Abstract
The antigenic cross-reactive characteristics of herpes B virus and herpes simplex virus (HSV) type 1 (HSV-1) and HSV-2 are responsible for false-positive diagnoses by serological assays in humans and macaques. In the present study, we developed a fluorometric indirect enzyme-linked immunosorbent assay (ELISA) with recombinant herpes B virus glycoprotein D (gD) and HSV-1 and HSV-2 gG (gG-1 and gG-2, respectively) to discriminate between the three primate herpesvirus infections. The secreted form of gD, gDdTM, was used to detect antibody to herpes B virus gD. Sera positive for herpes B virus, HSV-1, and HSV-2 showed specific reactions to gD, gG-1, and gG-2, respectively. Sera collected from humans and rhesus macaques were investigated for the presence of antibodies to the recombinant proteins of the three herpesviruses. The results suggested that the approach is able to discriminate between herpes B virus and HSV infections. The ELISA was also found to be able to detect infections with multiple primate herpesviruses and may have the potential to identify a subsequent infection in individuals that have already been infected with another herpesvirus. In addition, we found evidence of a greater cross-reactivity of herpes B virus with HSV-1 than with HSV-2. It is suggested that the ELISA with the recombinant antigens is useful not only for the serodiagnosis of primate herpesvirus infections but also for elucidation of the seroprevalence of herpesviruses in humans and primates.
Herpes B virus (Cercopithecine herpesvirus 1) infection is a fatal zoonosis characterized by acute encephalomyelitis (26, 27). The rate of mortality among individuals with the infection is high if such individuals are not given antiviral therapy in the early stages of infection. The natural hosts of the causative agent are Asian macaques, which are used in the medical field as models for humans. This suggests that laboratory workers in contact with the macaques could become exposed to virus-contaminated sources, such as saliva and urine from infected hosts (4). Therefore, the development of a rapid and accurate method for the detection of herpes B virus infection is required for both the early diagnosis of the infection in patients and the establishment of virus-free macaque colonies. Serological assays, including enzyme-linked immunosorbent assay (ELISA) and Western blotting (WB) analysis with a herpes B virus-infected cell antigen, are available for the detection of herpes B virus infections (2, 7, 12, 18).
The serodiagnosis of herpes B virus infections is difficult because of the antigenic cross-reactivity of herpes B virus with related herpesviruses. Herpes B virus is classified as a member of the subfamily Alphaherpesvirinae, which includes herpes simplex virus (HSV) type 1 (HSV-1) and HSV-2, and has been shown to share antigenic and biological characteristics with these human herpesviruses, such as a tropism for neurons and propagation and dissemination in natural hosts (6, 8, 21). The high seroprevalence of HSV in humans, which has been reported to be 60 to 88% for HSV-1 (3, 5, 28, 29), limits the detection of herpes B virus infection by serological tests in patients suspected of being infected with the virus. In addition, a biosafety level 4 laboratory is required for preparation of the virus-infected cell antigen. Therefore, an alternative antigen as a replacement for the infected cell antigen is needed for the serological diagnosis of herpes B virus infections.
Recombinant DNA techniques currently play an important role in the diagnosis of many viral infections. The recombinant proteins used as antigens in serological tests are particularly useful for the discrimination of antibodies to closely related viruses. Immunoassays with glycoprotein G (gG) of HSV-1 and HSV-2 (gG-1 and gG-2, respectively), which are known to be type-specific antigens (16, 25), have been developed for the typing of HSV (1, 9, 14, 15, 22) and are available commercially. These assays have been applied in epidemiological studies as well as to the serological diagnosis of infections in patients. In addition, the development of serological assays for the diagnosis of herpes B virus infection with the recombinant protein has been reported (20, 24). In an earlier study we produced the gD of herpes B virus in mammalian cells; and the resultant recombinant protein was evaluated for its antigenicity by WB, dot blotting, and immunoprecipitation analyses (24). Since a nonspecific reaction was observed by WB, we constructed the secretory form of gD, gDdTM, which lacked the transmembrane domain (TM) and cytoplasmic tail (CT). gDdTM showed a specific reaction with sera from herpes B virus-infected macaques and was confirmed to have the same sensitivity as the original gD antigen. Therefore, we concluded that the gDdTM antigen is useful for the detection of antibody to herpes B virus.
