Abstract
We examined sequential methicillin-susceptible Staphylococcus aureus isolates from a patient with mitral valve endocarditis recovered during persistent bacteremia on standard therapy and relapse after treatment with daptomycin. An isolate obtained after 5 days of antimicrobial therapy, but before exposure to daptomycin, showed subtle physiological changes in response to daptomycin, with significant regrowth in the daptomycin killing assay compared to the treatment-naive strain. Once daptomycin was started, the population became more heterogeneous and tested as nonsusceptible. These organisms were examined in a simulated-vegetation in vitro pharmacodynamic model, which confirmed progressive decreases in killing with daptomycin concentrations that simulate those attained in humans with 6-mg/kg of body weight daily dosing. Early surgical intervention or combination therapy or both might have prevented the loss of daptomycin susceptibility.
Daptomycin is a lipopeptide antibiotic which demonstrates potent bactericidal activity in vitro against methicillin-susceptible (MSSA) and methicillin-resistant (MRSA) Staphylococcus aureus, glycopeptide-intermediate S. aureus, vancomycin-resistant S. aureus, and other gram-positive bacteria (1, 7, 10, 15, 25). The mechanism of action appears to involve the disruption of the potential across the bacterial plasma membrane (2, 9, 11). Daptomycin was approved in 2003 for treatment of skin and skin structure infections due to MSSA, MRSA, and other organisms (3) and was found to be superior to vancomycin in a rat model of MRSA aortic valve endocarditis (21). Recently, clinical trials of daptomycin in cases of S. aureus bacteremia and right-sided endocarditis that showed noninferiority to comparator therapy were completed, resulting in approval by the Food and Drug Administration for use against these infections (6).
Several recent reports noted attenuated in vitro activity of daptomycin against some isolates of MRSA that had developed intermediate resistance to glycopeptides through in vitro or in vivo exposure to vancomycin as well as a correlation of daptomycin and vancomycin MICs among clinical S. aureus isolates (5, 16, 24). However, the clinical significance of these effects is unknown. Here we examined sequential bloodstream isolates from a patient with community onset endocarditis due to MSSA in which rapid loss of susceptibility to daptomycin (MIC > 1 μg/ml) accompanying treatment failure occurred. These isolates were further examined in an in vitro pharmacokinetic model of endocarditis.
MATERIALS AND METHODS
Bacterial isolates.
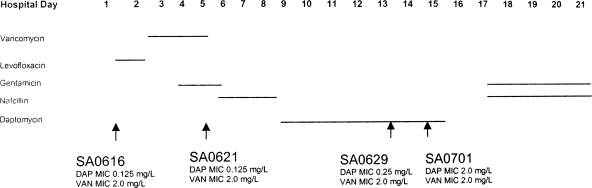
All MSSA isolates for this study were obtained from a man with native mitral valve endocarditis, confirmed by transesophageal echocardiography. The antimicrobial treatment he received in relation to the isolates studied is shown in Fig. 1. All organisms were confirmed to be PBP2a-negative by commercially available latex agglutination assays. The patient ultimately underwent mitral valve replacement on hospital day 26 and cultures and Gram stain of the mitral valve were negative. The patient received 4 weeks of postoperative nafcillin, has made a full recovery, and was alive and well 1 year after discharge.
FIG. 1.
MSSA isolates from a patient with mitral valve endocarditis on antibiotic therapy. The time of administration of each antibiotic is denoted by a horizontal line.
Susceptibility testing.
Daptomycin (Cubist Pharmacuticals, Lexington, MA) susceptibility testing was performed in duplicate using a final inoculum of 5 × 105 CFU/ml by a macrodilution method in cation-adjusted Mueller-Hinton broth (MHB) (Becton Dickinson, Cockeysville, MD) supplemented to contain a final calcium concentration of 50 μg/ml. Daptomycin and vancomycin susceptibility testing was performed in duplicate by gradient diffusion using E-test strips (AB Biodisk, Solna, Sweden) on Mueller-Hinton agar plates inoculated with a 0.5 McFarland suspension of organisms in 0.9% saline.
Population analysis.
