Abstract
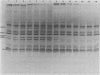
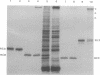
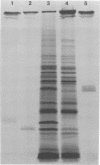
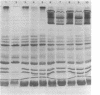
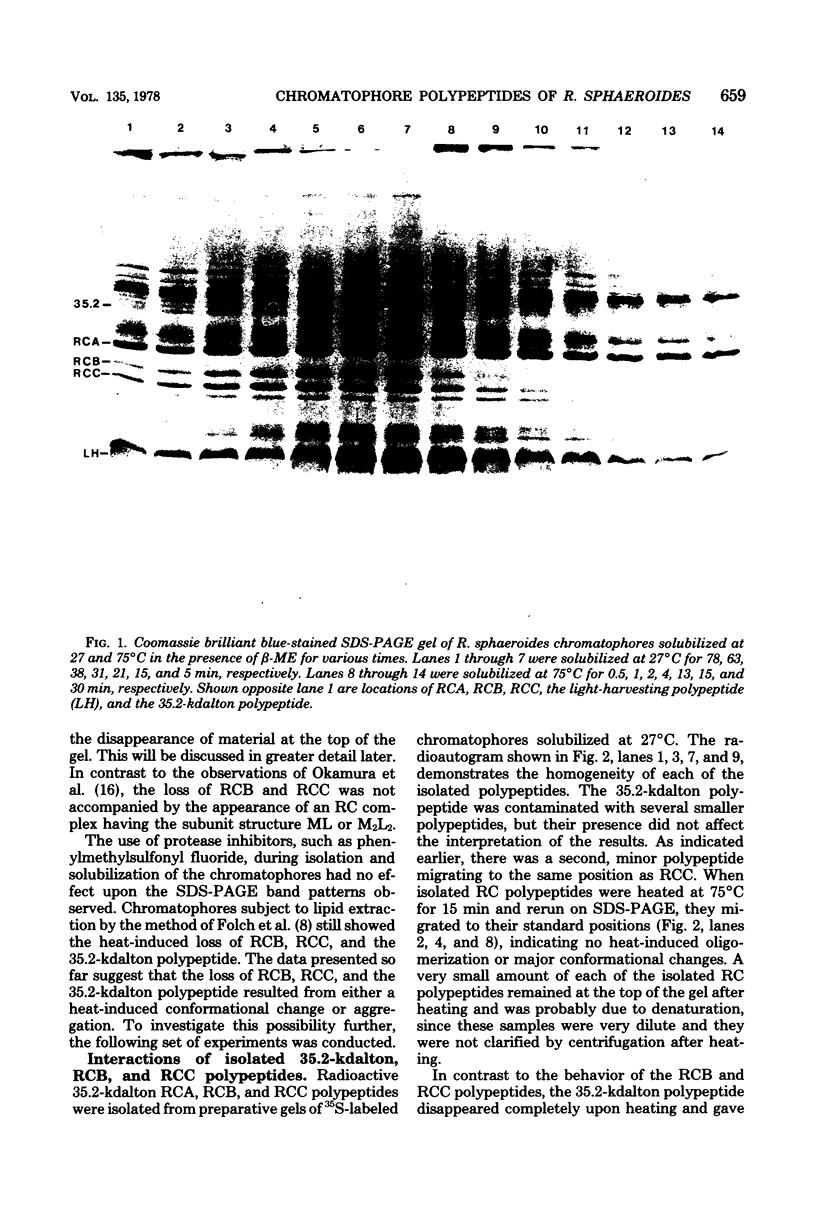
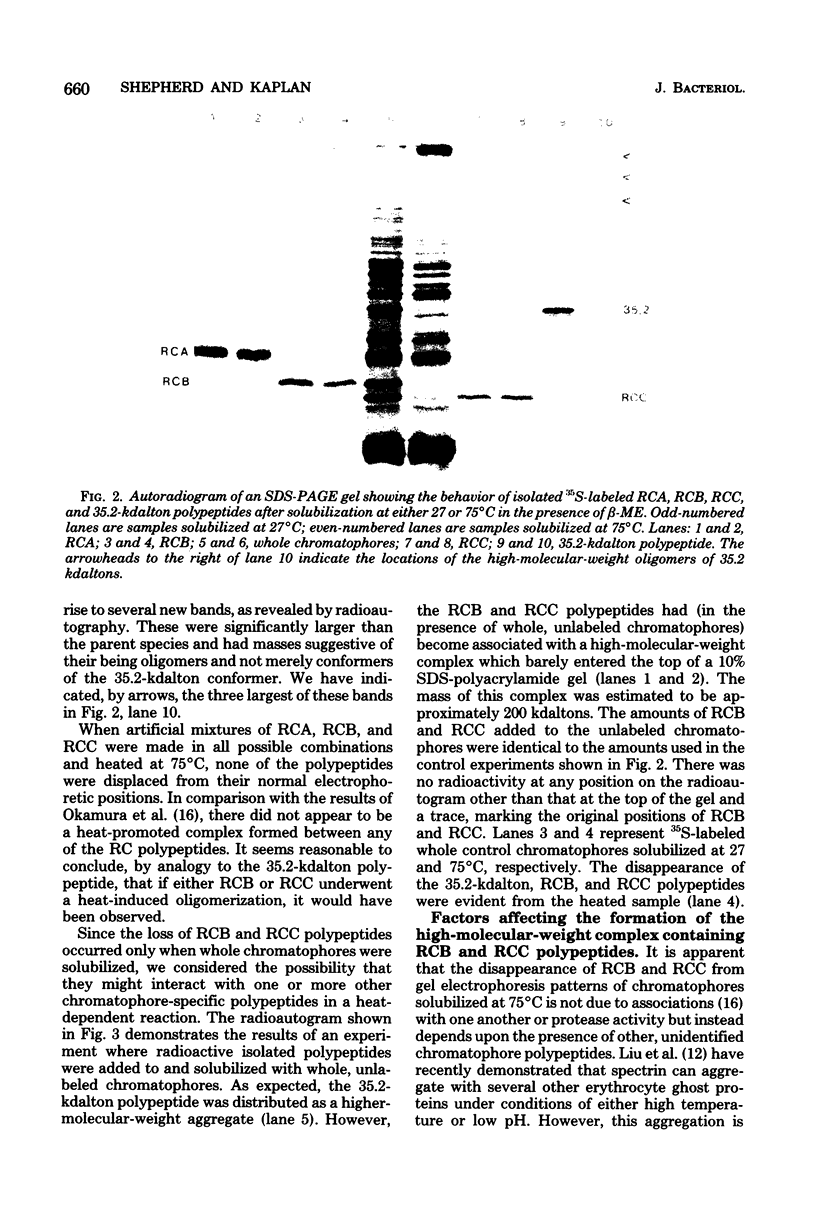
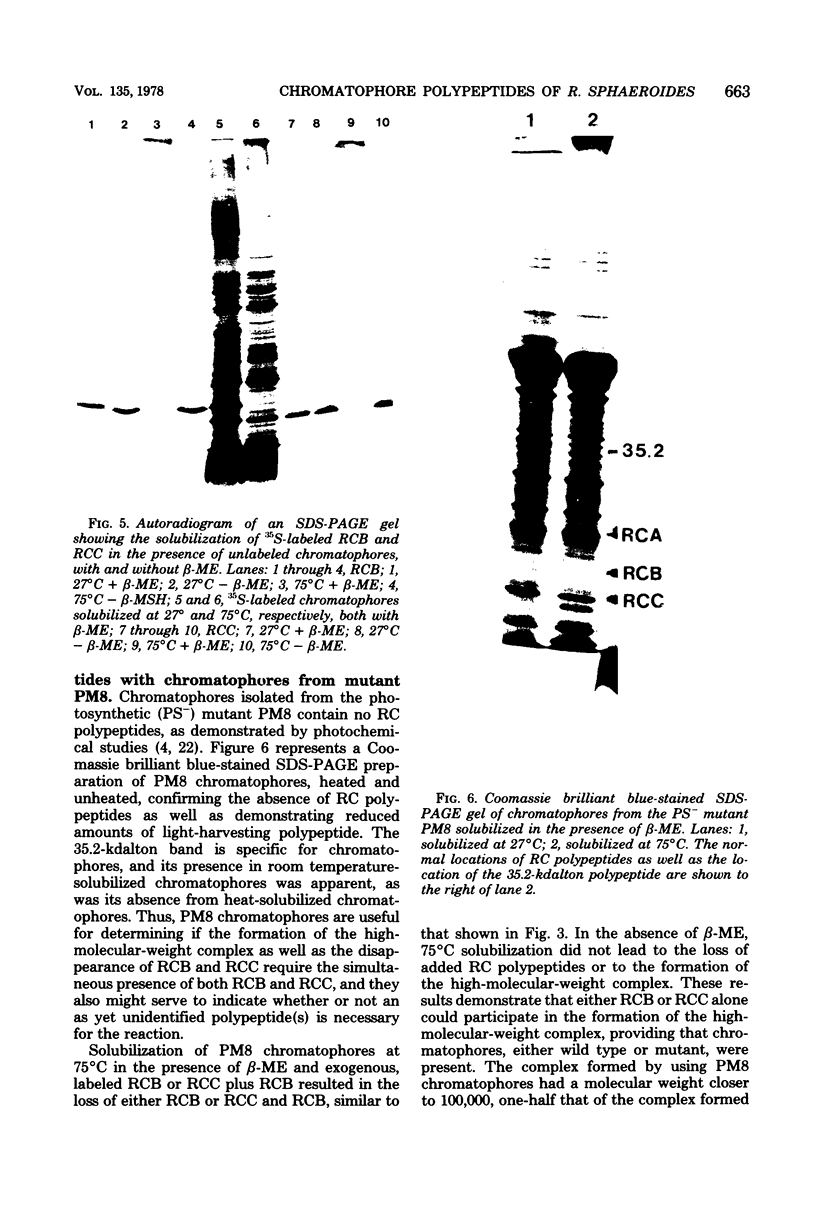
Solubilization at 75 degrees C of Rhodopseudomonas sphaeroides chromatophores in the presence of sodium dodecyl sulfate (SDS) and 2-mercaptoethanol (beta-ME) resulted in the selective absence of reaction center B and C polypeptides from SDS-polyacrylamide gel electrophoresis profiles. A newly identified, chromatophore-specific polypeptide, with a mass of 35.2 kdaltons, was also missing under these conditions of chromatophore solubilization. Solubilization at 27 degrees C in the presence of SDS and beta-ME also resulted in the disappearance of these three polypeptides, but at much slower rates. Disappearance of either endogenous or exogenously supplied reaction center polypeptides B and C during SDS solubilization of whole chromatophores at either 27 or 75 degrees C was shown to be entirely dependent upon the presence of beta-ME. After chromatophore solubilization in the presence of beta-ME and subsequent SDS-polyacrylamide gel electrophoresis, exogenously added reaction centers B and C could be localized in a complex of no less than 100 to 200 kdaltons. However, the precise size of the complex was influenced by the stoichiometry of the reacting components. The disappearance of the 35.2-kdalton polypeptide was neither dependent upon the presence of beta-ME nor dependent upon the presence of any additional chromatophore polypeptides. The 35.2-kdalton polypeptide underwent a heat-induced oligomerization to yield several high-molecular-weight species.
Full text
PDF








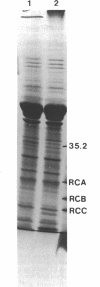
