Abstract
Active surveillance of invasive group A streptococcal (GAS) infections was conducted in Denmark during 2003 and 2004 as a part of the Strep-EURO initiative. The main objective was to improve understanding of the epidemiology of invasive GAS disease in Denmark. During the 2 years, 278 cases were reported, corresponding to a mean annual incidence of 2.6 cases per 100,000 inhabitants. The vast majority of isolates, 253 (91%), were from blood, with the remaining 25 (9%) being from cerebrospinal fluid, joints, or other normally sterile sites. The mean case fatality rate (CFR) was 20%, with the rate being higher in patients more than 70 years of age (36.5%). For streptococcal toxic shock syndrome (STSS) and necrotizing fasciitis the CFRs were 53% and 25%, respectively. Out of 16 T types recorded, three predominated: T28 (23%), T1 (22%), and the cluster T3/13/B3264 (14%). Among 29 different emm types, emm28 and emm1 accounted for 51% of strains, followed by emm3 (11%), emm89 (7%), and emm12 (5.5%). Low resistance rates were detected for macrolide-lincosamide-streptogramin B (MLSB) antibiotics (3%) and tetracycline (8%); two isolates exhibited coresistance to tetracycline and macrolides. Of nine pyrogenic exotoxin (superantigen) genes examined, speA and speC were identified in 58% and 40% of the strains, respectively; either of the genes was present in all strains causing STSS. Most strains harbored speG (99%). ssa was present in 14% of the isolates only. In Denmark, as in comparable countries, GAS invasive disease shows a sustained, high endemicity, with involvement of both established and emerging streptococcal emm and T types.
Streptococcus pyogenes (group A streptococci [GAS]) is a gram-positive, exclusively human pathogen causing common throat and skin infections but also severe invasive disease and the nonsuppurative complications acute rheumatic fever and poststreptococcal glomerulonephritis. In particular, the spectrum of acute invasive disease includes erysipelas, cellulitis, endometritis, pneumonia, septicemia, meningitis, and the severe manifestations necrotizing fasciitis (NF) and streptococcal toxic shock syndrome (STSS) (50).
The late 1980s marked a sudden rise of the severe infections and consequently an increased interest in this pathogen. Recent epidemiological studies from the United States and Europe have shown a sustained, high incidence of severe streptococcal infections (7, 14, 22, 42, 52) accounting for hundreds of thousands of cases each year (4) and thus currently a global concern.
Various surface structures of GAS, including M proteins, hyaluronic acid capsule, and matrix-binding proteins, play important roles in virulence by mediating adherence, colonization, and invasion of human skin and mucus membranes (3). In addition, a number of extracellular toxins and enzymes are produced by this organism. The antigenically variable M protein has been used since 1928 in a serotyping system, now comprising about 80 known types. Based on variability of the N-terminal end of the emm gene (encoding the M protein), about 250 defined emm types (31) and an increasing number of subtypes are recognized (http://www.cdc.gov/ncidod/biotech/strep/strepblast.htm). This is in contrast to traditional serological T typing, which comprises fewer than 30 different types (27). Of the extracellular factors produced by GAS, several superantigens (SAg), among these the pyrogenic exotoxins A and C (SpeA, and SpeC, respectively), have received particular attention in the context of invasive disease (6, 33). The number of described GAS SAgs as well as knowledge of their involvement in GAS pathogenicity is increasing continuously (3, 47). Use of the SAg genes, combined with more conventional methods (T typing, M/emm typing, and pulsed-field gel electrophoresis), may also be useful as an epidemiological tool (10).
In spite of more than half a century of extensive use of β-lactams, no resistance against these drugs among GAS has been reported; however, resistance to macrolide-lincosamide-streptogramin B (MLSB) antibiotics is common in many countries. A combination of a β-lactam and clindamycin is the recommended treatment for severe GAS disease (59). Additionally, though tetracycline is not used in the treatment of GAS disease, the susceptibility of GAS to tetracycline seems to be highly variable between countries (26, 32, 38).
Since invasive GAS disease is not notifiable by law in most countries, national incidence data have generally been obtained through enhanced surveillance during limited periods. The present paper, a part of the EU-funded action Strep-EURO (http://www.strep-euro.lu.se), concerns severe GAS disease in Denmark during 2003 and 2004, with special focus on epidemiological trends compared to previous national or regional reports. The results demonstrate a continued, high incidence and a potential need for prevention of invasive GAS disease, especially soft tissue infections, puerperal sepsis, and nosocomial infections.
MATERIALS AND METHODS
Case definition.
The study was conducted during 2003 and 2004 and included GAS isolates recovered from hospitalized patients with invasive disease. The case definition was that of the Working Group on Severe Streptococcal Infections, published in 1993 (57). The criteria for inclusion of patients in the study were based on the isolation of GAS from blood or another normally sterile body site or from nonsterile sites in the presence of a clinical diagnosis of STSS or NF.
Collection system.
GAS isolates were received at the Statens Serum Institut (SSI) from all 15 Danish clinical microbiological departments (15 different regions), with a national catchment area of ∼5.5 million inhabitants (http://www.dst.dk/HomeUK/Statistics/Key_indicators/Population/pop.aspx; accessed 10 July 2007). The reporting system from the departments to the Streptococcus Unit, SSI, has been the same since 1988, and since 1996 the unit has distributed a detailed questionnaire to the clinicians treating the patients, which is intended to collect basic and clinical patient data, e.g., age, gender, and site of bacterial isolation, as well as diagnosis, predisposing factors, and treatment. Dates of death or a confirmation that the person was alive was obtained from the Central Office of Civil Registration. The annual numbers of inhabitants in the respective areas were obtained from Statistics Denmark.
Bacterial isolates.
The clinical microbiology departments identified the GAS isolates by agglutination tests from 5% horse blood agar plates (SSI) that were incubated overnight, sent to SSI in Stuart transport medium, and subsequently cultured on 5% horse blood agar plates (SSI) and incubated overnight at 37°C in a 5% CO2 atmosphere. Bacterial strains were stored at −80°C on filtered broth with glycerin.
Typing procedures.