Population analyses for daptomycin were performed as described previously in duplicate on Mueller-Hinton agar (Becton Dickinson, Cockeysville, MD) supplemented to a final calcium concentration of 50 or 100 μg/ml using an inoculum of 109 CFU/ml (19, 20, 23, 24).
Daptomycin in vitro killing assays.
Approximately 107 CFU obtained after 12 to 16 h of growth of the test organism in cation-adjusted MHB were inoculated into 20 ml of fresh MHB, which was supplemented to a final calcium concentration of 50 to 55 μg/ml and which contained 8 μg/ml of daptomycin. A 200-μl aliquot was obtained at 0, 4, 8, and 24 h, serially diluted, and plated on antibiotic-free agar in duplicate to determine colony counts as previously described (24).
In vitro pharmacodynamic model.
An in vitro pharmacodynamic model with simulated endocardial vegetations (SEV) containing an inoculum of approximately 9.5 log10 CFU/g as previously described was utilized to determine the impact of vancomycin exposure on daptomycin pharmacokinetics and pharmacodynamics (12, 15, 25, 26). Briefly, SEV consist of a mixture of organism suspension, human cryoprecipitate antihemolytic factor, and platelet suspension from human volunteer donors (American Red Cross) in 1.5-ml siliconized Eppendorf tubes. After the addition of a monofilament line to each tube, bovine thrombin (5,000 U/ml) was added to solidify the mixture. The resulting SEV were removed from the tubes and placed in the in vitro pharmacodynamic model (17, 23). All model simulations were performed in MHB supplemented with calcium and 12.5 mg/liter magnesium. For daptomycin simulations, human albumin at 25% USP (American Red Cross, Detroit, MI) (NDC 52769-475-05) was incorporated into MHB to achieve a concentration of 4 g/dl. The calcium content was titrated to maintain physiologic concentrations (1.1 to 1.3 mmol/liter) due to daptomycin's dependency on calcium and to account for calcium binding to albumin (8, 11).
Antibiotic-simulated regimens.
Simulated regimens were administered as the following over 96 h: daptomycin (peak, 98.6 mg/liter; trough, 12.5 mg/liter; average half-life, 8 h), 6 mg/kg of body weight every 24 h; vancomycin (peak, 40 mg/liter; trough, 10 mg/liter; average half-life, 6 h) and rifampin (peak, 4 mg/liter; trough, 0.75 mg/liter; average half-life, 3 h), 300 mg every 8 h.
Pharmacodynamic analysis.
Two SEV were removed in duplicate (total of four) at 0, 4, 8, 24, 32, 48, 72, and 96 h as previously described (12, 15, 25). This method provides a lower limit of detection of 2.0 log10 CFU. Bactericidal activity was defined as a ≥3 log10 CFU/g reduction in colony count from the initial inoculum, and bacteriostatic activity was defined as a <3 log10 reduction. The time to achieve a 99.9% bacterial load reduction (T99) was determined by linear regression if r2 was ≥0.95 or by visual inspection.
Pharmacokinetic analysis.
Samples for pharmacokinetic analysis were obtained in duplicate from each model over 0 to 96 h (12, 15, 26). Vancomycin concentrations were determined by fluorescence polarization immunoassay (Abbott Diagnostics TDx) with a lower limit of detection of 2 μg/ml and an interday percent coefficient of variation (CV%) of ≤1%. The determination of daptomycin and rifampin concentrations was performed by microbioassay utilizing Micrococcus luteus ATCC 9341 as previously described (r = 0.98; intraday CV% of ≤8% and interday CV% of ≤10%; lower limit of detection of 1 μg/ml) (12, 25). The half-lives, areas under the concentration-time curve, and peak concentrations were determined by the trapezoidal method utilizing PK Analyst software (version 1.10; MicroMath Scientific Software, Salt Lake City, UT).
Resistance.
The evaluation of resistance was determined at multiple time points throughout the simulation at 0, 8, 24, 48, 72, and 96 h. Samples were plated on MHA plates containing four times the MIC of daptomycin and examined for growth after 48 h of incubation at 37°C. Colonies which exhibited growth on these plates were subsequently examined by E-test.
Statistical analysis.
