Abstract
Agouti lethal yellow (Ay) mice express agouti ectopically because of a genetic rearrangement at the agouti locus. The agouti peptide is a potent antagonist of the melanocortin 4 receptor (MC4R) expressed in neurons, and this leads to hyperphagia, hypoactivity, and increased fat mass. The MC4R signals through Gs and is thought to stimulate the production of cAMP and activation of downstream cAMP effector molecules such as PKA. Disruption of the RIIβ regulatory subunit gene of PKA results in release of the active catalytic subunit and an increase in basal PKA activity in cells where RIIβ is highly expressed. Because RIIβ is expressed in neurons including those in the hypothalamic nuclei where MC4R is prominent we tested the possibility that the RIIβ knockout might rescue the body weight phenotypes of the Ay mice. Disruption of the RIIβ PKA regulatory subunit gene in mice leads to a 50% reduction in white adipose tissue and resistance to diet-induced obesity and hyperglycemia. The RIIβ mutation rescued the elevated body weight, hyperphagia, and obesity of Ay mice. Partial rescue of the Ay phenotypes was even observed on an RIIβ heterozygote background. These results suggest that the RIIβ gene mutation alters adiposity and locomotor activity by modifying PKA signaling pathways downstream of the agouti antagonism of MC4R in the hypothalamus.
Keywords: adiposity, cAMP, hypothalamus
The rapid increase in obesity in the human population and the associated increased risk for ailments such as diabetes and cardiovascular disease has driven an intense effort to understand the physiological pathways that regulate body weight. Both induced and spontaneous mutations in the mouse genome have been instrumental in identifying the genes and cell types that play a role in energy balance in mammals. We have previously determined that mice lacking the RIIβ regulatory subunit of PKA exhibit a 10% reduction in body weight and a 50% decrease in white adipose tissue (WAT) and are resistant to diet-induced obesity and hyperglycemia (1, 2). RIIβ knockout (KO) mice have a 2-fold increase in their nocturnal locomotor activity and an increase in resting metabolic rate as measured by oxygen consumption (1, 3, 4). These studies have established that signaling through the RIIβ–PKA pathway can have dramatic effects on both body weight and energy expenditure in mice. The RIIβ subunit of PKA is highly expressed in WAT, brown adipose tissue (BAT), and brain (1, 5), but significant expression is also seen in several other tissues including thyroid, testis, and ovary (6–8). The contribution of each of these tissues to the overall RIIβ KO phenotype has not yet been resolved.
The arcuate nucleus region (ARC) of the hypothalamus serves as a center to integrate signals that control feeding and energy expenditure. Two anatomically distinct populations of leptin-responsive neurons exist in the ARC; those that express neuropeptide Y (NPY) and agouti-related protein (AgRP) and those that express proopiomelanocortin (POMC) (9, 10). The NPY/AgRP and POMC neurons project from the ARC to the paraventricular hypothalamus (PVH) and other regions that express the Gs-coupled melanocortin receptors MC3R and MC4R. Activation of the MC4R by α-melanocyte-stimulating hormone (α-MSH) released from POMC neurons is thought to stimulate the cAMP pathway and lead to a decrease in food intake and an increase in energy expenditure. AgRP acts to antagonize the action of α-MSH on both MC3R and MC4R. A knockout of the MC4R gene causes obesity (11), and disruption of NPY (12, 13) or AgRP (14) production leads to more subtle changes in food intake and body weight.
Agouti protein is normally expressed in the skin, but a chromosomal deletion in agouti lethal yellow (Ay) mice places the agouti gene next to a constitutively active promoter causing agouti to be expressed almost ubiquitously, including the hypothalamus (15–18). Mice carrying just a single Ay allele are obese, hypoactive, hyperphagic, hyperglycemic, and hyperinsulinemic (19, 20). Injection of a viral vector expressing agouti only in the PVH also caused mice to become hyperphagic and gain weight (21). These studies support the hypothesis that antagonism of melanocortin receptors in the PVH is at least partially responsible for the obesity syndrome in Ay mice (11, 22–24). Because agouti is a specific antagonist of MC4R but not MC3R, the MC4R is thought to be the key player in body weight regulation (22).
The intracellular signaling pathways through which melanocortin receptors exert their effects in the hypothalamus are not well understood. It is clear that cAMP levels rise dramatically after melanocortin receptor (MCR) stimulation and that PKA is activated; however, other signaling pathways including the MAP kinase pathway and the inositol phospholipid/Ca2+ pathways have been implicated in MCR signaling (25–30). RIIβ is expressed throughout the hypothalamus in many of the same regions that also contain MCRs including the ARC, PVH, dorsal medial hypothalamus (DMH), and ventral medial hypothalamus (VMH) (5, 31–33). Each of these regions has distinct effects on food intake and energy expenditure. Because RIIβ KO mice exhibit a phenotype that is consistent with increased anorexigenic tone downstream of MCR, we hypothesized that deletion of RIIβ might alleviate the obesity syndrome of Ay mice. We found that deletion of RIIβ reduces both food intake and adiposity in Ay mice. The deletion of RIIβ not only rescues the hypoactivity normally seen in Ay mice but increases nocturnal activity to levels that surpass WT controls and approach the hyperactivity phenotype of the RIIβ KO mice. These data suggest that the RIIβ gene mutation is modifying the signaling pathway downstream of the agouti antagonism of melanocortin receptors in the hypothalamus.
