Abstract
The ability to store fat in the form of cytoplasmic triglyceride droplets is conserved from Saccharomyces cerevisiae to humans. Although much is known regarding the composition and catabolism of lipid droplets, the molecular components necessary for the biogenesis of lipid droplets have remained obscure. Here we report the characterization of a conserved gene family important for lipid droplet formation named fat-inducing transcript (FIT). FIT1 and FIT2 are endoplasmic reticulum resident membrane proteins that induce lipid droplet accumulation in cell culture and when expressed in mouse liver. shRNA silencing of FIT2 in 3T3-LI adipocytes prevents accumulation of lipid droplets, and depletion of FIT2 in zebrafish blocks diet-induced accumulation of lipid droplets in the intestine and liver, highlighting an important role for FIT2 in lipid droplet formation in vivo. Together these studies identify and characterize a conserved gene family that is important in the fundamental process of storing fat.
Keywords: adipocytes, diabetes, FIT, obesity, triglyceride
The ability to store energy in the form of triglyceride (TG) is conserved from Saccharomyces cerevisiae to humans. TGs are stored in the cytoplasm surrounded by a monolayer of phospholipid in distinct structures or organelles given numerous names, such as lipid particles, oil bodies, adiposomes, eicosasomes, and, more commonly, lipid droplets (1). Under normal physiological conditions, lipid droplets are involved in maintaining energy balance at the cellular and whole-organism levels. Yet under conditions of extreme lipid droplet acquisition, as in obesity, the risk for acquiring common debilitating diseases such as type 2 diabetes and cardiovascular diseases is increased (2).
Despite their central role in energy homeostasis, only recently have the composition and functions of many of the components of lipid droplets from S. cerevisiae, Drosophila, and mammalian cells been revealed. In general, lipid droplets are composed of a core of neutral lipids, primarily TGs, surrounded by a monolayer of phospholipids and lipid droplet-associated proteins (3–7). In mammalian cells, the catabolism of lipid droplets is a highly regulated process involving hormonal signals, droplet-associated proteins, and lipases (8–10). Although much has been learned about the components and catabolism of lipid droplets, the molecular mechanism of lipid droplet biogenesis has remained unknown. The prevailing view is that lipid droplets are formed at the endoplasmic reticulum (ER) because the ER is the site of TG biosynthesis, and lipid droplets are often observed in close association with the cytoplasmic face of the ER (11–13). A widely accepted model of lipid droplet biogenesis involves the formation of a core or lens of newly synthesized TG between the leaflets of the ER membrane that buds off with the cytoplasmic leaflet of the ER surrounding the neutral lipid core and acquires exchangeable cytosolic lipid droplet-associated proteins (14). However, this view was recently challenged by observations suggesting that lipid droplets form on the cytosolic leaflet of the ER membrane (15). In any case, we surmised that proteins that mediate lipid droplet biogenesis are localized to the ER, are not directly involved in the biosynthesis of TG (an activity attributable to diacylglycerol acyltransferase enzymes), and are regulated by the peroxisome proliferator-activated receptor nuclear hormone receptors, PPARα and PPARγ, which play crucial roles in lipid metabolism. Here we describe the identification and characterization of a family of proteins that fit these characteristics and are important for the accumulation of lipid droplets.
Results
Identification of the FIT Family.
We sought to identify proteins involved in intracellular fatty acid transport and metabolism either through storage as TG in lipid droplets or catabolism by β-oxidation in mitochondria. Fenofibrate and other fibrate drugs are specific agonists for the peroxisome proliferator-activated receptor-α (PPARα) nuclear hormone receptor, and activation of PPARα leads to enhanced gene expression for much of the biochemical repertoire of β-oxidation (16). Genotype matched WT and PPARα-deficient mice were fed a diet containing fenofibrate for 7 days. RNA was purified from livers to generate cDNA probes used to query a gene array. Because many of the genes activated by PPARα have been identified, we focused exclusively on genes that were listed as ESTs or having unknown function. Our attention was directed toward two unknown transcripts: fat-inducing transcript 1 (FIT1) and FIT2. The fenofibrate-induced expression of both mouse FIT1 and FIT2 was confirmed to be PPARα-dependent in the liver and not induced by fenofibrate in the heart [supporting information (SI) Fig. 7]. FIT1 and FIT2 genes encode putative 292- and 262 amino acid proteins, respectively, which are 50% similar to each other (Fig. 1A). FIT1 and FIT2 contain multiple potential transmembrane domains (SI Fig. 8) that are highly expressed in heart and skeletal muscle according to gene array studies (determined by Novartis Gene Atlas). However, FIT1 and FIT2 do not have homology to known proteins or protein domains found in any species, indicating that FIT genes comprise a unique gene family. These characteristics indicated that these proteins are potentially involved in lipid metabolism in oxidative tissues.
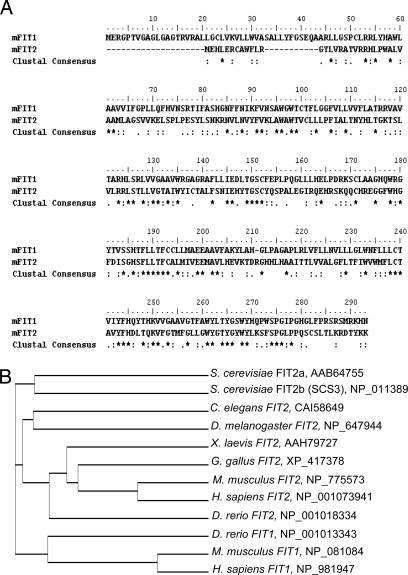
Fig. 1.
