Abstract
The hypothesis that early flowering plants were insect-pollinated could be tested by an examination of the pollination biology of basal angiosperms and the pollination modes of fossil angiosperms. We provide data to show that early fossil angiosperms were insect-pollinated. Eighty-six percent of 29 extant basal angiosperm families have species that are zoophilous (of which 34% are specialized) and 17% of the families have species that are wind-pollinated, whereas basal eudicot families and basal monocot families more commonly have wind and specialized pollination modes (up to 78%). Character reconstruction based on recent molecular trees of angiosperms suggests that the most parsimonious result is that zoophily is the ancestral state. Combining pollen ornamentation, size, and aperture characteristics and the abundance of single-species pollen clumps of Cenomanian angiosperm-dispersed pollen species from the Dakota Formation demonstrates a dominance of zoophilous pollination (76% versus 24% wind pollination). The zoophilous pollen species have adaptations for pollination by generalist insects (39%), specialized pollen-collecting insects (27%), and other specialized pollinators (10%). These data quantify the presences of more specialized pollination modes during the mid-Cretaceous angiosperm diversification.
Keywords: Cretaceous, pollen, pollination biology, floral evolution
Early steps in angiosperm–pollinator coevolution are best understood through research on Cretaceous fossil flowers and in situ pollen (1–5). A widely accepted hypothesis is that insect pollination was the dominant mode of angiosperm pollination during the Early Cretaceous (4) with specialization increasing by the mid-Cretaceous (3). Even ancient relatives of extant wind-pollinated taxa have been suggested to be initially insect-pollinated, such as Late Albian flowers of Platanus-like plants (2) and Campanian fagaceous flowers (6). By the mid-Cretaceous, showy bisexual flowers indicate specialized insect pollination (3, 7, 8).
Understanding pollination biology of fossil plants is often based on morphological interpretations (3, 7, 9) using traits of fossil flowers and pollen compared with similar floral and pollen morphologies of living plants known to have specific pollinators. For example, pollen from wind-pollinated flowers appears to be dry with a smooth surface, of moderate size, and produced in large quantities (10–12). In contrast, zoophilous flowers appear to have pollen that is sticky with pollenkitt or other substances, generally with ornate surfaces, of variable size, and are produced in variable quantities (10, 13–16).
Other evidence central to a discussion of early angiosperm pollination biology is the fossil insect record (17–21) and pollinator modes of basal extant angiosperms (22, 23). Both support the zoophilous ancestral hypothesis (22, 23), with well preserved Early Cretaceous fossil insect pollinators (17–21) and insect pollination found in the most basal angiosperm families (Table 1). Yet pollination modes have not been analyzed phylogenetically for angiosperms.
Table 1.
Comparison of pollination modes of basal angiosperm families (Table 2) and inferred modes for the Cenomanian pollen species (Table 3)
| Plant group | No. | Pollination mode |
|||
|---|---|---|---|---|---|
| Insect, % | Wind, % | Water, % | Specialized, % | ||
| Basal angiosperms | 29 | 86 | 17 | 3 | 34 |
| Basally placed monocots | 15 | 40 | 27 | 40 | 75 |
| Basally placed eudicots | 16 | 56 | 63 | 0 | 78 |
| Fossil monosulcate | 16 | 87 | 13 | NA | 29 |
| Fossil tricolpate | 25 | 68 | 32 | NA | 65 |
Wind, insect, and water percentages are based on the total number of taxa in a group that have the mode divided by the number of taxa and thus can be counted more than once. Specialized percentages for the extant taxa are based on the total number of families with hymenoptera and water divided by the number of nonwind-pollinated families; specialized percentages for the fossils are based on morphology and frequency. NA, not available.
We have taken two approaches that examine pollination systems in early angiosperms and their subsequent specialization. First, we looked at the phylogenetic distribution of pollination modes in extant basal angiosperms (dicots excluding the eudicots), basal monocots (families of Acorales and Alismatales), and basal eudicots (families of Ranunculales, Sabiales, Proteales, Trochodentrales, Buxales, and Gunnerales; refs. 24 and 25 and www.mobot.org/MOBOT/research/APweb; Table 2). This study allowed for the identification of the initial pollination mode and possible shifts in the pollination modes. Second, we examined dispersed angiosperm pollen grains and pollen clumps common in the mid-Cretaceous. Pollen clumps are found in zoophilous flowers in extant angiosperms (13, 15, 16), and this study provides evidence of extensive fossil pollen clumping. We suggest that this pollen-clumping character was a major step in angiosperm–pollinator coevolution. When such pollen clumps are found, they imply an increase in zoophilous pollination in the fossil record. We combine data on pollen clumping with data of pollen ornamentation, size, and aperture characters from single dispersed grains to provide frequencies of these characters as related to wind, general zoophilous, and specialized modes of pollination. Our data from the mid-Cretaceous (Middle Cenomanian) Dakota Formation (27) provides a test of early modes of pollination during a period of rapid angiosperm diversification (24, 28).
Table 2.