Results
Deletion of RIIβ Decreases the Body Weight and Linear Growth of Ay Mice.
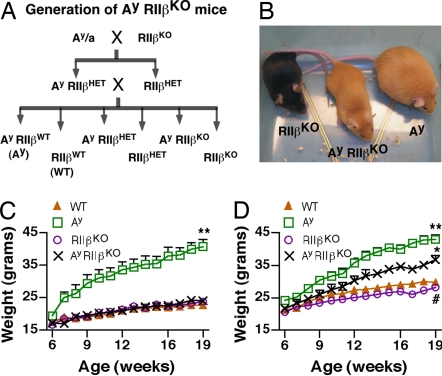
The PKA holoenzyme is composed of two catalytic subunits and two regulatory subunits that are either type I (RIα,β) or type II (RIIα,β). Previous studies in our laboratory have determined that in RIIβ KO mice there is an increase in basal PKA activity as well as a compensatory increase in the type I PKA holoenzyme that is half-maximally activated by lower cAMP levels than the type II holoenzyme (1, 5, 34, 35). The mating strategy depicted in Fig. 1A was used to derive Ay/RIIβ double mutants (Ay/RIIβ KO) and control animals. Previous studies have shown that Ay mice begin to significantly differ in their body weight relative to controls by 6–8 wk of age (24, 36, 37). We found that female Ay/RIIβ KO mice were visibly smaller than Ay (Fig. 1B), and they maintained a body weight similar to WT at least through 19 wk of age (Fig. 1C). A similar result was found in males, but the rescue was not as complete (Fig. 1D).
Fig. 1.
Generation of Ay/RIIβ KO mice and effects on body weight. (A) Mating scheme to generate Ay mice lacking RIIβ (Ay/RIIβ KO) and littermate controls. (B) Female Ay/RIIβ KO mouse and littermate controls at 20 wk of age. Ay/RIIβ KO mice are visibly smaller than Ay controls but still retain the yellow color of Ay mice. (C) Growth curves of females from 6 to 19 wk of age. **, P < 0.001 vs. all groups. (D) Growth curves of males from 6 to 19 wk of age. **, P < 0.001 vs. WT; *, P < 0.01 vs. WT and Ay; #, P < 0.05 vs. WT.
Ay mice also have increased linear growth that correlates with elevated circulating IGF-1 levels (38). We compared linear growth at the time that mice were killed and verified that female Ay mice were significantly longer than WT controls. We also determined that linear growth was normalized in Ay/RIIβ KO mice (Table 1). The rescue of both body weight and linear growth in Ay/RIIβ KO mice is consistent with our hypothesis that the RIIβ KO phenotype is due to increased activation of PKA downstream of the melanocortin receptors. Significant reductions in body weight were also found in male Ay/RIIβ KO mice compared with Ay controls (Fig. 1D). However, the extent of the rescue was not as complete as in females, and male Ay/RIIβ KO mice still weighed significantly more than WT mice at all ages examined. Linear length was not significantly different among the genotypes of males analyzed at 19–20 wk of age.
Table 1.
The RIIβ KO background rescues the Ay body weight phenotypes in female mice
| Mice | Body weight, g | Length, cm | BAT, mg | REPRO, mg | PSOAS, mg | ING, mg | WAT, %BW | Liver, g | Leptin, ng/ml |
|---|---|---|---|---|---|---|---|---|---|
| WT | 23.5 ± 0.9 | 9.37 ± 0.08 | 47 ± 5 | 417 ± 73 | 72 ± 20 | 273 ± 52 | 3.2 ± 0.5 | 1.04 ± 0.06 | 4.5 ± 2.0 |
| Ay | 41.9 ± 2.0 | 9.81 ± 0.04 | 287 ± 28 | 2,896 ± 267 | 428 ± 37 | 1,695 ± 114 | 12.0 ± 0.8 | 2.04 ± 0.21 | 55.6 ± 10.8 |
| RIIβ | 23.8 ± 0.5 | 9.45 ± 0.04 | 68 ± 12 | 239 ± 25 | 41 ± 5 | 222 ± 16 | 2.1 ± 0.2 | 1.17 ± 0.04 | 1.1 ± 0.2 |
| Ay/RIIβ | 27.0 ± 1.9* | 9.42 ± 0.12* | 104 ± 21* | 808 ± 237* | 154 ± 41* | 604 ± 146* | 5.3 ± 1.1* | 1.27 ± 0.08* | 12.1 ± 4.5 |
Animals were all littermates from the crosses indicated in Fig. 1A and were killed at 20–23 weeks of age. Length is the measurement in centimeters of the snout to anus distance. WAT is the combined weight of the reproductive (REPRO), retroperitoneal (PSOAS), and inguinal (ING) fat pads as a percentage of body weight (BW). Data were analyzed with one-way ANOVA and Tukey's multiple-comparison test.
*, P < 0.01 vs. Ay.
Deletion of RIIβ Increases Locomotor Activity and Decreases Adiposity, Food Intake, and Serum Leptin in Ay Mice.