In the present study, we developed a fluorometric indirect ELISA with a combination of recombinant herpes B virus gD, gG-1, and gG-2 as coating antigens. We used the gDdTM described above to detect antibody to herpes B virus. The three antigens were investigated for their cross-reactivities with sera confirmed to have antibody to herpes B virus or HSV. Sera from rhesus macaques and humans, including patients with meningitis or myelitis, were also examined for the presence of antibody to herpes B virus, HSV-1, or HSV-2. The results were used to evaluate the ability of the ELISA to discriminate between the three herpesvirus infections.
MATERIALS AND METHODS
Antigens.
The preparation of recombinant herpes B virus gDdTM has been described previously (24). A recombinant plasmid, pBgDdTM, was used to transfect COS7 cells. The supernatant containing the resultant gDdTM was used as a coating antigen, while the supernatant of the COS7 cells transfected with an empty vector, pcDNA3.1(−), was used as the negative coating antigen. The recombinant gG-1 and gG-2 antigens and the whole HSV-1 and HSV-2 antigens were purchased from Austral Biologicals and Biogenesis Ltd. (Poole, United Kingdom), respectively.
Serum samples.
Polyclonal antisera with antibodies to HSV-1 and HSV-2 were collected from rabbits experimentally immunized with HSV-1 and HSV-2 (11). The complement fixation titers to HSV for the anti-HSV-1 and anti-HSV-2 rabbit sera were 1:256 and 1:128, respectively (10). Human control sera confirmed to have HSV-1 or HSV-2 antibody were also used for evaluation of the ELISA developed in the present study. The control serum sample for HSV-1 was obtained from a person with no clinical symptoms. This serum sample was confirmed to have a complement fixation titer to HSV of 128 (10) and neutralizing antibody titers to HSV-1 and HSV-2 of 64 and 4, respectively (13). Serum obtained from a patient with meningitis was used as the control for HSV-2 (17). Antibody to gG-2 was qualitatively detected in this patient's serum by a type-specific ELISA, and amplified products of HSV were obtained from the cerebrospinal fluid of this patient, although the virus type was not determined. Control serum with antibody to herpes B virus was obtained from a rhesus macaque that was naturally infected with the virus. The antibody to herpes B virus in this serum was qualitatively detected by ELISA with inactivated herpes B virus antigen (23). In addition, 24 and 21 serum samples were collected from rhesus macaques and persons with no clinical symptoms, respectively. Five convalescent-phase serum samples were obtained from patients diagnosed with central nervous system HSV infections (17).
Fluorometric indirect ELISA.
Ninety-six-well microplates (Maxisorp immunoplate; Nalge Nunc, Tokyo, Japan) were coated with the recombinant or HSV antigens diluted in carbonate buffer overnight at 4°C. The supernatants of COS7 cells transfected with pBgDdTM or pcDNA3.1(−) were diluted 1:500 and were used as the coating antigen. Ten nanograms per well of the gG-1 or gG-2 antigen was used for the recombinant antigen-based ELISA, whereas 100 ng per well of HSV-1 or HSV-2 antigens was used for the whole-virus antigen-based ELISA. The prepared plates were blocked with blocking buffer (phosphate-buffered saline [PBS] containing 3% bovine serum albumin) for 2 h at room temperature. After each incubation step, the plates were washed three times with PBS containing 1% Tween 20 (PBST) and four times before the enzyme-substrate reaction step. The serum samples were serially diluted fourfold from 1:100 to 1:25,600 with dilution buffer (PBST containing 1% bovine serum albumin). One hundred microliters of the diluted serum was added to each well, and the plate was incubated for 2 h on a plate shaker at room temperature. Biotin-conjugated secondary antibodies were used in the present study. Donkey anti-rabbit immunoglobulin G (IgG; Chemicon International Inc.) diluted 1:100,000 in the dilution buffer, goat anti-monkey IgG γ chain (Rockland Immunochemicals Inc.) diluted to a concentration of 25 ng/ml, and goat anti-human IgG (Fc) (American Qualex International Inc., CA) diluted to a concentration of 6.25 ng/ml were used for the detection of rabbit, monkey, and human IgG, respectively. The secondary antibody reaction step was performed for 1 h on the plate shaker at room temperature. Streptavidin-conjugated β-galactosidase was diluted at 1:1,000 in the dilution buffer, and 100 μl was added to each well. The reaction was performed for 1 h on the plate shaker at room temperature. The enzyme-substrate reaction with a 0.2 mM 4-methylumbelliferyl-β-d-galactoside substrate solution was performed for 2 h at 37°C and was stopped by adding 0.1 M glycine (pH 10.3). The amount of fluorescent reactant was calculated as the number of fluorescence units (FUs) after measurement of the absorbance at 460 nm with a fluorometric microplate reader (Fluoroskan II; Labsystems, Tokyo, Japan). The FU values for the positive antigens subtracted from those for the negative antigens were used to evaluate the reaction in the ELISA. Reactions with values of less than 500 were considered negative. Antibody titers were taken as the reciprocal of the final dilutions on titration curves which gave positive reactions.
