cells without being motile, there is a delay in egress when parasites and cells are treated with the microfilament disruptor cytochalasin D or mycalolide B (though not for other inhibitors of induced egress and parasite motility, such as high extraparasitic [K+], BAPTA-AM, and U73122). One possible explanation for the delay in egress is that parasites divide at a lower rate upon parasite actin disruption. To explore this possibility, we determined the number of parasites per vacuole 24 h postinvasion in cells that were exposed to 3 μM mycalolide B for 1 h immediately after parasite invasion (Fig. 5). The proportion of vacuoles containing 2, 4, 8, or 16 parasites was not significantly different between the mycalolide B and control treatments. Therefore, the delay in egress caused by transient exposure to mycalolide B is not due to a disruption in parasite division.
FIG. 5.
Parasite development after transient exposure to mycalolide B. At 1 h after infection, infected cells were treated with either 3 μM mycalolide B or DMEM solvent (control) for 1 h. The number of parasites was determined for at least 100 vacuoles 23 h later. Data are expressed as the percentage of vacuoles containing the indicated number of parasites and are means from three independent experiments. Each error bar equals 1 standard deviation.
It is also possible that the delay in egress after treatment with mycalolide B could be due not to the disruption of parasite microfilaments but to the disruption of host cell microfilaments. In order to test this hypothesis, we pretreated host cells transiently with mycalolide B before parasite infection and monitored egress. In summary, HFFs were exposed to 3 μM mycalolide B for 10 min, and parasites were allowed to invade after the drug was washed off. Interestingly, these parasites, although not directly exposed to mycalolide B, showed a delay in egress relative to that of controls (Fig. 6), in a manner consistent with what was observed when both parasites and host cells had been exposed to mycalolide B after invasion (Fig. 4A). By 48 h postinvasion, 37% of vacuoles from mycalolide B-treated HFFs had exited, compared to 78% of controls. By 72 h, 78% of mycalolide B-treated vacuoles and 100% of control vacuoles had undergone egress. As with the cells exposed to mycalolide B after invasion, preinvasion exposure of HFFs to mycalolide B did not inhibit parasite division over 24 h (data not shown). Thus, disruption of host cell microfilaments does not affect the division rate of T. gondii.
FIG. 6.
Effect of disruption of host cell actin alone on parasite egress. HFFs were pretreated either with 3 μM mycalolide B or with DMEM solvent (control) for 10 min before parasites were allowed to invade. Percent egress is the percentage of lysed vacuoles at 48 h or 72 h postinvasion relative to the level at 30 h postinvasion. Data are means from three independent experiments. Each error bar equals 1 standard deviation.
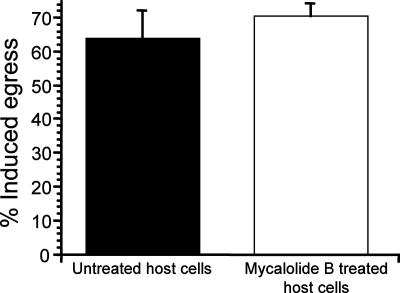
To verify that treatment of cells with mycalolide B prior to parasite infection did not disrupt the parasite's actin cytoskeleton, we examined the parasites' ability to undergo IIE. For this experiment, parasites were allowed to invade host cells that had been pretreated for 10 min with 3 μM mycalolide B. After 30 h of growth, parasites were exposed to 1 μM A23187 ionophore for 10 min. After this treatment, 63% of vacuoles in control cells had undergone egress, compared to 70% of vacuoles in mycalolide B-exposed cells (Fig. 7). This result confirmed that despite their delay in egress, parasites in the HFFs treated before invasion were not exposed to mycalolide B and possessed intact motility machinery. In addition, we exposed HFFs to 3 μM mycalolide B for 10 min, removed the drug, and then allowed parasites to invade the HFFs for 10 min. After 3 h, we recovered invading parasites by lysing the HFFs by passing them through a 27.5-gauge needle (referred to as syringe lysis). These recovered parasites were able to invade and develop in new HFFs, indicating that invasion of mycalolide B-exposed cells did not affect the parasites' ability to move and invade host cells (data not shown). In contrast, when parasites were allowed to invade HFFs for 10 min, were subsequently exposed along with host HFFs to 3 μM mycalolide B for 10 min, and were recovered by syringe lysis after 3 h, they were never able to reinvade control HFFs. Thus, this set of experiments demonstrates that disruption of the host actin cytoskeleton alone can account for the observed delay in parasite egress after treatment with microfilament disruptors.