Extant angiosperm families and their pollination modes, divided by wind, animal, and type of pollinator if known
| Family | Wind | Animal | Pollinator | Ref(s). |
|---|---|---|---|---|
| Amborellaceae | † | † | BDW | 29 |
| Nymphaeaceae | † | BDH | 23,54,55 | |
| Cabombaceae | † | BDH | 23,54 | |
| Austrobaileyaceae | † | BD? | 23,56 | |
| Illiciaceae | † | BD | 23,55 | |
| Schisandraceae | † | BD | 23,54 | |
| Trimeniaceae | † | † | BHW | 56 |
| Ceratophyllaceae | Wa | 23,55 | ||
| Chloranthaceae | † | † | TW | 23,54 |
| Myristicaceae | † | BT | 23,54 | |
| Magnoliaceae | † | BDTH | 23,54,55 | |
| Degeneriaceae | † | B | 23 | |
| Himantandraceae | † | Unknown | 57 | |
| Eupomatiaceae | † | B | 23,56 | |
| Annonaceae | † | BDT | 23,54,55 | |
| Atherospermataceae | Unknown | |||
| Calycanthaceae | † | B | 23,56 | |
| Gomortegaceae | † | Unknown | 56 | |
| Hernandiaceae | † | Unknown | 56 | |
| Lauraceae | † | BDTH | 23,55 | |
| Monimiaceae | † | BDTH | 23,54 | |
| Siparunaceae | Unknown | |||
| Canellaceae | † | BT | 23 | |
| Winteraceae | † | BDMT | 23,54,55 | |
| Aristolochiaceae | † | D | 23,55 | |
| Hydnoraceae | † | B | 56 | |
| Lactoridaceae | † | W | 23 | |
| Piperaceae | † | BDH | 23,55 | |
| Saururaceae | † | † | BDHTW | 23 |
| Acoraceae | Unknown | |||
| Tofieldiaceae | Unknown | |||
| Araceae | † | BDH | 23,54 | |
| Alismataceae | † | DH | 55 | |
| Aponogetonaceae | † | Unknown | 56 | |
| Butomaceae | † | DH | 58 | |
| Cymodoceaceae | Wa | 56 | ||
| Juncaginaceae | † | W | 56 | |
| Hydrocharitaceae | † | † | BDWWa | 55,59 |
| Limnocharitaceae | † | Unknown | 57 | |
| Posidoniaceae | Wa | 56 | ||
| Potamogetonaceae | † | WWa | 55 | |
| Ruppiaceae | Wa | 56 | ||
| Scheuchzeriaceae | † | W | 56 | |
| Zosteraceae | Wa | 56 | ||
| Remaining monocots | † | † | All | 55 |
| Berberidaceae | † | DH | 55,60 | |
| Eupteleaceae | † | W | 54,56 | |
| Circaeasteraceae | † | Unknown | 57 | |
| Lardizabalaceae | † | DH | 61 | |
| Menispermaceae | † | † | HW | 54,55 |
| Papaveraceae (inc. Fumarioideae Papaveroideae, Pteridophylloideae) | † | BDH | 55 | |
| Ranunculaceae | † | † | HWO | 54,55 |
| Sabiaceae | † | W | 54 | |
| Nelumbonaceae | † | BDH | 54,62 | |
| Platanaceae | † | W | 54,55 | |
| Proteaceae | † | BDH | 26,54,55 | |
| Trochodendraceae | † | † | DW | 54,56 |
| Buxaceae | † | W | 54 | |
| Didymelaceae | † | W | 54 | |
| Gunneraceae | † | W | 54 | |
| Myrothamnaceae | † | W | 56 | |
| Remaining eudicots | † | † | All | 55 |
† indicates presence; B, beetles (Coleoptera); D, Diptera (flies); H, Hymenoptera (mostly bees); M, Micropterigidae (basal family of Lepidoptera); T, thrips (Thysanoptera); W, wind; Wa, water; O, other.
Results and Discussion
Although the pollination modes of extant basal angiosperms have been summarized (23, 29), these occurrences have not been compared with those found in basal eudicot and basal monocot families. Three groups of angiosperm families (refs. 24 and 25 and www.mobot.org/MOBOT/research/APweb) were examined: basal angiosperms (Table 2, Amborellaceae to Saururaceae), basal monocot (Table 2, Acoraceae to remaining monocots), and basal eudicots (Table 2, Berberidaceae to remaining eudicots). The pollinators were assigned to the following pollination modes based on Thien et al. (23): Coleoptera (beetle), Diptera (fly), Hymenoptera (mostly bee), Micropterigidae (basal family of Lepidoptera), Thysanoptera (thrips), wind, water, other, or unknown (Table 2). Our data (Tables 1 and 2) show that 86% of the basal angiosperm families have species that are insect-pollinated, 17% of the families have wind-pollinated species, and 34% of the families with nonwind-pollinated species have specialized pollination modes, including water and Hymenoptera. In contrast, of the basal monocot families, only 40% have species that are insect-pollinated, 27% have wind-pollinated species, and 40% have water-pollinated species. However, specialized pollination is found in 75% of the basal monocot families with nonwind-pollinated species. Finally, in the basal eudicot families, 56% have insect-pollinated species (versus 63% wind) of which 78% have species with specialized pollination.