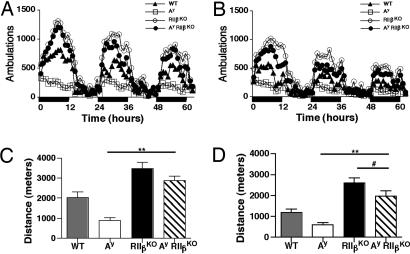
The Ay mice exhibited decreased levels of locomotor activity as previously published (36). However, deletion of RIIβ raised the locomotor activity of Ay mice above that of WT controls (Fig. 2), and Ay/RIIβ KO mice were ≈1.5 times as active as controls. In both females and males the locomotor activity of Ay mice was approximately half that of WT. In contrast, the RIIβ KO mice are twice as active as WT, as we have previously published (3, 4), and it appears that this phenotype of hyperactivity persisted in the Ay/RIIβ KO double mutants.
Fig. 2.
Locomotor activity. The number of ambulations per hour measured over 3 days for females (A) and males (B). Each ambulation is equivalent to 8 cm of distance traveled. Black bars on the x axis indicate lights-off. (C) The total distance traveled for females in meters over three consecutive 12-h dark cycles and two 12-h light cycles. **, P < 0.001, P < 0.05 (WT vs. Ay), and P < 0.01 (WT vs. RIIβ KO). (D) The total distance traveled for males as described in C. **, P < 0.001; #, P < 0.05 and P < 0.001 (WT vs. RIIβ KO).
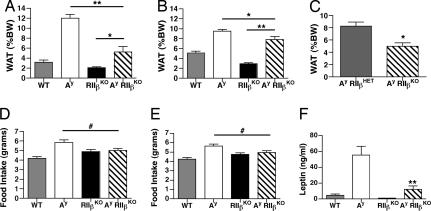
Deletion of RIIβ resulted in a 34% reduction in adiposity in RIIβ KO mice as compared with controls, consistent with our previous findings (1). Deletion of RIIβ also reduced the adiposity of Ay mice. Female Ay/RIIβ KO mice had a 56% reduction in adiposity compared with Ay (Fig. 3A). The weights of three major WAT pads were significantly reduced in Ay/RIIβ KO mice (Table 1). Liver weights were also significantly reduced, and visual inspection of the liver revealed that the fatty liver typical of Ay mice was rescued to a more normal size and color in Ay/RIIβ KO mice. Male Ay/RIIβ KO mice were also significantly leaner than Ay controls (Fig. 3B), with a 17% reduction in total adiposity. These reductions were more modest than those seen in females of similar age. However, in younger, 13- to 14-wk-old males we observed a 40% reduction in adiposity of Ay/RIIβ KO mice compared with Ay controls (Fig. 3C).
Fig. 3.
Adiposity, feeding, and leptin. (A) Female Ay/RIIβ KO mice are significantly leaner than Ay controls. **, P < 0.001; *, P < 0.01, P < 0.001 (WT vs. Ay), and P < 0.01 (WT vs. RIIβ KO, Student's t test). (B) Male Ay/RIIβ KO mice are significantly leaner than Ay mice but significantly more obese than WT mice. (C) Decreased adiposity in 13.5-wk-old male Ay/RIIβ KO mice compared with Ay/RIIβ HET. Shown is WAT pad weight as a percentage of body weight at 13.5 wk of age. *, P < 0.003 (Student's t test). (D) Average food intake at 18 wk of age in females. Ay mice consume more food than WT controls (P < 0.01), but food intake is decreased in Ay/RIIβ KO mice. #, P < 0.05. (E) Food intake in male Ay/RIIβ KO mice. Plotted is the average daily food intake over 7 consecutive days measured in grams per mouse per day. #, P < 0.05. (F) Serum leptin levels are decreased in female Ay/RIIβ KO mice. **, P < 0.001 (WT vs. Ay and Ay vs. Ay/RIIβ KO).
Ay mice were hyperphagic as expected, but food intake was significantly decreased in female Ay/RIIβ KO mice (Fig. 3D). A similar reduction in food intake was found in male Ay/RIIβ KO mice (Fig. 3E). Feeding in Ay/RIIβ KO mice was similar to RIIβ KO mice, and, consistent with previous reports (1, 4), the RIIβ KO mice tended to eat slightly more than controls.
Leptin is an adipose tissue-specific hormone that is secreted in proportion to body fat and is one of the key satiety signals (39). The hypothalamus normally responds to increased leptin levels by stimulating neuronal pathways that reduce food intake. In Ay mice and other mouse models of obesity there are very high levels of leptin, as shown in Fig. 3F, but the animals are leptin-insensitive, which contributes to their hyperphagia (24, 40). Because decreased serum leptin levels in RIIβ KO mice and other strains have been a good indicator of adiposity (1, 2, 41), we measured serum leptin levels in females and found that they were ≈80% lower in Ay/RIIβ KO compared with Ay mice (Fig. 3F and Table 1). This decrease in leptin is consistent with the decreased adiposity of the Ay/RIIβ KO mice.
RIIβ Haploinsufficiency Can Attenuate the Obesity Syndrome of Ay Mice.
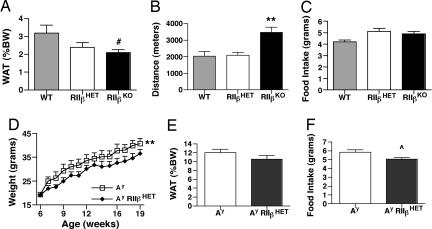
To test whether a heterozygous deficiency in RIIβ would be sufficient to partially rescue the Ay phenotype we first characterized the phenotype of the RIIβ heterozygotes in more detail. RIIβ heterozygous mice have a lean body weight phenotype that is intermediate between WT and KO. Female heterozygotes had a 25% reduction in WAT pad weight (Fig. 4A) and a 60% reduction in leptin levels (WT, 4.5 ± 2 ng/ml, n = 4; RIIβ heterozygotes, 1.8 ± 0.4 ng/ml, n = 6; RIIβ KO, 1.1 ± 0.2 ng/ml, n = 6). There was no increase in nocturnal locomotor activity (Fig. 4B). Male RIIβ heterozygotes were 14% leaner than WT controls. Both male and female RIIβ heterozygous mice also tended to be slightly hyperphagic, similar to RIIβ KO mice (Fig. 4C).
