Abstract
Ca2+/calmodulin-dependent protein kinase II (CaMKII) is a key regulator of synaptic responses in the postsynaptic density, but understanding of its mechanisms of action in the presynaptic neuron is incomplete. Here we show that CaMKII constitutively associates with and modulates voltage-gated calcium (CaV)2.1 channels that conduct P/Q type Ca2+ currents and initiate transmitter release. Both exogenous and brain-specific inhibitors of CaMKII accelerate voltage-dependent inactivation, cause a negative shift in the voltage dependence of inactivation, and reduce Ca2+-dependent facilitation of CaV2.1 channels. The modulatory effects of CaMKII are reduced by a peptide that prevents binding to CaV2.1 channels but not by a peptide that blocks catalytic activity, suggesting that binding rather than phosphorylation is responsible for modulation. Our results reveal a signaling complex formed by CaV2.1 channels and CaMKII that regulates P/Q-type Ca2+ current in neurons. We propose an “effector checkpoint” model for the control of Ca2+ channel fitness for function that depends on association with CaMKII, SNARE proteins, and other effectors of Ca2+ signals. This regulatory mechanism would be important in presynaptic nerve terminals, where CaV2.1 channels initiate synaptic transmission and CaMKII has noncatalytic effects on presynaptic plasticity.
Keywords: calcium channels, facilitation, phosphorylation, synaptic transmission
Ca2+ entry into neurons through presynaptic voltage-gated Ca2+ channels links membrane depolarization to integration of synaptic signals in cell bodies and dendrites and to exocytosis of neurotransmitters in the nerve terminal. At many synapses, transmission is mediated by P/Q type Ca2+ currents conducted by voltage-gated calcium (CaV)2.1 channels (1–3), which are located in high density in active zones of presynaptic terminals (4, 5). These channels consist of a pore-forming α12.1 subunit and auxiliary β, α2δ, and possibly γ subunits (6). The function of these channels is tightly regulated by interaction with G protein βγ subunits (7–9), soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins (10, 11), the ubiquitous Ca2+ sensor calmodulin (CaM) (12–14), and other related Ca2+-binding proteins (15, 16). Evidently, the intracellular domains of CaV2.1 channels form a signal transduction platform that supports local Ca2+ signaling by these proteins as part of a signaling complex.
Ca2+/CaM-dependent protein kinase II (CaMKII) is a multifunctional serine/threonine kinase expressed ubiquitously but highly enriched in brain tissue, where it is a major calcium signaling protein (17). Neuronal CaMKII is composed of 6–12 subunits, primarily the 52-kDa α isoform and the 60-kDa β isoform (18). Under basal conditions, the N-terminal autoinhibitory domain binds to the catalytic domain and blocks access of ATP and substrates. In response to Ca2+ signals, binding of Ca2+/CaM removes the autoinhibition of CaMKII and allows phosphorylation of substrates, including the neighboring subunit within the same holoenzyme (19). This autophosphorylation process enables the kinase to remain active even after the dissociation of Ca2+/CaM (20–22), which allows CaMKII to integrate signals from intracellular Ca2+ transients and oscillations (23). Sustained activation of CaMKII localized at the postsynaptic density results in phosphorylation of numerous substrates, including neurotransmitter-gated ion channels, other signaling molecules, and scaffolding proteins, to modulate synaptic transmission (24). Postsynaptic CaMKII activity is required for induction of long-term potentiation in the CA1 region of the hippocampus and for spatial learning and memory (25–28); mutant animals lacking αCaMKII display impaired hippocampus-dependent learning (27, 29, 30). Compared with the well documented role of CaMKII in regulating the postsynaptic response, the potential role of CaMKII in regulating presynaptic function is unclear, although it has been shown to regulate neurotransmission (31–35) and to alter the specificity of neurotransmitter release (36). Here we report that CaMKII constitutively binds to the α12.1 subunit of CaV2.1 channels and modulates their inactivation properties in both transfected cells and hippocampal neurons. Surprisingly, CaMKII exerts this modulatory effect by binding to the C-terminal domain of the channels rather than by phosphorylating them. The modulatory effect of CaMKII is inhibited by the brain-specific CaMKII inhibitor CaM-KIIN (37), suggesting dynamic, bidirectional regulation of CaV2.1 channels by CaMKII in brain neurons. We propose an “effector checkpoint” model for control of the functional fitness of Ca2+ channels by interacting effectors of the Ca2+ signal, which provides a common conceptual framework for distinct forms of Ca2+ channel regulation in different cell types.
Results
Endogenous CaMKII Modulates Inactivation of CaV2.1 Channels.
CaM associates with the α1 subunit of CaV2.1 channels at a bipartite site in the C-terminal domain and mediates Ca2+-dependent facilitation and inactivation (12–14, 38, 39). The C-terminal lobe of CaM initiates facilitation in response to local calcium transients by interaction with an IQ-like motif, whereas the N-terminal lobe primarily interacts with an adjacent CaM-binding motif (CBD) and initiates inactivation in response to more long lasting calcium transients (12, 14, 39). Mutations in these two motifs completely prevent binding and modulation of CaV2.1 channels by CaM.
