Abstract
Two percent of 1,973 pneumococcus strains isolated from carriers since 2001 in Portugal were found to be optochin resistant. These strains belonged to eight serotypes (and some were nontypeable), and they had diverse genetic backgrounds. Novel optochin-resistant lineages were detected over time, suggesting that there was a continuous, although sporadic, emergence of optochin resistance.
The accurate identification of pneumococcus isolates has traditionally relied on observations of typical colony morphology, α-hemolysis on sheep blood agar, and optochin (ethylhydrocupreine hydrochloride) susceptibility (16). Bile solubility tests, although very sensitive and simple to perform, are far from being widely used as routine tests in clinical microbiology laboratories (1, 5). Other identification methods based on the detection of specific DNA sequences have been recently proposed (2, 5, 19).
Optochin-resistant pneumococcal strains were first reported in Finland in 1987 (10), and since then, sporadic reports of isolates from diverse geographic areas have appeared in the literature (1, 3, 9, 12, 14, 15). In particular, Aguiar et al. have recently reported the emergence of optochin-resistant pneumococci in Portugal, which accounted for 3.2% of all clinical isolates recovered from 30 laboratories across the country (1). These observations prompted us to retrospectively review the detection of optochin resistance among isolates that were colonizing asymptomatic Portuguese carriers and that were recovered in studies conducted since 2001.
Between January and March of 2001, 2002, 2003, and 2006, a total of 717, 834, 766, and 571 nasopharyngeal samples, respectively, were obtained from children attending day-care centers in Lisbon and Oeiras, Portugal, by following previously described procedures (8, 13). The children's ages ranged from 4 months to 6 years. Pneumococcal isolation rates, antibiotypes, and molecular characterizations of antimicrobial-resistant pneumococci isolated in 2001, 2002, and 2003 have been described previously (11).
Pneumococci were isolated based on selective growth on gentamicin blood agar plates, optochin susceptibility, colony morphology, and α-hemolysis (16). Bile solubility tests were performed for isolates with reduced susceptibility to optochin that appeared to be pneumococci based on the other phenotypic observations (16). In particular, optochin susceptibility was performed by disk diffusion, using commercially available optochin discs (5 μg; 6 mm; Oxoid, Hampshire, England) applied onto blood agar plates (Trypticase soy agar supplemented with 5% sheep blood) that had been inoculated with a 0.5 McFarland standard suspension of the culture to be tested. Plates were incubated overnight at 37°C in a 5% CO2-enriched atmosphere. Isolates were considered to be resistant to optochin if they displayed inhibition zones smaller than 14 mm or larger than 14 mm but containing colonies inside the halo (16).
Antimicrobial susceptibility testing was assayed by the Kirby-Bauer disk diffusion method for susceptibility to erythromycin, clindamycin, tetracycline, chloramphenicol, sulfamethoxazole-trimethoprim, and levofloxacin according to CLSI guidelines (6) and by Etest (AB Biodisk, Solna, Sweden) for penicillin and ceftriaxone. Results were interpreted following CLSI criteria (6).
Capsular typing was done by multiplex PCR (4). For those isolates whose serotype could not be determined by this technique, the Quellung reaction was performed using specific antisera (Statens Serum Institute, Copenhagen, Denmark) (18).
Pulsed-field gel electrophoresis (PFGE) of macrorestriction DNA fragments was done after SmaI digestion, and a dendrogram was generated using Bionumerics Software (Applied Maths, Gent, Belgium) (17).
A total of 1,973 pneumococcal isolates were obtained during the four surveillance periods. Of these, 42 (2.1%) were optochin resistant and bile soluble. The prevalence of optochin resistance ranged from 1.3 to 3.2% depending on the year of isolation (Table 1).
TABLE 1.
Origin of optochin-resistant pneumococcus strainsa
| Year | Total no. of pneumococcus isolates | No. of Optr isolates (%) | No. of DCC with Optr isolates/total no. of DCC |
|---|---|---|---|
| 2001 | 465 | 15 (3.2) | 7/11 |
| 2002 | 559 | 10 (1.8) | 6/14 |
| 2003 | 557 | 12 (2.2) | 6/14 |
| 2006 | 392 | 5 (1.3) | 4/12 |
Optr, optochin-resistant; DCC, day-care centers.