FIT sequence analysis. (A) Amino acid sequence alignment of murine FIT1 and FIT2 (35% identical, 50% similar). (B) Sequence alignments of FIT orthologs in multiple species. Cladogram generated with ClustalW showing the amino acid sequence homologies among FIT proteins. Accession numbers for each FIT ortholog are indicated next to the cladogram.
Performing a BLAST search with the full-length mouse FIT1 amino acid sequence against the expressed database, we identified FIT1 and FIT2 orthologous genes in mammals, whereas only a single FIT gene can be identified in amphibians, birds, insects, and worms, which exhibit higher homology to FIT2 (Fig. 1B). In contrast, the zebrafish genome contains orthologs of both mammalian FIT1 and FIT2. S. cerevisiae has two FIT2 orthologs (designated here as FIT2a and FIT2b/SCS3), which is consistent with evidence that the ancestral S. cerevisiae underwent a genome-wide duplication (17).
Northern blot analysis of mouse tissues indicated that FIT1 is highly expressed in heart and skeletal muscle, as well as at lower levels in the liver, kidney, and testes (Fig. 2A). Western blot analysis of mouse tissues indicated that FIT1 protein was detected primarily in skeletal muscle, with lower levels in the heart (Fig. 2B). Mouse FIT2 mRNA was ubiquitously detected as two to three transcripts, with highest levels in white and brown adipose tissue, the heart, and skeletal muscle (Fig. 2A). Western blot analysis indicated that FIT2 protein was ubiquitously detected, with highest levels in white and brown adipose tissue (Fig. 2B). The levels of mFIT1 and mFIT2 mRNA do not correlate well with protein levels in the heart, indicating potential posttranscriptional regulation in this organ. An examination of human tissues showed that FIT1 was primarily expressed in heart and skeletal muscle, whereas FIT2 was expressed in all tissues represented on the Northern blot (Fig. 2C). Together these analyses indicated that both mouse and human FIT1 have an expression pattern more restricted to oxidative tissues, whereas FIT2 has a broader expression pattern, with highest levels in mouse adipose tissue (human adipose tissue was not examined).
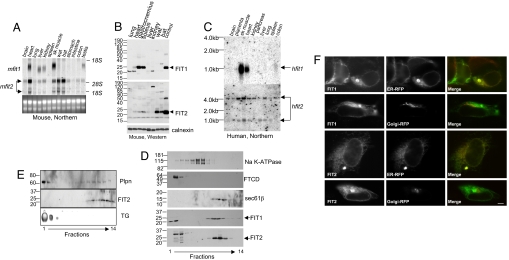
Fig. 2.
Analysis of FIT expression and localization. (A) Fifteen micrograms of total RNA from the mouse tissues shown was subjected to Northern blot analysis for murine FIT1 and FIT2 (mfit1 and mfit2). Sk muscle, skeletal muscle; wat, white adipose tissue; bat, brown adipose tissue. The ethidium bromide-stained gel indicates loading. (B) Eighty micrograms of total cell lysates from the selected mouse tissues shown was subjected to Western blot analysis. Calnexin served as a loading control. Other cross-reacting bands not indicated by arrows are nonspecific. Lysates from HEK293 cells expressing FIT1 or FIT2 served as positive controls. (C) A human RNA blot was analyzed for both fit1 and fit2 (hfit1 and hfit2) expression. (D) Total postnuclear membranes from mouse hearts were separated by continuous sucrose gradients (fractions shown from lowest to highest density). Fractions were subjected to Western blot analysis by using antibody markers for the plasma membrane (Na K-ATPase), Golgi apparatus (FTCD/Gogli 58-kDa protein), ER (Sec61-β), and FIT1 and FIT2. (E) Lipid droplets and membranes from mouse brown adipose tissue were fractionated on a continuous sucrose gradient, and fractions were subjected to Western blot analysis for FIT2 and perilipin (Plpn) and determination of TG in each fraction by TLC analysis. (F) Mouse FIT1-V5 and FIT2-V5 colocalized with the ER marker protein RFP-KDEL (ER-RFP) but not with the Golgi-specific marker GalTase-RFP (Golgi-RFP) in HEK293 cells. (Scale bar: 10 μm.)
FITs Are ER Resident Membrane Proteins.
Subcellular fractionation of mouse heart membranes and confocal immunolocalization studies were used to determine the subcellular localization of FITs. Fractionation of mouse heart membranes by sucrose density ultracentrifugation indicated that both FIT1 and FIT2 colocalized with the ER membrane resident protein Sec61-β (Fig. 2D) and slightly lighter membranes (fraction 9). The bands found in fractions 1 and 2 represent nonspecific antigens of the incorrect molecular weight and were observed by using these polyclonal antibodies to FIT1 and FIT2 in total cell lysates from mouse tissues and in HEK293 cells (Fig. 2B). To exclude the possibility that FIT proteins also localize to lipid droplets, we examined the localization of FIT2 in murine brown adipose tissue, a major tissue in which FIT2 is expressed (FIT1 is not expressed in adipose tissue or adipocytes in culture) (Fig. 2A). Separation of lipid droplets from membranes of brown adipose tissue indicated that FIT2 does not reside in the lipid droplet fraction enriched in TG and the lipid droplet-associated PAT family protein, perilipin (Fig. 2E). Expression of carboxyl-terminal V5 epitope-tagged FIT1 or FIT2 in HEK293 cells resulted in a clear reticular staining pattern that colocalized with a synthetic ER marker protein, red fluorescent protein (RFP) having the ER retention signal, KDEL, but not at all with the Golgi-specific marker protein, galactosyl-transferase-RFP (Fig. 2F). Also noted in some cells expressing FITs was the presence of single, large, oval-shaped fluorescent ER domains (colocalizing with ER-RFP) likely representing proliferated smooth ER that commonly occurs as a result of overexpression of ER membrane proteins (18, 19). Together these findings provide further support for exclusive ER localization of FIT proteins. The V5 epitope tag on FIT1 and FIT2 did not abolish its activity (see SI Fig. 9). In agreement with the ER localization of mouse FIT1 and FIT2, one of the S. cerevisiae FIT2 orthologs, FIT2b (FIT2a has not yet been localized), has been localized exclusively to the ER in a high-throughput attempt to localize all expressed ORFs in S. cerevisiae (20). Together the data indicate that FIT1 and FIT2 are ER resident membrane proteins, the established site for TG biosynthesis and the proposed site for lipid droplet biogenesis.