The isolates were T typed by slide agglutination using mono- and polyspecific sera (SSI, Copenhagen, Denmark, for isolates from 2003 and Sevapharma, Czech Republic, for isolates from 2004). Tests were performed at two laboratories in order to assess the concordance of results using the two brands of antisera. For emm typing the hypervariable region of the emm gene was amplified and sequenced as described previously (39). Sequence alignment and emm type assignment were performed using the Basic Local Alignment Search Tool (BLAST) at the National Center for Biotechnology Information (NCBI) (available at http://www.ncbi.nlm.nih.gov/BLAST/) and sequences listed at the Centers for Disease Control and Prevention (CDC) (http://www.cdc.gov/ncidod/biotech/strep/strepblast.htm). Uncommon T/emm combinations were retested at Department of Laboratory Medicine, Lund University, Lund, Sweden.
Detection of SAg genes.
For detection of nine SAg genes (Table 1), an initial multiplex PCR was developed. Bacterial DNA was isolated as described before (25). The PCR was performed in a 50-μl final volume containing 5 μl 10× PCR buffer, 1.25 μl deoxynucleoside triphosphates (10 mM), 2.5U Taq DNA polymerase (New England Biolabs), 5pmol/μl of each primer (Invitrogen, Sweden), 1 μl supernatant, and distilled water to 50 μl. The thermal scheme was as follows: denaturing for 2 min at 95°C; 35 cycles of denaturing for 1 min at 94°C, annealing for 50 s at 50°C, and elongation for 1.5 min at 72°C; and a final cycle of 5 min at 72°C. As noted from Table 1, some of the primers generated amplicons of similar size (speH and speJ [approximately 630 bp] and speI and ssa [approximately 680 bp]); for this reason, the isolates with positive bands of these sizes were subjected to single PCRs with corresponding primers.
TABLE 1.
Primers used for detection of SAg genes
| Gene | Primer sequence (5′→3′)
|
Amplicon size (bp) | |
|---|---|---|---|
| Forward | Reverse | ||
| speA | CTT AAG AAC CAA GAG ATG GC | ATA GGC TTT GGA TAC CAT C | 200 |
| speB | TTC TAG GAT ACT CTA CCA GC | ATT TGA GCA GTT GCA GTA GC | 300 |
| speC | CAT CTA TGG AGG AAT TAC GC | TGT GCC AAT TTC GAT TCT GC | 246 |
| speF | GCG AAA TTA GAA AAG AGG AC | GCT GAG CAA AAG TGT GTG | 1,193 |
| speG | TAT AAT ATT ACC CCA TGC GA | AAG GCT CCC CGA TG | 447 |
| speH | AAG CAA ATT CTT ATA ATA CAA CC | TTA GCT GAT TGA CAC ATC TAC A | 630 |
| speI | ATG AGT AGT GTG GGA GTT ATT AA | TTA TTT ATT AAA TTT AAC TAA GTA TAT ATC AAT A | 678 |
| speJ | AAA TCA GAT AGT GAA AAT ATT AAA GAC | TTA TTT AGT CCA AAG GTA AAT ATC | 629 |
| ssa | AGT AGT CAG CCT GAC CCT AC | TTT GGT AAG GTG AAC CTC TAT | 691 |
Antimicrobial susceptibility testing.
Susceptibility to tetracycline, erythromycin, and clindamycin was determined using disk diffusion according to Swedish Reference Group for Antibiotics guidelines (http://www.srga.org; accessed 17 February 2005). MICs for resistant isolates were determined by Etest (AB Biodisk, Solna, Sweden). Macrolide resistance phenotypes, i.e., macrolide (M), constitutive MLSB (cMLSB), and inducible MLSB (iMLSB) resistance, were determined by the double-disk test using erythromycin and clindamycin, as described previously (44).
Identification of antibiotic resistance genes.
Macrolide-resistant strains were subjected to a multiplex PCR for detection of the resistance determinants erm(A), erm(B), and mef(A), using primer sequences in accordance with previously described methodologies (12, 53). A pair of primers giving an 180-bp amplicon of the chromosomally encoded 16S RNA was introduced as an internal positive control (34). Tetracycline-resistant isolates were screened for presence of resistance determinants tet(M) and tet(O), using published primers (1).
Statistics.
For nominal data, the chi-square or Fisher's exact test was used when appropriate (significance, P < 0.05).
RESULTS
During the active surveillance period, a total of 278 GAS isolates from invasive infections were received at SSI. Completed questionnaires were received for 253 cases (91%).
Incidence.
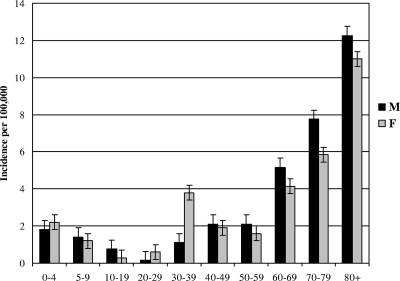
The values reported indicated a mean incidence of 2.6 cases per 100,000 inhabitants per year (2.9 in 2003 and 2.3 in 2004). The incidence increased with age, reaching 12/100,000 for those over 80 years of age (Fig. 1). The median and the mean ages of patients were 61 years (range, 0 to 100 years) and 56 years, respectively, with no differences between males and females. During 2003 a higher incidence was reported among the group comprising ages 30 to 39 compared to 2004 (3.6 and 1.2, respectively; P < 0.005). There were marked regional differences between incidence rates, ranging from 0.25/100,000 (Ribe County) to 5.4/100,000 (Copenhagen City). Among 13 cases in children between 0 and 4 years, 4 (31%) were from Storström County (total reported cases, 16). A seasonal fluctuation could be observed, with an increasing number of cases during the cold months (January to April), but for puerperal fever, most cases were reported during May to August of the first year.
FIG. 1.
Incidence of invasive GAS infections in Denmark during 2003 and 2004 as related to gender and age. Error bars indicate standard deviations.
Clinical presentations.
The majority of isolates (91%) were recovered from blood, with the remaining 9% being from cerebrospinal fluid (4 isolates), joints (3 isolates), and other normally sterile sites (18 isolates). Skin and soft tissue infections accounted for 26% of the cases, arthritis for 5.5%, puerperal sepsis for 5%, and meningitis for 3%. Eleven percent developed STSS, and 6% had NF. Finally, 19% had no focal symptoms, and 34% were reported as having other clinical presentations. According to the questionnaire, “other clinical presentations” included a variety of infections, e.g., wound sepsis, peritonitis and miscellaneous orthopedic diagnoses. As many as 55% of the patients had more than one clinical presentation (i.e., STSS and NF, skin/soft tissue infection and NF, meningitis and other clinical presentation, etc.).