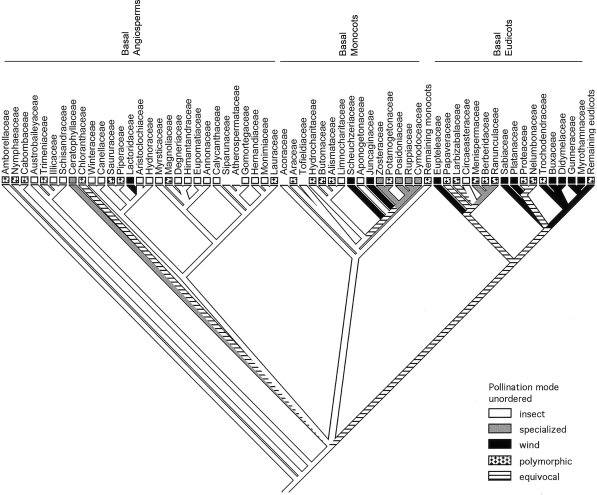
These pollination modes (Tables 1 and 2) are mapped on a mostly resolved conservative molecular tree (refs. 24 and 30–32 and www.mobot.org/MOBOT/research/Apweb; specifically figure 3.3 in ref. 24) and a fully resolved molecular tree (refs. 24, 30, 33, and 34; specifically figure 2.3 in ref. 24). As illustrated by the conservative tree (Fig. 1), using the most-parsimonious reconstruction (MPR) method (35), general insect pollination (most often beetles or flies) is initially present in all extant families of both the basal angiosperms (except water-pollinated Ceratophyllaceae and wind-pollinated Lactoridaceae), and the basal monocots. In contrast, the initial pollination mode for basal eudicots is equivocal, because of the increase of wind-pollinated clades (10 families; Table 2) early in eudicot evolution. With the MPR method, the resolved tree had the same result, whereas the delayed transformaton (DELTRAN) method also resolved the ancestral mode as insect pollination for the basal eudicots and the accelerated transformation (ACCTRAN) method likewise resolved the ancestral mode as wind. The families with wind and specialized pollination are clustered in the more derived families of basal monocots and basal eudicots, and these pollination modes appear to have evolved multiple times in these families in all analyses except for the eudicots in the ACCTRAN method. It is interesting to note that no bird, bat, or other more specialized pollination modes (10, 13) have been reported in these extant basal angiosperms and that finding is consistent with the appearance of bats and derived passerine birds during the Tertiary (36, 37). Molecular data suggest that early monocots originated and diverged between 147 million years ago (MYA) and 128 MYA, whereas the basal eudicot families originated and diverged between 125 MYA and 116 MYA (24). The fossil dates for the earliest evidence of each clade are 130 MYA and 125 MYA, respectively (38). Morphological, molecular, and fossil data show a similar Early Cretaceous age for the diversification of some insect pollinators (18). Thus, the distribution suggests increased wind and specialized pollination by the mid-Cretaceous and an increase of specialized pollination during the later Upper Cretaceous and Paleogene contemporaneously with continuing angiosperm radiations (1–7, 9).
Fig. 1.
MacClade reconstruction of the evolution of pollination modes based on molecular topology (refs. 24 and 30–32 and www.mobot.org/MOBOT/research/Apweb; specifically figure 3.3 in ref. 24). Missing rectangles by families indicate unknown pollination mode (Table 1). Insect pollination includes Coleoptera (beetle), Diptera (fly), and Thysanoptera (thrips). Specialized pollination includes water and Hymenoptera (mostly bee).
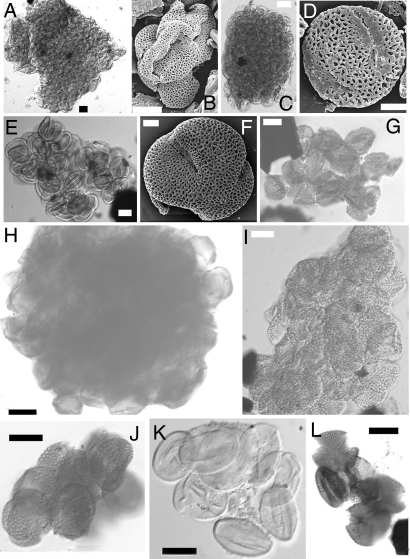
We examined pollen from the mid-Cretaceous (Cenomanian) Dakota Formation in southwest Minnesota (27, 39) and found 41 angiosperm pollen species (Table 3) of which nine species (22%) were clumped (Fig. 2). These clumps range in size from 20 to >150 μm and include ≈5–800 pollen grains. Three types of pollen clumps are found (Table 3). (i) Four species have pollen clumps that are composed of many grains, either individual pollen grains or pollen tetrads, and also were commonly dispersed either as single grains or tetrads (frequencies of 23–72%). These include Artiopollis indivisus (Fig. 2 A and B), Tricolpites sp. (Fig. 2 C and D), cf. Phimopollenites (Fig. 2 E and F), and Tricolpites cf. vulgaris (Fig. 2G). (ii) Two species have pollen clumps composed of >50 individual grains, but have few dispersed pollen grains (frequencies of 0.2–0.7%). These include cf. Psilatricolporites sp. (Fig. 2H) and Liliacidites cf. reticulatus (Fig. 2I). (iii) Three species have pollen clumps each of which are composed of ≈10 individual grains that are also found commonly dispersed as individual pollen grains (frequencies of 7–21%). These include Rousea cf. delicipollis (Fig. 2J), Cupuliferoidaepollenites sp. (Fig. 2K), and Dryadopollis sp.1 (Fig. 2L).
Table 3.
List of pollen taxa identified in the Cenomanian Dakota Formation, Minnesota (27)
| Pollen type | Ornamentation | Grain size, μm | Aperture type | Clumps |
Frequency, % |
Pollination mode | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Size, μm | No. of grains | No. | C | H | O | |||||
| Clavatipollenites tenellis | Reticulate | 28 | Monosulcate | NA | NA | NA | P | A | 0.1 | Zoo. gen. |
| Clavatipollenites sp.2 | Reticulate | 24 | Monosulcate | NA | NA | NA | P | A | 0.2 | Zoo. gen. |
| ?Clavatipollenites sp.3 | Microfoveolate | 27 | Monosulcate | NA | NA | NA | A | A | 0.9 | Wind (?) |