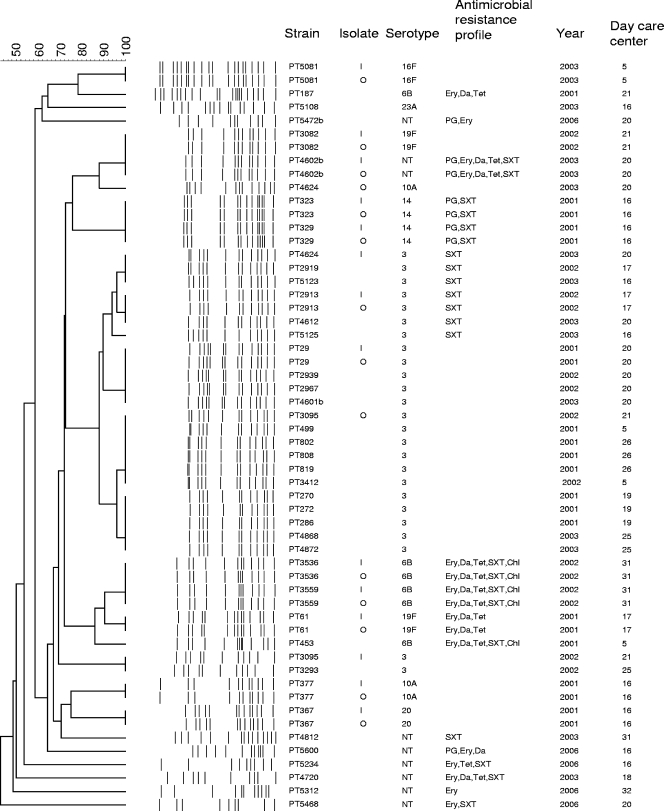
Two optochin resistance phenotypes were observed: 13 isolates had halos that were ≥14 mm, with subpopulations inside the inhibition zone, and 29 were uniformly optochin resistant. These phenotypes have also been described by Pikis et al. (15). Optochin resistance was confirmed for all isolates by picking one sample from the closest growth to the optochin disk and another from the farthest zone from the halo. For all 13 isolates with subpopulations, PFGE profiles were obtained for the two subpopulations. In all samples but two (PT3095 and PT4624), identical PFGE patterns were observed (Fig. 1). For the 29 isolates displaying uniform resistance to optochin, a single culture was grown for DNA extraction and PFGE profiling.
FIG. 1.
Dendrogram of optochin-resistant pneumococci. I, subpopulation isolated from the inhibition zone close to the optochin disk; O, subpopulation isolated from the farthest zone from the halo; NT, nontypeable; PG, penicillin; Ery, erythromycin; Da, clindamycin; Tet, tetracycline; SXT, trimethoprim-sulfamethoxazole; Chl, chloramphenicol.
Fifty percent of optochin-resistant isolates were susceptible to all antimicrobial agents tested, and 21% were multidrug resistant (defined as resistance to three or more antimicrobial agents). Fifty percent were of serotype 3. Other serotypes detected were 6B (4 strains), 10A (2 strains), 14 (2 strains), 19F (2 strains), 16F (1 strain), 20 (1 strain), and 23A (1 strain). Eight strains were nontypeable.
Seventeen PFGE clusters were identified, indicating genetic variability among the collection as was suggested by the serotyping results (Fig. 1). In particular, novel optochin-resistant genetic backgrounds were detected for all years surveyed. Furthermore, among optochin-resistant strains of serotypes 3, 6A, 10A, and 19F and among nontypeable isolates, more than one genetic background was identified and, specifically, all eight nontypeable isolates had unique genetic backgrounds. Among the clusters detected, the largest one was represented by 19 of the 21 serotype 3 optochin-resistant isolates.
Of interest, all optochin-resistant strains, with the exception of the single isolates of serotypes 20 and 23A, had genetic backgrounds that were also detected among optochin-susceptible pneumococci circulating in Portuguese day-care centers (data not shown). In addition, all clusters with two or more isolates included strains recovered from at least two day-care centers in different years (Fig. 1).
Finally, optochin-resistant strains of serotypes 3, 6B, 14, and 19F were found to be members of internationally disseminated clones: Netherlands3 ST180 (19 isolates), Poland6B ST315 (1 isolate), Greece6B ST273 (3 isolates), Spain9V ST156 (2 isolates of serotype 14), and Portugal19F ST177 (1 isolate).