Risk factors.
Seventy-five percent of all patients were reported to have at least one risk factor, of which skin lesions (42 cases) were registered as the most prevalent. Diabetes accounted for 13 cases, immunosuppression for 16, and injecting drug use for 6. Among 15 children aged 0 to 16 years, three had chicken pox; of those, two cases occurred in the same area (Århus) within 10 days. All three patients underwent surgery; two had NF (one developed STSS), and the third had septic arthritis. In 64% of the cases, risk factors other than those listed in the questionnaire were recorded, i.e., inflammatory bowel disease, pregnancy, and disseminated sclerosis. Hospital-acquired infections accounted for 11% of all the cases; of those, 17 were from 2003 and 9 were from 2004.
Outcome.
During the entire study, 35 patients were admitted to intensive care. A total of 64 patients underwent surgical interventions; among those 18 had STSS (case fatality rate [CFR], 44%), and 11 had NF (CFR, 9%).
The overall CFR within 7 days after hospital admission was 16%, compared to 20% within 30 days. As expected, the CFR was significantly higher among patients with STSS and NF (47% and 25%, respectively) than in the remaining patients (13%) (P < 0.00004). The CFR also varied among age groups, with 95% of fatal cases occurring among patients over 50 years of age (P < 0.0013) (31% among those 50 to 69 years, 33% among those 70 to 79 years, and 31% among those 80 years or older).
Type distribution of GAS.
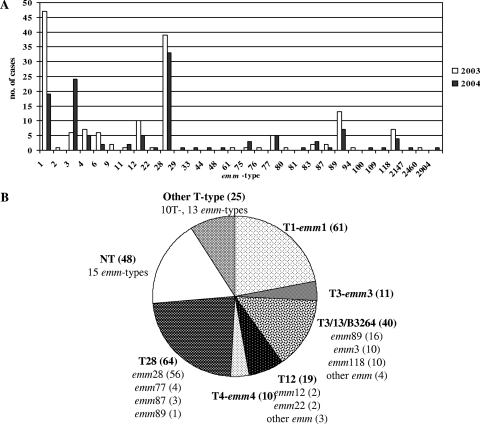
A broad range of 29 emm types was recorded (Fig. 2), with the five most common types being emm28 (26%), emm1 (24%), emm3 (11%), emm89 (7%), and emm12 (5.5%). The frequency of emm28 isolates was fairly constant, whereas that of emm1 decreased over time (from 47 in 2003 to 19 in 2004; P < 0.003). In contrast, 24 (80%) of the 30 emm3 isolates were registered in the second year (P < 0.005). Out of 16 different T types, T28, T1, and T3/13/B3264 accounted for 60% of all isolates, whereas 17% of strains were nontypeable (NT).
FIG. 2.
Invasive GAS isolated during 2003 and 2004. (A) emm type distribution; (B) emm type combinations for the most common T types.
T/emm type combinations.
As shown in Fig. 2B, of the total number of emm28 isolates, 78% (56 strains) were T28, 21% (15 strains) were NT, and 1 strain was T12. Almost all emm1 isolates (92%) were T1, with the remaining being NT (5 strains). The emm3 isolates were evenly distributed between T3, the cluster T3/13/B3264, and NT (37%, 33%, and 30%, respectively). The cluster T3/13/B3264 was present in 80% of emm89 strains (the remaining were T11, T13, T28, and TB3264 [one isolate each]), whereas emm12 strains were mainly T12 (93%), (the remaining were NT). Among the 48 NT isolates, 15 different emm types were found. Four unusual T/emm combinations were detected (T12/emm28, T12/emm6, T23/emm76, and T11/emm118), all of which were confirmed in two laboratories.
Disease manifestation and emm type.
Out of 253 patients for whom complete clinical data were available, 67 (26%) had skin or soft tissue infections (Table 2). The corresponding GAS isolates belonged to a range of 16 emm types, with the five leading types mentioned above accounting for 73% within this group. Most cases of cellulitis/erysipelas were caused by emm1, followed by emm3 and -28. STSS and NF were mostly (66% and 81%, respectively) caused by emm1 or -3. Nine of 16 NF patients developed STSS; the implicated strains were of emm3 (five strains) and 1 (four strains). Bacteremia cases (n = 51) belonged to 17 different emm types, with emm28 accounting for 33%. Three bacteremia cases (two emm3 and one emm77) were complicated by STSS.
TABLE 2.
emm types and clinical presentation of invasive GAS infections in Denmark in 2003 and 2004
| Clinical manifestation | No. of cases/CFR (%) | No (%) of STSS cases/CFR (%) | No. of cases of emm type/CFR (%):
|
|||||
|---|---|---|---|---|---|---|---|---|
| emm1 | emm3 | emm12 | emm28 | emm89 | Other | |||
| Bacteremia (with no focal symptom) | 48/15 | 3 (6)/33 | 5/40 | 6/25 | 2/0 | 17/18 | 4/0 | 17/6 |
| Necrotizing fasciitis | 16/27 | 9 (56)/33 | 8/29 | 5/40 | 0/0 | 0/0 | 0/0 | 3/0 |
| Skin and soft tissue infections | 67/19 | 8 (12)/88 | 20/10 | 10/20 | 4/25 | 12/42 | 3/0 | 18/17 |
| Arthritis | 14/21 | 3 (21)/67 | 3/33 | 1/100 | 2/0 | 3/33 | 1/0 | 4/0 |
| Puerperal sepsis | 12/8 | 1 (8)/100 | 1/0 | 1/100 | 0/0 | 9/0 | 0/0 | 1/0 |
| Meningitis | 8/0 | 0 | 1/0 | 1/0 | 1/0 | 1/0 | 1/0 | 3/0 |
| Other | 85/20 | 0 | 24/0 | 4/0 | 7/0 | 26/0 | 8/0 | 16/0 |
| Clinical data not available | 25/24 | 5/40 | 3/67 | 0/0 | 4/25 | 4/25 | 9/11 | |
| Non-STSS | 223/14 | 51/16 | 18/17 | 13/0 | 62/21 | 16/6 | 72/10 | |
| STSS | 30/53 | 10/50 | 9/56 | 2/50 | 6/50 | 0/0 | 3/66 | |
| Total | 66/23 | 30/33 | 15/7 | 72/24 | 20/10 | 75/13 | ||
The puerperal sepsis cases (n = 12) were caused primarily by emm28 (75%), with the remaining strains being emm1, -3, and -9 (one case each). STSS occurred in the case caused by the emm3 strain; this was the only fatal case.
