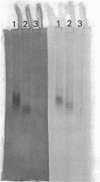
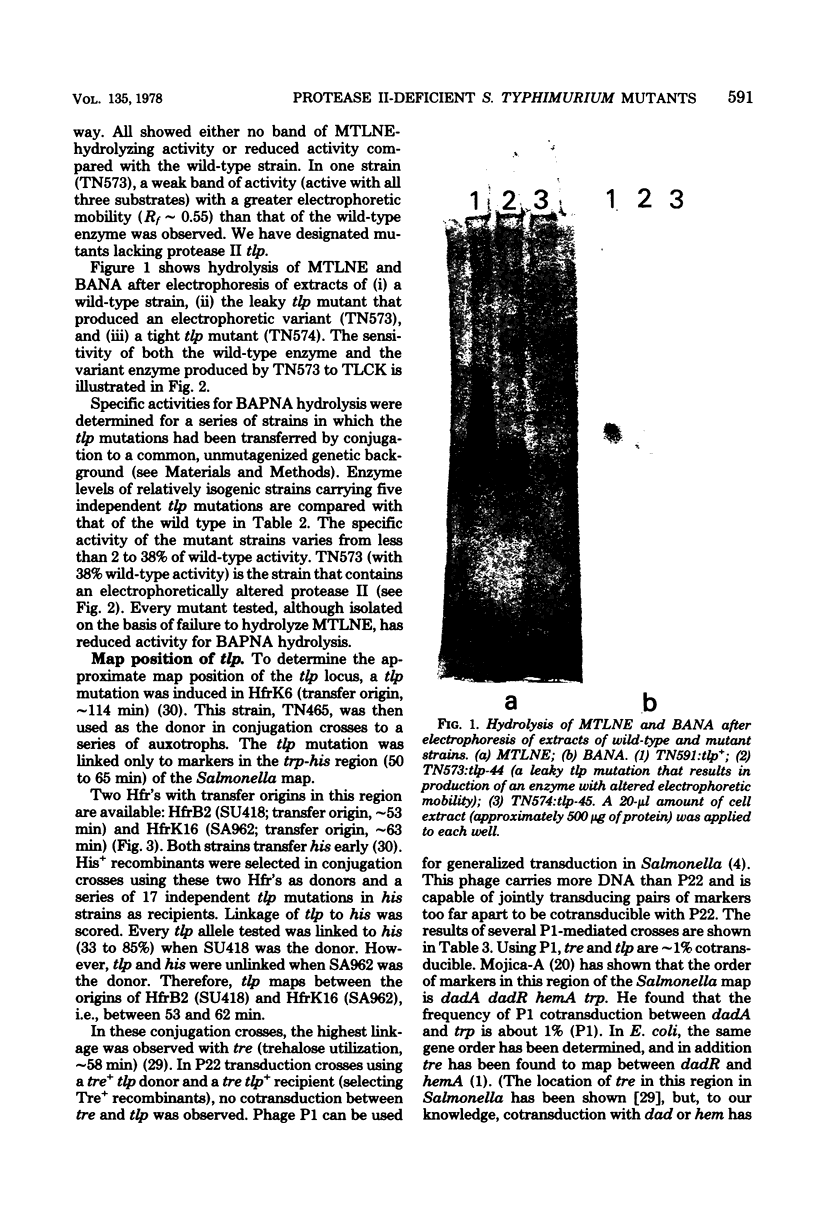
Abstract
Mutants of Salmonella typhimurium lacking protease II, an endoprotease with trypsin-like specificity, have been isolated. These mutants can be identified by using the chromogenic substrate N-methyl-N-p-toluenesulfonyl-L-lysine beta-naphthyl ester to screen colonies growing on agar for the presence of the enzyme. All of the mutations isolated map at locus tlp (typsin-like protease) which is cotransducible (approximately 1%) using phage P1 with tre (trehalose utilization) at approximately 58 min on the Salmonella map. Double mutants lacking both protease I and protease II have been constructed. These strains grew normally. They were able to degrade abnormal proteins and to carry out protein turnover during carbon starvation at the same rate as the wild type.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- ERLANGER B. F., KOKOWSKY N., COHEN W. The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys. 1961 Nov;95:271–278. doi: 10.1016/0003-9861(61)90145-x. [DOI] [PubMed] [Google Scholar]
- Elmore D. T., Smyth J. J. The behaviour of trypsin towards alpha-N-methyl-alpha-N-toluene-p-sulfonyl-L-lysine beta-naphthyl ester. A new method for determining the absolute molarity of solutions of trypsin. Biochem J. 1968 Mar;107(1):97–102. doi: 10.1042/bj1070097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto M., Stocker B. A. Transduction by phage P1kc in Salmonella typhimurium. Virology. 1974 Aug;60(2):503–514. doi: 10.1016/0042-6822(74)90344-4. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L. Degradation of abnormal proteins in Escherichia coli (protein breakdown-protein structure-mistranslation-amino acid analogs-puromycin). Proc Natl Acad Sci U S A. 1972 Feb;69(2):422–426. doi: 10.1073/pnas.69.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Inouye H., Beckwith J. Synthesis and processing of an Escherichia coli alkaline phosphatase precursor in vitro. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1440–1444. doi: 10.1073/pnas.74.4.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Chan R. K., Tye B. K., Botstein D. Mutagenesis by insertion of a drug-resistance element carrying an inverted repetition. J Mol Biol. 1975 Oct 5;97(4):561–575. doi: 10.1016/s0022-2836(75)80059-3. [DOI] [PubMed] [Google Scholar]
- Kowit J. D., Choy W. N., Champe S. P., Goldberg A. L. Role and location of "protease I" from Escherichia coli. J Bacteriol. 1976 Dec;128(3):776–784. doi: 10.1128/jb.128.3.776-784.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Meiss H. K., Brill W. J., Magasanik B. Genetic control of histidine degradation in Salmonella typhimurium, strain LT-2. J Biol Chem. 1969 Oct 10;244(19):5382–5391. [PubMed] [Google Scholar]