Fig. 4.
Partial rescue of Ay phenotypes on RIIβ heterozygous background. (A) Female adiposity. (B) Female locomotor activity. (C) Food intake. (D) Ay/RIIβ HET mice have decreased body weight. **, P < 0.001 (paired Student's t test). (E) Decreased white fat pad weight. (F) Decreased food intake. ⋀, P = 0.02. All data shown are for female mice.
The Ay mice were crossed onto an RIIβ heterozygous background, and parameters associated with the obesity of the Ay mice were measured. Ay/RIIβ heterozygote females had decreased body weight (Fig. 4D) but did not show the complete rescue of body weight seen for the Ay/RIIβ KO mice (Fig. 1C). WAT pad weight (Fig. 4E) and food intake (Fig. 4F) were partially rescued on the RIIβ heterozygous background compared with Ay (see Fig. 3 A and D for comparison with the KO rescue). Leptin levels were also decreased (data not shown), consistent with the change in adiposity. Activity levels of Ay/RIIβ heterozygotes were increased compared with Ay but were still less than WT. Adiposity and body weight were also significantly decreased in male Ay/RIIβ+/− mice compared with Ay mice (data not shown). These data suggest that a 50% reduction in RIIβ is enough to partially alleviate some of the obesity syndrome of Ay mice but does not elicit the hyperactivityphenotype seen with RIIβ KO and Ay/RIIβ KO mice.
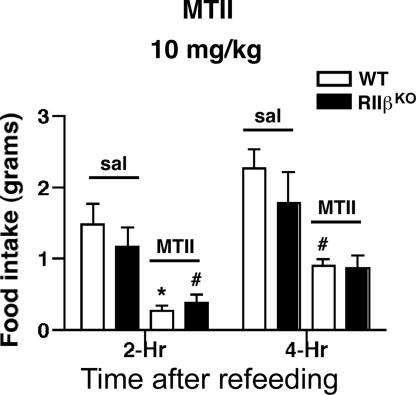
RIIβ KO Mice Respond Normally to Stimulation of Melanocortin Receptors with MTII.
Cell culture models have established that the melanocortin receptor agonist melanotan II (MTII) increases intracellular cAMP levels in cells transfected with either MC3R or MC4R. In vivo administration of MTII causes an acute decrease in food intake in animals that have been fasted and refed, and this response is thought to be mediated by activation of the MC4R on neurons in the PVH (22, 42). The rescue of hyperphagia in the Ay mice on an RIIβ KO background could be due to (i) an increased sensitivity to MCR ligands if neurons have compensated for the loss of RIIβ by forming more type I kinase, which activates at a lower threshold of cAMP (34, 43), or (ii) an increased constitutive PKA activity because a major regulatory subunit, RIIβ, is absent, and therefore a fraction of the C subunit is free in the cell. To distinguish between these possibilities, we tested for increased sensitivity to MTII in RIIβ KO mice. Mice were fasted overnight and then refed after i.p. injection of either saline or MTII. At a dose of 10 mg/kg MTII, both WT and RIIβ KO mice responded with a significant decrease in food intake during the first 4 h (Fig. 5). A low dose of 1 mg/kg MTII was tested to see whether the KO mice would respond to a dose that does not reduce feeding in WT mice, and the results showed that both WT and RIIβ KO mice had a similar small response that was not significant (data not shown). These results indicate that the MCR pathway continues to function in the absence of RIIβ–PKA and that there is not a substantial change in sensitivity to agonist. We favor the second possibility, that the constitutive tone of PKA activity is elevated in MCR-expressing neurons and that this decreases feeding and increases energy expenditure. Indeed, RIIβ KO mice are eating substantially less than would be expected for animals with a leptin level that is 1.1 ng/ml compared with the WT average of 4.5 ng/ml.
Fig. 5.
RIIβ KO mice have normal feeding response to the MC3/4R agonist MTII. Female WT and RIIβ KO mice responded similarly to an injection of 10 mg/kg MTII given i.p. in saline. Mice were fasted for 20 h before injection, and food was made available 15 min after injection.
Discussion
Although a great deal is known about the hormones, neuropeptides, and hypothalamic cell types that participate in body weight regulation, much less is understood about the interplay of intracellular signaling systems that underlie the regulation of feeding, energy expenditure, and fat storage. The MCR signaling system is a key regulator of body weight. Profound obesity develops in both mice and humans when MC4R is inactivated (11, 44), when the gene encoding the MCR ligand αMSH is deleted (45), or when the MC3/4R antagonist AgRP (23) or the MC4R antagonist agouti are overexpressed in the hypothalamus (19). Conversely, overexpression of αMSH leads to leanness (46), and AgRP KO mice remain relatively lean as they age compared with WT mice (14). Downstream signaling pathways from the melanocortin receptors have been studied in cell culture and nonhypothalamic tissue, and the results support the prediction for a Gs-coupled receptor: stimulation of cAMP production, activation of PKA, and changes in phosphorylation and gene expression. However, other signaling pathways have also been shown to be activated, resulting in activation of the inositol phospholipid pathway and release of Ca2+ (26), and activation of MAPK (27, 30). In addition, cAMP can activate Rap-1 guanine nucleotide exchange factors (Epacs) and cyclic nucleotide-gated ion channels, and any of these non-PKA signaling pathways could play a role in melanocortin receptor signaling.