In terms of CFR, three emm types were overrepresented; the first was emm28 (31% of total fatal cases after 30 days), followed by emm1 and emm3 (27 and 18%, respectively).
SAg gene distribution.
The multiplex PCR revealed the presence of three to six SAg genes in the various isolates. As expected, speB, speF, and speG were detected in most or all tested isolates. The phage-associated genes speC and speA, and ssa, were detected in 58%, 40%, 14% of the isolates, respectively (Table 3). In 78% of the strains, four of the nine SAg genes studied were detected, whereas in 14% of the strains five genes were found and in 4% only three genes were found. No strain exhibited speI or speJ, and speH was detected in two strains only.
TABLE 3.
Presence of SAg genes among the most represented emm types
| emm type | Total
|
speA
|
speC
|
speG
|
speH
|
ssa
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of isolates | % | No. of isolates | % | No. of isolates | % | No. of isolates | % | No. of isolates | % | No. of isolates | % | |
| 1 | 66 | 24 | 48 | 73 | 20 | 30 | 66 | 100 | 2 | 3 | ||
| 3 | 30 | 11 | 15 | 50 | 15 | 50 | 30 | 100 | 15 | 50 | ||
| 4 | 12 | 4 | 4 | 33 | 8 | 67 | 12 | 100 | 8 | 67 | ||
| 12 | 15 | 5 | 6 | 40 | 9 | 60 | 15 | 100 | 1 | 7 | ||
| 28 | 72 | 26 | 21 | 29 | 49 | 68 | 72 | 100 | 1 | 1 | ||
| 89 | 20 | 7 | 6 | 30 | 12 | 60 | 19 | 95 | 1 | 5 | 1 | 5 |
| 118 | 11 | 4 | 2 | 18 | 8 | 73 | 11 | 100 | 2 | 18 | ||
| 77 | 10 | 4 | 3 | 30 | 8 | 80 | 10 | 100 | ||||
| 6 | 8 | 3 | 7 | 88 | 8 | 100 | 1 | 13 | 1 | 13 | ||
| Other (n < 5) | 34 | 12 | 6 | 18 | 25 | 74 | 32 | 94 | 7 | 19 | ||
| Total | 278 | 100 | 111 | 40 | 161 | 58 | 275 | 99 | 2 | 0.7 | 38 | 14 |
emm types and SAg genes.
Among emm28 strains, 68% were speA negative and speC positive, whereas 30% were speA positive and speC negative. Among emm1 strains, 67% were speA positive and speC negative, whereas 24% were speA negative and speC positive. Among emm3 strains, 47% were speA negative and speC positive, and 47% were speA positive and speC negative. Of the remaining types, speA was detected in 25% and speC in 70%. Only six strains were speA positive and speC positive (emm1, four strains; emm3 and emm77, one strain each).
Antibiotic resistance.
The rate of resistance to erythromycin was 3%, with all eight strains being recovered from blood. The strains exhibited the following MLSB phenotype distribution: four showed the iMLSB, one the cMLSB, and three the M phenotype. The genotypes detected were erm(A) (five strains, with either iMLSB or cMLSB) and mef(A) (three strains with the M phenotype). No strain harbored erm(B). All erm(A)-positive strains were T28; four of them were emm28 and one was emm77, showing coresistance to tetracycline and harboring the tet(O) gene. mef(A)-positive isolates were T11/emm61, T4/emm4, and NT/emm75.
Resistance to tetracycline was encountered in 8% of the strains, with MICs ranging between 4 and 48 mg/liter. Out of the 23 tetracycline-resistant strains, 16 harbored tet(M) and the remaining 7 harbored tet(O). Notably, all strains harboring tet(O) were emm77, whereas the tet(M)-positive strains exhibited 12 different emm types.
Of the 23 tetracycline-resistant isolates, questionnaires were available for 18 cases. Six isolates were from patients with no focal symptoms: two were emm77 (T28 and T13/28), and the other four were T11/emm61, NT/emm83, NT/emm33, and NT/emmst2904. Eight isolates were from cases of cellulitis, with one complicated by NF: three isolates were T28/emm77 (one of them was the NF case), and the remaining isolates (one each) were of types T13/emm77, T13/13/B3264/emm2460, T5/27/44/emm2147, NT/emm83, and T6/emm109. Two isolates were from patients with arthritis (T13/emm77 and T11/emm44), whereas two were from patients with clinical manifestations other than those listed in the questionnaire (NT/emm9 and -1). Erythromycin-resistant isolates were from two patients with cellulitis (one without focal symptoms), whereas five had other clinical presentations.
DISCUSSION
We present here results of 2 years of surveillance of invasive GAS disease in Denmark. The mean incidence during the study period was 2.6 cases per 100,000 inhabitants, which was similar to that during the last two decades in Denmark (16) and to other reported Scandinavian data (10, 16, 19, 54). Population-based surveillance studies from the United States, Canada, and The Netherlands have shown rates ranging between 1.5 and 5 cases per 100,000 inhabitants per year (30, 36, 37, 56). A seasonal fluctuation could be observed, with an elevated number of cases during January to April, which could be explained by the well-known influence of indoor overcrowding on transmission of streptococci (46). Regional differences in incidence rates were observed; in particular, more densely populated regions had higher incidence rates (e.g., Copenhagen city, 5.0 and 5.8/100,000, compared to 2.6 and 1.9/100,000 in suburban Copenhagen areas) during the 2 years, which is conceivably due to population density determining incidence rates. Finally, in the rural district of Bornholm (43,956 inhabitants), the only four reported cases were from 2003, yielding a misleadingly high incidence of 9.1; however, this did not represent an outbreak (three different emm types), though three infections were hospital acquired.
Skin and soft tissue infections accounted for 26% of all cases. These infections are reported to be the most common focal manifestations of invasive GAS disease (16, 19, 60). However, this rate was much lower than that in the close neighbor Sweden (63% during 2002 to 2004) (10).