RESULTS
Antigenic specificity of recombinant herpes B virus gD.
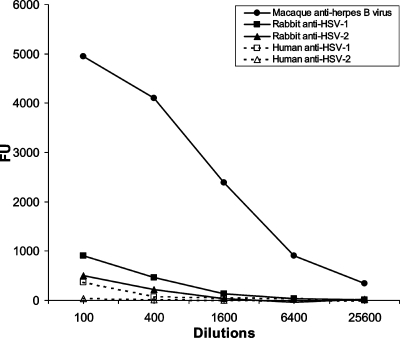
The reactivities of control sera for herpes B virus, HSV-1, or HSV-2 against the secretory form of herpes B virus gD lacking TM and CT (gDdTM) were investigated. Serum collected from rhesus macaques naturally infected with herpes B virus showed high levels of reactivity to the recombinant antigen (Fig. 1). The FU values for serial dilutions of the serum samples were almost linear, suggesting that specific binding between the coated antigen and the antibody in the serum occurred. The titer was 6,400. Sera from an uninfected macaque, rabbit, and human had low FU values (less than 500), suggesting no reactivity to the positive antigen (data not shown). Although the serum sample from a rabbit immunized with HSV-1 showed a slight cross-reaction with a titer of 100 (Fig. 1), the other HSV-1- or HSV-2-infected rabbit and human serum samples showed no reactivity to the antigen of herpes B virus.
FIG. 1.
Reactivity of the control sera with the herpes B virus gDdTM antigen. The results obtained with rhesus macaque serum which contained antibody to herpes B virus, rabbit sera immunized with HSV-1 or HSV-2, and human serum which contained antibody to HSV-1 or HSV-2 are shown. Sera were serially diluted fourfold from 1:100 to 1:25,600, and the FU values for each dilution were obtained by the fluorometric indirect ELISA with herpes B virus gDdTM. The FU values were plotted against each dilution of serum. The resulting titration curves are shown.
Antigenic specificity of recombinant gG-1 and gG-2.
Anti-herpes B virus macaque serum and anti-HSV-1 and anti-HSV-2 rabbit and human sera were investigated for their reactivities to gG-1 or gG-2 by the fluorometric indirect ELISA. The results were compared with those obtained by the ELISA with the HSV-1 or the HSV-2 antigen. Virus-uninfected macaque, rabbit, and human sera did not react with any recombinant or whole-virus antigen (data not shown). Sera from the rabbits infected with HSV-1 or HSV-2 reacted not only with the homologous antigens but also with the heterologous antigens in the gG- and HSV-based ELISAs (Fig. 2A and B). The antibody titers obtained under the homologous antigen-antibody conditions, however, were higher than those obtained under the heterologous antigen-antibody conditions. Under the homologous conditions, the titers obtained by the gG-based ELISA were higher than those obtained by the HSV-based ELISA, while under the heterologous conditions, the titers showing the reaction to gG were lower than those showing the reactions to the virus antigens. In addition, the human control serum for HSV-1 reacted only with the homologous antigens, and the reactivity to gG-1 was higher than that to the HSV-1 antigen (data not shown). The human control serum for HSV-2 showed a notably higher reaction to gG-2 than to any of the other antigens tested (data not shown). A macaque anti-herpes B virus serum was found to cross-react with the whole-virus antigens but not with gG (Fig. 2C).
FIG. 2.
Reactivity of the control sera with HSV gG-1 or gG-2 and whole-virus (HSV-1 or HSV-2) antigens. The results for rabbit anti-HSV-1 sera (A), rabbit anti-HSV-2 sera (B), and rhesus macaque anti-herpes B virus sera (C) are shown. The titration curves were obtained as described in the legend to Fig. 1, except that gG or HSV was used as the coating antigen. In each panel, four titration curves show the reactivity of the serum with the gG-1, gG-2, HSV-1, and HSV-2 antigens.