FIT-Mediated Lipid Droplet Accumulation.
To determine whether FIT proteins play a role in lipid metabolism, we overexpressed FIT proteins in HEK293 cells and examined the presence of lipid droplets by using the fluorescent lipid droplet stain (neutral lipid stain) BODIPY493/503. Overexpression of FIT1 or FIT2, at similar levels (SI Fig. 10) resulted in the accumulation of lipid droplets (Fig. 3A). As a positive control, cells expressing DGAT1, one of two acyltransferases important in the committed step in TG biosynthesis (11), showed multiple lipid droplets per cell (Fig. 3A). Compared with mock-transfected cells, overexpression of DGAT1 or DGAT2, a second acyltransferase important in the committed step in TG biosynthesis (11), resulted in a 7- to 10-fold increase in levels of cellular TG, whereas TG levels increased only a modest 2-fold in FIT1- and FIT2-expressing cells (Fig. 3B). Overexpression of FIT1 or FIT2 did not change the levels of phospholipids (SI Fig. 11). Cholesteryl esters (CEs) in FIT1-, FIT2-, or DGAT2-overexpressing cells significantly increased, compared with control cells (SI Fig. 11). Because CEs also increased in DGAT2-expressing cells and DGAT2 does not use cholesterol as a substrate, being highly specific for diacyglycerol (12), we concluded that moderate increases in CEs in HEK293 cells is an indirect consequence of lipid droplet formation.
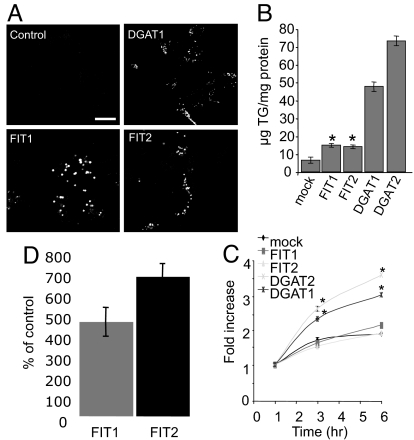
Fig. 3.
Lipid droplet formation induced by FIT1 and FIT2. (A) HEK293 cells were transiently transfected with mouse FIT1, FIT2, or DGAT1, and lipid droplets were visualized by using confocal fluorescence microscopy by staining with BODIPY 493/503. (Scale bar: 5 μm.) (B) TG mass measurements from transiently transfected HEK293 cells with the indicated constructs. Data are represented as the mean ± SD. *, Mock versus FIT1 or FIT2 (P < 0.001) (n = 4 transfections per construct; four independent experiments). (C) TG biosynthesis was determined in transfected HEK293 cells for the indicated times. DGATs served as positive controls for TG biosynthesis. Data are represented as the mean ± SD. *, Mock versus DGAT1 or DGAT2 (P < 0.0001) (n = 4 transfections per construct; three independent experiments). (D) Newly synthesized TG in the buoyant lipid droplet fractions from FIT1- and FIT2-expressing HEK293 cells was significantly enriched compared with mock-transfected cells. Data are represented as the mean ± SD of the percentage of mock-transfected (control) cells. Control versus FIT1 or FIT2 (P = 0.0001) (n = 4 per transfection; two independent experiments).
We next tested whether FIT1 and FIT2 enhance TG biosynthesis by quantifying the rate of TG biosynthesis by using radiolabeled glycerol as a precursor for TG. Overexpression of positive controls DGAT1 and DGAT2 led to a significant increase in the rate of TG biosynthesis (Fig. 3C) above mock-transfected cells as previously shown (11, 12). In contrast, cells expressing FIT1 or FIT2 resulted in similar rates of TG biosynthesis, compared with mock-transfected cells (Fig. 3C). In addition, the mRNAs of genes important in fatty acid and TG biosynthesis were not changed in cells expressing FIT1 or FIT2 (SI Fig. 12). Taken together, the data indicate that FIT1 and FIT2 do not enhance TG biosynthesis, but rather increase the partitioning of cellular TG into lipid droplets. To provide evidence that FIT1 and FIT2 increase the partitioning of TG into lipid droplets, HEK293 cells were transfected with FIT1 or FIT2 and treated for 3 h with radiolabeled glycerol to label newly synthesized TG; then lipid droplets were isolated by ultracentrifugation and radioactive TG-quantified. Expression of FIT1 and FIT2 significantly increased (between 4- and 6-fold) the amount of labeled, newly synthesized TG in lipid droplets, compared with control cells (Fig. 3D), supporting the conclusion that FIT1 and FIT2 do not affect TG biosynthesis (Fig. 3C), but rather partitioned TG into lipid droplets. To rule out the possibility that FIT1 and FIT2 inhibit lipolysis of TG leading to increased lipid droplets, HEK293 cells were transfected with FIT1 or FIT2 and labeled for 18 h with14C-oleic acid–BSA complex to label TG pools, and then TG biosynthesis was inhibited with triacsin C (21). The decrease in TG levels was quantified as a measure of TG lipolysis (21). Expression of ADRP in cells served as a positive control for inhibition of lipolysis (6). Lipolysis of TG was similar in cells expressing FIT1 or FIT2, compared with control HEK293 cells (SI Fig. 13), and moderately reduced in cells expressing ADRP, indicating that FIT1 and FIT2 do not increase lipid droplet accumulation by inhibiting lipolysis of TG (SI Fig. 13).