Differences between regimens in log10 CFU/g at 4, 24, 48, 72, and 96 h and in times to achieve a 99.9% bacterial load reduction were determined using analysis of variance with Tukey's test for multiple comparisons. For all experiments, a P value of ≤0.05 was considered statistically significant. All statistical analyses were performed using SPSS statistical software (version 14.0; SPSS, Inc., Chicago, IL).
RESULTS
The daptomycin susceptibilities of the bloodstream isolates studied in this report and their relationship to the administered antimicrobials are shown in Table 1. All isolates were isogenic by pulsed-field gel electrophoresis (data not shown) and had a vancomycin MIC by E-test of 2 μg/ml. The initial MSSA isolate, SA0616, was susceptible to daptomycin by E-test and broth macrodilution methods. Isolates obtained prior to the initial clearance of the bacteremia remained susceptible to daptomycin, without significant changes in MIC, including those obtained up to 3 days after initiation of daptomycin therapy. After the bacteremia had cleared during hospital days 12 and 13, SA0629 was detected in one blood culture on hospital day 14. This isolate also was susceptible to daptomycin. However, isolate SA0701, obtained on hospital day 16 during treatment with daptomycin, was no longer susceptible to daptomycin by E-test and broth dilution methods, with a MIC of 2 μg/ml.
TABLE 1.
Killing activity of daptomycin at 8 μg/ml in calcium-supplemented MHB
| Isolate | Mean log10 CFU/ml (SD) of surviving bacteriaa at:
|
|||
|---|---|---|---|---|
| 0 h | 4 h | 8 h | 24 h | |
| SA0616 | 6.70 (0.26) | 2.21 (0.33) | 1.62 (0.04) | 1.92 (0.51) |
| SA0621 | 6.61 (0.16) | 2.14 (0.36) | 2.05 (0.67) | 3.12 (1.18)* |
| SA0701 | 6.62 (0.20) | 3.39 (0.05)** | 2.00 (0.26) | 2.10 (0.89) |
| S. aureus ATCC 29213 | 6.48 (0.26) | 2.91 (0.21) | 1.6 (0) | 1.6 (0) |
Means and standard deviations were calculated from data on six experiments. The level of detection of the assay is to a log10 CFU/ml of 1.6. *, P = 0.045 versus SA0616 at 24 h using the Wilcoxon two sample test; **, P = 0.002 versus SA0616 and SA0621 at 4 h using the Wilcoxon two-sample test.
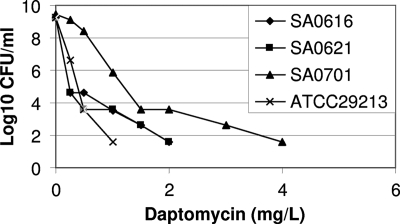
Daptomycin population analyses are shown in Fig. 2. Daptomycin-nonsusceptible isolate SA0701demonstrated increased subpopulations that were able to grow in media containing daptomycin at concentrations above the susceptible range compared to the susceptible progenitor isolates SA0616 and SA0621.
FIG. 2.
Daptomycin population analysis of initial bloodstream MSSA SA0616, isolate SA0621 obtained after 5 days of vancomycin therapy, and daptomycin-nonsusceptible SA0701, obtained after 8 days of daptomycin therapy. The experiment was performed twice, with results of one representative experiment shown. S. aureus strain ATCC 29213 is included in the analysis as a reference.
In vitro daptomycin killing assay results are shown in Table 1. Isolate 0621, obtained after administration of vancomycin, nafcillin, and levofloxacin, showed regrowth at 24 h, resulting in a significantly higher bacterial cell density at that time point in the killing assay compared to SA0616, the initial isolate from the patient (3.5 versus 4.7 log10 CFU/ml reduction). Daptomycin retained bactericidal activity against the nonsusceptible isolate SA0701. There was a significant decrease in the magnitude of the killing noted at 4 h compared to those for the susceptible isolates SA0616 and SA0621. However, levels of killing at 8 and 24 h were not different for susceptible isolates SA0616 and SA0621 and nonsusceptible isolate SA0701.