FIG. 7.
Effect of disruption of host cell actin alone on induced egress. Parasites were allowed to infect host cells that had been pretreated with either 3 μM mycalolide B or DMEM solvent alone (control) for 10 min. At 30 h postinvasion, cultures were exposed to 1 μM A23187 for 10 min. Percent egress is the percentage of lysed vacuoles after ionophore exposure relative to the preexposure level. Data are mean percentages for three independent experiments. Each error bar equals 1 standard deviation.
Egress is delayed by hypertonic stress on the host cell and is promoted by hypotonic stress.
Disruption of the actin cytoskeleton in fibroblasts causes a loss of intracellular tension (42), and the normally flattened cells round up (Fig. 3). If parasite egress is dependent on stretching and ultimate rupture of the host cell membrane, then forces that change the tension of the host cell membrane, such as disruption of the cytoskeleton, should affect the timing of egress. Thus, it is our hypothesis that the observed delay in egress caused by disruption of the host cells' actin cytoskeleton is due to the resulting change in host cell morphology and membrane tension. To explore this possibility, we examined the effects of changing host membrane tension independently of the actin cytoskeleton. For this purpose we chose to expose infected cells to osmotic stress, since a hypertonic medium is expected to release membrane tension while a hypotonic medium would increase it.
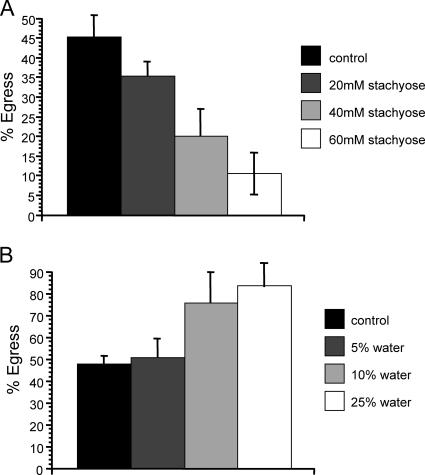
To test the effects of hypertonic stress on egress, at 30 h postinvasion the medium was switched to growth medium containing 20 mM, 40 mM, or 60 mM stachyose, which increases the solute concentration and promotes the loss of water from the cell. The molality of the growth medium with 20 mM stachyose was 344 mmol/kg, that for 40 mM stachyose was 365 mmol/kg, that for 60 mM stachyose was 381 mmol/kg, and that for normal growth medium was 326 mmol/kg. When these cells were examined at 42 h postinvasion, 43% of control vacuoles had lysed compared to 35% for 20 mM stachyose, 20% for 40 mM stachyose, and 10% for 60 mM stachyose (Fig. 8A). Again, this represented a delay, and not a block, of egress; 100% of the vacuoles in cells exposed to all the hypertonic medium concentrations had undergone egress by 72 h. Similarly, we examined the effect of hypotonic stress on egress, by switching the culture medium to medium plus 5%, 10%, or 25% H2O at 30 h postinvasion, which results in swelling of cells. The molality for the culture medium with 5% H2O was 307 mmol/kg, that for 10% H2O was 293 mmol/kg, that for 25% H2O was 243 mmol/kg, and that for normal culture medium was 326 mmol/kg. After 42 h postinvasion, vacuoles in cells exposed to the 5%, 10%, or 25% H2O medium showed 50%, 75%, or 83% egress, respectively, compared to 47% egress for control cells, demonstrating that hypotonic stress results in premature parasite egress and that this effect becomes greater as the tension is increased (Fig. 8B). This effect is not a direct effect of the hypotonic medium, since cells loaded with the membrane-impermeant dye CFSE did not leak dye or lyse over the 12-h exposure to the hypotonic medium (data not shown).
