Abstract
Acanthamoeba amoebae of genotype T2 were identified as the causative agent of Acanthamoeba skin lesions and granulomatous amoebic encephalitis (GAE) in a human immunodeficiency virus-negative patient with underlying tuberculosis. To our knowledge this, is the first case of GAE involving genotype T2.
Acanthamoebae are the causative agents of Acanthamoeba keratitis and granulomatous amebic encephalitis (GAE), a condition that predominantly occurs in immunocompromised individuals. In GAE the skin and the olfactory neuroepithelium probably function as portals of entry, and occasionally, inflammation can be observed at these primary foci. Descriptions of approximately 150 cases of GAE caused by Acanthamoeba have been published worldwide, and less than 10 of these patients have survived (9). Recently, the first case of GAE in Austria was diagnosed (A. Aichelburg et al., submitted for publication). The patient was a 25-year-old man from India with respiratory and neurological symptoms and widespread granulomatous dermal lesions. He had a CD4+ count of 182 cells/μl but was negative for human immunodeficiency virus (HIV). The final diagnosis included miliary tuberculosis, tuberculous meningitis, GAE, and Acanthamoeba skin lesions. The patient did not improve after tuberculostatic treatment or treatment with streptomycin, fluconazole, trimethoprim-sulfamethoxazole, amphotericin B, flucytosine, and sulfadiazine, which are drugs that are potentially effective against Acanthamoeba. However, he was finally treated successfully with oral and topical miltefosine and intrathecal and intravenous amikacin.
Altogether six cerebrospinal fluid (CSF) samples, two dermal punch biopsy specimens, one bronchoalveolar lavage (BAL) specimen, one lung tissue biopsy specimen, one liver tissue biopsy specimen, and one biopsy specimen from the main brain lesion were investigated for acanthamoebae by lactophenol cotton blue (LPCB) staining (15), culture, and PCR. For culture, specimens were inoculated onto the centers of nonnutrient agar plates (1.5%) precoated with Escherichia coli. The plates were sealed with Parafilm, incubated at 30°C for 14 days, and examined every 48 h. For PCR, whole-cell DNA was isolated as described previously (19) and a fragment of the 18S rRNA gene was amplified with primers JDP1 and JDP2 (8). Acanthamoeba sp. strain ATCC PRA-105, genotype T4, was used as a positive control. The amplicons were sequenced with a 310 ABI Prism automated sequencer (Applied Biosystems, Langen, Germany), and the sequences of both strands were obtained. Multiple-sequence alignment was performed with the Clustal X application (16) but with the primer sites excluded from the analysis. Genotypes were assessed with the model assumption of a <5% sequence dissimilarity within one genotype (5). The lung, liver, and brain biopsy specimens were also examined by immunofluorescence staining with polyclonal rabbit anti-Acanthamoeba castellanii serum at dilutions of 1:50 and 1:100. Sequential patient serum samples at dilutions ranging from 1:5 to 1:4,000 were tested for anti-Acanthamoeba immunoglobulin G (IgG) and IgM antibodies by immunoblotting, as described previously (20), by using the antigens of all three Acanthamoeba groups (for group I, strain Am23; for group II, strain ATCC PRA-105; for group III, strain ATCC 50704). Pooled serum from 10 healthy individuals was used as a negative control. Drug susceptibility assays were carried out at 30°C in microtiter plates with 105 trophozoites (strain ATCC PRA-105) per well. Antimicrobials were used at the following final concentrations (corresponding to the concentrations suggested for treatment): 250 mg/ml for amikacin, 5 mg/ml for amphotericin B, 7 mg/ml for caspofungin, 2 mg/ml for fluconazole, 10 mg/ml for flucytosine, 60 mg/ml for rifampin, 250 mg/ml for streptomycin, 100 mg/ml for sulfadiazine, and 10 mg/ml for voriconazole. The viability of the amoebae, as determined by phase-contrast microscopy, was recorded after 24 h; and 100% eradication was confirmed by transferring the respective suspension to E. coli-coated plates and recording the amoebal growth for 14 days. Assays were performed in two independent experiments, each time in triplicate.