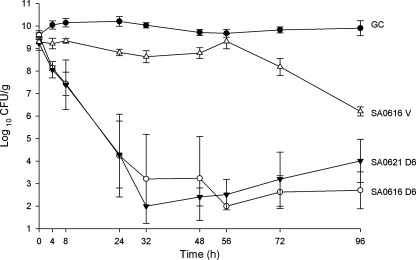
To simulate conditions of bacterial endocarditis, we assayed the activity of daptomycin against isolates SA0616, SA0621, SA0629, and SA0701 using an established in vitro pharmacodynamic model with simulated vegetations. The observed pharmacokinetic parameters of the different antibiotics in this model are shown in Table 2. Daptomycin demonstrated bactericidal activity against SA0616 and 0621 and achieved greater killing against initial isolate SA0616 than vancomycin. There was slight regrowth of SA0621 at the end of 96 h (Fig. 3). However, this did not reach statistical significance. This experiment was repeated (n = 4) and achieved identical results.
TABLE 2.
Measured mean pharmacokinetics of antibiotics applied to the in vitro pharmacodynamic modela
| Antibiotic | Isolate(s) evaluated | Cmax (μg/ml) | Cmin (μg/ml) | t1/2 (h) | AUC at 24 h (μg/ml·h) |
|---|---|---|---|---|---|
| Vancomycin | SA0616 | 43.2 | 6 | 7.4 | 98 |
| Daptomycin | SA0616, SA0621, SA0629, SA0701 | 99.6 | 13.7 | 8.7 | 1,267 |
| Rifampin | SA0629 | 6.7 | 0.49 | 3.4 | 98 |
Cmax, maximum concentration; Cmin, minimum concentration; t1/2, half-life; AUC, area under the concentration-time curve.
FIG. 3.
Activity of daptomycin at 6 mg/kg every 24 h (q24h) versus prevancomycin isolate SA0616 and postvancomycin isolate SA0621 in an in vitro pharmacodynamic model over 96 h in duplicate. D6, daptomycin in 6-mg/kg doses given q24h; V, vancomycin in 1,000-mg doses given q12h; GC, growth control.
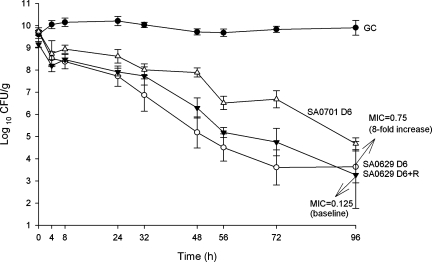
In the vegetation model, the first breakthrough isolate on daptomycin therapy, SA0629, showed a 2-dilution increase in MIC at 96 h (Fig. 4). Compared to the initial isolate, SA0616, the breakthrough isolate SA0629 exhibited significantly reduced killing at 24 h (P = 0.014). Daptomycin achieved 99.9% kill by 24 h of both SA0616 and postvancomycin strain SA0621, but this time was significantly prolonged to 48 h for the daptomycin breakthrough isolate (SA0629) (P = 0.01 and 0.03, respectively). Daptomycin displayed further reduced activity against nonsusceptible isolate SA0701, which was statistically different from all other strains tested (P ≤ 0.002).
FIG. 4.
Activity of daptomycin with or without rifampin at 300 mg every 8 h (q8h) against breakthrough isolate SA0629 and daptomycin-nonsusceptible isolate SA0701 in an in vitro pharmacodynamic model over 96 h in duplicate. D6, daptomycin in 6-mg/kg doses given q24h; R, rifampin at 300-mg doses given q8h; GC, growth control. MICs are in μg/ml.
To determine the impact of the addition of rifampin to daptomycin on the emergence of subpopulations with higher daptomycin MICs for isolate SA0629, the addition of rifampin to daptomycin at a dose of 300 mg every 8 h was investigated. Almost identical levels of killing were observed, but the emergence of isolates with higher daptomycin MICs was suppressed. (Fig. 4).