Application to rhesus macaque sera.
Twenty-four serum samples from rhesus macaques in a laboratory facility were examined for the presence of antibodies to the five antigens gDdTM, gG-1, gG-2, HSV-1, and HSV-2. The antibody titers were calculated for each antigen (Table 1). Twelve of the 24 macaque serum samples were found to have antibody to the herpes B virus gD. Among these 12 serum samples, 1 showed reactivity to the gG-1 antigen and none showed reactivity to the gG-2 antigens, whereas 11 had antibodies to HSV-1 and 5 had antibodies to HSV-2. The titer of antibody to HSV-1 was higher than that to HSV-2 in five serum samples (serum samples 1386, 1401, 1402, 1413, and 1416) in which antibodies to both HSV-1 and HSV-2 were detected.
TABLE 1.
Titers of antibodies to recombinant or whole primate herpesvirus antigens in rhesus macaque sera
| Monkey no. | Antibody titera
|
||||
|---|---|---|---|---|---|
| Anti-gD | Anti-gG-1 | Anti-gG-2 | Anti-HSV-1 | Anti-HSV-2 | |
| 7 | UD | UD | UD | UD | UD |
| 8 | UD | UD | UD | UD | UD |
| 9 | UD | UD | UD | UD | UD |
| 10 | UD | UD | UD | UD | UD |
| 11 | UD | UD | UD | UD | UD |
| 1308 | 200 | UD | UD | 200 | UD |
| 1309 | UD | UD | UD | UD | UD |
| 1333 | 3,200 | UD | UD | 800 | UD |
| 1371 | 3,200 | UD | UD | UD | UD |
| 1373 | 800 | UD | UD | 800 | UD |
| 1376 | UD | UD | UD | UD | UD |
| 1379 | 3,200 | UD | UD | 400 | UD |
| 1381 | NDa | ND | ND | ND | ND |
| 1383 | UD | UD | UD | UD | UD |
| 1385 | UD | UD | UD | UD | UD |
| 1386 | 3,200 | UD | UD | 3,200 | 800 |
| 1395 | UD | UD | UD | UD | UD |
| 1401 | 6,400 | 1,600 | UD | 3,200 | 800 |
| 1402 | 1,600 | UD | UD | 1,600 | 400 |
| 1403 | 1,600 | UD | UD | 1,600 | UD |
| 1404 | UD | UD | UD | UD | UD |
| 1413 | 3,200 | UD | UD | 3,200 | 200 |
| 1416 | 1,600 | UD | UD | 1,600 | 400 |
| 1417 | 800 | UD | UD | 800 | UD |
Abbreviations: UD, under the detection limit (titer, <100); ND, not determined.
Assessment of human control and patient sera.
Twenty-one serum samples collected from human controls were investigated for antibodies to the five antigens (Table 2). None of the serum samples had antibody to gDdTM. Of the 21 serum samples, 9 were found to have antibody to gG-1 and 1 was found to have antibody to gG-2. The results obtained by the ELISA with gG-1 were identical to those obtained by the ELISA with HSV-1, whereas the results of the ELISAs with gG-2 and HSV-2 were not identical.
TABLE 2.
Titers of antibodies to herpes B virus, HSV-1, and HSV-2 in humans with no clinical symptoms
| Control subject no. | Antibody titer
|
||||
|---|---|---|---|---|---|
| Anti-gD | Anti-gG-1 | Anti-gG-2 | Anti-HSV-1 | Anti-HSV-2 | |
| 1 | UDa | 3,200 | UD | 3,200 | UD |
| 2 | UD | 6,400 | UD | 3,200 | 200 |
| 3 | UD | UD | UD | UD | UD |
| 4 | UD | UD | UD | UD | UD |
| 5 | UD | UD | UD | UD | UD |
| 6 | UD | 800 | 800 | 400 | UD |
| 7 | UD | 3,200 | UD | 3,200 | 200 |
| 8 | UD | 3,200 | UD | 3,200 | 200 |
| 9 | UD | 3,200 | UD | 3,200 | UD |
| 10 | UD | UD | UD | UD | UD |
| 11 | UD | UD | UD | UD | UD |
| 12 | UD | 6,400 | UD | 3,200 | 400 |
| 13 | UD | UD | UD | UD | UD |
| 14 | UD | UD | UD | UD | UD |
| 15 | UD | UD | UD | UD | UD |
| 16 | UD | UD | UD | UD | UD |
| 17 | UD | UD | UD | UD | UD |
| 18 | UD | UD | UD | UD | UD |
| 19 | UD | UD | UD | UD | UD |
| 20 | UD | 6,400 | UD | 6,400 | 400 |
| 21 | UD | 3,200 | UD | 6,400 | 400 |
UD, under the detection limit (<100).