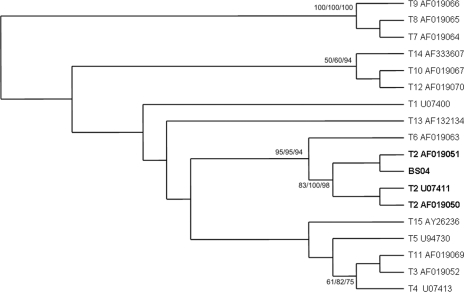
The LPCB staining, culture, and immunofluorescence results were consistently negative. However, the first four CSF samples, dermal specimens, the BAL specimen, and the lung and brain tissue biopsy specimens were positive by the Acanthamoeba-specific PCR. Sequencing identified the causative agent (“strain” BS04) as Acanthamoeba genotype T2. All sequences obtained were identical. In cluster analysis, BS04 clusters with strain CCAP 1501/3c (GenBank accession no. AF019051) (Fig. 1). The liver biopsy sample and the two final CSF samples, obtained 5 and 9 months after admission to the hospital, respectively (both after therapy), were negative. Serological testing revealed a high IgG titer (1:2,000) and, in particular, a high IgM titer (1:1,000) in a serum sample obtained 3 days after admission to the hospital; the titers then gradually declined in consecutive serum samples and became comparable to those in the negative control sample (IgM titer, 1:125) after 1 year. Interestingly, the patient showed the highest immunoreactivity against Acanthamoeba morphological group III (corresponding to genotype T2). Since the patient did not show any improvement even after treatment with drugs that had been shown to be useful in previously published cases of successful treatment, miltefosine (18) and amikacin were finally used to successfully treat our patient.
FIG. 1.
Maximum-likelihood cluster analysis of the sequence obtained and the sequences of various Acanthamoeba strains, with special reference to genotype T2 and by the use of Acanthamoeba genotypes 7, 8, and 9 as outgroups. BS04 is the sequence from the case presented here. Bootstrap values are based on 100 replicates and are given at the nodes (for maximum likelihood/neighbor joining/maximum parsimony). The designations after the genotype or strain names are GenBank accession numbers. The asterisk indicates values below 50%.
In the present case, three aspects are of particular interest: (i) the coinfection with Mycobacterium tuberculosis, (ii) the fact that acanthamoebae were detectable only by PCR while the culture result remained negative, and (iii) the fact that the acanthamoebae were genotype T2 and not genotype T4, the genotype commonly associated with disease.
Coinfection with M. tuberculosis not only is important as the reason for the severe immunosuppression of our patient (thus having contributed to the establishment of the Acanthamoeba infection) but also is important because acanthamoebae can act as hosts for mycobacteria, protecting them from antibiotics and disinfectants and even increasing their virulence (1, 4, 7). Other cases of Acanthamoeba-Mycobacterium coinfections have been reported, but these affected AIDS patients (13).
The fact that the culture result was consistently negative is not entirely unexpected, as acanthamoebae usually reside deep in the tissue, mainly around blood vessels, and their isolation from CSF is generally uncommon (2, 13). It is tempting to speculate that this is particularly true for non-AIDS patients, where amoebae cannot disseminate ad libitum. When the biopsy sample was obtained from the present case, the CD4+ counts had already reached 421 cells/μl. As the location of the lesion obviated a total excision, the site of active infection was probably missed. This would also explain the negative immunofluorescence test results and highlights the importance of molecular methods in the diagnosis of Acanthamoeba infections. In a recent case of disseminated Acanthamoeba infection in an HIV-negative patient, even detailed histological analysis of multiple biopsy specimens and culture did not enable a premortem diagnosis (14).