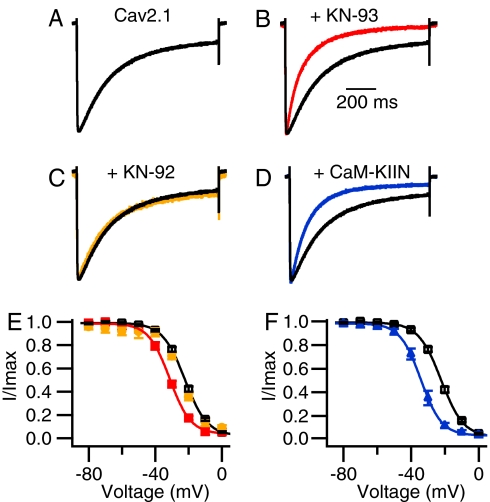
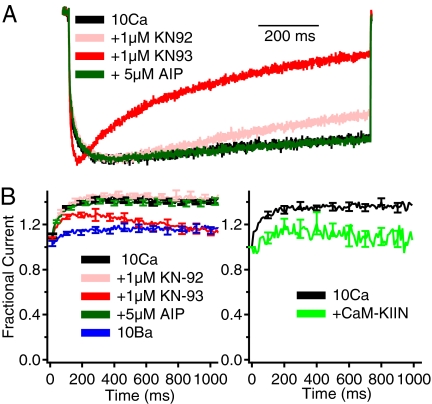
To determine the functional impact of CaMKII independent of regulation of CaV2.1 channels by CaM, we analyzed ion currents conducted by CaV2.1 channels expressed in transfected tsA-201 cells by using the whole-cell patch-clamp configuration with intracellular recording solutions containing a high concentration (10 mM) of EGTA and extracellular recording solutions with Ba2+ as the permeant ion. CaMKII is a ubiquitous enzyme present in essentially every tissue. Therefore, we examined the effect of endogenous CaMKII in tsA-201 cells by use of KN-93, a selective antagonist of CaMKII, which blocks the activation and autophosphorylation of the kinase (40). In the presence of 3 μM KN-93, Ba2+ currents conducted by CaV2.1 channels during a 1-s pulse to +20 mV inactivated much faster than in control conditions or in the presence of the inactive analog KN-92 (Fig. 1 A–C, compare black and colored traces). In addition, KN-93 caused a hyperpolarizing shift of ≈10 mV in the voltage dependence of inactivation of CaV2.1 channels, whereas KN-92 had no effect (Fig. 1E). These effects of KN-93 were not accompanied by a significant reduction in amplitude of the Ba2+ current [supporting information (SI) Fig. 7], indicating that KN-93 does not act as a blocker of CaV2.1 channels under our experimental conditions.
Fig. 1.
Inhibition of CaV2.1 channels by the CaMKII inhibitors KN-93 and CaM-KIIN. (A–D) Ba2+ currents were evoked by 1-s depolarizations to +20 mV from a holding potential of −80 mV. The current traces are the means of the normalized individual current traces and are scaled for comparison. (A) Black trace, CaV2.1 channels alone (τ = 231 ± 7 ms; n = 67). (B) Red trace, 3 μM KN-93 (τ = 124 ± 8 ms; n = 20; P < 0.001). (C) Orange trace, 3 μM KN-92 (τ = 216 ± 10 ms; n = 16). (D) Blue trace, CaV2.1 channels coexpressed with CaM-KIIN (τ = 126 ± 7 ms; n = 27; P < 0.001). (E and F) Ba2+ currents were recorded during 5-ms test pulses to +30 mV after 5-s conditioning prepulses to the indicated potentials. Cells were held at −80 mV, and the tail currents from the test pulse were normalized to the largest tail current in each series and plotted as means ± SEM vs. conditioning prepulse voltage. (E) CaV2.1, black open squares (Vh = −22.4 ± 0.7 mV; n = 42); KN-93, red filled squares (Vh = −31.6 ± 0.7 mV; n = 13); and KN-92, orange filled circles (Vh = −23.7 ± 0.7 mV; n = 9). (F) CaV2.1, black open squares as in E; CaV2.1 coexpressed with CaM-KIIN, blue filled triangles (Vh = −34.2 ± 0.7 mV; n = 23).
CaM-KIIN is a brain-specific protein of 79-aa residues that inhibits CaMKII noncompetitively, like KN-93 (37, 41). Similar effects were observed when CaM-KIIN was coexpressed with CaV2.1 channels. CaM-KIIN induced a significant acceleration of the inactivation rate (Fig. 1D) as well as a hyperpolarizing shift of ≈10 mV in the voltage dependence of inactivation of CaV2.1 channels (Fig. 1F). These results show that noncompetitive inhibition of CaMKII by either a small molecule inhibitor (KN-93) or an endogenous brain-specific protein regulator (CaM-KIIN) enhances inactivation of CaV2.1 channels. KN-93, KN-92, and CaM-KIIN did not affect the voltage dependence of activation of CaV2.1 channels (data not shown). Thus, inhibition of CaMKII in tsA-201 cells selectively increases the rate of voltage-dependent inactivation of CaV2.1 channels and negatively shifts its voltage dependence, thereby making fewer channels available for activation. These results indicate that CaMKII itself slows inactivation and positively shifts the voltage dependence of inactivation of CaV2.1 channels. These effects would both substantially increase Ca2+ entry into neurons and presynaptic nerve terminals.
CaMKII Constitutively Binds to CaV2.1 Channels.
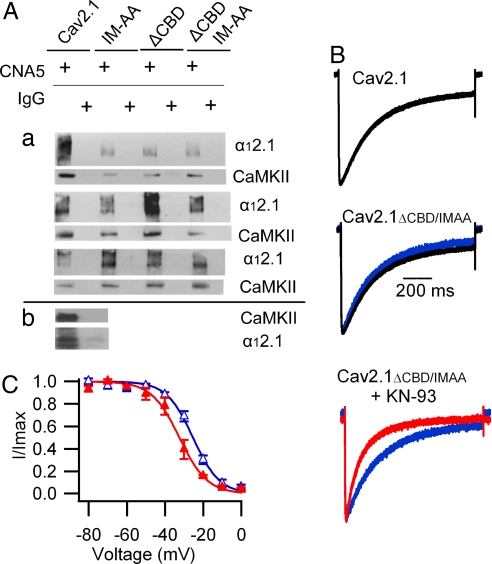
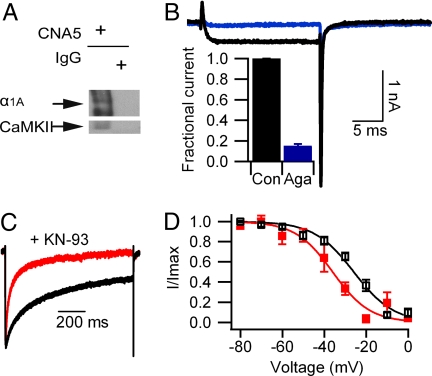
To test whether CaMKII directly interacts with the CaV2.1 channels, we immunoprecipitated the α12.1 subunit from extracts of transfected tsA-201 cells and probed the precipitated protein for associated CaMKII by using a broad-specificity antibody. CaMKII was associated with CaV2.1 channels under our basal growth conditions for tsA-201 cells, as indicated by specific coimmunoprecipitation of CaMKII with an antibody against the α12.1 subunit (Fig. 2Aa, lanes 1 and 2) and coimmunoprecipitation of α12.1 subunit with an antibody against CaMKII (Fig. 2Ab). Mutations that block CaM binding to α12.1 did not block binding of CaMKII (Fig. 2Aa). Moreover, KN-93 induced a significant acceleration of voltage-dependent inactivation and a negative shift of the voltage dependence of inactivation of the CaV2.1ΔCBD/IM-AA mutant (Fig. 2 B and C), indicating that both the binding and modulatory effect of endogenous CaMKII persisted in the absence of CaM bound to CaV2.1 channels.