| Liliacidites giganteus | Reticulate | 76 | Monosulcate | NA | NA | NA | P | A | A | Zoo. spec. oth. |
| Liliacidites cf. inaequalis | Reticulate | 24 | Monosulcate | NA | NA | NA | A | 1.0 | A | Zoo. gen. |
| Liliacidites cf. reticulatus | Reticulate | 24 | Monosulcate | 110 × 64 | 50 | 1, type 2 | P | 0.7 | P | Zoo. gen. |
| Liliacidites sp.1 | Reticulate | 34 | Monosulcate | NA | NA | NA | A | A | 0.9 | Zoo. gen. |
| Liliacidites sp.2 | Reticulate | 21 | Monosulcate | NA | NA | NA | A | A | P | Zoo. spec. oth. |
| Liliacidites sp.3 | Reticulate | 30 | Monosulcate | NA | NA | NA | A | A | 3.0 | Zoo. gen. |
| Liliacidites sp.4 | Reticulate | 37 | Monosulcate | NA | NA | NA | A | A | 0.7 | Zoo. gen. |
| Liliacidites sp.5 | Reticulate | 24 | Monosulcate | NA | NA | NA | A | 0.3 | P | Zoo. gen. |
| Retimonocolpites dividuus | Reticulate | 35 | Monosulcate | NA | NA | NA | P | A | 0.7 | Zoo. gen. |
| ? Spinizonocolpites sp. | Scabrate | 25 | Monosulcate | NA | NA | NA | P | A | A | Wind (?) |
| Stellatopollis largissimus | Reticulate | 123 | Monosulcate | NA | NA | NA | P | A | A | Zoo. spec. oth. |
| Stellatopollis sp. | Reticulate | 53 | Monosulcate | NA | NA | NA | P | A | A | Zoo. spec. oth. |
| Doyleipollenites robbinsiae | Reticulate to foveolate | 27 | Trichotomosulcate | NA | NA | NA | 0.5 | A | 0.5 | Zoo. gen. |
| Artiopollis indivisus (tetrad) | Microreticulate | 13 | Tricolpate | 36 × 33–151 × 142 | 24–800 | 3, type 1 | A | A | 30 | Zoo. spec. p. c. |
| Cupuliferoidaepollenites sp. | Scabrate | 16 | Tricolpate | 47 × 38–35 × 26 | 8–9 | 2, type 3 | 21 | A | 9.0 | Zoo. spec. p. c. |
| Foveotricolpites sp. | Foveolate | 38 | Tricolpate | NA | NA | NA | A | A | 0.4 | Wind |
| Fraxinoipollenites constrictus | Microfoveolate | 38 | Tricolpate | NA | NA | NA | 5.0 | 5.0 | 0.9 | Wind |
| Rousea cf. delicipollis | Reticulate to foveolate | 23 | Tricolpate | 52 × 30–46 × 31 | 10 | 2, type 3 | 7.0 | P | A | Zoo. spec. p. c. |
| Satishia sp. | Microreticulate | 28 | Tricolpate | NA | NA | NA | P | A | A | Wind (?) |
| Striatopollis paraneus | Striato-reticulate | 21 | Tricolpate | NA | NA | NA | 2.0 | A | A | Zoo. spec. p. c. |
| Tricolpites sp. | Reticulate | 14 | Tricolpate | 27 × 19–76 × 62 | 10–200 | 7, type 1 | 13 | A | 23 | Zoo. spec. p. c. |
| Tricolpites nemejci | Microreticulate | 27 | Tricolpate | NA | NA | NA | 0.3 | A | 3.0 | Wind (?) |
| Tricolpites cf. vulgaris | Reticulate | 20 | Tricolpate | 37 × 23–82 × 70 | 5–100 | 5, type 1 | 39 | 21 | 12 | Zoo. spec. p. c. |
| Tricolpate sp.4 | Reticulate | 22 | Tricolpate | NA | NA | NA | 4.0 | A | 4.0 | Zoo. spec. p. c. |
| Tricolpate sp.7 | Microfoveolate | 18 | Tricolpate | NA | NA | NA | P | A | 0.5 | Zoo. gen. |
| Tricolpate sp.8 | Scabrate | 26 | Tricolpate | NA | NA | NA | A | A | 0.2 | Wind (?) |
| Tricolpate sp.10 | Microreticulate to microfoveolate | 29 | Tricolpate | NA | NA | NA | A | A | 1.0 | Wind |
| Tricolpate sp.11 | Reticulate to foveolate | 15 | Tricolpate | NA | NA | NA | P | A | 0.5 | Zoo. gen. |
| Tricolpate sp.12 | Reticulate | 19 | Tricolpate | NA | NA | NA | A | A | 3.0 | Zoo. spec. p. c. |
| Tricolpate sp.14 | Microfoveolate | 18 | Tricolpate | NA | NA | NA | A | A | 0.9 | Zoo. gen. |
| cf. Phimopollenites sp. | Microreticulate | 19 | Tricolporoidate | 20 × 13–107 × 75 | 7–50 | 3, type 1 | P | 72 | 2.0 | Zoo. spec. p. c. |
| cf. Psilatricolporites sp. | Scabrate | 14 | Tricolporoidate | 91 × 88 | 100 | 1, type 2 | A | A | 0.2 | Zoo. gen. |
| Dryadopollis sp.1 | Microreticulate | 19 | Tricolporate | 66 × 38–62 × 30 | 7–15 | 2, type 3 | 7.0 | A | A | Zoo. spec. p. c. |
| Dryadopollis sp.2 | Microreticulate | 10 | Tricolporate | NA | NA | NA | 0.3 | A | A | Zoo. gen. |
| Foveotricolporites rhombohedralis | Microfoveolate | 47 | Tricolporate | NA | NA | NA | 0.5 | A | 0.5 | Zoo. gen. |
| cf. Foveotricolporites sp. | Microfoveolate | 30 | Tricolporate | NA | NA | NA | P | A | A | Wind (?) |
| Nyssapollenites sp. | Microfoveolate | 14 | Tricolporate | NA | NA | NA | 0.8 | A | 2.0 | Zoo. spec. p. c. |
| Tricolporate sp.2 | Striate | 25 | Tricolporate | NA | NA | NA | A | A | P | Wind (?) |
Monosulcate pollen grains are listed first followed by tricolpate and tricolporate. Grain size is average of largest dimension. Frequencies were calculated based only on the dispersed grains found (nonangiospermous taxa were excluded) at Courtland Clay Pit (C), Highway 4 Clay Pit (H), Ochs Clay Pit (O). Present (P) indicates species found but not in random counts of grains used to calculate frequencies. A, absent. Modes of pollination are based on Table 4: Zoo., zoophilous; gen., general; spec., specialized; p. c., pollen collecting; oth., other; NA, not available.
Fig. 2.