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. R., Davis J. L., Carraway K. L. Calcium-promoted changes of the human erythrocyte membrane. Involvement of spectrin, transglutaminase, and a membrane-bound protease. J Biol Chem. 1977 Oct 10;252(19):6617–6623. [PubMed] [Google Scholar]
- Chopra I., Howe G. B., Ball P. R. Lysozyme-promoted association of protein I molecules in the outer membrane of Escherichia coli. J Bacteriol. 1977 Nov;132(2):411–418. doi: 10.1128/jb.132.2.411-418.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton R. K., Haselkorn R. Protein components of bacterial photosynthetic membranes. J Mol Biol. 1972 Jul 14;68(1):97–105. doi: 10.1016/0022-2836(72)90265-3. [DOI] [PubMed] [Google Scholar]
- Clayton R. K., Sistrom W. R., Zaugg W. S. The role of a reaction center in photochemical activities of bacterial chromatophores. Biochim Biophys Acta. 1965 Jul 22;102(2):341–348. doi: 10.1016/0926-6585(65)90123-8. [DOI] [PubMed] [Google Scholar]
- Collins M. L., Niederman R. A. Membranes of Rhodospirillum rubrum: physicochemical properties of chromatophore fractions isolated from osmotically and mechanically disrupted cells. J Bacteriol. 1976 Jun;126(3):1326–1338. doi: 10.1128/jb.126.3.1326-1338.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Firsow N. N., Drews G. Differentiation of the intracytoplasmic membrane of Rhodopseudomonas palustris induced by variations of oxygen partial pressure or light intensity. Arch Microbiol. 1977 Dec 15;115(3):299–306. doi: 10.1007/BF00446456. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Kaplan S. Isolation and fractionation of the photosynthetic membranous organelles from Rhodopseudomonas spheroides. J Bacteriol. 1971 Oct;108(1):465–473. doi: 10.1128/jb.108.1.465-473.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolchine G., Reiss-Husson F. Proteins of R. spheroides Y reaction center: gel electrophoresis and electrofocusing studies. Biochem Biophys Res Commun. 