Five serum samples from patients diagnosed with central nervous system HSV infections were examined for the presence of antibodies to the five antigens (Table 3). None of the serum samples had antibody to gDdTM. The results obtained by the gG-based ELISA developed in the present study were the same as those obtained by the previous ELISA (17): two serum samples (serum samples P-1 and P-4) had antibodies to both gG-1 and gG-2, two (serum samples P-2 and P-3) had only anti-HSV-2 antibody, and the fifth (serum sample P-5) did not have antibody to either gG-1 or gG-2. However, three serum samples (serum samples P-1, P-2, and P-4) showed reactivity to both HSV antigens, whereas the other two serum samples did not react with any viral antigens.
TABLE 3.
Titers of antibodies to recombinant or whole primate herpesvirus antigens in patient sera
| Patient no. | Diagnosis | Virus(es) detected bya:
|
Antibody titer
|
|||||
|---|---|---|---|---|---|---|---|---|
| PCR | ELISA | Anti-gD | Anti-gG-1 | Anti-gG-2 | Anti-HSV-1 | Anti-HSV-2 | ||
| P-1 | Myelitis | HSV-2 | HSV-1 and HSV-2 | UDb | 3,200 | 3,200 | 200 | 200 |
| P-2 | Meningitis | HSVc | HSV-2 | UD | UD | 800 | 400 | 400 |
| P-3 | Meningitis | NDd | HSV-2 | UD | UD | 800 | UD | UD |
| P-4 | Meningitis | ND | HSV-1 and HSV-2 | UD | 800 | 400 | 3,200 | 200 |
| P-5 | Meningitis | HSV-1 | ND | UD | UD | UD | UD | UD |
DNA and detection of antibodies to HSV-1 and HSV-2 were performed by PCR and a gG-based ELISA, respectively, in a previous study (19).
UD, under the detection limit (<100).
DNA was amplified from the cerebrospinal fluid of patient P-2, but the type could not be determined (17).
ND, not detected.
DISCUSSION
Sensitive reactions to recombinant antigen gDdTM, gG-1, and gG-2 were shown by the control sera from rhesus macaques, rabbits, and humans. Although a slight cross-reaction was observed, the ELISA with the recombinant antigens was confirmed to show specificity for the detection of herpes B virus and HSV infections. The specificity of herpes B virus gD was also demonstrated, and it is proposed that gD may be a valuable diagnostic reagent for the identification of herpes B virus infections (19, 20). In our study, all macaque sera except the serum from one individual that reacted to the HSV antigen were also confirmed to have antibody to gDdTM but not to gG. In contrast, all human control and patient serum samples positive for the whole-virus antigen had antibody to gG-1 and/or gG-2 but did not have antibody to gDdTM. The limited detection of antibody to the recombinant proteins in the only natural hosts supports the specificity of the recombinant antigen-based ELISA. Taken together, we suggest that use of the combination of the recombinant proteins from herpes B virus and HSV is suitable for the discrimination of herpes B virus infection from HSV infection.
The herpes B virus recombinant antigen, gDdTM, does not contain the TM and CT regions, in which a linear B-cell epitope spanning residues 362 to 370 was found (19). In our previous study, we found that some serum samples seropositive for herpes B virus failed to react to gDdTM by WB analysis but could be found to have antibody to this secretory form of the protein by dot blot analysis (24). In accordance with our findings, the recombinant gD lacking the linear epitope was found to have a reduced reactivity to anti-herpes B virus serum under denatured conditions, suggesting the presence of conformation-dependent epitopes in the extracellular domain (19). In the present study, gDdTM was used under nondenatured ELISA conditions. The results showed that an anti-herpes B virus macaque serum reacted strongly with the secreted form of herpes B virus gD, whereas a negative serum did not. In addition, the investigation of rhesus macaque sera showed that the gD-based ELISA did not fail to detect antibody in any serum sample which cross-reacted with the HSV antigens. Thus, it is suggested that the ELISA developed is able to detect antibodies by recognizing the epitopes in the extracellular domain.