Fig. 2.
Binding of CaMKII to α12.1 subunits expressed in tsA-201 cells. (A) Lysates from cells transfected with WT CaV2.1, CaV2.1IM-AA, CaV2.1ΔCBD, or CaV2.1ΔCBD/IM-AA were subjected to immunoprecipitation and analyzed as described in Materials and Methods. (a) After immunoprecipitation with anti-CNA5 α12.1-specific antibody or control IgG, blots from three separate experiments were probed with α12.1-specific antibodies (upper blots) or CaMKII-specific antibodies (lower blots). When transfected with the same amount of DNA, the expression levels of mutant channels were lower than that of the WT channel (top blots). However, with a comparable level of channel expression, the amounts of CaMKII that coimmunoprecipitate with CaV2.1 were not different (middle and bottom blots). (b) In a separate experiment, lysates from cells transfected with WT CaV2.1 were subjected to immunoprecipitation with CaMKII antibodies or control IgG before blots were probed with CaMKII-specific (upper blot) or α12.1-specific (lower blot) antibodies. (B) Effect of KN-93 on voltage-dependent inactivation of CaV2.1ΔCBD/IM-AA channels. CaV2.1, black trace as in Fig. 1; CaV2.1ΔCBD/IM-AA, blue trace (τ = 240 ± 23 ms; n = 6); CaV2.1ΔCBD/IM-AA plus 3 μM KN-93, red trace (τ = 119 ± 5 ms; n = 6; P < 0.001). (C) Effect of KN-93 on the voltage dependence of inactivation of CaV2.1ΔCBD/IM-AA channels. CaV2.1ΔCBD/IM-AA, blue open triangles (Vh = −25.1 ± 1.4 mV; n = 7); CaV2.1ΔCBD/IM-AA plus 3 μM KN-93, red trace (Vh = −32.8 ± 2.2 mV; n = 5; P < 0.001).
CaMKII Binding Is Necessary for Modulation of CaV2.1 Channels.
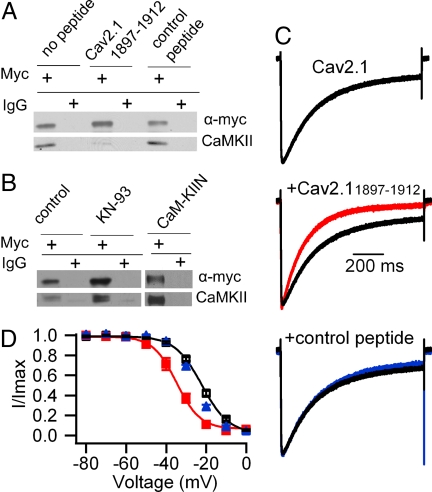
In cardiac myocytes, the α1 subunit of cardiac CaV1.2 channels binds CaMKIIδ at a site in the C-terminal domain (42), and CaMKII enhances activation of L-type Ca2+ currents (43). We expressed the C-terminal domain of α12.1 with a lipid anchor and myc epitope tag and measured coimmunoprecipitation of CaMKII. CaMKII was coimmunoprecipitated by an anti-myc antibody but not by control IgG (Fig. 3A, no peptide). The amino acid sequence of the CaMKII-binding site in the C-terminal domain of CaV1.2 channels is conserved in the segment containing amino acid residues 1897–1912 in CaV2.1 channels (42). A competing peptide containing this sequence from CaV2.1 channels blocked coimmunoprecipitation of CaMKII, whereas a control peptide had no effect (Fig. 3A).
Fig. 3.
Requirement for CaMKII binding to the C-terminal fragment of α12.1 subunit for modulation of CaV2.1 channels. (A and B) Lysates from cells transfected with the myc-tagged C-terminal construct of α12.1 (CaV2.11784–2211) were subjected to immunoprecipitation with myc-specific antibody or control IgG as indicated. Blots were probed with myc-specific (upper blot) or CaMKII-specific (lower blot) antibodies. (A) Immunoprecipitation in the absence (lanes 1 and 2) and presence (lanes 3–6) of competing peptide (CaV2.11897–1912, lanes 3 and 4) or control peptide (lanes 5 and 6). (B) Immunoprecipitation in the absence (lanes 1 and 2) and presence (lanes 3 and 4) of 50 μM KN-93 or coexpression of CaM-KIIN (lanes 5 and 6). (C) Effect of competing peptide CaV2.11897–1912 on voltage-dependent inactivation. (Top) No peptide control, repeated as black traces in the other panels (τ = 231 ± 7 ms, n = 67). (Middle) Peptide CaV2.11897–1912 (100 μM), red trace (τ = 163 ± 15 ms; n = 9; P < 0.001). (Bottom) Control peptide (100 μM), blue trace (τ = 241 ± 20 ms; n = 8). (D) Effect of competing peptide CaV2.11897–1912 on the voltage dependence of inactivation. No peptide, black open squares; competing peptide, red filled squares (Vh = −34.4 ± 1.4 mV; n = 10; P < 0.001); control peptide, blue filled triangles (Vh = −25.6 ± 0.7 mV; n = 7).