Pollen species from the Cenomanian Dakota Formation, Minnesota (27, 39) that shows clumping. (A and B) Artiopollis indivisus Agasie, 1969. (A) 046535-PY02A, S32, midfocus. Pollen clump. (B) SEM, 046533 stub3. Individual dispersed grain. (C and D) Tricolpites sp. (C) 046533-PY03A, R35/1, midfocus. Pollen clump. (D) SEM, 046533 stub3. Individual dispersed grain. (E and F) cf. Phimopollenites sp. (E) 046517-A1, + 10 μm, F14, midfocus. Pollen clump. (F) SEM, 046517 stub 1, polar view. Individual dispersed grain. (G) Tricolpites cf. vulgaris (Pierce) Srivastava, 1969, 036690 + 10 μm, Y42/2, midfocus. Pollen clump. (H) cf. Psilatricolporites sp., 046522-PY01A, N29/3, midfocus. Pollen clump. (I) Liliacidites cf. reticulatus (Brenner) Singh, 1971, 036716-A5 + 10 μm, M16, midfocus. Pollen clump. (J) Rousea cf. delicipollis Srivastava, 1975, 18297 + 10 μm, V33/2, midfocus. Pollen clump. (K) Cupuliferoidaepollenites sp., 046522-PY01A, Q18/4, midfocus. Pollen clump. (L) Dryadopollis sp.1, 036708 + 10 μm, N36, midfocus. Pollen clump. (Scale bars: A, C, E, and G–L, 10 μm; B, 5 μm; D and F, 2 μm.)
Although two pollen species have been reported in clumps from the Early Cretaceous of Eastern North American (40, 41), the importance of clumping was not appreciated until recently (11, 15, 16). Clumping may be caused by several methods including viscous fluids (tryphine, pollenkitt, elastoviscin), tangling, and common walls, resulting in clumps of variable sizes or some with uniform sizes and numbers of grains in permanent polyads and pollinia (15). Clusters of more than a few grains are rare in wind-pollinated plants because clumped pollen falls faster (11, 15, 16). The sticky surface of the pollen that causes clumping plays several roles in pollen dispersal and pollination, including retaining pollen in the anther, increasing adhesion to the pollinator, and transporting multiple pollen grains to the stigma (15). Multiple pollen grains on the stigma appear to increase fitness through pollen tube competition (42), are advantageous when pollinators are in low numbers or nonspecific in behavior (43), and lower the chance of individual pollen grains becoming dehydrated (44). Because of the important role pollen clumps can play in the reproductive biology of animal-pollinated plants, their occurrence is a significant step in early flowering plant evolution (11, 15, 16).
Clumped fossil pollen may occur naturally or result from the remains of anthers, insect pellets, and insect packaging (40, 45, 46). Cretaceous pollen clumps have been suggested to be the contents of immature fossil anther fragments included in the preparation of dispersed grains (40). Our data show that clumped and dispersed grains of the same type have the same size and ornamentation and are not arranged in immature tetrads (Fig. 2), and each specific pollen type may be in clumps that are of variable sizes and shapes. In addition, no flower or anther mesofossils were found, although other mesofossils were discovered at the same localities (27, 39), suggesting that the clumps contain mature grains and are not derived from fragmented immature anthers. Pollen clumps in insect pellets have also been reported as products of insect activity (45, 46). However, the possibility that the pollen clumps reported here are from fecal pellets seems unlikely as the pollen grains in the fossil clumps are complete and show no signs of damage originating from insect chewing and digestion, and the entire clumps are not regular in shape with smooth margins as would be expected in fecal pellets. Moreover, fecal pellet mesofossils were found at the same localities, but these did not contain pollen grains (S.H., personal observation). Modern bees often make pollen packages, although they usually have a mix of pollen types (47). None of the fossil clumps have more than one pollen type, so insect packaging is unlikely.
The fossil clumps are likely caused by pollen stickiness and originated either from mature fossil anthers or as dispersal events. In either case, the stickiness that forms the pollen clumps indicates an adaptation for zoophilous pollination. Additionally, none of the characters associated with wind pollination (including being dry when dispersed) were found in the fossil clumps (Table 3). Finally, clumping has been observed in fossil flowers. One of these fossil flowers shows pollen grains clustered in the anthers and clumps of identical pollen on the stigma (9). There is strong evidence that these flowers were pollinated by bees (9, 48). We propose that fossil pollen clumping is evidence for sticky pollen and that this character evolved by the mid-Cretaceous.
We define here four modes of pollination: wind, zoophilous general, zoophilous-specialized pollen collecting, and zoophilous-specialized other, based on literature about extant plants (Table 4). Pollen from wind-pollinated species has smooth ornamentation (e.g., scabrate, microreticulate, microfoveolate, striate), a dry surface, a size range of 25 to 40 μm, with little (two to three grains) or no clumping, and pollen produced in large quantities (10–12). Zoophilous-general flowers have grains that are ornamented, may be sticky, are 10–300 μm in size, can be clumped, and are produced at variable numbers (4, 10, 13, 14). Pollen grains from zoophilous-specialized pollen-collecting flowers are usually clumped, <24 μm in size, and produced at high numbers (13, 49, 50). Pollen grains classified as zoophilous-specialized other are usually of large size and produced in low numbers (14, 49, 50).
Table 4.