1972 Jul 25;48(2):333–340. doi: 10.1016/s0006-291x(72)80055-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu S. C., Fairbanks G., Palek J. Spontaneous, reversible protein cross-linking in the human erythrocyte membrane. Temperature and pH dependence. Biochemistry. 1977 Sep 6;16(18):4066–4074. doi: 10.1021/bi00637a020. [DOI] [PubMed] [Google Scholar]
- MacGregor C. H. Solubilization of Escherichia coli nitrate reductase by a membrane-bound protease. J Bacteriol. 1975 Mar;121(3):1102–1110. doi: 10.1128/jb.121.3.1102-1110.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael J. C., Ou J. T. Metal ion dependence of a heat-modifiable protein from the outer membrane of Escherichia coli upon sodium dodecyl sulfate-gel electrophoresis. J Bacteriol. 1977 Oct;132(1):314–320. doi: 10.1128/jb.132.1.314-320.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D. R., Agin P. P. Platelet plasma membrane glycoproteins. Evidence for the presence of nonequivalent disulfide bonds using nonreduced-reduced two-dimensional gel electrophoresis. J Biol Chem. 1977 Mar 25;252(6):2121–2126. [PubMed] [Google Scholar]
- Reithmeier R. A., Bragg P. D. Molecular characterization of a heat-modifiable protein from the outer membrane of Escherichia coli. Arch Biochem Biophys. 1977 Jan 30;178(2):527–534. doi: 10.1016/0003-9861(77)90223-5. [DOI] [PubMed] [Google Scholar]
- SISTROM W. R. A requirement for sodium in the growth of Rhodopseudomonas spheroides. J Gen Microbiol. 1960 Jun;22:778–785. doi: 10.1099/00221287-22-3-778. [DOI] [PubMed] [Google Scholar]
- SISTROM W. R., CLAYTON R. K. STUDIES ON A MUTANT OF RHODOPSEUDOMONAS SPHEROIDES UNABLE TO GROW PHOTOSYNTHETICALLY. Biochim Biophys Acta. 1964 Jul 29;88:61–73. doi: 10.1016/0926-6577(64)90154-8. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. 3. Evidence that the major protein of Escherichia coli O111 outer membrane consists of four distinct polypeptide species. J Bacteriol. 