To determine whether binding of CaMKII to this C-terminal site is required for Ca2+ channel regulation, we studied the effects of the CaV2.1(1897–1912) competing peptide on CaV2.1 channels expressed in tsA-201 cells. Dialysis of this competing peptide into tsA-201 cells transfected with CaV2.1 channels caused a substantial acceleration of the voltage-dependent inactivation of CaV2.1 channels (Fig. 3C) and a −12-mV shift of the voltage dependence of inactivation (Fig. 3D) without changing the voltage dependence of activation (data not shown). In contrast, the control peptide had no effect on the inactivation properties of CaV2.1 channels (Fig. 3 C and D). These results indicate that the binding of CaMKII to CaV2.1 channels is necessary for modulation and support the hypothesis that CaMKII binding to CaV2.1(1897–1912) is required for its effect. When we applied both KN-93 and the competing peptide to CaV2.1 channels, their combined effects were similar to those of KN-93 alone (data not shown), indicating both agents exert their effects through a common mechanism.
Although KN-93 and CaM-KIIN were effective in preventing modulation of CaV2.1 channels, neither KN-93 nor CaM-KIIN interfered with the binding of CaMKII to the C-terminal of α12.1 (Fig. 3B). These results suggest that in addition to binding, CaMKII may need to be in a specific activated conformation to modulate CaV2.1 channels. The noncompetitive inhibitors KN-93 and CaM-KIIN may prevent the conformational change of CaMKII that is required for its modulatory effects or make amino acid sequences that are required for modulation inaccessible for interaction with CaV2.1 channels.
CaMKII Modulates CaV2.1 Channels by a Noncatalytic Mechanism.
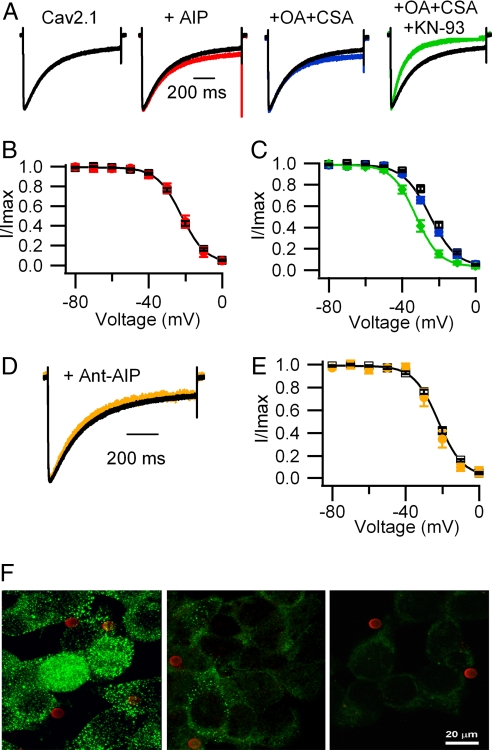
Our results indicate that CaMKII modulates CaV2.1 channels under conditions in which intracellular Ca2+ transients are prevented by use of extracellular Ba2+ and intracellular EGTA, suggesting that Ca2+-dependent phosphorylation by CaMKII may not be required for these effects. To determine directly whether CaMKII exerts its modulatory effects by phosphorylating CaV2.1 channels, we inhibited the catalytic activity of endogenous CaMKII by using a competitive pseudosubstrate inhibitor of CaMKII [autocamtide-2 related inhibitory peptide (AIP) (44)]. Dialysis of a concentration of AIP (5 μM) that exceeded its Ki by 100-fold did not alter the kinetics or voltage dependence of inactivation of CaV2.1 channels (Fig. 4 A and B) under exactly the same conditions that the CaV2.1(1897–1912) competing peptide was effective. Higher concentrations (50 or 100 μM) of AIP also had no effect (data not shown). These results suggest that the modulation of CaV2.1 channels may not be caused by phosphorylation by CaMKII.
Fig. 4.
Role of protein phosphorylation in the modulation of CaV2.1 channels by CaMKII. (A) Comparison of voltage-dependent inactivation. CaV2.1, black trace (τ = 231 ± 7 ms; n = 67); AIP (5 μM), red trace (τ = 217 ± 14 ms; n = 6); 1 μM okadaic acid (OA) plus 1 μM cyclosporin A (CSA), blue trace (τ = 200 ± 6 ms; n = 8); 1 μM OA plus 1 μM CSA plus 3 μM KN-93, green trace (τ = 134 ± 8 ms; n = 7; P < 0.001). (B and C) Comparison of the voltage dependence of inactivation. (B) Control, black open squares (Vh = −22.4 ± 0.7 mV; n = 42); AIP, red filled circles (Vh = −21.4 ± 1.7 mV; n = 6). (C) Control, black open circles; OA plus CSA, blue filled circles (Vh = −24.7 ± 0.8 mV; n = 9); OA plus CSA plus KN-93, green filled diamonds (Vh = −32.9 ± 1.3 mV; n = 5; P < 0.001). (D) Effect of Ant-AIP II (100 μM) on voltage-dependent inactivation. CaV2.1, black trace as in A; orange trace: τ = 211 ± 13 ms; n = 10. (E) Effect of Ant-AIP II on the voltage dependence of inactivation. Orange filled circles: Vh = −24.2 ± 1.8 mV, n = 5. (F) Effect of Ant-AIP II on the autophosphorylation of CaMKII in tsA-201 cells. (Left) Untreated control cells. (Center) Ant-AIP II (100 μM). (Right) No primary antibody. The red dots are CD8 beads recognizing cells transfected with CaV2.1 channels.