Three of the puerperal fever cases appeared to be nosocomial, showing a need for a constant focus on hospital-acquired infections, which were unexpectedly common in our study (11%). This number is similar to that in a report from the GAS study group in Ontario (9). However, a European perspective on this problem is still missing.
The majority of fatalities (95%) occurred in patients older than 50 years. However, 33% were in the group comprising ages 50 to 69, which was not considered a risk group for invasive GAS infection. It is interesting to note that bacteremia with emm1, NF with emm3, and soft tissue infections with emm28 were more often fatal than other emm type/disease combinations (Table 2).
There were no significant differences regarding gender, but during 2003 a peak in incidence was noted among patients between 30 and 39 years of age, and of those (29 cases), 23 patients (80%) were female. Puerperal sepsis was the most common disease (35%); interestingly, five of these cases were from Copenhagen area, accounting for 20% of invasive cases from this region in 2003. Elevated rates of invasive GAS infections were previously reported among this age group, due mainly to puerperal sepsis among females and arthritis among males (11). However, the gender difference in 2003 was not only due to puerperal sepsis, since of 23 females in this age group, only 8 had puerperal fever. Thus, excluding puerperal sepsis from this age group, the proportion of females to males (15/6) still was more than double, and no significant difference in specific disease manifestations and gender was observed.
Fifty percent of all cases were caused by emm28 and emm1, followed in frequency by emm3, -89, -12, and -4. These same types were the most prevalent during 1999 to 2002. Previous reports of invasive infections from Denmark (15) showed a gradual increase in frequency of emm1 from 1999 to 2002 (15% to 40%). During 2003, emm1 declined to 30%, and during 2004, it declined to 15%. In the same time, emm3 increased from 2% in 2002 to 20% in 2004. Thus, the distribution of emm1 and emm3 was changing over time, which could be at least in part related to epidemic waves (2) and to type substitution due to herd immunity or population mobility (5). Most emm1 isolates (62%) were reported from Jutland (41 out of 66 emm1 strains). Unexpectedly, there was only partial agreement between the present types and those recorded in Sweden during 2002 to 2004, where, besides emm89 (the most prevalent type), emm81 and -77 were common (10).
During the study period, there was a sustained, high prevalence of emm28 (24% in 2003 and 26% in 2004), a type showing a steady increase from 15% in 1999 to 24% in 2002 (17). A prominent role of emm28 strains in invasive GAS disease was also reported in Sweden and Finland, as well as in the United States (19, 36, 40, 45). Of the present emm28 strains, as many as nine were isolated from cases of puerperal fever. These isolates are under investigation for clonal relatedness. Also in France, type emm28 strains were implicated in a puerperal sepsis outbreak (40). Notably, it has been shown that M/emm28 strains exhibit a surface protein (R protein) closely related to the group B streptococcal Rib protein, which confers adhesion to vaginal epithelial cells (48). Recently it was shown that M28 GAS strains contain a 37.4-kb region (region of difference 2) that is similar in gene content and organization to a genomic island from group B streptococci and encodes Rib and some other putative virulence factors. It was suggested that this element, acquired by horizontal gene transfer, has enabled type M28 to expand its pathogenic features and be overrepresented among puerperal sepsis cases (21, 58).
In addition to the predominant types mentioned, a broad spectrum of other emm types was found, e.g., emm118. Since this uncommon type, in particular, affected only subjects over 30 years of age, it is interesting to know its prevalence in throat infections of children, raising immunity to this type. Our finding of new T/emm type combinations is in line with reports from others; e.g., in a recent paper reviewing more than 40,000 GAS isolates, many uncommon T/emm combinations were reported (28). Obviously, the surveillance of circulating emm types is important in the ongoing attempts to develop an M protein-based GAS vaccine (24).
Several studies described the potential involvement of streptococcal pyrogenic exotoxin SpeA in severe streptococcal disease (18, 33, 51, 55), while others reported an association with SpeC (13, 22). Nevertheless, some cases of STSS were reportedly not associated with either of these (23). In the present study, speA was detected in 17 and speC in 13 out of 30 strains causing STSS, whereas the combination of both was not found in any of those isolates. Similarly, out of 51 isolates from bacteremia with no focal symptoms, one had both speA and speC, 20 had speA, and 28 had speC, thus not differing from STSS isolates. Either one of these two factors, though also common in noninvasive isolates (10), could hypothetically be required in order for severe GAS disease to be manifested. The occurrence of ssa among tested isolates was in line with other reports (41).
In the present study, a low level of macrolide resistance was found, possibly related to low macrolide consumption in Denmark (around two defined daily doses per 1,000 inhabitants per day in the last few years [49]). In the beginning of the 1990s, high macrolide resistance rates in GAS occurred in several European countries (i.e., Finland, Italy, Spain, and France) (8, 20, 29, 43, 49), whereas in the last years high rates have been reported in Asia (26). Though resistance against clindamycin, a potential threat in the treatment of invasive GAS disease, is still uncommon, surveillance of MLSB phenotypes is important for prevention of resistance.
Prior to this study, high rates of tetracycline resistance among GAS (approximately 30%) were reported in Denmark (35) and were attributed to a high use of tetracycline in livestock. In our study only 8% of tested isolates were tetracycline resistant. The present tetracycline-resistant strains harbored either the tet(M) or the tet(O) determinant. As many as 7 out of 10 emm77 strains were tetracycline resistant, all of which harbored tet(O), suggesting a clonal spread. In contrast, tet(M) strains were more heterogeneous, with 16 isolates belonging to 12 different emm types.
In conclusion, the present national Danish study demonstrated a high incidence of invasive diseases, in particular among the elderly, who showed a high fatality rate. Furthermore, many cases of hospital-acquired GAS infections and puerperal sepsis were noted. In addition to a wide range of T/emm types recorded, minor changes of predominant types compared to those in previous Danish reports were found. Characterization of GAS by different typing methods helps to improve understanding of the epidemiology of invasive disease, with impact on disease control, notification of outbreaks, detection of changes in population immunity, and vaccine development.
Acknowledgments
We thank Kirsten Burmaiser from the Streptococcus Unit at SSI for excellent technical support, the clinicians and the clinical microbiological laboratories for great help in collecting strains and clinical data, Claes Schalén for valuable comments on the study, Fredrik Norberg for statistical help, and the Strep-EURO group for inspiring collaboration.
Footnotes
Published ahead of print on 24 October 2007.