FIG. 8.
Effect of osmotic stress on egress. Parasites were allowed to invade for 1 h and to develop in HFFs for 30 h. At 30 h postinvasion, the normal tissue culture medium was switched to either a hypertonic medium consisting of normal medium plus 20 mM, 40 mM, or 60 mM stachyose (A), a hypotonic medium consisting of either 95% normal medium plus 5% H2O, 90% normal medium plus 10% H2O, or 75% normal medium plus 25% H2O (B), or normal medium as a control (solid bars in both panels). Percent egress is the percentage of lysed vacuoles at 42 h relative to the level at 30 h postinvasion. Data are means from three independent experiments. Each error bar equals 1 standard deviation.
We also loaded CFSE into HFFs, parasitized the cells, and switched the medium to the hypotonic medium at 30 h postinvasion. At 42 h postinvasion, there was no apparent loss of CFSE from intact cells in control or hypotonic medium, again indicating that there is no permeabilization of host cells due to exposure to the hypotonic medium. In addition, the kinetics of egress from hypotonically stressed HFFs is very different from that of permeabilization-induced egress. By 10 min after treatment with 0.005% saponin, the rate of egress of 30-h-postinvasion parasites from HFFs is 100%. However, even when parasites were exposed to hypotonic medium for 30 min after 30 h postinvasion, there was no detectable egress. The results of these control experiments indicate that the premature egress seen upon hypotonic stress is not due to a direct permeabilization of the host cell membrane. Thus, while hypertonic stress and the accompanying decrease in tension on the cell membrane delay egress, hypotonic stress promotes premature parasite egress, most likely as a result of the concomitant increase in membrane tension.
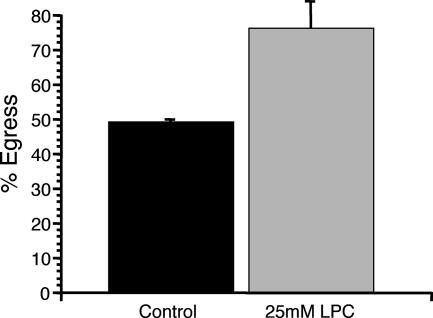
Another factor that can affect the stability of cell membranes is the incorporation of lipids that alter membrane curvature. Lipids that impart positive curvature to the cell membrane, such as LPC, reduce the energetic barrier for membrane rupture (5, 20) and have been shown to cause premature egress by Plasmodium merozoites from host red blood cells (RBCs) (19). LPC should likewise affect the timing of egress by T. gondii if parasite-generated internal pressure is required for egress. At 30 h postinvasion, the culture medium was switched to medium containing 25 mM LPC or control medium. When vacuoles were monitored at 42 h postinvasion, 76% of LPC-exposed vacuoles had undergone egress, compared to 49% of controls (Fig. 9). This result confirms that affecting the tension or structure of the host membrane affects the timing of egress.
FIG. 9.
Effect of LPC on egress. Parasites were allowed to invade for 1 h and to develop in HFFs for 30 h. At 30 h postinvasion, the normal tissue culture medium was switched to either AIM-V plus Albumax medium (control) or AIM-V plus Albumax medium plus 25 mM LPC. Percent egress is the percentage of lysed vacuoles at 42 h relative to the level at 30 h postinvasion. Data are means from three independent experiments. Each error bar equals 1 standard deviation.
DISCUSSION
In order for obligately intracellular parasites to infect new cells and complete their life cycles, they must be able to exit their current host cell. Despite the importance of egress in the intracellular parasite's life cycle, this process is often far less understood than the mechanisms by which the parasite invades the cell. Lysis of the host cell is a common means of parasite egress. This is true of the egress of Plasmodium merozoites from host RBCs. In this case, host cell lysis requires the action of parasite-derived proteases (7-9, 38), which may act by weakening the host cell cytoskeleton or integral membrane proteins. In the trypanosomatid parasite Leishmania amazonensis, a parasite-released pore-forming protein may be responsible for osmotic lysis of the host macrophage and consequent egress of the parasites (32, 33). Other intracellular parasites do not require lysis of the host cell for egress. For example, for the apicomplexan Cryptosporidium parvum, the host cell remains intact for several hours after parasite egress (12), while the intracellular bacterium Actinobacillus actinomycetemcomitans exits host cells via microtubule-dependent host cell protrusions (29).