To determine whether expression of FIT proteins leads to lipid droplet accumulation in vivo, an adenovirus-expressing FIT2 or a control adenovirus was injected into mice. At 7 days after injection, livers were examined for lipid droplets and TG was quantified. We focused on FIT2 because FIT2 protein is more abundant than FIT1 in the liver (Fig. 2B). The histological examination of livers from FIT2 adenovirus-injected mice indicated increased lipid droplets, compared with control livers (arrows indicate lipid droplets) (Fig. 4A). In agreement with the appearance of increased lipid droplets, FIT2-overexpressing livers (Fig. 4C) had a significant increase in TG, but normal levels of cholesterol, compared with controls (Fig. 4B).
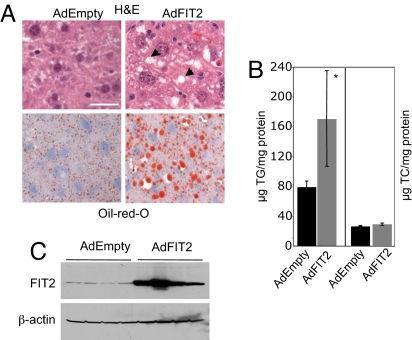
Fig. 4.
Expression of FIT2 in mouse liver. (A) Mice were injected with control adenovirus (Adempty) or adenovirus-expressing FIT2 (AdFIT2). AdFIT2 mice after 7 days had increased hepatic lipid droplets as judged by both H&E staining showing cleared spaces that indicated the presence of lipid droplets within hepatocytes (examples indicated with arrows) and Oil red O staining, indicating that these cleared spaces are TG-rich lipid droplets. These images are representative of observations made on six mice per group. (Scale bar: 12 μm.) (B) TG, but not cholesterol, was significantly increased in livers of mice expressing FIT2 (n = 6). Data represented as mean ± SD. *, AdEmpty versus AdFIT2 (P < 0.001). The large range in TG values correlated with the range in FIT2 expression shown in C. (C) Western blot analysis of FIT2 expression in livers of mice, indicating increased expression in AdFIT2-injected mice. β-actin served as a loading control.
shRNA Knockdown of FIT2 in Adipocytes.
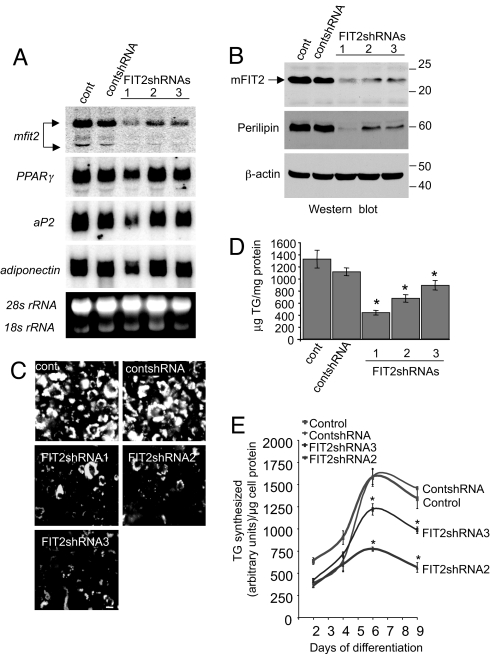
The requirement of FIT proteins for lipid droplet biogenesis was investigated by using shRNA technology. We surmised that if FIT proteins were essential for lipid droplet formation, then FITs should be expressed during adipogenesis at the onset of lipid droplet accumulation. We used the 3T3-L1 adipocyte cell line, a classic adipocyte differentiation cell model that produces large amounts of lipid droplets during differentiation of preadipocytes into adipocytes. Abundant levels of FIT2 mRNA and protein were detected during adipogenesis at the onset of formation of visible lipid droplets (SI Fig. 14). FIT1 was not detectable by Northern or Western blot analysis in 3T3-L1 adipocytes (data not shown), consistent with findings from mouse adipose tissues (Fig. 2 A and B). Therefore, we hypothesized that if FIT2 is indeed essential for droplet formation, then suppression of FIT2 expression should abolish the accumulation of lipid droplets. Preadipocytes infected with lentivirus expressing one of three individual shRNAs against FIT2 (FIT2shRNA1 to 3), a nonspecific control shRNA, or no virus were induced to differentiate into adipocytes. FIT2 mRNA and protein was significantly suppressed in adipocytes infected with lentivirus-expressing FIT2 shRNA (Fig. 5 A and B). Examination of these adipocytes for lipid droplets showed that cells having suppressed FIT2 expression had a dramatic reduction of lipid droplets and a significant reduction in total cellular TG (Fig. 5 C and D). Because these cells have suppressed FIT2 expression and not a complete deficiency, the remaining amount of lipid droplets in FIT2 knockdown adipocytes may be because of residual amounts of FIT2 (Fig. 5 A and B). Perilipin levels strongly correlate with lipid droplet numbers and TG levels in adipocytes (14, 22, 23). As expected, Perilipin was reduced in all FIT2shRNA-expressing adipocytes (Fig. 5B). We next sought to determine whether the knockdown of FIT2 in adipocytes affected the process of differentiation and TG biosynthesis. Examination of FIT2-suppressed cells showed that the expression of PPARγ, a nuclear hormone receptor essential for adipocyte differentiation, was similar in the FIT2shRNA2 and FIT2shRNA3 adipocytes, compared with controls, with moderate variation in levels between samples, but was decreased in FIT2shRNA1 adipocytes, which had the most reduced levels of FIT2 and TG. Expression of the PPARγ target genes, aP2, and adiponectin/ACRP30 followed a similar trend, with decreased levels in the FIT2shRNA1 adipocytes (Fig. 5A).