REFERENCES
- 1.Aarestrup, F. M., Y. Agerso, P. Gerner-Smidt, M. Madsen, and L. B. Jensen. 2000. Comparison of antimicrobial resistance phenotypes and resistance genes in Enterococcus faecalis and Enterococcus faecium from humans in the community, broilers, and pigs in Denmark. Diagn. Microbiol. Infect. Dis. 37127-137. [DOI] [PubMed] [Google Scholar]
- 2.Beres, S. B., G. L. Sylva, D. E. Sturdevant, C. N. Granville, M. Liu, S. M. Ricklefs, A. R. Whitney, L. D. Parkins, N. P. Hoe, G. J. Adams, D. E. Low, F. R. DeLeo, A. McGeer, and J. M. Musser. 2004. Genome-wide molecular dissection of serotype M3 group A Streptococcus strains causing two epidemics of invasive infections. Proc. Natl. Acad. Sci. USA 10111833-11838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bisno, A. L., M. O. Brito, and C. M. Collins. 2003. Molecular basis of group A streptococcal virulence. Lancet Infect. Dis. 3191-200. [DOI] [PubMed] [Google Scholar]
- 4.Carapetis, J. R., A. C. Steer, E. K. Mulholland, and M. Weber. 2005. The global burden of group A streptococcal diseases. Lancet Infect. Dis. 5685-694. [DOI] [PubMed] [Google Scholar]
- 5.Cleary, P., D. Johnson, and L. Wannamaker. 1979. Genetic variation in the M antigen of group A streptococci: reassortment of type-specific markers and possible antigenic drift. J. Infect. Dis. 140747-757. [DOI] [PubMed] [Google Scholar]
- 6.Cleary, P. P., E. L. Kaplan, J. P. Handley, A. Wlazlo, M. H. Kim, A. R. Hauser, and P. M. Schlievert. 1992. Clonal basis for resurgence of serious Streptococcus pyogenes disease in the 1980s. Lancet 339518-521. [DOI] [PubMed] [Google Scholar]
- 7.Colman, G., A. Tanna, A. Efstratiou, and E. T. Gaworzewska. 1993. The serotypes of Streptococcus pyogenes present in Britain during 1980-1990 and their association with disease. J. Med. Microbiol. 39165-178. [DOI] [PubMed] [Google Scholar]
- 8.Cornaglia, G., M. Ligozzi, A. Mazzariol, M. Valentini, G. Orefici, R. Fontana, et al. 1996. Rapid increase of resistance to erythromycin and clindamycin in Streptococcus pyogenes in Italy, 1993-1995. Emerg. Infect. Dis. 2339-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daneman, N., A. McGeer, D. E. Low, G. Tyrrell, A. E. Simor, M. McArthur, B. Schwartz, P. Jessamine, R. Croxford, and K. A. Green. 2005. Hospital-acquired invasive group A streptococcal infections in Ontario, Canada, 1992-2000. Clin. Infect. Dis. 41334-342. [DOI] [PubMed] [Google Scholar]
- 10.Darenberg, J., B. Luca-Harari, A. Jasir, A. Sandgren, H. Pettersson, C. Schalen, M. Norgren, V. Romanus, A. Norrby-Teglund, and B. H. Normark. 2007. Molecular and clinical characteristics of invasive group A streptococcal infection in Sweden. Clin. Infect. Dis. 45450-458. [DOI] [PubMed] [Google Scholar]
- 11.Davies, H. D., A. McGeer, B. Schwartz, K. Green, D. Cann, A. E. Simor, D. E. Low, et al. 1996. Invasive group A streptococcal infections in Ontario, Canada. N. Engl. J. Med. 335547-554. [DOI] [PubMed] [Google Scholar]
- 12.De Azavedo, J. C., R. H. Yeung, D. J. Bast, C. L. Duncan, S. B. Borgia, and D. E. Low. 1999. Prevalence and mechanisms of macrolide resistance in clinical isolates of group A streptococci from Ontario, Canada. Antimicrob. Agents Chemother. 432144-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demers, B., A. E. Simor, H. Vellend, P. M. Schlievert, S. Byrne, F. Jamieson, S. Walmsley, and D. E. Low. 1993. Severe invasive group A streptococcal infections in Ontario, Canada: 1987-1991. Clin. Infect. Dis. 16792-802. [DOI] [PubMed] [Google Scholar]
- 14.Efstratiou, A. 2000. Group A streptococci in the 1990s. J. Antimicrob. Chemother. 45(Suppl.)3-12. [DOI] [PubMed] [Google Scholar]
- 15.Ekelund, K., J. Darenberg, A. Norrby-Teglund, S. Hoffmann, D. Bang, P. Skinhoj, and H. B. Konradsen. 2005. Variations in emm type among group A streptococcal isolates causing invasive or noninvasive infections in a nationwide study. J. Clin. Microbiol. 433101-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ekelund, K., P. Skinhoj, J. Madsen, and H. B. Konradsen. 2005. Invasive group A, B, C and G streptococcal infections in Denmark 1999-2002: epidemiological and clinical aspects. Clin. Microbiol. Infect. 11569-576. [DOI] [PubMed] [Google Scholar]
- 17.Ekelund, K., P. Skinhoj, J. Madsen, and H. B. Konradsen. 2005. Reemergence of emm1 and a changed superantigen profile for group A streptococci causing invasive infections: results from a nationwide study. J. Clin. Microbiol. 431789-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eriksson, B. K., J. Andersson, S. E. Holm, and M. Norgren. 1999. Invasive group A streptococcal infections: T1M1 isolates expressing pyrogenic exotoxins A and B in combination with selective lack of toxin-neutralizing antibodies are associated with increased risk of streptococcal toxic shock syndrome. J. Infect. Dis. 180410-418. [DOI] [PubMed] [Google Scholar]
- 19.Eriksson, B. K., M. Norgren, K. McGregor, B. G. Spratt, and B. Henriques-Normark. 2003. Group A streptococcal infections in Sweden: a comparative study of invasive and noninvasive infections and analysis of dominant T28 emm28 isolates. Clin. Infect. Dis. 371189-1193. [DOI] [PubMed] [Google Scholar]
- 20.Granizo, J. J., L. Aguilar, J. Casal, R. Dal-Re, and F. Baquero. 2000. Streptococcus pyogenes resistance to erythromycin in relation to macrolide consumption in Spain (1986-1997). J. Antimicrob. Chemother. 46959-964. [DOI] [PubMed] [Google Scholar]