As early as 1961, Lund and colleagues reported that in vitro egress of the intracellular parasite Toxoplasma gondii occurred by rupture of the host cell membrane, due, they believed, to exhaustion of the elastic capacity of the cell membrane as a consequence of parasite replication (28). Nevertheless, since the discovery that parasite egress can be induced by ionic fluxes in the host and parasite (3, 13, 21), attention has been focused on egress as an active process. Moreover, since various chemical inhibitors of motility that are known to completely disrupt induced egress have been identified, egress has been thought likely to require parasite motility (2, 18, 30) and in various ways to parallel the events involved in invasion (21). Nonetheless, the relevance of parasite motility and of ionic fluxes during egress that is not experimentally induced has not been assessed. Our experiments described here show that, interestingly, parasite motility is not necessary for egress. A number of motility-disrupting agents failed to inhibit egress, and the complete and irreversible disruption of parasite motility by mycalolide B delayed, but did not block, egress.
The delay in egress observed with either mycalolide B or cytochalasin D appears to be due to a disruption of the host cytoskeleton and not to an effect on parasite motility. This is evident in experiments in which only the cell actin is disrupted yet egress is delayed. In addition, while we observe a delay in egress when parasites and cells are treated with 1 μM cytochalasin D, this effect is not observed with 0.1 μM cytochalasin D, a concentration that effectively blocks parasite motility (data not shown). This result further underscores the fact that parasite motility is not required for egress. The lack of an effect at the lower concentration of cytochalasin D is the reason we were unable to utilize the previously described cytochalasin D-resistant parasites (10) in our experiments, since they are not resistant to the higher concentrations of the drug needed to observe the delay in egress. Interestingly, the lower concentrations of cytochalasin D that do not cause delayed egress also do not induce the morphological changes observed with higher concentrations of the drug, a finding consistent with the idea that it is the changes in the shape and tension of the host cell that result in delayed egress. In agreement with this hypothesis, we have observed that egress either from unmutagenized KB cells (a tumor line derived from HeLa cells) or from a cytochalasin B/D-resistant KB mutant cell line (41) is not affected or delayed by cytochalasin D concentrations as high as 10 μM (data not shown). Although phalloidin staining indicated a disruption of the actin cytoskeleton in cytochalasin D-treated unmutagenized KB cells, there was not a large disruption of morphology in these cells, probably because these cells already have a much more rounded shape than HFFs. Thus, it appears that it is not disruption of the actin cytoskeleton itself, but the effect this disruption has on cell morphology, that is significant in causing a delay in egress for a particular cell type.
While we find that parasite motility is not essential for parasite egress, it is likely that the induction of motility is sufficient to cause egress, and thus, it can be speculated that the parasite possesses the means to actively exit the host cell. It is thus possible that under some circumstances (e.g., parasite-independent damage to the host cell), parasites may use a motility-based egress mechanism, while under other circumstances, egress is motility independent. We have tried, as yet unsuccessfully, to produce physiologically relevant conditions, short of artificial permeabilization or treatment with ionophores, under which parasites actively exit host cells before they would naturally. For example, it may be beneficial for parasites to exit the host cell if it becomes superinfected, or if rising temperatures become hazardous to parasite survival. However, infection of previously parasitized cells with human cytomegalovirus did not alter the timing of egress, and slow heating of parasitized cells from 37°C to 42°C resulted in the parasites dying in place in the parasitophorous vacuole in the host HFF (data not shown). Further experiments are thus required to determine if and when the mechanisms observed during induced egress are important to parasite survival. Nonetheless, studies using induced egress are important and valid in that they allow us to elucidate the mechanisms involved in calcium signaling and the activation of motility. Indeed, mutants with defects in IIE are defective in the establishment of in vivo infections (25), which indicates that the mechanisms involved in induced egress are relevant to the normal biology of the parasite.