Although we did not compare the sensitivity and specificity of the recombinant gD antigen with those of the whole herpes B virus antigens, the gG antigen was evaluated by comparison with the HSV antigen in experiments with anti-HSV-1 and anti-HSV-2 sera. The titers obtained by the gG-based ELISA were higher than those obtained by the HSV-based ELISA under the homogeneous antigen-antibody conditions, suggesting that the sensitivities of the recombinant proteins were higher than those of the whole-virus antigens. In contrast to this finding, the titers obtained by the recombinant antigen-based ELISA were lower than those obtained by the whole-virus-antigen-based ELISA under the cross-reactive conditions between HSV-1 and HSV-2, suggesting that the specificity for the recombinant antigens was greater than that for the whole-virus antigens.
A sample of macaque serum (serum sample 1401) was found to have antibody not only to gG-1 but also to herpes B virus gD. The other macaque serum sample with herpes B virus infection, however, did not have antibody to either gG-1 or gG-2. These results suggest a specific reaction of the serum sample (serum sample 1401) to gG-1. We concluded that this macaque had multiple virus infections (i.e., it was infected with HSV-1 as well as herpes B virus), although we could not determine which virus affected this individual first. The macaques investigated had opportunities to be exposed to HSV-1 and HSV-2 from laboratory workers. However, no animals had antibody to gG-2. Macaques in laboratory facilities might have more frequent opportunities to be exposed to HSV-1 than to HSV-2 because of the higher prevalence of HSV-1 infection in humans (3, 5, 28, 29).
We examined the existence of antibodies to the recombinant herpes B virus or HSV proteins in sera from patients diagnosed with HSV meningitis or myelitis, since the clinical symptoms caused by HSV infections are almost the same as those caused by herpes B virus infection. We did not detect antibody to gDdTM in these samples, whereas most of the patients were found to have antibody to gG-1 and/or gG-2. Although we could not examine serum from patients with herpes B virus infections, the identification of multiple infections in a macaque serum sample suggests that the ELISA developed can detect antibody to herpes B virus even in patients who have already been infected with HSV. On the other hand, serum taken from one patient (patient P-5) was not found to contain antibody to gG-1 or gG-2, even though HSV-1 DNA was amplified from the patient's cerebrospinal fluid. No antibody to gG was detected in the serum of this patient in the previous study either (17). In addition, this serum sample was also found not to have antibody to either HSV-1 or HSV-2. Therefore, it appears that this patient did not produce IgG antibody in the serum. Further investigation, such as tests for the detection of IgM antibody, would be required.
Eleven of 12 macaque serum samples confirmed to have antibody to herpes B virus were found to show cross-reactivity with HSV-1, whereas only 5 showed cross-reactivity with HSV-2. In addition, in all HSV-1- and HSV-2-seropositive macaques, the titers of antibodies to HSV-1 were higher than those to HSV-2. These results suggest that herpes B virus has more antigenic cross-reactivity with HSV-1 than with HSV-2. This suggestion could be supported by the findings in a report by Eberle et al. (6), in which the cross-neutralization titers of anti-herpes B virus serum to HSV-1 were shown to be higher than those to HSV-2. Complete genomic sequence analysis of herpes B virus showed that there are 20 proteins which are more similar to HSV-1 proteins, including capsid proteins, whereas another 46 proteins are more similar to HSV-2 proteins and include DNA cleavage and packaging proteins (21). Therefore, the higher degrees of similarity of the structural proteins recognized by the humoral immune system might explain the higher cross-reactivity of herpes B virus with HSV-1 than with HSV-2. However, gD and gG are not likely to contribute to the cross-reaction between herpes B virus and HSV.
In summary, the fluorometric indirect ELISA with recombinant herpes B virus gD and HSV gG was shown to have the potential to discriminate between herpes B virus infection and HSV-1 and HSV-2 infections in humans and macaques. In addition to the clinical aspect, this ELISA would contribute to the assessment of the seroprevalence of alphaherpesvirus infections in humans and primates, including the natural hosts of herpes B virus.