- 21.Green, N. M., S. Zhang, S. F. Porcella, M. J. Nagiec, K. D. Barbian, S. B. Beres, R. B. LeFebvre, and J. M. Musser. 2005. Genome sequence of a serotype M28 strain of group A streptococcus: potential new insights into puerperal sepsis and bacterial disease specificity. J. Infect. Dis. 192760-770. [DOI] [PubMed] [Google Scholar]
- 22.Holm, S. E., A. Norrby, A. M. Bergholm, and M. Norgren. 1992. Aspects of pathogenesis of serious group A streptococcal infections in Sweden, 1988-1989. J Infect. Dis. 16631-37. [DOI] [PubMed] [Google Scholar]
- 23.Hsueh, P. R., J. J. Wu, P. J. Tsai, J. W. Liu, Y. C. Chuang, and K. T. Luh. 1998. Invasive group A streptococcal disease in Taiwan is not associated with the presence of streptococcal pyrogenic exotoxin genes. Clin. Infect. Dis. 26584-589. [DOI] [PubMed] [Google Scholar]
- 24.Hu, M. C., M. A. Walls, S. D. Stroop, M. A. Reddish, B. Beall, and J. B. Dale. 2002. Immunogenicity of a 26-valent group A streptococcal vaccine. Infect. Immun. 702171-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jasir, A., A. Tanna, A. Efstratiou, and C. Schalen. 2001. Unusual occurrence of M type 77, antibiotic-resistant group A streptococci in southern Sweden. J. Clin. Microbiol. 39586-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jing, H. B., B. A. Ning, H. J. Hao, Y. L. Zheng, D. Chang, W. Jiang, and Y. Q. Jiang. 2006. Epidemiological analysis of group A streptococci recovered from patients in China. J. Med. Microbiol. 551101-1107. [DOI] [PubMed] [Google Scholar]
- 27.Johnson, D. R., E. Kaplan, J. Sramek, E. Bicova, J. Havlicek, H. Havlickova, J. Motlova, and P. Kriz. 1996. Laboratory diagnosis of group A streptococcal infections. World Health Organization. Geneva, Switzerland.
- 28.Johnson, D. R., E. L. Kaplan, A. VanGheem, R. R. Facklam, and B. Beall. 2006. Characterization of group A streptococci (Streptococcus pyogenes): correlation of M-protein and emm-gene type with T-protein agglutination pattern and serum opacity factor. J. Med. Microbiol. 55157-164. [DOI] [PubMed] [Google Scholar]
- 29.Jones, M. E., J. A. Karlowsky, D. C. Draghi, C. Thornsberry, D. F. Sahm, and D. Nathwani. 2003. Epidemiology and antibiotic susceptibility of bacteria causing skin and soft tissue infections in the USA and Europe: a guide to appropriate antimicrobial therapy. Int. J. Antimicrob. Agents 22406-419. [DOI] [PubMed] [Google Scholar]
- 30.Laupland, K. B., T. Ross, D. L. Church, and D. B. Gregson. 2006. Population-based surveillance of invasive pyogenic streptococcal infection in a large Canadian region. Clin. Microbiol. Infect. 12224-230. [DOI] [PubMed] [Google Scholar]
- 31.Li, Z., V. Sakota, D. Jackson, A. R. Franklin, and B. Beall. 2003. Array of M protein gene subtypes in 1064 recent invasive group A streptococcus isolates recovered from the active bacterial core surveillance. J. Infect. Dis. 1881587-1592. [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto, M., K. Sakae, M. Ohta, M. Endo, R. Okuno, S. Murayama, K. Hirasawa, R. Suzuki, J. Isobe, D. Tanaka, C. Katsukawa, A. Tamaru, M. Tomita, K. Ogata, T. Yasuoka, T. Ikebe, and H. Watanabe. 2005. Molecular mechanisms of high level tetracycline-resistance in group A streptococcal isolates, T serotypes 4 and 11. Int. J. Antimicrob. Agents 25142-147. [DOI] [PubMed] [Google Scholar]
- 33.Musser, J. M., A. R. Hauser, M. H. Kim, P. M. Schlievert, K. Nelson, and R. K. Selander. 1991. Streptococcus pyogenes causing toxic-shock-like syndrome and other invasive diseases: clonal diversity and pyrogenic exotoxin expression. Proc. Natl. Acad. Sci. USA 882668-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nielsen, H. U., A. M. Hammerum, K. Ekelund, D. Bang, L. V. Pallesen, and N. Frimodt-Moller. 2004. Tetracycline and macrolide co-resistance in Streptococcus pyogenes: co-selection as a reason for increase in macrolide-resistant S. pyogenes? Microb. Drug Resist. 10231-238. [DOI] [PubMed] [Google Scholar]
- 35.Nielsen, H. U., H. J. Kolmos, and N. Frimodt-Moller. 2002. Beta-hemolytic streptococcal bacteremia: a review of 241 cases. Scand. J. Infect. Dis. 34483-486. [DOI] [PubMed] [Google Scholar]
- 36.O'Brien, K. L., B. Beall, N. L. Barrett, P. R. Cieslak, A. Reingold, M. M. Farley, R. Danila, E. R. Zell, R. Facklam, B. Schwartz, and A. Schuchat. 2002. Epidemiology of invasive group A streptococcus disease in the United States, 1995-1999. Clin. Infect. Dis. 35268-276. [DOI] [PubMed] [Google Scholar]
- 37.Passaro, D. J., D. S. Smitht, E. C. Hett, A. L. Reingold, P. Daily, C. A. van Beneden, and D. J. Vugia. 2002. Invasive group A streptococcal infections in the San Francisco Bay area, 1989-99. Epidemiol. Infect 129471-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pires, R., D. Rolo, L. Gama-Norton, A. Morais, L. Lito, M. J. Salgado, C. Johansson, G. Mollerberg, B. Henriques-Normark, J. Goncalo-Marques, and I. Santos-Sanches. 2005. Group A streptococci from carriage and disease in Portugal: evolution of antimicrobial resistance and T antigenic types during 2000-2002. Microb. Drug Resist. 11360-370. [DOI] [PubMed] [Google Scholar]
- 39.Podbielski, A., B. Melzer, and R. Lutticken. 1991. Application of the polymerase chain reaction to study the M protein(-like) gene family in beta-hemolytic streptococci. Med. Microbiol. Immunol. 180213-227. [DOI] [PubMed] [Google Scholar]