Our results suggest that egress by T. gondii is initiated by parasite-generated internal pressure that results in stretching and rupture of the host cell membrane. Thus, factors that release tension on the host cell membrane, such as a decrease in intracellular osmotic pressure or disruption of the actin cytoskeleton in fibroblasts, delay parasite egress. Factors that weaken the membrane or exert tension on the membrane, such as incorporation of the positive amphiphile LPC into the membrane or stretching of the membrane through an increase in intracellular osmotic pressure, cause parasites to exit sooner. Membrane rupture under tension is probably a stochastic process, dependent on localized weaknesses that may form due to membrane thinning or lateral movement of integral membrane proteins in the fluid lipid bilayer (16, 31). Decreased tension on the membrane could still cause rupture but would require greater, and hence less frequently occurring, membrane defects. In fact, it is not yet clear how the parasite generates the intracellular force that ruptures the membrane. A simple explanation would be that the force is generated by the increasing volume of the vacuole within the cell as the parasite divides. Therefore, it is also possible that in some cases, when membrane tension is decreased in host cells, extra divisions of the parasite may generate additional tension on the cell membrane, thus aiding in eventual cell rupture. Nevertheless, we have not been able to unequivocally assess whether the parasites divide more times when the host cytoskeleton is disrupted.
Our data indicate that egress from host cells by T. gondii may in some respects resemble egress by the related apicomplexan parasite Plasmodium falciparum from host RBCs. In the erythrocytic stage of Plasmodium, the nonmotile merozoites exit upon explosive rupture of the RBCs, which rapidly disperses the parasites (19). This rupture appears to be a consequence of internal pressure and membrane stretching in the RBC. As with T. gondii, the exact mechanism by which the parasites rupture the membrane is not known, but as mentioned above, Plasmodium egress has been shown to be dependent on the action of parasite proteases (7-9, 38), which may weaken the cytoskeleton or integral membrane proteins (8, 9). Other parasite-derived proteins speculated to aid in generating internal pressure and weakening the RBC membrane include pore-forming and membrane curvature-forming proteins (19). Video microscopy indicates that lysis of host RBCs by Plasmodium merozoites is explosive, leaving no RBC ghost (in contrast to osmotic lysis) and rapidly propelling the nonmotile merozoites from the site of egress (19). The action of the Plasmodium proteases may be important in causing this explosive lysis (8, 9, 19). Proteases have also been shown to be required for invasion by T. gondii (4, 6, 34), but we have as yet been unable to find evidence that they have a role in egress, and in general, experimental evidence on whether proteases or other parasite-generated proteins play a role in T. gondii egress is currently lacking. Our own unpublished video microscopy observations of noninduced egress by T. gondii seem to indicate that lysis of the host cell is not explosive like that with Plasmodium. It may be that internal pressure generated by T. gondii causes rupture of the host cell in a discrete spot(s), as would be expected for membrane rupture due simply to internal pressure rather than the protease-dependent explosive rupture seen with Plasmodium merozoites. The disruption of the host cell membrane in this manner would result in the activation of parasite motility due to the accompanying loss of ions from the host cell. This scenario is consistent with the observations of Moudy et al. (30), who recorded leakage of the calcium indicator dye Fura-PE3(AM) shortly before noninduced parasites exit from host cells. Thus, a model arises in which the parasites divide until the internal pressure disrupts the host cell membrane, and motility is then activated due to the ensuing ionic changes.
Since the discovery that experimentally induced fluxes in host cell [K+] and parasitic [Ca2+] could activate motility in intracellular T. gondii and rapidly result in egress from the host cell, a model of motility-based egress by T. gondii has been dominant (21). Our observations that motility is not required for parasite egress and that egress is influenced by tension on the host cell membrane are, to our knowledge, the first experimental evidence for a mechanism of egress that is not activated by the investigator for this important parasite. Furthermore, our results indicate that T. gondii invasion and egress proceed by distinct mechanisms. Future work on how the parasite might actively influence the host membrane and on the role of nonmotile egress in in vivo infections will shed light on this crucial step in the pathogenesis and propagation of T. gondii.