Inhibition of the catalytic activity of CaMKII would prevent its effects on CaV2.1 channels only if both phosphorylation is required and active phosphoprotein phosphatases are present to remove previously incorporated phosphate. In the presence of okadaic acid (1 μM) and cyclosporin A (1 μM) to inhibit phosphoprotein phosphatases I, IIA, and calcineurin, KN-93 still induced an acceleration of voltage-dependent inactivation (Fig. 4A) and a negative shift of the voltage dependence of inactivation (Fig. 4C) similar to the effect on CaV2.1 channels alone.
To confirm that AIP was effective in inhibiting CaMKII, we used a membrane-permeant form of AIP [Ant-AIP-II (45)] and tested its effects on autophosphorylation of CaMKII with a phosphospecific antibody (Fig. 4 D–F). The membrane-permeant AIP peptide (100 μM) was effective in reducing CaMKII autophosphorylation in intact tsA-201 cells, as assessed by immunocytochemistry with a phosphospecific antibody (Fig. 4F), but had no effect on modulation of CaV2.1 channels (Fig. 4 D and E). Loss of specific labeling with this phosphospecific antibody after treatment with AIP confirmed that the antibody recognized only the phosphorylated form of CaMKII. Therefore, these results provide further support for the conclusion that binding of CaMKII per se rather than channel phosphorylation modulates CaV2.1 channels.
Sequential Ca2+- and CaMKII-Dependent Facilitation and Inactivation.
To determine the significance of CaMKII-dependent modulation of CaV2.1 channels during physiological stimuli, we analyzed currents elicited by 100-Hz trains of 5-ms depolarizations with either Ca2+ or Ba2+ as the permeant ion. Because CaV2.1 channels containing the β1b subunit exhibit more rapid voltage-dependent inactivation that can occlude Ca2+-dependent facilitation (13), we tested CaV2.1 channels containing β2a subunits, which confer slow voltage-dependent inactivation (46) and are widely expressed in brain neurons that also express CaV2.1 channels (47–49). Facilitation requires only a brief local Ca2+ increase that is unaffected by 10 mM EGTA in the intracellular solution, whereas this level of chelator blocks Ca2+-dependent inactivation (13). Therefore, we included 10 mM EGTA in the recording pipette to record facilitation in isolation. KN-93 (1 μM) significantly accelerated the inactivation of Ca2+ currents (ICa) compared with the control cells or cells treated with KN-92 (1 μM) or AIP (5 μM) (Fig. 5A). When Ba2+ was used as the permeant ion, little facilitation or inactivation was observed (Fig. 5B), as expected (13). In contrast, in the presence of extracellular Ca2+ as permeant ion, the amplitude of ICa conducted by CaV2.1 channels increased by ≈40% because of Ca2+-dependent facilitation during the first 300 ms and then reached a plateau (Fig. 5B). KN-93 (1 μM) reduced the facilitation of ICa, whereas neither KN-92 nor AIP produced appreciable changes from control (Fig. 5B). Similarly, coexpression of CaM-KIIN with CaV2.1 channels significantly reduced facilitation (Fig. 5B). These results indicate that bound CaMKII in an activated conformation is necessary to support Ca2+/CaM-dependent facilitation of P/Q-type Ca2+ current in response to bursts of action potentials.
Fig. 5.
Modulation of facilitation of CaV2.1 channels by binding of CaMKII. CaV2.1 channels with β2a subunits were expressed in tsA-201 cells. (A) Normalized CaV2.1 currents under the indicated conditions during 1-s depolarizations from −80 to +20 mV (or 0 mV for Ba2+) (n = 6–10). (B) Facilitation of ICa during a train of 5-ms pulses at 100 Hz from −80 to +20 mV (or 0 mV for Ba2+) (n = 7–11). Current amplitudes are normalized to the initial current amplitude in each train.
CaMKII Modulates P/Q-Type Currents in Brain Neurons.
To determine whether CaMKII is associated with CaV2.1 channels in vivo, proteins isolated from rat brain membranes were solubilized and analyzed by immunoprecipitation with anti-CNA5 antibodies against α12.1. The presence of CaMKII in the immunoprecipitate was detected with antibodies against CaMKII (Fig. 6A), indicating specific association between CaMKII and α12.1 in the brain.
Fig. 6.
Binding and modulation of P/Q type channels in adult rat hippocampal pyramidal neurons by CaMKII. (A) Solubilized rat brain membrane was subjected to immunoprecipitation with anti-CNA5 α12.1-specific antibodies or control IgG as indicated. Blots were probed with α12.1-specific (Upper) or CaMKII-specific (Lower) antibodies. (B) Representative Ba2+ currents remaining after pretreatment with nimodipine (5 μM) and ω-conotoxin GVIA (1 μM) (black trace) are largely blocked by further application of ω-agatoxin IVA (1 μM) (blue trace). (Inset) Percentage of inhibition caused by ω-agatoxin IVA. (C) Effect of KN-93 on voltage-dependent inactivation of P/Q currents in hippocampal pyramidal neurons. Ba2+ currents were recorded and analyzed the same way as in tsA-201 cells. Control, black trace (τ = 260 ± 16.4 ms; n = 30); KN-93, red trace (τ = 77.8 ± 16.2 ms; n = 10; P < 0.001). (D) Effect of KN-93 on the voltage dependence of inactivation of P/Q currents in hippocampal pyramidal neurons. Control, black open squares (Vh = −24.5 ± 1.3 mV; n = 12); KN-93, red filled squares (Vh = −37.3 ± 2.4 mV; n = 4; P < 0.001).
Functional effects of CaMKII on P/Q-type currents were measured in hippocampal pyramidal neurons. To measure regulation by CaMKII in a similar Ca2+-independent manner, P/Q-type Ba2+ currents were recorded after preincubation with nimodipine (5 μM) and ω-conotoxin GVIA (1 μM) to block L- and N-type Ba2+ currents, respectively. As shown in Fig. 6B, ω-agatoxin IVA (1 μM, n = 4) inhibited 85 ± 2% of the remaining Ba2+ current elicited by a 20-ms step pulse to +20 mV, indicating that primarily P/Q-type currents remained. In the presence of KN-93, the voltage-dependent inactivation of neuronal P/Q-type currents was significantly accelerated compared with the control currents (Fig. 6C). In addition, KN-93 also caused a shift of −12.8 mV in the voltage dependence of inactivation (Fig. 6D). These data indicate that P/Q-type Ca2+ currents in hippocampal neurons are also subject to constitutive modulation by CaMKII.