Fig. 5.
shRNA-mediated knockdown of FIT2 in adipocytes. 3T3-L1 cells were not infected or were infected with lentivirus-expressing shRNA sequences targeting murine FIT2 (FIT2shRNA1,2,3) or control shRNA (contshRNA) and differentiated for 7 days. (A) Northern blot analysis shows that FIT2shRNAs significantly reduced FIT2 mRNA levels compared with noninfected control (cont) and contshRNA-infected cells. Expression of adipocyte differentiation markers PPARγ, aP2, and adiponectin/ACRP30 are shown. The ethidium bromide-stained RNA gel serves to indicate loading. (B) Western blot analysis shows that FIT2 and Perilipin were reduced in FIT2shRNA cells, compared with controls. (C) FIT2shRNAs reduced lipid droplet accumulation in differentiated 3T3-L1 cells as visualized by BODIPY493/503 staining of lipid droplets. (Scale bar: 10 μm.) (D) Quantification of cellular TG in differentiated 3T3-L1 cells shows reduced TG levels in the FIT2 knockdown cells. Data represented as mean ± SD. *, FIT2shRNA1–3 versus controls P < 0.0001. (E) TG synthesis measurements were performed at the indicated time points during differentiation. Each time point represents three independent samples for each time point and is shown as mean ± SD. *, FIT2shRNA2,3 versus controls (P < 0.001). A–D are representative of three independent experiments. E is representative of two independent experiments.
The decrease in TG levels in FIT2shRNA cells could be because of decreased TG biosynthesis. To test this possibility, the rate of TG biosynthesis was examined in FIT2shRNA and control cells 2, 4, 6, and 9 days after induction of differentiation. TG biosynthesis was significantly decreased in FIT2shRNA cells (Fig. 5E). In contrast, TG lipase activity was similar in FIT2shRNA cells, compared with controls (SI Fig. 15). In summary, the data indicate that FIT2 knockdown in 3T3-L1 adipocytes resulted in decreased lipid droplet accumulation and significantly decreased TG biosynthesis.
Knockdown of FIT2 in Zebrafish.
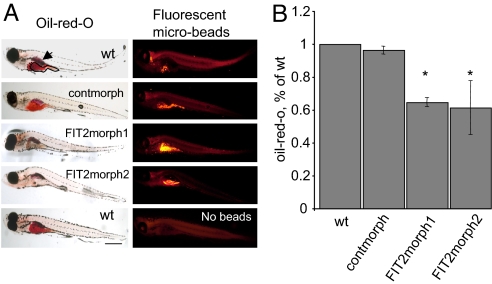
To extend these findings to a whole-animal model, we turned to the zebrafish model system. The zebrafish has orthologs to FIT1 and FIT2 (Fig. 1B) and, as a vertebrate, has conserved lipid metabolic pathways, compared with mammals (24), and transient gene knockdowns in embryos can be generated by using morpholino antisense oligonucleotides. Similar to mouse and human FIT2 expression patterns, expressed sequence tags for zebrafish FIT2 are ubiquitously found in tissues. Zebrafish larvae have low levels of TG and must therefore be fed a high-fat diet to induce lipid droplet accumulation, which occurs primarily in liver and intestine (25). We first tested the effect of FIT2 knockdown by using a morpholino that targets the ATG codon (FIT2morph1) and is expected to inhibit translation of FIT2. Embryos were injected at the one-cell stage with up to 4 ng of FIT2morph1, which was well tolerated and did not cause any overt obvious phenotype. To determine whether FIT2 knockdown in zebrafish results in decreased lipid droplet accumulation in liver and intestine in vivo, free-swimming 6-day-old larvae derived from morphant or control embryos were fed a high-fat diet for 6 h, fixed, and compared for lipid droplet accumulation by using Oil red O staining. The intestine, liver, and swim bladder of control and control morpholino zebrafish stained red for TGs after 6 h of high-fat feeding (Fig. 6A), which was similar to previously published data (25). The FIT2 morphant fish showed a near absence of intestinal and hepatic lipid droplet staining, although feeding behavior was normal as determined by ingestion of nonabsorbable fluorescent microbeads (Fig. 6A). In the FIT2 morphant fish, whole-body Oil red O staining as a measurement of TG levels was significantly decreased, compared with controls (Fig. 6B). To determine that reduced lipid droplets and TG are specifically because of morpholino-directed knockdown of FIT2, we compared the effects of a second morpholino that targets the 5′ splice site of FIT2 mRNA (FIT2morph2). When injected into embryos, FIT2morph2 significantly inhibits splicing of its single intron, resulting in the introduction of four in-frame stop codons (SI Fig. 16), and is therefore predicted to abolish FIT2 expression. Indeed, treatment with FIT2morph2 resulted in a similar decrease in lipid droplets in the liver and intestine and whole-body Oil red O staining, compared with FIT2morph1 (Fig. 6 A and B). Moreover, overexpression of zebrafish FIT2 in HEK293 cells confirmed that zebrafish FIT2 functions to produce lipid droplets and increase TG levels in mammalian cells (SI Fig. 17 A and B), further indicating conservation of function. Together these data show that FIT2 is important for lipid droplet accumulation in mouse adipocytes and during embryogenesis in zebrafish liver and intestine.