Acknowledgments
We thank John Boothroyd for providing us with the SAG1 antibody, Rolf Ingermann for the use of lab equipment, and Laura Knoll for input into our manuscript.
This work was supported by NIH grants from the NCRR Center of Biomedical Research Excellence (P20 RR15587, to G.A.) and the NIAID K22 program (AI061293-01, to G.A.). M.D.L. was supported by an Idaho IDEA Network of Biomedical Research Excellence grant from the NIH/NCRR (P20 RR016454).
Footnotes
Published ahead of print on 9 November 2007.
REFERENCES
- 1.Alexander, D. L., J. Mital, G. E. Ward, P. Bradley, and J. C. Boothroyd. 2005. Identification of the moving junction complex of Toxoplasma gondii: a collaboration between distinct secretory organelles. PLoS Pathog. 1e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black, M. W., G. Arrizabalaga, and J. C. Boothroyd. 2000. Ionophore-resistant mutants of Toxoplasma gondii reveal host cell permeabilization as an early event in egress. Mol. Cell. Biol. 209399-9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black, M. W., and J. C. Boothroyd. 2000. Lytic cycle of Toxoplasma gondii. Microbiol. Mol. Biol. Rev. 64607-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buitrago-Rey, R., J. Olarte, and J. E. Gomez-Marin. 2002. Evaluation of two inhibitors of invasion: LY311727 [3-(3-acetamide-1-benzyl-2-ethyl-indolyl-5-oxy)propane phosphonic acid] and AEBSF [4-(2-aminoethyl)-benzenesulphonyl fluoride] in acute murine toxoplasmosis. J. Antimicrob. Chemother. 49871-874. [DOI] [PubMed] [Google Scholar]
- 5.Chernomordik, L. V., M. M. Kozlov, G. B. Melikyan, I. G. Abidor, V. S. Markin, and Y. A. Chizmadzhev. 1985. The shape of lipid molecules and monolayer membrane-fusion. Biochim. Biophys. Acta 812643-655. [Google Scholar]
- 6.Conseil, V., M. Soete, and J. F. Dubremetz. 1999. Serine protease inhibitors block invasion of host cells by Toxoplasma gondii. Antimicrob. Agents Chemother. 431358-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dasaradhi, P. V., A. Mohmmed, A. Kumar, M. J. Hossain, R. K. Bhatnagar, V. S. Chauhan, and P. Malhotra. 2005. A role of falcipain-2, principal cysteine proteases of Plasmodium falciparum in merozoite egression. Biochem. Biophys. Res. Commun. 3361062-1068. [DOI] [PubMed] [Google Scholar]
- 8.Deguercy, A., M. Hommel, and J. Schrevel. 1990. Purification and characterization of 37-kilodalton proteases from Plasmodium falciparum and Plasmodium berghei which cleave erythrocyte cytoskeletal components. Mol. Biochem. Parasitol. 38233-244. [DOI] [PubMed] [Google Scholar]
- 9.Dhawan, S., M. Dua, A. H. Chishti, and M. Hanspal. 2003. Ankyrin peptide blocks falcipain-2-mediated malaria parasite release from red blood cells. J. Biol. Chem. 27830180-30186. [DOI] [PubMed] [Google Scholar]
- 10.Dobrowolski, J. M., and L. D. Sibley. 1996. Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell 84933-939. [DOI] [PubMed] [Google Scholar]
- 11.Dubey, J. P. 1993. Toxoplasma, Neospora, sarcocystis and other tissue cyst-forming coccidia of humans and animals, p. 1-158. In J. P. Kreier (ed.) Parasitic protozoa, vol. 6. Academic Press, San Diego, CA. [Google Scholar]
- 12.Elliott, D. A., and D. P. Clark. 2003. Host cell fate on Cryptosporidium parvum egress from MDCK cells. Infect. Immun. 715422-5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Endo, T., K. K. Sethi, and G. Piekarski. 1982. Toxoplasma gondii: calcium ionophore A23187-mediated exit of trophozoites from infected murine macrophages. Exp. Parasitol. 53179-188. [DOI] [PubMed] [Google Scholar]