Our initial reports on the lean and hyperactive phenotypes of RIIβ KO mice were not able to conclude which specific tissues were responsible for the phenotypes. A role for the induced expression of UCP1 in BAT was recently ruled out when we demonstrated that the lean and hyperactive phenotypes of RIIβ KO mice were not rescued in a UCP-1 KO background (3) and that reexpression of RIIβ in BAT does not reverse the phenotypes (M.A.S. and G.S.M., unpublished data). A cross between the RIIβ KO and ob/ob mice demonstrated that the RIIβ lean and hyperactive phenotype partially overcame the loss of leptin and suggested that the phenotypes of RIIβ KO mice arise from specific changes in leptin-responsive neuronal pathways (4). The results reported here show that the RIIβ KO background reverses the hyperphagia of Ay mice, stimulates locomotor activity to levels above WT, and reduces adiposity significantly in both males and females. We found that even the heterozygous RIIβ background was sufficient to partially reduce hyperphagia and body weight of the Ay mice. The rescue of the Ay phenotype suggests that the regulation of feeding and adiposity by the melanocortin receptor signaling pathway depends on PKA in vivo and that the phenotypes of the RIIβ KO mouse are likely due to increased PKA activity in the MC4R-expressing neurons of the hypothalamus that are the targets for agouti antagonism. However, it is also possible that the PKA mutation is affecting another pathway in the complex neuronal circuitry regulating energy homeostasis to effectively rescue the agouti phenotype. The neuronal basis of the RIIβ body weight phenotype was recently confirmed by preliminary results in our laboratory showing that reexpression of RIIβ only in neurons using a Cre recombinase-dependent approach reverses both the lean phenotype and the hyperlocomotor activity (M.A.S. and G.S.M., unpublished data).
Several transgenic and mutant mouse lines have been crossed to Ay mice to examine their ability to modify the Ay phenotypes. Overexpression of αMSH decreased body weight in female Ay mice (46), whereas deletion of NPY had no effect in Ay mice (47). Crosses of Ay with attractin (Atrn)- and mahogunin (Mgrn1)-deficient mice have had more dramatic rescue effects, similar to the Ay/RIIβ mice. Ay/Atrn and Ay/Mgrn1 double mutants have reduced body weight and adiposity and also exhibit increased locomotor activity compared with Ay mice (36, 37, 48), suggesting that attractin (a transmembrane protein that binds agouti) and mahogunin (an E3 ubiquitin ligase) act to block the action of agouti on MC4R-expressing neurons.
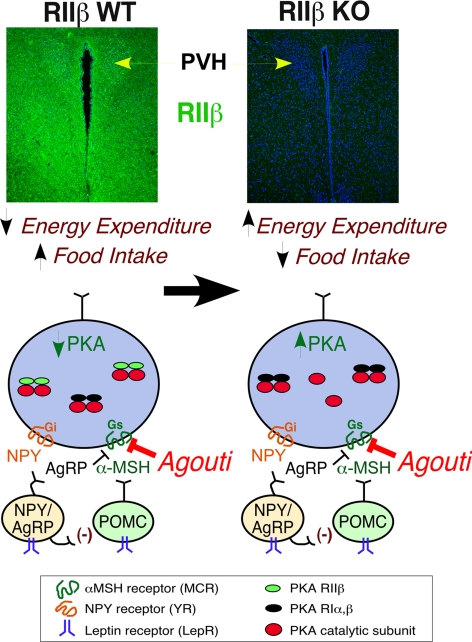
The rescue of hyperphagia and obesity in Ay mice by deletion of RIIβ suggests that in the absence of the regulatory subunit RIIβ there is more unregulated PKA catalytic subunit in MCR-expressing neurons in the hypothalamus (Fig. 6). We have previously reported a compensatory increase in the type I PKA holoenzyme in RIIβ KO tissues, and the type I PKA was found to be more sensitive to cAMP activation than the type II holoenzyme (1, 5, 34, 35). This compensatory increase in type I PKA occurs robustly in BAT and WAT, but a weaker compensation also occurs in brain. The net effect of having unregulated C subunit as well as a more sensitive type I PKA would be an overall increase in the basal activity of PKA signaling in hypothalamic neurons that normally respond to the anorexigenic peptide α-MSH. We predict that this could be responsible for the lean phenotype and increased energy expenditure in RIIβ KO mice. Ay mice ectopically express the MC4R antagonist agouti in hypothalamic nuclei, as shown in Fig. 6, and this is expected to prevent the MC4R-dependent stimulation of cAMP production and subsequent PKA activation. We propose that the RIIβ mutation acts downstream of MC4R and provides a direct stimulation of PKA activity even in the presence of agouti, thereby causing a rescue of agouti-dependent phenotypes.
Fig. 6.