Fig. 6.
Morpholino-mediated knockdown of FIT2 in zebrafish. (A) FIT2 morphants showed decreased staining for Oil red O in liver (outlined by dashed line in wild type) and intestine (outlined by solid line in wild type), compared with control fish (no morpholino control, WT; and nonspecific control morpholino, contmorph). Swim bladders of zebrafish stain with Oil red O (indicated by arrow). FIT2 morphants did not exhibit defects in feeding as judged by ingestion of nonabsorbable fluorescent microbeads. A WT control fed a high-fat diet without microbeads is shown (no beads) to demonstrate that fluorescence is because of ingested microbeads, not autofluorescence of fish. These images are representative of n = 400 fish, three independent experiments. (Scale bar: 0.4 mm.) (B) Quantification of Oil red O staining by spectrophotometric analysis in WT, contmorph, FIT2morph1, and FIT2morph2 fish (n = 20 fish per group, averaged over four independent experiments). *, WT versus morphants (P < 0.001) and shown as mean ± SD.
Discussion
The present study describes a highly conserved family of proteins that are important for the accumulation of lipid droplets. Four major lines of evidence support our conclusion that FIT proteins are important for the accumulation of lipid droplets: (i) FIT proteins are evolutionarily conserved and exclusively located in the ER, the site of TG biosynthesis and incidentally the long-proposed site of lipid droplet biogenesis; (ii) overexpression of FIT proteins in cultured cells or in mouse liver in vivo results in the accumulation of TG-rich lipid droplets; (iii) unlike DGATs, FIT proteins do not mediate the biosynthesis of TG, but enhance the partitioning of TG into lipid droplets, placing FIT proteins functionally downstream of DGATs; and (iv) shRNA-mediated depletion of FIT2 in 3T3-L1 adipocytes or knockdown of FIT2 in zebrafish embryos dramatically reduced the accumulation of lipid droplets. Curiously, the yeast FIT2 ortholog FIT2b was identified 13 years ago in a genetic screen as one of many genes when mutated resulted in myo-inositol auxotrophy in the presence of choline and named SCS3 (26). Hosaka and coworkers (26) speculated that SCS3 is involved in regulating inositol synthesis, but did not quantify levels of inositol phospholipids in SCS3 mutants or provide evidence that SCS3 regulates this pathway (26). Thus, a role of SCS3 in regulating inositol phospholipid biosynthesis is purely speculative. Moreover, FIT2a was not identified from this genetic screen. Since this original publication of SCS3, no further studies on SCS3 (FIT2b) have been reported, but the proteins involved in regulating phospholipid biosynthesis in S. cerevisiae have been identified and SCS3 is not among them (27, 28). In our studies, we have not found changes in levels of phospholipids, including inositol phospholipids in HEK293 cells overexpressing FIT1 or FIT2 (SI Fig. 11).
We initially identified FIT genes as cDNAs up-regulated by PPARα in liver. On the one hand, this finding is somewhat perplexing given that activation of PPARα is associated with increased expression of genes involved in the oxidation of fatty acids (16). On the other hand, activation of PPARα in the liver also up-regulates genes involved in lipogenesis, including malic enzyme, acetyl-coA carboxylase, fatty acid synthase, steroyl-CoA desaturase 1, glycerol-3-phosphate acyltransferase (29, 30), and two lipid droplet-associated proteins that are part of the PAT family, PAT-1/MLDP/OXPAT/LSDP5 (31, 32) and ADRP (33), indicating that liver PPARα regulates both lipid catabolic and anabolic pathways. Notably, FIT2 expression is up-regulated during 3T3-L1 differentiation similarly as PPARγ at a time when lipid droplets are known to accumulate, giving 3T3-L1 cells their adipocyte phenotype. Indeed, we found that FIT2 expression is up-regulated by the treatment of 3T3-L1 cells with rosiglitazone, a specific PPARγ agonist, further supporting the notion that FIT2 is additionally regulated by PPARγ (B.K., D.M., and D.L.S., unpublished data).