Criteria for wind and zoophilous modes of pollination based on extant plants
| Pollination mode | Surface feature | Pollen size, μm | Dispersal method | Pollen production |
|---|---|---|---|---|
| Wind-pollinated | Smooth and dry | 25–40 | Individually | Large quantities |
| Animal-pollinated | ||||
| Zoophilous general | Ornamental, may be sticky and oily | 10–300 | Individually or clumped | Variable quantities |
| Zoophilous specialized pollen collecting | Ornamental, sticky, and oily | <24 (?) | Usually clumped | Large quantities |
| Zoophilous specialized other | Ornamental, sticky, and oily | Usually large | Individually, clumped (?) | Low quantities |
Accordingly, each of the 41 fossil species of clumped and dispersed pollen is assigned to one of these four suggested modes of pollination. The first group of fossil pollen has characteristics of the wind mode including “wind?” in Table 3, designated for grains with wind morphology but low abundance, includes 24% of species, is always found dispersed, and is never in clumps. Ten species in this wind mode type range from being rare to having frequencies up to 5%. Based on the variation of abundance between sites, frequency of grains is probably not the best trait for distinguishing the wind mode from zoophilous modes in the fossil record. The remaining 31 species are classified as zoophilous based on ornamentation and size and are divided into three modes. Sixteen fossil pollen species have the traits of the general zoophilous pollination mode including low frequencies (0.1–3%, generally lower than the wind-pollinated species) and variable size (39% of the dispersed species, two species with type 2 clumps; Table 3). Nevertheless, the fact that two of these species have clumped pollen indicates advances in the evolution of sticky pollen. Eleven fossil pollen species that occur in frequencies from 2–72% and are <24 μm in size (27% of the dispersed species, seven species with type 1 or 3 clumps; Table 3) are typical of pollen grains produced for the zoophilous-specialized pollen-collecting mode. In these cases stickiness is important for pollinator transport and the majority of our fossil species were found as type 1 or 3 clumps (Table 3). High pollen production is typical in modern insect-pollinated angiosperms that use pollen as a primary attractant (13), with flowers adapted for bee pollination known to produce large quantities of pollen (51). There is fossil evidence of pollen feeders from a mid-Cretaceous flower that produced large quantities of pollen (46). Finally, four fossil species that are extremely rare and usually have large pollen grains (10% of the dispersed species) are similar to those with zoophilous-specialized other modes. Large pollen grains are found in some derived lepidopterian pollination systems (49).
The diversity including generalized to specialized insect pollination observed in extant angiosperms is also found in the fossil pollen species reported here (Table 1). Monosulcate pollen is found in extant basal angiosperms and monocots, and we infer that 87% of the fossil pollen species reported here were insect-pollinated and that 29% of the fossil pollen species had specialized modes of pollination (Table 1), mostly in the monosulcate (putatively monocot) species (Table 3). This finding is in contrast to fossil tricolpate-derived species (putatively eudicots) of which 65% are inferred to have specialized modes (Tables 1 and 3). The number of wind-pollinated species also increases from 13% in fossil monosulcate to 32% in fossil tricolpate species (Table 1).
During the Early Cretaceous, angiosperm pollen production was low (4), and apparently clumping was rare (40). Our data strongly suggest that by the mid-Cretaceous there is evidence of adaptations to permit pollen clumping and increases in specialized pollinators, which is consistent with insect molecular phylogeny showing bees originated between 110 MYA and 90 MYA (18), and the earliest fossil bee is reported from Early Cretaceous (21). This is a period of major radiation for the angiosperms (24, 28). Thus, the increase in specialized pollination modes may be linked to bee pollination.
Our reconstruction of the evolution of pollination modes supports the hypothesis that insect pollination is the initial pollination mode for angiosperms and suggests that more specialized animal pollination modes are derived. Fossil data from the mid-Cretaceous pollen record also provide evidence for specialized pollination modes and indicates that pollenkitt and other compounds that permit pollen clumping appeared later. Together, the pollination modes of extant basal angiosperms, coupled with dispersed and clumped pollen data, support the hypothesis that zoophilous pollination was common during the mid-Cretaceous and that specialization had begun to occur. These hypotheses on the evolution of specific modes of pollination and pollen stickiness need to be tested further with studies specifically looking for angiosperm fossil pollen clumping during the Early Cretaceous.
Materials and Methods
Three groups of angiosperm families (refs. 24 and 25, www.mobot.org/MOBOT/research/Apwe, and Table 2) were examined: noneudicots and nonmonocots (Amborellales, Nymphaeales, Austrobaileyales, Ceratophyllales, Chloranthales, Magnoliales, Laurales, Canellales, Piperales), basal monocot clades (Acorales, Alismatales), and basal eudicot clades (Ranunculales, Sabiales, Proteales, Trochodendrales, Buxales, Gunnerales). Pollinators were identified for the species of each family and placed in the following pollinator groups: Coleoptera (beetle), Diptera, Hymenoptera, Micropterigidae (basal family of Lepidoptera), Thysanoptera (thrips), wind, and water (refs. 23 and 29 and Table 2). Data were summarized as follows. First, pollination modes (52) for the species from each family were identified as wind, insect, or water. Families with more than one mode were counted multiple times. Percentages were based on the number of families with the mode divided by the total number of families in the group; thus, many families were counted more than once. Second, the number of families with specialized pollination (water and Hymenoptera) were counted, and percentages were calculated by dividing by the number of nonwind-pollinated families.
The MacClade (35) reconstruction of the pollination modes was based on two molecular trees, a conservative unresolved tree (refs. 24 and 30–32 and www.mobot.org/MOBOT/research/Apweb; specifically figure 3.3 in ref. 24, shown in Fig. 1), and a less-supported resolved tree (refs. 24, 30, 33, and 34, specifically figure 2.3 in ref. 24 and data not shown). The states were considered unordered and reversible, and polymorphic families were coded for all possible pollinator states. The unresolved tree could only be analyzed by the MPR method, whereas the resolved trees were also analyzed by DELTRAN and ACCTRAN. The definition of insect and specialized states is as above.
Three localities of the Cenomanian Dakota Formation in southwest Minnesota (27, 39) were investigated: Courtland Clay Pit (latitude 44°16′29″ N, longitude 94°23′13″W), Highway 4 Clay Pit (latitude 44°26′05″ N, longitude 94°43′37″W), and Ochs Clay Pit (latitude 44°13′26″ N, longitude 95°00′42″W). Pollen samples were collected vertically at 30-cm intervals from each of the sections sampled. Abundant palynomorphs where found in eight samples at the Courtland Clay Pit, three samples at Highway 4 Clay Pit, and seven samples at Ochs Clay Pit.