Model of the interactions of PKA–RIIβ with agouti. In the absence of the predominant neuronal regulatory subunit RIIβ, it is proposed that there is unregulated PKA activity and an increase in type I kinase in key hypothalamic neurons that are also expressing MC4R. The result is an overall increase in PKA signaling downstream of the melanocortin receptors resulting in decreased feeding and increased energy expenditure in Ay/RIIβ KO mice. Immunohistochemistry detection of RIIβ expression in the hypothalamic areas of the PVH and surrounding structures is shown in green with DNA counterstained in blue (To-Pro-3).
Our experiments do not directly address the specific brain regions that are most important for the observed interaction between the RIIβ KO and Ay. Although agouti is an antagonist for both the type 1 and type 4 MCR, the MC4R is most closely associated with the regulation of body weight, feeding, energy expenditure, and body length, which are the parameters affected by ectopic agouti expression in Ay mice. The MC4R KO mouse and to a lesser extent the heterozygotes display a phenotype similar to that seen for Ay. One area of high expression of MC4R is the paraventricular nucleus (PVH) of the hypothalamus. Lesions of the PVH lead to hyperphagia (49, 50), and stereotaxic injection of MC4R ligands in this region regulate both feeding and energy expenditure (51, 52). The RIIβ protein is clearly expressed in the PVH, as shown by immunohistochemistry in Fig. 6, and we have also recently shown that a Sim1-Cre recombinase transgenic (gift from Bradford Lowell, Harvard University, Boston) will activate RIIβ expression in the PVH specifically using the RIIβneolox animals described in Methods [see supporting information (SI) Fig. 7]. Ectopic agouti expression is likely to be affecting this neuronal pathway by antagonizing the response of the MC4R to αMSH, as depicted in Fig. 6, and this would lead to the Ay body weight phenotypes. In a recent report Balthasar et al. (53) developed an MC4R null mouse line in which the gene could be reactivated by Cre recombinase. Using Sim1-Cre to reactivate the MC4R in the PVH they showed that this completely reversed the hyperphagia and body length increase associated with the MC4R KO and partially rescued the obesity. Remarkably, the reexpression of MC4R in the PVH did not rescue the diminished energy expenditure of the MC4R KO, suggesting that POMC neuronal projections to other regions of the brain are involved in the energy expenditure phenotype. It is likely that the reversal of agouti-induced hyperphagia and body length increase seen in Ay mice by inactivation of RIIβ is due to increased PKA function in the PVH. The elevation of locomotor activity we see in RIIβ KO and Ay/RIIβ KO mice may depend on other sites of RIIβ expression in the hypothalamus or elsewhere. Cell-type specific reexpression of RIIβ will be helpful to resolve this question in the future.
Methods
Generation of Ay/RIIβ KO Mice.
Homozygous RIIβneolox mice contain a loxed neomycin resistance cassette in the 5′ untranslated region of Prkar2b, are phenotypically identical to RIIβ null mice (1, 5), show no expression of RIIβ, and were used in the above crosses to Ay mice (M.A.S., unpublished data). Homozygous RIIβneolox mice are referred to as RIIβ KO mice in this study. Ay mice on a C57BL/6J background (strain B6.Cg-Ay/J) were purchased from The Jackson Laboratory. Mice for all experiments were littermates generated from mating Ay/RIIβ HET and RIIβ HET mice (Fig. 1A). Mice were maintained on a rodent chow diet (Picolab 20, 4.5% fat). The Ay allele was identified by coat color at the time of weaning (48). All procedures were approved by the Institutional Care and Use Committee of the School of Medicine of the University of Washington.
Body Weight and Adiposity.
Mice were killed at 20–23 wk of age, and adiposity was determined by weighing the three major WAT pads (inguinal, reproductive, and retroperitoneal). In addition, we weighed interscapular BAT located on the dorsal side of the mouse near the neck region. Body length was measured as the distance from the nose to the anus.
Locomotor Activity and Food Intake.
Locomotor activity was measured at 16 wk in a plastic activity chamber (47 × 25 × 21 cm) equipped with photobeams. Daily food intake was measured in home cages every 24 h for 7 consecutive days and averaged.
Leptin Measurements.
Mice were killed with CO2 gas. Whole blood was collected between 1200 and 1500 hours by cardiac puncture. The serum was collected and assayed for leptin concentration by ELISA.
MTII Treatment.
Mice were individually housed for 2 wk before injection. Mice were fasted for 20 h and then given an i.p. injection of either saline or MTII (Bachem) at a dose of 1–10 mg/kg of body weight. Food was made available 15 min after injection, and food intake was monitored at 2, 4, 8, and 24 h after refeeding.
Data Analysis and Statistics.
Values shown in all figures and in the table are the means ± SEM for each genotype. Statistical analyses were conducted with Prism 4 (GraphPad). Comparisons among the four genotypes (WT, Ay, RIIβ KO, and Ay/RIIβ KO) were done with one-way ANOVA and Newman–Keuls multiple-comparison test for post hoc analysis unless noted otherwise. Growth curves were analyzed with a one-way ANOVA with repeated measures and Newman–Keuls multiple-comparison test, or a two-tailed, paired Student t test when Ay and Ay/RIIβ HET mice were compared. Comparisons of Ay and Ay/RIIβ HET mice were analyzed with a two-tailed, unpaired Student t test.
Supplementary Material
ACKNOWLEDGMENTS.
We thank the Clinical Nutrition Research Unit Laboratory Core at the University of Washington for leptin assays, Michelle Matias for help with fat pad analysis, and Sara Shum for assistance with mouse breeding. This work was supported by National Institutes of Health Grants 5 T32 NS 07332 (to T.A.C.) and GM32875 (to G.S.M.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0710607105/DC1.