Discussion
CaV2.1 Channels Bind CaMKII Constitutively.
CaMKII binds to CaV2.1 channels in the absence of Ca2+, and bound CaMKII modulates Ba2+ currents recorded with 10 mM EGTA in the intracellular solution. These results indicate that CaMKII binds constitutively to CaV2.1 channels. The interaction between CaMKII and the C-terminal fragment of α12.1 that we have observed is consistent with the previous report (42) that CaMKII associates with CaV1.2 channels via a peptide sequence that is highly conserved in CaV2.1 channels. CaMKII likely binds to this conserved kinase-binding site in CaV2.1 channels, because a competing peptide designed from this amino acid sequence inhibits CaMKII binding and modulation. Even though CaMKII binds constitutively to CaV2.1 channels in the absence of a Ca2+ signal, the ability of KN-93 and CaM-KIIN to prevent the modulation by CaMKII without disrupting the interaction of CaMKII with CaV2.1 channels suggests that modulation requires an activated state of CaMKII. Evidently, noncompetitive inhibition of CaMKII by KN-93 and CaM-KIIN induces a conformation of the kinase that is inactive in modulation of CaV2.1 channels.
Binding of CaMKII Enhances CaV2.1 Channel Activity.
Our results show that binding of CaMKII in an activated conformation enhances the activity of CaV2.1 channels by slowing voltage-dependent inactivation, positively shifting the voltage dependence of inactivation, and increasing facilitation during trains of stimuli. It is surprising that CaMKII binding per se is sufficient for regulation of CaV2.1 channels. This form of modulation of CaV2.1 channel activity takes place at resting Ca2+ levels. Therefore, its regulatory impact is to increase the probability and duration of opening of CaV2.1 channels and thereby increase Ca2+ entry in response to all depolarizing signals. This regulatory outcome could not be achieved by the Ca2+-dependent catalytic activity of the enzyme because the time required for the rise in intracellular Ca2+, activation of the enzyme, and phosphorylation of the Ca2+ channel would not allow enhancement of activation of CaV2.1 channels in response to single action potentials or short trains of action potentials. Thus, constitutive modulation by binding of CaMKII and Ca2+-dependent modulation of CaV2.1 channels by CaM may coordinately act as molecular switches to control CaV2.1 channel activity under basal conditions and also to regulate it in response to activity-dependent alterations in intracellular Ca2+ levels.
Previous studies in cardiac myocytes have shown that CaMKII mediates facilitation of L-type Ca2+ currents by promoting a gating mode characterized by frequent, long openings (43). This facilitation of ICa is thought to require direct binding of CaMKII to the C-terminal fragment of CaV1.2 and phosphorylation of CaV1.2 channels (42). Similarly, constitutively bound CaMKII enhances the activity of CaV2.1 channels by slowing voltage-dependent inactivation, positively shifting the voltage dependence of inactivation and increasing Ca2+-dependent facilitation. These results suggest a general mechanism whereby CaMKII amplifies the function of associated Ca2+ channels in the cascade of molecular signals that regulate intracellular Ca2+ in brain and heart.
Regulation of CaMKII/CaV2.1 Channel Interaction by CaM-KIIN.
Our results provide evidence for a novel functional role of CaM-KIIN. Interaction of CaM-KIIN with CaMKII noncompetitively inhibits CaMKII activity and prevents enhancement and facilitation of CaV2.1 channel activity by CaMKII. In this situation, CaM-KIIN would be effective in inhibiting activation of CaMKII by local Ca2+ signals that require CaMKII bound to CaV2.1 channels to be sensed effectively. Moreover, the effect of CaM-KIIN to reduce CaV2.1 channel activity and its facilitation would inhibit the response of all other Ca2+-dependent signaling proteins to local elevations of Ca2+. The presence of CaM-KIIN may therefore enforce a requirement for global elevation of Ca2+ near CaV2.1 channels for effective regulation of CaMKII and other Ca2+ effectors.
An Effector Checkpoint Model for Control of Ca2+ Channel Function.
Our results show that an effector of the Ca2+ signal, CaMKII, up-regulates the activity of CaV2.1 channels when bound. This regulation increases the activity of those Ca2+ channels with Ca2+ signals that would be used physiologically by CaMKII. Could this be a more general mechanism of Ca2+ channel regulation? Two previous examples suggest that this is a general mechanism. Skeletal muscle CaV1.1 channels in transverse tubules interact directly with the ryanodine-sensitive Ca2+ release channels in the sarcoplasmic reticulum, which serve as their effectors in excitation–contraction coupling (50). Deletion of the gene for the ryanodine-sensitive Ca2+ release channel dramatically reduces the activity of the CaV1.1 channels (51). Thus, effector interaction enhances Ca2+ channel activity in this case.
SNARE proteins that are involved in docking and exocytosis of neurotransmitter vesicles are the effectors of the Ca2+ signal conducted by presynaptic Ca2+ channels. Moreover, the SNARE proteins regulate CaV2.1 and CaV2.2 channels in a biphasic manner (10, 11, 52, 53). The plasma membrane SNARE proteins syntaxin and SNAP-25 inhibit Ca2+ channel activity by shifting the voltage dependence of inactivation to more negative membrane potentials, but formation of a complete SNARE complex with synaptobrevin and synaptotagmin relieves the inhibition and enhances Ca2+ channel activation. Evidently, formation of a complete SNARE complex, which serves as the effector of the Ca2+ signal in synaptic transmission, increases both CaV2.1 and CaV2.2 channel activity. In all of these examples, the regulation is organized to enhance Ca2+ entry through channels that have appropriate effectors bound in place. Thus, this mechanism serves as an effector checkpoint to ensure the functional fitness of individual Ca2+ channel molecules for signal transduction to bound effector proteins like ryanodine receptors, SNARE proteins, and CaMKII before they are allowed to open with high efficiency. This regulatory process serves to focus Ca2+ entry on those Ca2+ channels that are poised to use the Ca2+ signal most effectively and thereby limits unproductive Ca2+ entry that might be deleterious.