DISCUSSION
The decreased susceptibility of S. aureus to both old and new antimicrobials has raised concern among clinicians, microbiologists, and pharmacists (13, 14, 27). In order to further explore the means to prevent or reduce the rate of the development of antimicrobial resistance, we examined sequential bloodstream isolates from a patient with prolonged MSSA bacteremia resulting from native valve endocarditis where loss of daptomycin susceptibility occurred rapidly. These isolates were subjected to in vitro susceptibility testing and killing assays and applied in a pharmacodynamic model simulation of endocardial vegetation.
That global changes can occur in staphylococci exposed to antibiotics has long been recognized. For example, small-colony variants of staphylococci that are more resistant to antimicrobial killing can arise in patients receiving antibiotics, particularly aminoglycosides (17). Vancomycin has also been shown to alter the expression of global regulators, which may have implications for virulence and resistance to other agents (4, 18-20, 22). For example, it is well established that the resistance of S. aureus to platelet microbicidal proteins is associated with prolonged bacteremia and development of metastatic foci of infection. This phenotype has been selected in vitro and in vivo by vancomycin exposure (23).
In this investigation, isolate SA0621, obtained after 5 days of antimicrobial therapy but before the initiation of daptomycin therapy, showed decreased daptomycin killing at 24 h by traditional assays with daptomycin-containing broth compared to the initial isolate, SA0616, obtained on the day of admission. A similar, but not statistically significant, trend was also observed using a simulated-vegetation model. Reduced killing was observed before a rise in MIC of daptomycin to the nonsusceptible range, and this would not be detected by routine laboratory investigation. The clinical significance of this in vitro observation is unclear, but one hypothesis might be that pretreatment with antibiotics like vancomycin and quinolones alters S. aureus genetically or metabolically so as to allow the persistence of a higher bacterial inoculum when daptomycin is utilized in situations when appropriate surgical treatment principles are not followed in the management of S. aureus infection. This could increase the probability of subsequent loss of daptomycin susceptibility. In this case, the patient was ultimately cured by surgical intervention together with antibiotic therapy. It is very likely that loss of daptomycin susceptibility would not have occurred had valve replacement been performed earlier in the hospital course.
Previous studies carried out using an experimental rat model of endocarditis demonstrated a greater reduction in surviving bacteria on cardiac vegetations when rifampin was added to daptomycin (21). Although we did not observe enhanced killing by the addition of rifampin to daptomycin against breakthrough isolate SA0629, the emergence of colonies with higher daptomycin MICs was suppressed by the addition of rifampin (Fig. 4). Therefore, combination antimicrobial therapy may be considered in the management of serious MRSA infections, not only to enhance antimicrobial activity but also to reduce the likelihood of resistance to monotherapy with agents such as daptomycin.
The observation of breakthrough bacteremia accompanying an increase in daptomycin MIC to 2 μg/ml (isolate SA0701) supports the current CLSI breakpoints, where S. aureus isolates with daptomycin MICs of ≥1 μg/ml are considered nonsusceptible. Decreased daptomycin killing of SA0701 was demonstrated in the in vitro simulated-vegetation model at 24 h. However, the finding that daptomycin retained bactericidal activity against nonsusceptible isolate SA0701 in conventional killing assays using daptomycin at 8 μg/ml (log10 CFU/ml reduction of 4.52 at 24 h) suggests that the rate of killing may be as important as the ultimate magnitude of killing in predicting clinical response.
This study is limited by the fact that it considers only one strain from a single patient with bacteremia. Given the likelihood that there may be strain-specific differences in S. aureus with regards to response to therapy and proclivity for the development of antimicrobial resistance, the extrapolation of these findings to other strains should be done with caution. Furthermore, data acquired through in vitro simulation of in vivo conditions must be placed in the appropriate context.
In conclusion, we believe that these findings support considerations of the evaluation of the medical treatment of S. aureus bacteremia, including aggressive incorporation of the newer antimicrobials, use of higher doses of antimicrobial therapy, and/or use of combination antimicrobial therapy. Such practices may be particularly useful in cases where susceptibility to vancomycin is reduced (e.g., MIC > 1 μg/ml). This case underscores the increasing recognition of the benefits of early surgery in the management of S. aureus native valve endocarditis, not only for improving patient outcome but also for preventing antimicrobial resistance. This study of sequential isogenic isolates of MSSA recovered from a patient failing medical therapy of mitral valve endocarditis demonstrates subtle changes in daptomycin inhibition and killing that may evade detection by routine monitoring measures in the clinical laboratory. The most resistant isolate (daptomycin MIC of 2 μg/ml) recovered during therapy with this agent was slowly killed by the lipopeptide in vitro at concentrations that should have been allowable at the doses employed. Therefore, reasons for failure remain elusive. The roles of combination therapy (daptomycin and rifampin) and early surgery to minimize the risk of resistance should be explored.