References
- 1.Cummings DE, Brandon EP, Planas JV, Motamed K, Idzerda RL, McKnight GS. Nature. 1996;382:622–626. doi: 10.1038/382622a0. [DOI] [PubMed] [Google Scholar]
- 2.Schreyer SA, Cummings DE, McKnight GS, LeBoeuf RC. Diabetes. 2001;50:2555–2562. doi: 10.2337/diabetes.50.11.2555. [DOI] [PubMed] [Google Scholar]
- 3.Nolan MA, Sikorski MA, McKnight GS. Mol Endocrinol. 2004;18:2302–2311. doi: 10.1210/me.2004-0194. [DOI] [PubMed] [Google Scholar]
- 4.Newhall KJ, Cummings DE, Nolan MA, McKnight GS. Mol Endocrinol. 2005;19:982–991. doi: 10.1210/me.2004-0343. [DOI] [PubMed] [Google Scholar]
- 5.Brandon EP, Logue SF, Adams MR, Qi M, Sullivan SP, Matsumoto AM, Dorsa DM, Wehner JM, McKnight GS, Idzerda RL. J Neurosci. 1998;18:3639–3649. doi: 10.1523/JNEUROSCI.18-10-03639.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calebiro D, de Filippis T, Lucchi S, Martinez F, Porazzi P, Trivellato R, Locati M, Beck-Peccoz P, Persani L. Mol Endocrinol. 2006;20:3196–3211. doi: 10.1210/me.2005-0493. [DOI] [PubMed] [Google Scholar]
- 7.Carr DW, DeManno DA, Atwood A, Hunzicker-Dunn M, Scott JD. J Biol Chem. 1993;268:20729–20732. [PubMed] [Google Scholar]
- 8.Landmark BF, Oyen O, Skalhegg BS, Fauske B, Jahnsen T, Hansson V. J Reprod Fertil. 1993;99:323–334. doi: 10.1530/jrf.0.0990323. [DOI] [PubMed] [Google Scholar]
- 9.Cone RD. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 10.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 11.Huszar D, Lunch CA, Fairchild-Huntress V, Dunmore JH, Smith FJ, Kesterson RA, Boston BA, Fang Q, Berkemeier LR, Gu W. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 12.Erickson JC, Clegg KE, Palmiter RD. Nature. 1996;381:415–421. doi: 10.1038/381415a0. [DOI] [PubMed] [Google Scholar]
- 13.Segal-Lieberman G, Trombly DJ, Juthani V, Wang X, Maratos-Flier E. Am J Physiol. 2003;284:E1131–E1139. doi: 10.1152/ajpendo.00491.2002. [DOI] [PubMed] [Google Scholar]
- 14.Wortley KE, Anderson KD, Yasenchak J, Murphy A, Valenzuela D, Diano S, Yancopoulos GD, Wiegand SJ, Sleeman MW. Cell Metab. 2005;2:421–427. doi: 10.1016/j.cmet.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Bultman SJ, Michaud EJ, Woychik RP. Cell. 1992;71:1195–1204. doi: 10.1016/s0092-8674(05)80067-4. [DOI] [PubMed] [Google Scholar]
- 16.Miller MW, Duhl DM, Vrieling H, Cordes SP, Ollmann MM, Winkes BM, Barsh GS. Genes Dev. 1993;7:454–467. doi: 10.1101/gad.7.3.454. [DOI] [PubMed] [Google Scholar]
- 17.Michaud EJ, Bultman SJ, Klebig ML, van Vugt MJ, Stubbs LJ, Russell LB, Woychik RP. Proc Natl Acad Sci USA. 1994;91:2562–2566. doi: 10.1073/pnas.91.7.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdel-Malek ZA, Scott MC, Furumura M, Lamoreux ML, Ollmann M, Barsh GS, Hearing VJ. J Cell Sci. 2001;114:1019–1024. doi: 10.1242/jcs.114.5.1019. [DOI] [PubMed] [Google Scholar]
- 19.Yen TT, Gill AM, Frigeri LG, Barsh GS, Wolff GL. FASEB J. 1994;8:479–488. doi: 10.1096/fasebj.8.8.8181666. [DOI] [PubMed] [Google Scholar]
- 20.Manne J, Argeson AC, Siracusa LD. Proc Natl Acad Sci USA. 1995;92:4721–4724. doi: 10.1073/pnas.92.11.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kas MJ, Tiesjema B, van Dijk G, Garner KM, Barsh GS, Brake OT, Verhaagen J, Adan RA. J Neurosci. 2004;24:10176–10181. doi: 10.1523/JNEUROSCI.3442-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 23.Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 24.Boston BA, Blaydon KM, Varnerin J, Cone RD. Science. 1997;278:1641–1644. doi: 10.1126/science.278.5343.1641. [DOI] [PubMed] [Google Scholar]
- 25.Kim CS, Lee S-H, Kim RY, Kim B-J, Li S-Z, Lee IH, Lee EJ, Lim S-K, Bae Y-S, Lee W, Baik J-H. J Biol Chem. 2002;277:31310–31317. doi: 10.1074/jbc.M112085200. [DOI] [PubMed] [Google Scholar]