1974 May;118(2):442–453. doi: 10.1128/jb.118.2.442-453.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. I. Effect of preparative conditions on the migration of protein in polyacrylamide gels. Arch Biochem Biophys. 1973 Aug;157(2):541–552. doi: 10.1016/0003-9861(73)90673-5. [DOI] [PubMed] [Google Scholar]
- Steiner L. A., Okamura M. Y., Lopes A. D., Moskowitz E., Feher G. Characterization of reaction centers from photosynthetic bacteria. II. Amino acid composition of the reaction center protein and its subunits in Rhodopseudomonas spheroides R-26. Biochemistry. 1974 Mar 26;13(7):1403–1410. doi: 10.1021/bi00704a014. [DOI] [PubMed] [Google Scholar]
- Steiner L. A., Okamura M. Y., Lopes A. D., Moskowitz E., Feher G. Characterization of reaction centers from photosynthetic bacteria. II. Amino acid composition of the reaction center protein and its subunits in Rhodopseudomonas spheroides R-26. Biochemistry. 1974 Mar 26;13(7):1403–1410. doi: 10.1021/bi00704a014. [DOI] [PubMed] [Google Scholar]
- Takemoto J., Huang Kao M. Y. Effects of incident light levels on photosynthetic membrane polypeptide composition and assembly in Rhodopseudomonas sphaeroides. J Bacteriol. 1977 Feb;129(2):1102–1109. doi: 10.1128/jb.129.2.1102-1109.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto J., Lascelles J. Function of membrane proteins coupled to bacteriochlorophyll synthesis. Studies with wild type and mutant strains of Rhodopseudomonas spheroides. Arch Biochem Biophys. 1974 Aug;163(2):507–514. doi: 10.1016/0003-9861(74)90508-6. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yoch D. C., Carithers R. P., Arnon D. I. Isolation and characterization of bound ion-sulfur proteins from bacterial photosynthetic membranes. I. Ferredoxins III and IV from Rhodospirillum rubrum chromatophores. J Biol Chem. 1977 Nov 10;252(21):7453–7460. [PubMed] [Google Scholar]