- 14.Endo, T., H. Tokuda, K. Yagita, and T. Koyama. 1987. Effects of extracellular potassium on acid release and motility initiation in Toxoplasma gondii. J. Protozool. 34291-295. [DOI] [PubMed] [Google Scholar]
- 15.Endo, T., and K. Yagita. 1990. Effect of extracellular ions on motility and cell entry in Toxoplasma gondii. J. Protozool. 37133-138. [DOI] [PubMed] [Google Scholar]
- 16.Evans, E. A., R. Waugh, and L. Melnik. 1976. Elastic area compressibility modulus of red cell membrane. Biophys. J. 16585-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang, J., N. Marchesini, and S. N. Moreno. 2006. A Toxoplasma gondii phosphoinositide phospholipase C (TgPI-PLC) with high affinity for phosphatidylinositol. Biochem. J. 394417-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fruth, I. A., and G. Arrizabalaga. 2007. Toxoplasma gondii: induction of egress by the potassium ionophore nigericin. Int. J. Parasitol. 371559-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glushakova, S., D. Yin, T. Li, and J. Zimmerberg. 2005. Membrane transformation during malaria parasite release from human red blood cells. Curr. Biol. 151645-1650. [DOI] [PubMed] [Google Scholar]
- 20.Hamai, C., T. Yang, S. Kataoka, P. S. Cremer, and S. M. Musser. 2006. Effect of average phospholipid curvature on supported bilayer formation on glass by vesicle fusion. Biophys. J. 901241-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoff, E. F., and V. B. Carruthers. 2002. Is Toxoplasma egress the first step in invasion? Trends Parasitol. 18251-255. [DOI] [PubMed] [Google Scholar]
- 22.Hori, M., S. Saito, Y. Z. Shin, H. Ozaki, N. Fusetani, and H. Karaki. 1993. Mycalolide-B, a novel and specific inhibitor of actomyosin ATPase isolated from marine sponge. FEBS Lett. 322151-154. [DOI] [PubMed] [Google Scholar]
- 23.Joiner, K. A., and J. F. Dubremetz. 1993. Toxoplasma gondii: a protozoan for the nineties. Infect. Immun. 611169-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kafsack, B. F., C. Beckers, and V. B. Carruthers. 2004. Synchronous invasion of host cells by Toxoplasma gondii. Mol. Biochem. Parasitol. 136309-311. [DOI] [PubMed] [Google Scholar]
- 25.Lavine, M. D., L. J. Knoll, P. J. Rooney, and G. Arrizabalaga. 2007. A Toxoplasma gondii mutant defective in responding to calcium fluxes shows reduced in vivo pathogenicity. Mol. Biochem. Parasitol. 155113-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lovett, J. L., and L. D. Sibley. 2003. Intracellular calcium stores in Toxoplasma gondii govern invasion of host cells. J. Cell Sci. 1163009-3016. [DOI] [PubMed] [Google Scholar]
- 27.Luft, B. J., and J. S. Remington. 1992. Toxoplasmic encephalitis in AIDS. Clin. Infect. Dis. 15211-222. [DOI] [PubMed] [Google Scholar]
- 28.Lund, E., E. Lycke, and P. Sourander. 1961. A cinematographic study of Toxoplasma gondii in cell cultures. Br. J. Exp. Pathol. 42357-362. [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer, D. H., J. E. Rose, J. E. Lippmann, and P. M. Fives-Taylor. 1999. Microtubules are associated with intracellular movement and spread of the periodontopathogen Actinobacillus actinomycetemcomitans. Infect. Immun. 676518-6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moudy, R., T. J. Manning, and C. J. Beckers. 2001. The loss of cytoplasmic potassium upon host cell breakdown triggers egress of Toxoplasma gondii. J. Biol. Chem. 27641492-41501. [DOI] [PubMed] [Google Scholar]