Our study shows that optochin-resistant strains were already present in Portugal in 2001 and continue to circulate and emerge among asymptomatic carriers. We are unable to conclude whether optochin-resistant strains were already in the community before 2001, since at that time, pneumococcus-like cultures exhibiting an optochin resistance phenotype were not further characterized or preserved in our laboratory. Full resistance to optochin was the most abundant phenotype (69%) in our study, in contrast to the study by Aguiar et al. (1), which reported that all optochin-resistant clinical isolates were a mixture of subpopulations. These observations suggest that different mechanisms leading to optochin resistance are disseminated in Portugal. At present, these mechanisms remain uncharacterized.
We found that optochin-resistant colonizing strains from Portugal are associated with several different serotypes and genetic backgrounds, including internationally disseminated clones. They were present in several day-care centers, suggesting they were not confined geographically. Similar conclusions were reached by Aguiar et al. (1), although the two collections, colonizing versus disease isolates, included mostly different serotypes and genetic backgrounds. In our study, 86% of the optochin-resistant strains had capsular types not targeted by the 7-valent pneumococcal conjugate vaccine.
The majority of optochin-resistant colonizing isolates had genetic backgrounds that were also detected among optochin-susceptible pneumococci, and novel optochin-resistant genetic backgrounds were detected in all years, suggesting that there was a continuous, although sporadic, emergence of optochin resistance. The driving forces for such selection are currently unknown, but compounds similar to optochin, such as quinine and mefloquine, are used for treatment of and prophylaxis against malaria (7). Contacts between Portugal and African countries with endemic malaria are frequent due to tourism and immigration. Whether optochin resistance mechanisms in pneumococcus isolates from healthy children in Portugal can be linked to this flow has not been investigated.
Still, clonal expansion of a serotype 3 clone accounted for 50% of all optochin-resistant isolates and, additionally, represented 16% of all serotype 3 isolates recovered during the 4 years of surveillance (regardless of their optochin susceptibility pattern).
In summary, optochin-resistant pneumococci were detected from asymptomatic carriers in Portugal since 2001 but might have been present before. Optochin resistance is associated with different clones, most of which express serotypes that are not included in the current 7-valent pneumococcal conjugate vaccine. Therefore, optochin susceptibility should be complemented with other pneumococcal identification tests, such as bile solubility tests or PCR-based techniques, when suspected pneumococcal cultures exhibiting resistance to optochin are isolated. Accurate identification of pneumococci is important not only for the diagnosis and treatment of infections but also for colonization studies, such as those aimed to evaluate the impact of pneumococcal conjugate vaccines.
Acknowledgments
This work was supported by projects EURIS (contract QKL2-CT-2000-01020) and PREVIS (contract LSHM-CT-2003-503413) from the European Commission. S.N. was supported by grants from EURIS (011/BIC/01) and PREVIS (010/BIC/04), and R.S.-L. was supported by Fundação para a Ciência e Tecnologia (SFRH/BPD/14596/2003 and SFRH/BPD/30871/2006).
We thank Rosario Mato, Nelson Frazão, Natacha Sousa, Carla Simas, Inês Crisóstomo, Alexandra Simões, Ilda Santos Sanches, António Brito-Avô, Joana Saldanha, and Anabela Gonçalves for participating in studies that led to the isolation of the strains described in the study.
Footnotes
Published ahead of print on 21 November 2007.
REFERENCES
- 1.Aguiar, S. I., M. J. Frias, L. Santos, J. Melo-Cristino, and M. Ramirez. 2006. Emergence of optochin resistance among Streptococcus pneumoniae in Portugal. Microb. Drug Resist. 12239-245. [DOI] [PubMed] [Google Scholar]
- 2.Arbique, J. C., C. Poyart, P. Trieu-Cuot, G. Quesne, M. D. G. S. Carvalho, A. G. Steigerwalt, R. E. Morey, D. Jackson, R. J. Davidson, and R. R. Facklam. 2004. Accuracy of phenotypic and genotypic testing for identification of Streptococcus pneumoniae and description of Streptococcus pseudopneumoniae sp. nov. J. Clin. Microbiol. 424686-4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borek, A. P., D. C. Dressel, J. Hussong, and L. R. Peterson. 1997. Evolving clinical problems with Streptococcus pneumoniae: increasing resistance to antimicrobial agents, and failure of traditional optochin identification in Chicago, Illinois, between 1993 and 1996. Diagn. Microbiol. Infect. Dis. 29209-214. [DOI] [PubMed] [Google Scholar]
- 4.Brito, D. A., M. Ramirez, and H. de Lencastre. 2003. Serotyping Streptococcus pneumoniae by multiplex PCR. J. Clin. Microbiol. 412378-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chandler, L. J., B. S. Reisner, G. L. Woods, and A. K. Jafri. 2000. Comparison of four methods for identifying Streptococcus pneumoniae. Diagn. Microbiol. Infect. Dis. 37285-287. [DOI] [PubMed] [Google Scholar]
- 6.CLSI. 2006. Performance standards for antimicrobial disk susceptibility tests, 9th ed. Approved standard. CLSI publication M2-A9. Clinical and Laboratory Standards Institute, Wayne, PA.