- Miller C. G., Heiman C., Yen C. Mutants of Salmonella typhimurium deficient in an endoprotease. J Bacteriol. 1976 Jul;127(1):490–497. doi: 10.1128/jb.127.1.490-497.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. G., Mackinnon K. Peptidase mutants of Salmonella typhimurium. J Bacteriol. 1974 Oct;120(1):355–363. doi: 10.1128/jb.120.1.355-363.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. G. Peptidases and proteases of Escherichia coli and Salmonella typhimurium. Annu Rev Microbiol. 1975;29:485–504. doi: 10.1146/annurev.mi.29.100175.002413. [DOI] [PubMed] [Google Scholar]
- Miller C. G., Zipser D. Degradation of Escherichia coli beta-galactosidase fragments in protease-deficient mutants of Salmonella typhimurium. J Bacteriol. 1977 Apr;130(1):347–353. doi: 10.1128/jb.130.1.347-353.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojica T. Transduction by phage P1CM clr-100 in Salmonella typhimurium. Mol Gen Genet. 1975;138(2):113–126. doi: 10.1007/BF02428116. [DOI] [PubMed] [Google Scholar]
- Pacaud M. Purification of protease II from Escherichia coli by affinity chromatography and separation of two enzyme species from cells harvested at late log phase. Eur J Biochem. 1976 Apr 15;64(1):199–204. doi: 10.1111/j.1432-1033.1976.tb10288.x. [DOI] [PubMed] [Google Scholar]
- Pacaud M., Richaud C. Protease II from Escherichia coli. Purification and characterization. J Biol Chem. 1975 Oct 10;250(19):7771–7779. [PubMed] [Google Scholar]
- Pacaud M., Sibilli S., Bras G. Protease I from Escherichia coli. Some physicochemical properties and substrate specificity. Eur J Biochem. 1976 Oct 1;69(1):141–151. doi: 10.1111/j.1432-1033.1976.tb10867.x. [DOI] [PubMed] [Google Scholar]
- Pacaud M., Uriel J. Isolation and some propeties of a proteolytic enzyme from Escherichia coli (protease I). Eur J Biochem. 1971 Dec 10;23(3):435–442. doi: 10.1111/j.1432-1033.1971.tb01638.x. [DOI] [PubMed] [Google Scholar]
- Pine M. J. Turnover of intracellular proteins. Annu Rev Microbiol. 1972;26:103–126. doi: 10.1146/annurev.mi.26.100172.000535. [DOI] [PubMed] [Google Scholar]
- Regnier P., Thang M. N. Subcellular distribution and characterization of endo and exo-cellular proteases in E. coli. Biochimie. 1972;54(10):1227–1236. doi: 10.1016/s0300-9084(72)80063-4. [DOI] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W. Proteolytic cleavage of bacteriophage lambda repressor in induction. Proc Natl Acad Sci U S A. 1975 Jan;72(1):147–151. doi: 10.1073/pnas.72.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E. Linkage map of Salmonella typhimurium, edition IV. Bacteriol Rev. 1972 Dec;36(4):558–586. doi: 10.1128/br.36.4.558-586.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E., Ross H., Ziegler L., Mäkelä P. H. F + , Hfr, and F' strains of Salmonella typhimurium and Salmonella abony. Bacteriol Rev. 1972 Dec;36(4):608–637. doi: 10.1128/br.36.4.608-637.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieger H. Phage P22-mutants with increased or decreased transduction abilities. Mol Gen Genet. 1972;119(1):75–88. doi: 10.1007/BF00270447. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- de Lares L. B., Ratouchniak J., Casse F. Chromosomal location of gene governing the trehalose utilization in Escherichia coli K12. Mol Gen Genet. 1977 Mar 28;152(1):105–108. doi: 10.1007/BF00264946. [DOI] [PubMed] [Google Scholar]