- 26.Newman EA, Chai BX, Zhang W, Li JY, Ammori JB, Mulholland MW. J Surg Res. 2006;132:201–207. doi: 10.1016/j.jss.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Patten CS, Daniels D, Suzuki A, Fluharty SJ, Yee DK. Regul Pept. 2007;142:111–122. doi: 10.1016/j.regpep.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Shinyama H, Masuzaki H, Fang H, Flier JS. Endocrinology. 2003;144:1301–1314. doi: 10.1210/en.2002-220931. [DOI] [PubMed] [Google Scholar]
- 29.Vongs A, Lynn NM, Rosenblum CI. Regul Pept. 2004;120:113–118. doi: 10.1016/j.regpep.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 30.Sutton GM, Duos B, Patterson LM, Berthoud HR. Endocrinology. 2005;146:3739–3747. doi: 10.1210/en.2005-0562. [DOI] [PubMed] [Google Scholar]
- 31.Cadd G, McKnight GS. Neuron. 1989;3:71–79. doi: 10.1016/0896-6273(89)90116-5. [DOI] [PubMed] [Google Scholar]
- 32.Bagnol D, Lu XY, Kaelin CB, Day HE, Ollmann M, Gantz I, Akil H, Barsh GS, Watson SJ. J Neurosci. 1999;19:RC26. doi: 10.1523/JNEUROSCI.19-18-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu H, Kishi T, Roseberry AG, Cai X, Lee CE, Montez JM, Friedman JM, Elmquist JK. J Neurosci. 2003;23:7143–7154. doi: 10.1523/JNEUROSCI.23-18-07143.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amieux PS, Cummings DE, Motamed K, Brandon EP, Wailes LA, Le K, Idzerda RL, McKnight GS. J Biol Chem. 1997;272:3993–3998. doi: 10.1074/jbc.272.7.3993. [DOI] [PubMed] [Google Scholar]
- 35.Planas JV, Cummings DE, Idzerda RL, McKnight GS. J Biol Chem. 1999;274:36281–36287. doi: 10.1074/jbc.274.51.36281. [DOI] [PubMed] [Google Scholar]
- 36.Dinulescu DM, Fan W, Boston BA, McCall K, Lamoreux ML, Moore KJ, Montagno J, Cone RD. Proc Natl Acad Sci USA. 1998;95:12707–12712. doi: 10.1073/pnas.95.21.12707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phan LK, Chung WK, Leibel RL. Am J Physiol. 2006;291:E611–E620. doi: 10.1152/ajpendo.00034.2006. [DOI] [PubMed] [Google Scholar]
- 38.Martin NM, Houston PA, Patterson M, Sajedi A, Carmignac DF, Ghatei MA, Bloom SR, Small CJ. Int J Obes (London) 2006;30:430–438. doi: 10.1038/sj.ijo.0803076. [DOI] [PubMed] [Google Scholar]
- 39.Seeley RJ, Woods SC. Nat Rev Neurosci. 2003;4:901–909. doi: 10.1038/nrn1245. [DOI] [PubMed] [Google Scholar]
- 40.Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 41.Tortoriello DV, McMinn JE, Chua SC. Int J Obes (London) 2007;31:395–402. doi: 10.1038/sj.ijo.0803392. [DOI] [PubMed] [Google Scholar]
- 42.Marsh DJ, Hollopeter G, Huszar D, Laufer R, Yagaloff KA, Fisher SL, Burn P, Palmiter RD. Nat Genet. 1999;21:119–122. doi: 10.1038/5070. [DOI] [PubMed] [Google Scholar]
- 43.Cadd GG, Uhler MD, McKnight GS. J Biol Chem. 1990;265:19502–19506. [PubMed] [Google Scholar]
- 44.MacKenzie RG. Peptides. 2006;27:395–403. doi: 10.1016/j.peptides.2005.03.064. [DOI] [PubMed] [Google Scholar]
- 45.Yaswen L, Diehl N, Brennan MB, Hochgeschwender U. Nat Med. 1999;5:1066–1070. doi: 10.1038/12506. [DOI] [PubMed] [Google Scholar]
- 46.Savontaus E, Breen TL, Kim A, Yang LM, Chua SC, Jr, Wardlaw SL. Endocrinology. 2004;145:3881–3891. doi: 10.1210/en.2004-0263. [DOI] [PubMed] [Google Scholar]
- 47.Hollopeter G, Erickson JC, Palmiter RD. Int J Obes Relat Metab Disord. 1998;22:506–512. doi: 10.1038/sj.ijo.0800615. [DOI] [PubMed] [Google Scholar]
- 48.Miller KA, Gunn TM, Carrasquillo MM, Lamoreux ML, Galbraith DB, Barsh GS. Genetics. 1997;146:1407–1415. doi: 10.1093/genetics/146.4.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gold RM. Science. 1973;182:488–490. doi: 10.1126/science.182.4111.488. [DOI] [PubMed] [Google Scholar]
- 50.Weingarten HP, Chang PK, McDonald TJ. Brain Res Bull. 1985;14:551–559. doi: 10.1016/0361-9230(85)90104-2. [DOI] [PubMed] [Google Scholar]
- 51.Giraudo SQ, Billington CJ, Levine AS. Brain Res. 1998;809:302–306. doi: 10.1016/s0006-8993(98)00837-3. [DOI] [PubMed] [Google Scholar]
- 52.Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD. Neuron. 1999;24:155–163. doi: 10.1016/s0896-6273(00)80829-6. [DOI] [PubMed] [Google Scholar]
- 53.Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.