shRNA-mediated knockdown of FIT2 in 3T3-L1 adipocytes resulted in a dramatic decrease in lipid droplets and TG levels. Interestingly, this decrease, with the exception of FIT2shRNA1 adipocytes, was not associated with a significant decrease in PPARγ and the PPARγ target genes aP2 or adiponectin/ACRP30, indicating that FIT2-depleted adipocytes differentiate similarly to control cells. This finding suggests that the primary effect of FIT2 knockdown is on TG lipid droplet accumulation and not on differentiation. Interestingly, the decrease in TG levels in FIT2 knockdown adipocytes was associated with decreased TG biosynthesis. This finding is in contrast to our findings that overexpression of FITs in HEK293 cells does not enhance TG biosynthesis. One possible explanation for these findings is that an inhibition in the ability to produce lipid droplets, and therefore store TG in FIT2 knockdown adipocytes, results in a partial feedback inhibition in TG biosynthesis without an entire blockade in adipogenesis. This scenario would limit the buildup of lipotoxic lipid intermediates from the glycerol-3-phosphate pathway, such as diacylglycerol (34). Nonetheless, knocking down FIT2 in zebrafish resulted in decreased neutral lipid accumulation in liver and intestine, providing confirmatory in vivo evidence in a whole-animal vertebrate model that FIT2 is important for lipid droplet accumulation. Moreover, overexpression of FIT2 in mouse liver in vivo increased levels of TG-rich lipid droplets. Presently, the mechanism by which FIT proteins mediate lipid droplet accumulation is not known. We have found no evidence by using immunoprecipitation techniques that FIT proteins interact with the ubiquitously expressed lipid droplet-associated protein ADRP (data not shown) that plays a role in lipid droplet metabolism.
The sum of our data support the conclusion that FIT proteins are necessary for lipid droplet accumulation. The identification of FIT proteins should facilitate the development of reagents to regulate FIT expression or activity to treat diseases associated with excessive lipid droplet accumulation, such as obesity, type 2 diabetes, and atherosclerosis.
Materials and Methods
Reagents.
Rabbit polyclonal antibodies were raised against peptides corresponding to the C-terminal 15 amino acids of murine FIT1 and FIT2 and validated by using lysates from HEK293 cells expressing murine FIT1 and murine FIT2 (SI Fig. 18).
Membrane Fractionations and Isolation of Lipid Droplets.
See SI Materials and Methods for details.
TLC Assays and TG and Cholesterol Measurements.
TG or cholesterol quantified by enzymatic assay (Infinity triglyceride kit and Infinity cholesterol; ThermoElectron). See SI Materials and Methods for details.
Confocal Immunofluorescence Microscopy.
HEK293 cells were transiently transfected with expression plasmids for mFIT1-V5, mFIT2-V5, or RFP with the ER-retention signal KDEL or with the live cell Gogli marker protein galactosyl-transferase-Tag-RFP (35, 36) and processed for confocal microscopy as described in SI Materials and Methods.
TG Hydrolysis and Biosynthesis Assays.
For a detailed description see SI Materials and Methods.
Lentivirus shRNA.
A detailed description of shRNA sequences and lentivirus production can be found in SI Materials and Methods. 3T3-L1 preadipocytes were infected with a MOI of 10–50 and selected for 7 days with blasticidin. Cells were consequently plated and differentiated for 7 days to produce mature adipocytes. shRNAi experiments were repeated two independent times for FIT2shRNA1 or three independent times for FIT2shRNA2 and FIT2shRNA3.
Morpholino Knockdown of FIT2 in Zebrafish.
Sequences of morpholinos and experimental details can be found in SI Materials and Methods.
Supplementary Material
ACKNOWLEDGMENTS.
This work was supported in part by National Institutes of Health Grants P30 DK41296 (through the Marion Bessin Liver Research Center) (to D.L.S.) and R01 HL064282 (to T.E.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. K.A. is a guest editor invited by the Editorial Board.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession nos. GSM136099 and GSM136100).
This article contains supporting information online at www.pnas.org/cgi/content/full/0708579105/DC1.
References
- 1.Martin S, Parton RG. Nat Rev Mol Cell Biol. 2006;7:373–378. doi: 10.1038/nrm1912. [DOI] [PubMed] [Google Scholar]
- 2.Spiegelman BM, Flier JS. Cell. 2001;104:531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 3.Brasaemle DL, Dolios G, Shapiro L, Wang R. J Biol Chem. 2004;279:46835–46842. doi: 10.1074/jbc.M409340200. [DOI] [PubMed] [Google Scholar]
- 4.Liu P, Ying Y, Zhao Y, Mundy DI, Zhu M, Anderson RG. J Biol Chem. 2004;279:3787–3792. doi: 10.1074/jbc.M311945200. [DOI] [PubMed] [Google Scholar]
- 5.Beller M, Riedel D, Jansch L, Dieterich G, Wehland J, Jackle H, Kuhnlein RP. Mol Cell Proteomics. 2006;5:1082–1094. doi: 10.1074/mcp.M600011-MCP200. [DOI] [PubMed] [Google Scholar]