- 31.Nichol, J. A., and O. F. Hutter. 1996. Tensile strength and dilatational elasticity of giant sarcolemmal vesicles shed from rabbit muscle. J. Physiol. 493187-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noronha, F. S., J. S. Cruz, P. S. Beirao, and M. F. Horta. 2000. Macrophage damage by Leishmania amazonensis cytolysin: evidence of pore formation on cell membrane. Infect. Immun. 684578-4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noronha, F. S., F. J. Ramalho-Pinto, and M. F. Horta. 1996. Cytolytic activity in the genus Leishmania: involvement of a putative pore-forming protein. Infect. Immun. 643975-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Que, X., H. Ngo, J. Lawton, M. Gray, Q. Liu, J. Engel, L. Brinen, P. Ghosh, K. A. Joiner, and S. L. Reed. 2002. The cathepsin B of Toxoplasma gondii, toxopain-1, is critical for parasite invasion and rhoptry protein processing. J. Biol. Chem. 27725791-25797. [DOI] [PubMed] [Google Scholar]
- 35.Saito, S., and H. Karaki. 1996. A family of novel actin-inhibiting marine toxins. Clin. Exp. Pharmacol. Physiol. 23743-746. [DOI] [PubMed] [Google Scholar]
- 36.Saito, S., S. Watabe, H. Ozaki, N. Fusetani, and H. Karaki. 1994. Mycalolide B, a novel actin depolymerizing agent. J. Biol. Chem. 26929710-29714. [PubMed] [Google Scholar]
- 37.Saito, S. Y., S. Watabe, H. Ozaki, M. Kobayashi, T. Suzuki, H. Kobayashi, N. Fusetani, and H. Karaki. 1998. Actin-depolymerizing effect of dimeric macrolides, bistheonellide A and swinholide A. J. Biochem. (Tokyo) 123571-578. [DOI] [PubMed] [Google Scholar]
- 38.Salmon, B. L., A. Oksman, and D. E. Goldberg. 2001. Malaria parasite exit from the host erythrocyte: a two-step process requiring extraerythrocytic proteolysis. Proc. Natl. Acad. Sci. USA 98271-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sibley, L. D., S. Hakansson, and V. B. Carruthers. 1998. Gliding motility: an efficient mechanism for cell penetration. Curr. Biol. 8R12-R14. [DOI] [PubMed] [Google Scholar]
- 40.Stommel, E. W., K. H. Ely, J. D. Schwartzman, and L. H. Kasper. 1997. Toxoplasma gondii: dithiol-induced Ca2+ flux causes egress of parasites from the parasitophorous vacuole. Exp. Parasitol. 8788-97. [DOI] [PubMed] [Google Scholar]
- 41.Toyama, S., and S. Toyama. 1984. A variant form of beta-actin in a mutant of KB cells resistant to cytochalasin B. Cell 37609-614. [DOI] [PubMed] [Google Scholar]
- 42.Trzewik, J., A. Artmann-Temiz, P. T. Linder, T. Demirci, I. Digel, and G. M. Artmann. 2004. Evaluation of lateral mechanical tension in thin-film tissue constructs. Ann. Biomed. Eng. 321243-1251. [DOI] [PubMed] [Google Scholar]
- 43.Wada, S., S. Matsunaga, S. Saito, N. Fusetani, and S. Watabe. 1998. Actin-binding specificity of marine macrolide toxins, mycalolide B and kabiramide D. J. Biochem. (Tokyo) 123946-952. [DOI] [PubMed] [Google Scholar]
- 44.Wetzel, D. M., L. A. Chen, F. A. Ruiz, S. N. Moreno, and L. D. Sibley. 2004. Calcium-mediated protein secretion potentiates motility in Toxoplasma gondii. J. Cell Sci. 1175739-5748. [DOI] [PubMed] [Google Scholar]