These samples were processed at the Paleobotany and Palynology Laboratory of the Florida Museum of Natural History, using standard pollen processing methods for siliciclastic and lignite samples (27, 53). All samples were coarsely crushed and sieved with a tea strainer before chemical processing. At least two slides were scanned to find pollen clumps and build a catalog of pollen and spore types for each sample. At least 300 palynomorphs were randomly counted to calculate frequencies. Frequencies in Table 3 were calculated based on dispersed grains identified, excluding nonangiospermous taxa. A Zeiss Axiophot microscope and an AxioCam digital camera and imaging capturing software were used for palynomorph identification and photography. Slides are stored in the Paleobotany and Palynology Collection of the Florida Museum of Natural History.
ACKNOWLEDGMENTS.
We thank R. Hunt, C. Labanderia, D. Soltis, and D. Grimaldi for helpful comments and T. Lott, S. Jarzen, and P. Zeigler for assistance with the manuscript. This project was supported by the 2004 Evolving Earth Foundation, 2004 Sigma Xi Grant in Aid of Research, and University of Florida Becker–Dilcher Research Fund (S.H.) This is University of Florida Contribution to Paleobiology paper no. 606.
Footnotes
The authors declare no conflict of interest.
References
- 1.Dilcher DL. Rev Palaeobot Palynol. 1979;27:291–328. [Google Scholar]
- 2.Crane PR, Friis EM, Pedersen KR. Science. 1986;232:852–854. doi: 10.1126/science.232.4752.852. [DOI] [PubMed] [Google Scholar]
- 3.Crepet WL, Nixon KC. In: The Anther: Form, Function, and Phylogeny. D'Arcy WG, Keating RC, editors. Cambridge, UK: Cambridge Univ Press; 1996. pp. 25–57. [Google Scholar]
- 4.Friis EM, Pedersen KR, Crane PR. Ann Mo Bot Gard. 1999;86:259–296. [Google Scholar]
- 5.Friis EM, Pedersen KR, Crane PR. Int J Plant Sci. 2000;161(Suppl 6):169–182. doi: 10.1086/314248. [DOI] [PubMed] [Google Scholar]
- 6.Herendeen PS, Crane PR, Drinnan AN. Int J Plant Sci. 1995;156:93–116. [Google Scholar]
- 7.Basinger JF, Dilcher DL. Science. 1984;224:511–513. doi: 10.1126/science.224.4648.511. [DOI] [PubMed] [Google Scholar]
- 8.Dilcher DL, Crane PR. Ann Mo Bot Gard. 1984;71:351–383. [Google Scholar]
- 9.Crepet WL, Taylor DW. Am J Bot. 1986;73:548–563. [Google Scholar]
- 10.Proctor M, Yeo P, Lack A. The Natural History of Pollination. Portland, OR: Timber Press; 1996. [Google Scholar]
- 11.Ackerman JD. In: Pollen and Pollination. Dafni A, Hesse M, Pacini E, editors. New York: Springer Wien; 2000. pp. 167–185. [Google Scholar]
- 12.Linder HP. In: Reproductive Biology. Owens SJ, Rudall PJ, editors. Kew, London: Royal Botanical Gardens; 1998. pp. 123–135. [Google Scholar]
- 13.Faegri K, Pijl L Van Der. The Principles of Pollination Ecology. Oxford: Pergamon; 1979. [Google Scholar]
- 14.Cruden RW. In: Pollen and Pollination. Dafni A, Hesse M, Pacini E, editors. New York: Springer Wien; 2000. pp. 143–165. [Google Scholar]
- 15.Pacini E. In: Pollen and Pollination. Dafni A, Hesse M, Pacini E, editors. New York: Springer Wien; 2000. pp. 19–43. [Google Scholar]
- 16.Pacini E, Franchi GG. In: Reproductive Biology. Owens SJ, Rudall PJ, editors. Kew, London: Royal Botanical Gardens; 1998. pp. 183–195. [Google Scholar]
- 17.Grimaldi D. Ann Mo Bot Gard. 1999;86:373–406. [Google Scholar]
- 18.Grimaldi D, Engel MS. Evolution of the Insects. Cambridge, UK: Cambridge Univ Press; 2005. [Google Scholar]
- 19.Labandeira CC. In: Phanerozoic Terrestrial Ecosystems. Gastaldo RA, Di Michele WA, editors. Lancaster, PA: Paleontological Society; 2000. pp. 233–269. [Google Scholar]
- 20.Labandeira CC. In: Plant-Animal Interactions. Herrera CM, Pellmyr O, editors. Malden, MA: Blackwell; 2002. pp. 26–74.pp. 248–261. [Google Scholar]
- 21.Poinar GO, Danforth BN. Science. 2006;314:614. doi: 10.1126/science.1134103. [DOI] [PubMed] [Google Scholar]
- 22.Pellmyr O. In: Plant-Animal Interactions. Herrera CM, Pellmyr O, editors. Malden, MA: Blackwell; 2002. pp. 157–184. [Google Scholar]
- 23.Thien LB, Azuma H, Kawano S. Int J Plant Sci. 2000;161:S225–S235. [Google Scholar]
- 24.Soltis DE, Soltis PS, Endress PK, Chase MW. Phylogeny and Evolution of Angiosperms. Sunderland, MA: Sinauer; 2005. [Google Scholar]
- 25.Angiosperm Phylogeny Group. Bot J Linn Soc. 2003;141:399–436. [Google Scholar]