Footnotes
Published ahead of print on 14 November 2007.
REFERENCES
- 1.Akins, R. L., and M. J. Rybak. 2001. Bactericidal activities of two daptomycin regimens against clinical strains of glycopeptide intermediate-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus faecium, and methicillin-resistant Staphylococcus aureus isolates in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 45454-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alborn, W. E., Jr., N. E. Allen, and D. A. Preston. 1991. Daptomycin disrupts membrane potential in growing Staphylococcus aureus. Antimicrob. Agents Chemother. 352282-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arbeit, R. D., D. Maki, F. P. Tally, E. Campanaro, and B. I. Eisenstein. 2004. The safety and efficacy of daptomycin for the treatment of complicated skin and skin structure infections. Clin. Infect. Dis. 381673-1681. [DOI] [PubMed] [Google Scholar]
- 4.Bischoff, M., and B. Berger-Bachi. 2001. Teicoplanin stress-selected mutations increasing σB activity in Staphylococcus aureus. Antimicrob. Agents Chemother. 451714-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui, L., E. Tominaga, H. Neoh, and K. Hiramatsu. 2006. Correlation between reduced daptomycin susceptibility and vancomycin resistance in vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 501079-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fowler, V. G., Jr., H. W. Boucher, G. R. Corey, et al. 2006. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N. Engl. J. Med. 355653-665. [DOI] [PubMed] [Google Scholar]
- 7.Fuchs, P. C., A. L. Barry, and S. D. Brown. 2002. In vitro bactericidal activity of daptomycin against staphylococci. J. Antimicrob. Chemother. 49467-470. [DOI] [PubMed] [Google Scholar]
- 8.Hanberger, H., L. E. Nilsson, R. Maller, and B. Isaksson. 1991. Pharmacodynamics of daptomycin and vancomycin on Enterococcus faecalis and Staphylococcus aureus demonstrated by studies of initial killing and postantibiotic effect and influence of Ca2+ and albumin on these drugs. Antimicrob. Agents Chemother. 351710-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hancock, R. E. 2005. Mechanisms of action of newer antibiotics for gram-positive pathogens. Lancet Infect. Dis. 5209-218. [DOI] [PubMed] [Google Scholar]
- 10.Jevitt, L. A., A. J. Smith, P. P. Williams, P. M. Raney, J. E. McGowan, Jr., and F. C. Tenover. 2003. In vitro activities of daptomycin, linezolid, and quinupristin-dalfopristin against a challenge panel of staphylococci and enterococci, including vancomycin-intermediate Staphylococcus aureus and vancomycin-resistant Enterococcus faecium. Microb. Drug Resist. 9389-393. [DOI] [PubMed] [Google Scholar]
- 11.Lamp, K. C., M. J. Rybak, E. M. Bailey, and G. W. Kaatz. 1992. In vitro pharmacodynamic effects of concentration, pH, and growth phase on serum bactericidal activities of daptomycin and vancomycin. Antimicrob. Agents Chemother. 362709-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LaPlante, K. L., and M. J. Rybak. 2004. Impact of high-inoculum Staphylococcus aureus on the activities of nafcillin, vancomycin, linezolid, and daptomycin, alone and in combination with gentamicin, in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 484665-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mangili, A., I. Bica, D. R. Snydman, and D. H. Hamer. 2005. Daptomycin-resistant, methicillin-resistant Staphylococcus aureus bacteremia. Clin. Infect. Dis. 401058-1060. [DOI] [PubMed] [Google Scholar]
- 14.Marty, F. M., W. W. Yeh, C. B. Wennersten, L. Venkataraman, E. Albano, E. P. Alyea, H. S. Gold, L. R. Baden, and S. K. Pillai. 2006. Emergence of a clinical daptomycin-resistant Staphylococcus aureus isolate during treatment of methicillin-resistant Staphylococcus aureus bacteremia and osteomyelitis. J. Clin. Microbiol. 44595-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGrath, B. J., S. L. Kang, G. W. Kaatz, and M. J. Rybak. 1994. Bactericidal activities of teicoplanin, vancomycin, and gentamicin alone and in combination against Staphylococcus aureus in an in vitro pharmacodynamic model of endocarditis. Antimicrob. Agents Chemother. 382034-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel, J. B., L. A. Jevitt, J. Hageman, L. C. McDonald, and F. Tenover. 2006. An association between reduced susceptibility to daptomycin and reduced susceptibility to vancomycin in Staphylococcus aureus. Clin. Infect. Dis. 421652-1653. [DOI] [PubMed] [Google Scholar]
- 17.Proctor, R. A. 2000. Respiration and small-colony variants of Staphylococcus aureus, p. 345-350. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. American Society for Microbiology, Washington, DC.