Regulation of CaV2.1 Channels by CaMKII in a Signaling Complex in Presynaptic Nerve Terminals.
CaV2.1 channels are most highly concentrated in presynaptic terminals, where they form a signaling complex and initiate release of neurotransmitters and synaptic transmission (54). The potential significance of regulation of presynaptic CaV2.1 channels by CaMKII in synaptic plasticity is considered in the SI Text and SI Fig. 8.
Materials and Methods
α12.1 mutants were constructed as described previously (38). tsA-201 cells, a subclone of HEK293 human embryonic kidney cells, were transfected (16, 38) with cDNAs encoding CaV2.1 channel subunits plus the cell surface marker CD8, and transfected cells were identified by CD8 labeling and studied by whole-cell voltage clamp and immunocytochemistry 24–48 h later by using methods described previously (15, 16, 38). For immunoprecipitation experiments, transfected cells were lysed in detergent, and CaV2.1 channels were immunoprecipitated and immunoblotted as described previously (16). Details of these experimental procedures are given in the SI Text.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Dr. Amy Lee (Emory University, Atlanta) for providing the α12.1ΔCBD/IM-AA construct and Dr. Tom Soderling (Vollum Institute, Oregon Health and Science University, Portland, OR) for providing the CaM-KIIN construct. This work was supported by National Institutes of Health Research Grant R01 NS22625 (to W.A.C.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0710213105/DC1.
References
- 1.Wheeler DB, Randall A, Tsien RW. Science. 1994;264:107–111. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]
- 2.Mintz IM, Sabatini BL, Regehr WG. Neuron. 1995;15:675–688. doi: 10.1016/0896-6273(95)90155-8. [DOI] [PubMed] [Google Scholar]
- 3.Doroshenko PA, Woppmann A, Miljanich G, Augustine GJ. Neuropharmacology. 1997;36:865–872. doi: 10.1016/s0028-3908(97)00032-4. [DOI] [PubMed] [Google Scholar]
- 4.Westenbroek RE, Sakurai T, Elliott EM, Hell JW, Starr TV, Snutch TP, Catterall WA. J Neurosci. 1995;15:6403–6418. doi: 10.1523/JNEUROSCI.15-10-06403.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu LG, Westenbroek RE, Borst JG, Catterall WA, Sakmann B. J Neurosci. 1999;19:726–736. doi: 10.1523/JNEUROSCI.19-02-00726.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catterall WA. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 7.Ikeda SR. Nature. 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- 8.Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. Nature. 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- 9.De Waard M, Liu H, Walker D, Scott VE, Gurnett CA, Campbell KP. Nature. 1997;385:446–450. doi: 10.1038/385446a0. [DOI] [PubMed] [Google Scholar]
- 10.Bezprozvanny I, Scheller RH, Tsien RW. Nature. 1995;378:623–626. doi: 10.1038/378623a0. [DOI] [PubMed] [Google Scholar]
- 11.Zhong H, Yokoyama CT, Scheuer T, Catterall WA. Nat Neurosci. 1999;2:939–941. doi: 10.1038/14721. [DOI] [PubMed] [Google Scholar]
- 12.Lee A, Wong ST, Gallagher D, Li B, Storm DR, Scheuer T, Catterall WA. Nature. 1999;399:155–159. doi: 10.1038/20194. [DOI] [PubMed] [Google Scholar]
- 13.Lee A, Scheuer T, Catterall WA. J Neurosci. 2000;20:6830–6838. doi: 10.1523/JNEUROSCI.20-18-06830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeMaria CD, Soong TW, Alseikhan BA, Alvania RS, Yue DT. Nature. 2001;411:484–489. doi: 10.1038/35078091. [DOI] [PubMed] [Google Scholar]
- 15.Lee A, Westenbroek RE, Haeseleer F, Palczewski K, Scheuer T, Catterall WA. Nat Neurosci. 2002;5:210–217. doi: 10.1038/nn805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lautermilch NJ, Few AP, Scheuer T, Catterall WA. J Neurosci. 2005;25:7062–7070. doi: 10.1523/JNEUROSCI.0447-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulman H, Greengard P. Nature. 1978;271:478–479. doi: 10.1038/271478a0. [DOI] [PubMed] [Google Scholar]
- 18.Miller SG, Kennedy MB. J Biol Chem. 1985;260:9039–9046. [PubMed] [Google Scholar]
- 19.Hudmon A, Schulman H. Biochem J. 2002;364:593–611. doi: 10.1042/BJ20020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller SG, Patton BL, Kennedy MB. Neuron. 1988;1:593–604. doi: 10.1016/0896-6273(88)90109-2. [DOI] [PubMed] [Google Scholar]
- 21.Schworer CM, Colbran RJ, Keefer JR, Soderling TR. J Biol Chem. 1988;263:13486–13489. [PubMed] [Google Scholar]
- 22.Lou LL, Schulman H. J Neurosci. 1989;9:2020–2032. doi: 10.1523/JNEUROSCI.09-06-02020.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Koninck P, Schulman H. Science. 1998;279:227–230. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- 24.Soderling TR. Curr Opin Neurobiol. 2000;10:375–380. doi: 10.1016/s0959-4388(00)00090-8. [DOI] [PubMed] [Google Scholar]
- 25.Malenka RC, Kauer JA, Perkel DJ, Mauk MD, Kelly PT, Nicoll RA, Waxham MN. Nature. 1989;340:554–557. doi: 10.1038/340554a0. [DOI] [PubMed] [Google Scholar]