- 26.Wallace HM, Maynard GV, Trueman SJ. Aust J Entomol. 2002;41:55–59. [Google Scholar]
- 27.Hu S. PhD dissertation. Gainesville: University of Florida; 2006. [Google Scholar]
- 28.Lidgard S, Crane PR. Nature. 1988;331:344–346. [Google Scholar]
- 29.Thien LB, Sage TL, Jaffre T, Bernhardt P, Pontieri V, Weston PH, Malloch D, Azuma H, Graham SW, McPherson MA, et al. Ann Mo Bot Gard. 2003;90:466–490. [Google Scholar]
- 30.Zanis M, Soltis DE, Soltis PS, Mathews S, Donoghue MJ. Proc Natl Acad Sci USA. 2002;99:6848–6853. doi: 10.1073/pnas.092136399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nickrent DL. In: Plantas Parásites de la Peninsula Ibérica e Islas Baleares. López-Sáez JA, Catalán P, Sáez L, editors. Madrid: Mundi-Prensa Libros; 2002. pp. 29–56. [Google Scholar]
- 32.Qiu YL, Lee JY, Bernasconi-Quadroni F, Soltis DE, Soltis PS, Zanis M, Chen Z, Savolainen V, Chase MW. Nature. 1999;402:404–407. doi: 10.1038/46536. [DOI] [PubMed] [Google Scholar]
- 33.Soltis DE, Soltis PS, Chase MW, Mort M, Albach D, Zanis M, Savolainen V, Hahn W, Hoot S, Fay M, et al. Bot J Linn Soc. 2000;133:381–461. [Google Scholar]
- 34.Soltis DE, Senters AE, Zanis M, Kim S, Thompson JD, Soltis PS, Ronse De Craene LP, Endress PK, Farris JS. Am J Bot. 2003;90:461–470. doi: 10.3732/ajb.90.3.461. [DOI] [PubMed] [Google Scholar]
- 35.Maddison WP, Maddison DR. MacClade: Analysis of Phylogeny and Character Evolution. Sunderland, MA: Sinauer; 1992. [DOI] [PubMed] [Google Scholar]
- 36.Teeling EC, Springer MS, Madsen O, Bates P, O'Brien SJ, Murphy WJ. Science. 2005;307:580–584. doi: 10.1126/science.1105113. [DOI] [PubMed] [Google Scholar]
- 37.Ericson PGP, Irestedt M, Johansson US. J Avian Biol. 2003;34:3–15. [Google Scholar]
- 38.Brenner GJ. In: Flowering Plant Origin, Evolution, and Phylogeny. Taylor DW, Hickey LJ, editors. New York: Chapman & Hall; 1996. pp. 91–115. [Google Scholar]
- 39.Hu S, Dilcher DL, Schneider H, Jarzen DM. Int J Plant Sci. 2006;167:579–589. [Google Scholar]
- 40.Doyle JA, Van Campo M, Lugardon B. Pollen Spores. 1975;17:429–486. [Google Scholar]
- 41.Brenner GJ. The Spores and Pollen of the Potomac Group of Maryland. Baltimore: Waverly; 1963. [Google Scholar]
- 42.Erbar C. Int J Plant Sci. 2003;164:S265–S277. [Google Scholar]
- 43.Harder LD, Barrett SCH. In: Floral Biology: Studies on Floral Evolution in Animal-Pollinated Plants. Lloyd DG, Barrett SCH, editors. New York: Chapman & Hall; 1995. pp. 140–190. [Google Scholar]
- 44.Dafni A, Firmage D. In: Pollen and Pollination. Dafni A, Hesse M, Pacini E, editors. New York: Springer Wien; 2000. pp. 113–132. [Google Scholar]
- 45.Davis OK, Buchmann SL. AASP Contrib Ser. 1994;29:63–73. [Google Scholar]
- 46.Lupia R, Herendeen PS, Keller JA. Int J Plant Sci. 2002;163:675–686. [Google Scholar]
- 47.Klungness LM, Peng Y. J Apicult Res. 1983;22:264–271. [Google Scholar]
- 48.Taylor DW, Crepet WL. Am J Bot. 1987;74:274–286. [Google Scholar]
- 49.Baker HG, Baker I. Am J Bot. 1979;66:591–600. [Google Scholar]
- 50.Harder LD. Biol J Linn Soc. 1998;64:513–525. [Google Scholar]
- 51.Harder LD. Ecology. 1990;71:1110–1125. [Google Scholar]
- 52.Gomez MJ, Zamora R. In: Plant-Pollinator Interactions: From Specialization to Generalization. Waser NM, Ollerton J, editors. Chicago: Univ of Chicago Press; 2006. pp. 145–166. [Google Scholar]
- 53.Traverse A. Paleopalynology. Boston: Unwin Hyman; 1988. [Google Scholar]
- 54.Eriksson O, Bremer B. Evolution (Lawrence, Kans) 1992;46:258–266. doi: 10.1111/j.1558-5646.1992.tb02000.x. [DOI] [PubMed] [Google Scholar]
- 55.Judd WS, Campbell CS, Kellogg EA, Stevens PF, Donoghue MJ. Plant Systematics. Sunderland, MA: Sinauer; 2002. [Google Scholar]
- 56.Judd WS. Advanced Plant Taxonomy: Freaky Families. Gainesville: Univ of Florida; 2006. [Google Scholar]
- 57.Watson L, Dallwitz MJ. Delta: Description Language for Taxonomy. Albany, Australia: Commonwealth Scientific and Industrial Research Organization; 1992. [Google Scholar]
- 58.Bhardwaj M, Eckert CG. Am J Bot. 2001;88:2204–2213. [PubMed] [Google Scholar]
- 59.Tanaka N, Uehara K, Murata J. J Plant Res. 2004;117:265–276. doi: 10.1007/s10265-004-0155-5. [DOI] [PubMed] [Google Scholar]
- 60.Hannan GL, Prucher HA. Am Midl Naturalist. 1996;136:267–277. [Google Scholar]
- 61.Kawagoe T, Suzuki N. Ecol Res. 2002;17:295–303. [Google Scholar]
- 62.Sohmer SH, Sefton DF. Brittonia. 1978;30:355–364. [Google Scholar]