- 7.de la Campa, A. G., E. Garcia, A. Fenoll, and R. Munoz. 1997. Molecular bases of three characteristic phenotypes of pneumococcus: optochin-sensitivity, coumarin-sensitivity, and quinolone-resistance. Microb. Drug Resist. 3177-193. [DOI] [PubMed] [Google Scholar]
- 8.De Lencastre, H., K. G. Kristinsson, A. Brito-Avo, I. S. Sanches, R. Sa-Leao, J. Saldanha, E. Sigvaldadottir, S. Karlsson, D. Oliveira, R. Mato, M. Aires de Sousa, and A. Tomasz. 1999. Carriage of respiratory tract pathogens and molecular epidemiology of Streptococcus pneumoniae colonization in healthy children attending day care centers in Lisbon, Portugal. Microb. Drug Resist. 519-29. [DOI] [PubMed] [Google Scholar]
- 9.Dias, C. A., G. Agnes, A. P. G. Frazzon, F. D. Kruger, P. A. d'Azevedo, M. D. G. S. Carvalho, R. R. Facklam, and L. M. Teixeira. 2007. Diversity of mutations in the atpC gene coding for the c subunit of F0F1 ATPase in clinical isolates of optochin-resistant Streptococcus pneumoniae from Brazil. J. Clin. Microbiol. 453065-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kontiainen, S., and A. Sivonen. 1987. Optochin resistance in Streptococcus pneumoniae strains isolated from blood and middle ear fluid. Eur. J. Clin. Microbiol. 6422-424. [DOI] [PubMed] [Google Scholar]
- 11.Mato, R., I. S. Sanches, C. Simas, S. Nunes, J. A. Carriço, N. G. Sousa, N. Frazão, J. Saldanha, A. Brito-Avô, J. S. Almeida, and H. De Lencastre. 2005. Natural history of drug-resistant clones of Streptococcus pneumoniae colonizing healthy children in Portugal. Microb. Drug Resist. 11309-322. [DOI] [PubMed] [Google Scholar]
- 12.Munoz, R., A. Fenoll, D. Vicioso, and J. Casal. 1990. Optochin-resistant variants of Streptococcus pneumoniae. Diagn. Microbiol. Infect. Dis. 1363-66. [DOI] [PubMed] [Google Scholar]
- 13.O'Brien, K. L., H. Nohynek, et al. 2003. Report from a WHO Working Group: standard method for detecting upper respiratory carriage of Streptococcus pneumoniae. Pediatr. Infect. Dis. J. 22e1-e11. [DOI] [PubMed] [Google Scholar]
- 14.Phillips, G., R. Barker, and O. Brogan. 1988. Optochin-resistant Streptococcus pneumoniae. Lancet ii281. [DOI] [PubMed] [Google Scholar]
- 15.Pikis, A., J. M. Campos, W. J. Rodriguez, and J. M. Keith. 2001. Optochin resistance in Streptococcus pneumoniae: mechanism, significance, and clinical implications. J. Infect. Dis. 184582-590. [DOI] [PubMed] [Google Scholar]
- 16.Rouff, K., R. A. Whiley, and D. Beighton. 2003. Streptococcus, p. 405-421. In P. R. Murray, E. J. Barron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. ASM Press, Washington, DC.
- 17.Sá-Leão, R., A. S. Simões, S. Nunes, N. G. Sousa, N. Frazão, and H. de Lencastre. 2006. Identification, prevalence and population structure of non-typable Streptococcus pneumoniae in carriage samples isolated from preschoolers attending day-care centres. Microbiology 152367-376. [DOI] [PubMed] [Google Scholar]
- 18.Sørensen, U. B. 1993. Typing of pneumococci by using 12 pooled antisera. J. Clin. Microbiol. 312097-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verhelst, R., T. Kaijalainen, T. De Baere, G. Verschraegen, G. Claeys, L. Van Simaey, C. De Ganck, and M. Vaneechoutte. 2003. Comparison of five genotypic techniques for identification of optochin-resistant pneumococcus-like isolates. J. Clin. Microbiol. 413521-3525. [DOI] [PMC free article] [PubMed] [Google Scholar]