Acknowledgments
This study was supported by a grant-in-aid for the Emerging and Re-emerging Disease project from the Ministry of Health, Labor, and Welfare of Japan.
Footnotes
Published ahead of print on 7 November 2007.
REFERENCES
- 1.Ashley, R. L., J. Militoni, F. Lee, A. Nahmias, and L. Corey. 1988. Comparison of Western blot (immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J. Clin. Microbiol. 26662-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blewett, E. L., J. T. Saliki, and R. Eberle. 1999. Development of a competitive ELISA for detection of primates infected with monkey B virus (herpesvirus simiae). J. Virol. Methods 7759-67. [DOI] [PubMed] [Google Scholar]
- 3.Bünzli, D., V. Wietlisbach, F. Barazzoni, R. Sahli, and P. R. Meylan. 2004. Seroepidemiology of herpes simplex virus type 1 and 2 in western and southern Switzerland in adults aged 25-74 in 1992-93: a population-based study. BMC Infect. Dis. 410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen, J. I., D. S. Davenport, J. A. Stewart, S. Deitchman, J. K. Hilliard, L. E. Chapman, and the B Virus Working Group. 2002. Recommendations for prevention of and therapy for exposure to B virus (Cercopithecine herpesvirus 1). Clin. Infect. Dis. 351191-1203. [DOI] [PubMed] [Google Scholar]
- 5.Cowan, F. M., R. S. French, P. Mayaud, R. Gopal, N. J. Robinson, S. A. de Oliveira, T. Faillace, A. Uusküla, M. Nygård-Kibur, S. Ramalingam, G. Sridharan, R. El Aouad, K. Alami, M. Rbai, N. P. Sunil-Chandra, and D. W. Brown. 2003. Seroepidemiological study of herpes simplex virus types 1 and 2 in Brazil, Estonia, India, Morocco, and Sri Lanka. Sex. Transm. Infect. 79286-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eberle, R., D. Black, and J. K. Hilliard. 1989. Relatedness of glycoproteins expressed on the surface of simian herpes-virus virions and infected cells to specific HSV glycoproteins. Arch. Virol. 109233-252. [DOI] [PubMed] [Google Scholar]
- 7.Eichberg, J. W., R. L. Heberling, J. E. Guajardo, and S. S. Kalter. 1980. Detection of primate herpesvirus antibodies including Herpesvirus simiae by enzyme immunoassay. Dev. Biol. Stand. 4561-66. [PubMed] [Google Scholar]
- 8.Hilliard, J. K., D. Black, and R. Eberle. 1987. Simian alphaherpesviruses and their relation to the human herpes simplex viruses. Arch. Virol. 10983-102. [DOI] [PubMed] [Google Scholar]
- 9.Ho, D. W., P. R. Field, E. Sjögren-Jansson, S. Jeansson, and A. L. Cunningham. 1992. Indirect ELISA for the detection of HSV-2 specific IgG and IgM antibodies with glycoprotein G (gG-2). J. Virol. Methods 36249-264. [DOI] [PubMed] [Google Scholar]
- 10.Hondo, R. 1974. A seroepidemiological study of herpes simplex virus. Jpn. J. Med. Sci. Biol. 27205-213. [DOI] [PubMed] [Google Scholar]
- 11.Hondo, R., T. Kurata, S. Sato, A. Oda, and Y. Aoyama. 1982. Enzymatic treatment of formalin-fixed and paraffin-embedded specimens for detection of antigens of herpes simplex, varicella-zoster and human cytomegaloviruses. Jpn. J. Exp. Med. 5217-25. [PubMed] [Google Scholar]
- 12.Katz, D., J. K. Hilliard, R. Eberle, and S. L. Lipper. 1986. ELISA for detection of group-common and virus-specific antibodies in human and simian sera induced by herpes simplex and related simian viruses. J. Virol. Methods 1499-109. [DOI] [PubMed] [Google Scholar]
- 13.Kawana, T., and K. Yoshino. 1980. Estimation of type-specific neutralizing antibody to herpes simplex virus type 2 in uterine cervical cancer patients by a new absorption method. Microbiol. Immunol. 241163-1174. [DOI] [PubMed] [Google Scholar]