- 26.Malinow R, Schulman H, Tsien RW. Science. 1989;245:862–866. doi: 10.1126/science.2549638. [DOI] [PubMed] [Google Scholar]
- 27.Silva AJ, Stevens CF, Tonegawa S, Wang Y. Science. 1992;257:201–206. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- 28.Silva AJ, Paylor R, Wehner JM, Tonegawa S. Science. 1992;257:206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- 29.Elgersma Y, Fedorov NB, Ikonen S, Choi ES, Elgersma M, Carvalho OM, Giese KP, Silva AJ. Neuron. 2002;36:493–505. doi: 10.1016/s0896-6273(02)01007-3. [DOI] [PubMed] [Google Scholar]
- 30.Hinds HL, Tonegawa S, Malinow R. Learn Mem. 1998;5:344–354. [PMC free article] [PubMed] [Google Scholar]
- 31.Llinas R, McGuinness TL, Leonard CS, Sugimori M, Greengard P. Proc Natl Acad Sci USA. 1985;82:3035–3039. doi: 10.1073/pnas.82.9.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin JW, Sugimori M, Llinas RR, McGuinness TL, Greengard P. Proc Natl Acad Sci USA. 1990;87:8257–8261. doi: 10.1073/pnas.87.21.8257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Llinas R, Gruner JA, Sugimori M, McGuinness TL, Greengard P. J Physiol. 1991;436:257–282. doi: 10.1113/jphysiol.1991.sp018549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chapman PF, Frenguelli BG, Smith A, Chen CM, Silva AJ. Neuron. 1995;14:591–597. doi: 10.1016/0896-6273(95)90315-1. [DOI] [PubMed] [Google Scholar]
- 35.Lu FM, Hawkins RD. Proc Natl Acad Sci USA. 2006;103:4264–4269. doi: 10.1073/pnas.0508162103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slonimsky JD, Mattaliano MD, Moon JI, Griffith LC, Birren SJ. Proc Natl Acad Sci USA. 2006;103:2915–2919. doi: 10.1073/pnas.0511276103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang BH, Mukherji S, Soderling TR. Proc Natl Acad Sci USA. 1998;95:10890–10895. doi: 10.1073/pnas.95.18.10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee A, Zhou H, Scheuer T, Catterall WA. Proc Natl Acad Sci USA. 2003;100:16059–16064. doi: 10.1073/pnas.2237000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erickson MG, Alseikhan BA, Peterson BZ, Yue DT. Neuron. 2001;31:973–985. doi: 10.1016/s0896-6273(01)00438-x. [DOI] [PubMed] [Google Scholar]
- 40.Sumi M, Kiuchi K, Ishikawa T, Ishii A, Hagiwara M, Nagatsu T, Hidaka H. Biochem Biophys Res Commun. 1991;181:968–975. doi: 10.1016/0006-291x(91)92031-e. [DOI] [PubMed] [Google Scholar]
- 41.Wayman GA, Kaech S, Grant WF, Davare M, Impey S, Tokumitsu H, Nozaki N, Banker G, Soderling TR. J Neurosci. 2004;24:3786–3794. doi: 10.1523/JNEUROSCI.3294-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hudmon A, Schulman H, Kim J, Maltez JM, Tsien RW, Pitt GS. J Cell Biol. 2005;171:537–547. doi: 10.1083/jcb.200505155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dzhura I, Wu Y, Colbran RJ, Balser JR, Anderson ME. Nat Cell Biol. 2000;2:173–177. doi: 10.1038/35004052. [DOI] [PubMed] [Google Scholar]
- 44.Ishida A, Kameshita I, Okuno S, Kitani T, Fujisawa H. Biochem Biophys Res Commun. 1995;212:806–812. doi: 10.1006/bbrc.1995.2040. [DOI] [PubMed] [Google Scholar]
- 45.Mauceri D, Cattabeni F, Di Luca M, Gardoni F. J Biol Chem. 2004;279:23813–23821. doi: 10.1074/jbc.M402796200. [DOI] [PubMed] [Google Scholar]
- 46.Stea A, Tomlinson W, Soong T, Bourinet E, Dubel S, Vincent S, Snutch T. Proc Natl Acad Sci USA. 1994;91:10576–10580. doi: 10.1073/pnas.91.22.10576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanaka O, Sakagami H, Kondo H. Brain Res Mol Brain Res. 1995;30:1–16. doi: 10.1016/0169-328x(94)00265-g. [DOI] [PubMed] [Google Scholar]
- 48.Ludwig A, Flockerzi V, Hofmann F. J Neurosci. 1997;17:1339–1349. doi: 10.1523/JNEUROSCI.17-04-01339.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Day NC, Volsen SG, McCormack AL, Craig PJ, Smith W, Beattie RE, Shaw PJ, Ellis SB, Harpold MM, Ince PG. Brain Res Mol Brain Res. 1998;60:259–269. doi: 10.1016/s0169-328x(98)00186-7. [DOI] [PubMed] [Google Scholar]
- 50.Numa S, Tanabe T, Takeshima H, Mikami A, Niidome T, Nishimura S, Adams BA, Beam KG. Cold Spring Harb Symp Quant Biol. 1990;55:1–7. doi: 10.1101/sqb.1990.055.01.003. [DOI] [PubMed] [Google Scholar]
- 51.Nakai J, Dirksen RT, Nguyen HT, Pessah IN, Beam KG, Allen PD. Nature. 1996;380:72–75. doi: 10.1038/380072a0. [DOI] [PubMed] [Google Scholar]
- 52.Wiser O, Tobi D, Trus M, Atlas D. FEBS Lett. 1997;404:203–207. doi: 10.1016/s0014-5793(97)00130-0. [DOI] [PubMed] [Google Scholar]
- 53.Sutton KG, McRory JE, Guthrie H, Murphy TH, Snutch TP. Nature. 1999;401:800–804. doi: 10.1038/44586. [DOI] [PubMed] [Google Scholar]
- 54.Sheng Z-H, Lee A, Catterall WA. In: Structure and Function of the Synapse. Ehlers M, Hell JW, editors. Norwell, MA: Springer; 2008. in press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.