- 18.Renzoni, A., P. Francois, D. Li, W. L. Kelley, D. P. Lew, P. Vaudaux, and J. Schrenzel. 2004. Modulation of fibronectin adhesions and other virulence factors in a teicoplanin-resistant derivative of methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 482958-2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakoulas, G., G. M. Eliopoulos, R. C. Moellering, Jr., C. Wennersten, L. Venkataraman, R. P. Novick, and H. S. Gold. 2002. Accessory gene regulator (agr) locus in geographically diverse Staphylococcus aureus isolates with reduced susceptibility to vancomycin. Antimicrob. Agents Chemother. 461492-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakoulas, G., G. M. Eliopoulos, R. C. Moellering, Jr., R. P. Novick, L. Venkataraman, C. Wennersten, P. C. DeGirolami, M. J. Schwaber, and H. S. Gold. 2003. Staphylococcus aureus accessory gene regulator (agr) group II: is there a relationship to the development of intermediate-level glycopeptide resistance? J. Infect. Dis. 187929-938. [DOI] [PubMed] [Google Scholar]
- 21.Sakoulas, G., G. M. Eliopoulos, J. Alder, and C. Thauvin-Eliopoulos. 2003. Daptomycin in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 471714-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakoulas, G., P. A. Moise-Broder, J. Schentag, A. Forrest, R. C. Moellering, Jr., and G. M. Eliopoulos. 2004. Relationship of vancomycin minimum inhibitory concentration (MIC) and bactericidal activity to the treatment efficacy of vancomycin in methicillin-resistant Staphylococcus aureus bacteremia. J. Clin. Microbiol. 422398-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakoulas, G., G. M. Eliopoulos, V. G. Fowler, Jr., R. C. Moellering, Jr., R. P. Novick, N. Lucindo, M. R. Yeaman, and A. S. Bayer. 2005. Reduced susceptibility of Staphylococcus aureus to vancomycin and platelet microbicidal protein correlate with defective autolysis and loss of accessory gene regulator (agr) function. Antimicrob. Agents Chemother. 492687-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakoulas, G., J. Alder, C. Thauvin-Eliopoulos, R. C. Moellering, Jr., and G. M. Eliopoulos. 2006. Induction of daptomycin heterogeneous susceptibility in Staphylococcus aureus by exposure to vancomycin. Antimicrob. Agents Chemother. 501581-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Streit, J. M., R. N. Jones, and H. S. Sader. 2004. Daptomycin activity and spectrum: a worldwide sample of 6737 clinical gram-positive organisms. J. Antimicrob. Chemother. 53669-674. [DOI] [PubMed] [Google Scholar]
- 26.Tsuji, B. T., and M. J. Rybak. 2005. Short-course gentamicin in combination with daptomycin or vancomycin against Staphylococcus aureus in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 492735-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang, G., J. F. Hindler, K. W. Ward, and D. A. Bruckner. 2006. Increased vancomycin MICs for Staphylococcus aureus clinical isolates from a university hospital during a 5-year period. J. Clin. Microbiol. 443883-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]