- 14.Lee, F. K., R. M. Coleman, L. Pereira, P. D. Bailey, M. Tatsuno, and A. J. Nahmias. 1985. Detection of herpes simplex virus type 2-specific antibody with glycoprotein G. J. Clin. Microbiol. 22641-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee, F. K., L. Pereira, C. Griffin, E. Reid, and A. Nahmias. 1986. A novel glycoprotein for detection of herpes simplex virus type 1-specific antibodies. J. Virol. Methods 14111-118. [DOI] [PubMed] [Google Scholar]
- 16.Liljeqvist, J. A., E. Trybala, B. Svennerholm, S. Jeansson, E. Sjogren-Jansson, and T. Bergstrom. 1998. Localization of type-specific epitopes of herpes simplex virus type 2 glycoprotein G recognized by human and mouse antibodies. J. Gen. Virol. 791215-1224. [DOI] [PubMed] [Google Scholar]
- 17.Nishimura, Y., M. Ayabe, H. Shoji, H. Hashiguchi, Y. Eizuru, and T. Kawana. 2001. Differentiation of herpes simplex virus types 1 and 2 in sera of patients with HSV central nervous system infections by type-specific enzyme-linked immunosorbent assay. J. Infect. 43206-209. [DOI] [PubMed] [Google Scholar]
- 18.Norcott, J. P., and D. W. Brown. 1993. Competitive radioimmunoassay to detect antibodies to herpes B virus and SA8 virus. J. Clin. Microbiol. 31931-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perelygina, L., H. Zurkuhlen, I. Patrusheva, and J. K. Hilliard. 2002. Identification of a herpes B virus-specific glycoprotein d immunodominant epitope recognized by natural and foreign hosts. J. Infect. Dis. 186453-461. [DOI] [PubMed] [Google Scholar]
- 20.Perelygina, L., I. Patrusheva, S. Hombaiah, H. Zurkuhlen, M. J. Wildes, N. Patrushev, and J. Hilliard. 2005. Production of herpes B virus recombinant glycoproteins and evaluation of their diagnostic potential. J. Clin. Microbiol. 43620-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perelygina, L., L. Zhu, H. Zurkuhlen, R. Mills, M. Borodovsky, and J. K. Hilliard. 2003. Complete sequence and comparative analysis of the genome of herpes B virus (Cercopithecine herpesvirus 1) from a rhesus monkey. J. Virol. 776167-6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Svennerholm, B., S. Olofsson, S. Jeansson, A. Vahlne, and E. Lycke. 1984. Herpes simplex virus type-selective enzyme-linked immunosorbent assay with Helix pomatia lectin-purified antigens. J. Clin. Microbiol. 19235-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takano, J., T. Narita, K. Fujimoto, R. Mukai, and A. Yamada. 2001. Detection of B virus infection in cynomolgus monkeys by ELISA using simian agent 8 as alternative antigen. Exp. Anim. 50345-347. [DOI] [PubMed] [Google Scholar]
- 24.Tanabayashi, K., R. Mukai, and A. Yamada. 2001. Detection of B virus antibody in monkey sera using glycoprotein D expressed in mammalian cells. J. Clin. Microbiol. 393025-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tunbäck, P., J. A. Liljeqvist, G. B. Löwhagen, and T. Bergström. 2000. Glycoprotein G of herpes simplex virus type 1: identification of type-specific epitopes by human antibodies. J. Gen. Virol. 811033-1040. [DOI] [PubMed] [Google Scholar]
- 26.Weigler, B. J. 1992. Biology of B virus in macaque and human hosts: a review. Clin. Infect. Dis. 14555-567. [DOI] [PubMed] [Google Scholar]
- 27.Whitley, R. J., and J. K. Hilliard. 2001. Cercopithecine herpesvirus (B virus), p. 2835-2848. In D. M. Knipe (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 28.Wutzler, P., H. W. Doerr, I. Färber, U. Eichhorn, B. Helbig, A. Sauerbrei, A. Brandstädt, and H. F. Rabenau. 2000. Seroprevalence of herpes simplex virus type 1 and type 2 in selected German populations—relevance for the incidence of genital herpes. J. Med. Virol. 61201-207. [DOI] [PubMed] [Google Scholar]
- 29.Xu, F., M. R. Sternberg, B. J. Kottiri, G. M. McQuillan, F. K. Lee, A. J. Nahmias, S. M. Berman, and L. E. Markowitz. 2006. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 296964-973. [DOI] [PubMed] [Google Scholar]