- 40.Raymond, J., L. Schlegel, F. Garnier, and A. Bouvet. 2005. Molecular characterization of Streptococcus pyogenes isolates to investigate an outbreak of puerperal sepsis. Infect. Control Hosp. Epidemiol. 26455-461. [DOI] [PubMed] [Google Scholar]
- 41.Reda, K. B., V. Kapur, D. Goela, J. G. Lamphear, J. M. Musser, and R. R. Rich. 1996. Phylogenetic distribution of streptococcal superantigen SSA allelic variants provides evidence for horizontal transfer of ssa within Streptococcus pyogenes. Infect. Immun. 641161-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwartz, B., R. R. Facklam, and R. F. Breiman. 1990. Changing epidemiology of group A streptococcal infection in the USA. Lancet 3361167-1171. [DOI] [PubMed] [Google Scholar]
- 43.Seppala, H., A. Nissinen, H. Jarvinen, S. Huovinen, T. Henriksson, E. Herva, S. E. Holm, M. Jahkola, M. L. Katila, T. Klaukka, et al. 1992. Resistance to erythromycin in group A streptococci. N. Engl. J. Med. 326292-297. [DOI] [PubMed] [Google Scholar]
- 44.Seppala, H., A. Nissinen, Q. Yu, and P. Huovinen. 1993. Three different phenotypes of erythromycin-resistant Streptococcus pyogenes in Finland. J. Antimicrob. Chemother. 32885-891. [DOI] [PubMed] [Google Scholar]
- 45.Siljander, T., M. Toropainen, A. Muotiala, N. P. Hoe, J. M. Musser, and J. Vuopio-Varkila. 2006. emm typing of invasive T28 group A streptococci, 1995-2004, Finland. J. Med. Microbiol. 551701-1706. [DOI] [PubMed] [Google Scholar]
- 46.Smith, A., T. L. Lamagni, I. Oliver, A. Efstratiou, R. C. George, and J. M. Stuart. 2005. Invasive group A streptococcal disease: should close contacts routinely receive antibiotic prophylaxis? Lancet Infect. Dis. 5494-500. [DOI] [PubMed] [Google Scholar]
- 47.Sriskandan, S., L. Faulkner, and P. Hopkins. 2007. Streptococcus pyogenes: insight into the function of the streptococcal superantigens. Int. J. Biochem. Cell. Biol. 3912-19. [DOI] [PubMed] [Google Scholar]
- 48.Stalhammar-Carlemalm, M., T. Areschoug, C. Larsson, and G. Lindahl. 1999. The R28 protein of Streptococcus pyogenes is related to several group B streptococcal surface proteins, confers protective immunity and promotes binding to human epithelial cells. Mol. Microbiol. 33208-219. [DOI] [PubMed] [Google Scholar]
- 49.Statens Serum Institut, Danish Veterinary and Food Administration, Danish Medicines Agency, and Danish Institute for Food and Veterinary Research. 2004. DANMAP 2003—use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, foods and humans in Denmark. Statens Serum Institut, Copenhagen, Denmark.
- 50.Stevens, D. L. 1996. Invasive group A streptococcal disease. Infect. Agents Dis. 5157-166. [PubMed] [Google Scholar]
- 51.Stevens, D. L., M. H. Tanner, J. Winship, R. Swarts, K. M. Ries, P. M. Schlievert, and E. Kaplan. 1989. Severe group A streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N. Engl. J. Med. 3211-7. [DOI] [PubMed] [Google Scholar]
- 52.Stromberg, A., V. Romanus, and L. G. Burman. 1991. Outbreak of group A streptococcal bacteremia in Sweden: an epidemiologic and clinical study. J. Infect. Dis. 164595-598. [DOI] [PubMed] [Google Scholar]
- 53.Sutcliffe, J., T. Grebe, A. Tait-Kamradt, and L. Wondrack. 1996. Detection of erythromycin-resistant determinants by PCR. Antimicrob. Agents Chemother. 402562-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Svensson, N., S. Oberg, B. Henriques, S. Holm, G. Kallenius, V. Romanus, and J. Giesecke. 2000. Invasive group A streptococcal infections in Sweden in 1994 and 1995: epidemiology and clinical spectrum. Scand. J. Infect. Dis. 32609-614. [DOI] [PubMed] [Google Scholar]
- 55.Talkington, D. F., B. Schwartz, C. M. Black, J. K. Todd, J. Elliott, R. F. Breiman, and R. R. Facklam. 1993. Association of phenotypic and genotypic characteristics of invasive Streptococcus pyogenes isolates with clinical components of streptococcal toxic shock syndrome. Infect. Immun. 613369-3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vlaminckx, B. J., W. van Pelt, L. M. Schouls, A. van Silfhout, E. M. Mascini, C. P. Elzenaar, T. Fernandes, A. Bosman, and J. F. Schellekens. 2005. Long-term surveillance of invasive group A streptococcal disease in The Netherlands, 1994-2003. Clin. Microbiol. Infect. 11226-231. [DOI] [PubMed] [Google Scholar]
- 57.Working Group on Severe Streptococcal Infections. 1993. Defining the group A streptococcal toxic shock syndrome. Rationale and consensus definition. JAMA. 269390-391. [PubMed] [Google Scholar]
- 58.Zhang, S., N. M. Green, I. Sitkiewicz, R. B. Lefebvre, and J. M. Musser. 2006. Identification and characterization of an antigen I/II family protein produced by group A Streptococcus. Infect. Immun. 744200-4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zimbelman, J., A. Palmer, and J. Todd. 1999. Improved outcome of clindamycin compared with beta-lactam antibiotic treatment for invasive Streptococcus pyogenes infection. Pediatr. Infect. Dis. J. 181096-1100. [DOI] [PubMed] [Google Scholar]
- 60.Zurawski, C. A., M. Bardsley, B. Beall, J. A. Elliott, R. Facklam, B. Schwartz, and M. M. Farley. 1998. Invasive group A streptococcal disease in metropolitan Atlanta: a population-based assessment. Clin. Infect. Dis. 27150-157. [DOI] [PubMed] [Google Scholar]