- 6.Chang BH, Chan L. Am J Physiol. 2007;292:G1465–G1468. doi: 10.1152/ajpgi.00566.2006. [DOI] [PubMed] [Google Scholar]
- 7.Mullner H, Daum G. Acta Biochim Pol. 2004;51:323–347. [PubMed] [Google Scholar]
- 8.Zechner R, Strauss JG, Haemmerle G, Lass A, Zimmermann R. Curr Opin Lipidol. 2005;16:333–340. doi: 10.1097/01.mol.0000169354.20395.1c. [DOI] [PubMed] [Google Scholar]
- 9.Londos C, Brasaemle DL, Schultz CJ, Segrest JP, Kimmel AR. Semin Cell Dev Biol. 1999;10:51–58. doi: 10.1006/scdb.1998.0275. [DOI] [PubMed] [Google Scholar]
- 10.Duncan RE, Ahmadian M, Jaworski K, Sarkadi-Nagy E, Sul HS. Annu Rev Nutr. 2007;27:79–101. doi: 10.1146/annurev.nutr.27.061406.093734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cases S, Smith SJ, Zheng YW, Myers HM, Lear SR, Sande E, Novak S, Collins C, Welch CB, Lusis AJ, et al. Proc Natl Acad Sci USA. 1998;95:13018–13023. doi: 10.1073/pnas.95.22.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cases S, Stone SJ, Zhou P, Yen E, Tow B, Lardizabal KD, Voelker T, Farese RV., Jr J Biol Chem. 2001;276:38870–38876. doi: 10.1074/jbc.M106219200. [DOI] [PubMed] [Google Scholar]
- 13.Novikoff AB, Novikoff PM, Rosen OM, Rubin CS. J Cell Biol. 1980;87:180–196. doi: 10.1083/jcb.87.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolins NE, Brasaemle DL, Bickel PE. FEBS Lett. 2006;580:5484–5491. doi: 10.1016/j.febslet.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 15.Robenek H, Hofnagel O, Buers I, Robenek MJ, Troyer D, Severs NJ. J Cell Sci. 2006;119:4215–4224. doi: 10.1242/jcs.03191. [DOI] [PubMed] [Google Scholar]
- 16.Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart JC. Circulation. 1998;98:2088–2093. doi: 10.1161/01.cir.98.19.2088. [DOI] [PubMed] [Google Scholar]
- 17.Wolfe KH, Shields DC. Nature. 1997;387:708–713. doi: 10.1038/42711. [DOI] [PubMed] [Google Scholar]
- 18.Almsherqi ZA, Kohlwein SD, Deng Y. J Cell Biol. 2006;173:839–844. doi: 10.1083/jcb.200603055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snapp EL, Hegde RS, Francolini M, Lombardo F, Colombo S, Pedrazzini E, Borgese N, Lippincott-Schwartz J. J Cell Biol. 2003;163:257–269. doi: 10.1083/jcb.200306020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huh WK, Falvo JV, Gerke LC, Carroll AS, Howson RW, Weissman JS, O'Shea EK. Nature. 2003;425:686–691. doi: 10.1038/nature02026. [DOI] [PubMed] [Google Scholar]
- 21.Brasaemle DL, Rubin B, Harten IA, Gruia-Gray J, Kimmel AR, Londos C. J Biol Chem. 2000;275:38486–38493. doi: 10.1074/jbc.M007322200. [DOI] [PubMed] [Google Scholar]
- 22.Xu G, Sztalryd C, Londos C. Biochim Biophys Acta. 2006;1761:83–90. doi: 10.1016/j.bbalip.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Brasaemle DL, Barber T, Kimmel AR, Londos C. J Biol Chem. 1997;272:9378–9387. doi: 10.1074/jbc.272.14.9378. [DOI] [PubMed] [Google Scholar]
- 24.Tocher D. In: Biochemistry and Molecular Biology of Fishes. Hochachka P, Mommsen T, editors. New York: Elsevier; 1995. pp. 119–157. [Google Scholar]
- 25.Schlegel A, Stainier DY. Biochemistry. 2006;45:15179–15187. doi: 10.1021/bi0619268. [DOI] [PubMed] [Google Scholar]
- 26.Hosaka K, Nikawa J, Kodaki T, Ishizu H, Yamashita S. J Biochem (Tokyo) 1994;116:1317–1321. doi: 10.1093/oxfordjournals.jbchem.a124681. [DOI] [PubMed] [Google Scholar]
- 27.Chen M, Hancock LC, Lopes JM. Biochim Biophys Acta. 2007;1771:310–321. doi: 10.1016/j.bbalip.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 28.Loewen CJ, Gaspar ML, Jesch SA, Delon C, Ktistakis NT, Henry SA, Levine TP. Science. 2004;304:1644–1647. doi: 10.1126/science.1096083. [DOI] [PubMed] [Google Scholar]
- 29.Castelein H, Gulick T, Declercq PE, Mannaerts GP, Moore DD, Baes MI. J Biol Chem. 1994;269:26754–26758. [PubMed] [Google Scholar]
- 30.Knight BL, Hebbachi A, Hauton D, Brown AM, Wiggins D, Patel DD, Gibbons GF. Biochem J. 2005;389:413–421. doi: 10.1042/BJ20041896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolins NE, Quaynor BK, Skinner JR, Tzekov A, Croce MA, Gropler MC, Varma V, Yao-Borengasser A, Rasouli N, Kern PA, et al. Diabetes. 2006;55:3418–3428. doi: 10.2337/db06-0399. [DOI] [PubMed] [Google Scholar]
- 32.Yamaguchi T, Matsushita S, Motojima K, Hirose F, Osumi T. J Biol Chem. 2006;281:14232–14240. doi: 10.1074/jbc.M601682200. [DOI] [PubMed] [Google Scholar]
- 33.Dalen KT, Ulven SM, Arntsen BM, Solaas K, Nebb HI. J Lipid Res. 2006;47:931–943. doi: 10.1194/jlr.M500459-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Schaffer JE. Curr Opin Lipidol. 2003;14:281–287. doi: 10.1097/00041433-200306000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Cole NB, Smith CL, Sciaky N, Terasaki M, Edidin M, Lippincott-Schwartz J. Science. 1996;273:797–801. doi: 10.1126/science.273.5276.797. [DOI] [PubMed] [Google Scholar]
- 36.Merzlyak EM, Goedhart J, Shcherbo D, Bulina ME, Shcheglov AS, Fradkov AF, Gaintzeva A, Lukyanov KA, Lukyanov S, Gadella TW, et al. Nat Methods. 2007;4:555–557. doi: 10.1038/nmeth1062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.