The Acanthamoeba strain recovered from the present case was identified as genotype T2. It has been proposed that genotype T2 be subdivided into a highly pathogenic cluster and a weakly pathogenic cluster (6). The sequence of strain BS04 clusters with the weaker pathogenic subgroup, which might also have contributed to the survival of our patient. It has been shown that of the 15 Acanthamoeba genotypes, genotype T4 is the predominant genotype in GAE (as well as in Acanthamoeba keratitis), followed by genotypes T1, T10, and T12 (3). The last two genotypes also correspond to morphological group III, as does genotype T2. A recent study revealed that while in the United Kingdom genotype T4 is the most abundant genotype, genotype T2 predominates in environmental samples in Iran (6). Our patient, who originated from India, might have acquired his infection outside Europe, and in this respect it is interesting to note that several GAE cases in HIV-negative patients have been reported from India, while in Europe and the United States GAE is mostly linked to AIDS (10-12, 17).
Nucleotide sequence accession number.
The sequence data were deposited in the GenBank database and are available under accession number EF428121.
Acknowledgments
We thank Susanne Glöckl and Iveta Häfeli (Department of Medical Parasitology, Clinical Institute of Hygiene and Medical Microbiology, Vienna, Austria) and Rama Sriram (CDC, Atlanta, GA) for excellent technical assistance.
Footnotes
Published ahead of print on 14 November 2007.
REFERENCES
- 1.Adekambi, T., S. Ben Salah, M. Khlif, D. Raoult, and M. Drancourt. 2006. Survival of environmental mycobacteria in Acanthamoeba polyphaga. Appl. Environ. Microbiol. 725974-5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloch, K. C., and F. L. Schuster. 2005. Inability to make a premortem diagnosis of Acanthamoeba species infection in a patient with fatal granulomatous amebic encephalitis. J. Clin. Microbiol. 433003-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Booton, G. C., G. S. Visvesvara, T. J. Byers, D. J. Kelly, and P. A. Fuerst. 2005. Identification and distribution of Acanthamoeba species genotypes associated with nonkeratitis infections. J. Clin. Microbiol. 431689-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cirillo, J. D., S. Falkow, L. S. Tompkins, and L. E. Bermudez. 1997. Interaction of Mycobacterium avium with environmental amoebae enhances virulence. Infect. Immun. 653759-3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gast, R. J., D. R. Ledee, P. A. Fuerst, and T. J. Byers. 1996. Subgenus systematics of Acanthamoeba: four nuclear 18S rDNA sequence types. J. Eukaryot. Microbiol. 43498-504. [DOI] [PubMed] [Google Scholar]
- 6.Maghsood, A. H., J. Sissons, M. Rezaian, D. Nolder, D. Warhurst, and N. A. Khan. 2005. Acanthamoeba genotype T4 from the UK and Iran and isolation of the T2 genotype from clinical isolates. J. Med. Microbiol. 54755-759. [DOI] [PubMed] [Google Scholar]
- 7.Mura, M., T. J. Bull., H. Evans, K. Sidi-Boumedine, L. McMinn, G. Rhodes, R. Pickup, and J. Hermon-Taylor. 2006. Replication and long-term persistence of bovine and human strains of Mycobacterium avium subsp. paratuberculosis within Acanthamoeba polyphaga. Appl. Environ. Microbiol. 72854-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schroeder, J. M., G. C. Booton, J. Hay, I. A. Niszl, D. V. Seal, M. B. Markus, P. A. Fuerst, and T. J. Byers. 2001. Use of subgenic 18S ribosomal DNA PCR and sequencing for genus and genotype identification of acanthamoebae from humans with keratitis and from sewage sludge. J. Clin. Microbiol. 391903-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schuster, F. L., and G. S. Visvesvara. 2004. Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int. J. Parasitol. 341001-1027. [DOI] [PubMed] [Google Scholar]
- 10.Sharma, P. P., P. Gupta, M. V. Murali, and V. G. Ramachandran. 1993. Primary amebic meningoencephalitis caused by Acanthamoeba: successfully treated with co-trimoxazole. Indian Pediatr. 301219-1222. [PubMed] [Google Scholar]
- 11.Shirwadkar, C. G., R. Samant, M. Sankhe, R. Deshpande, S. Yagi, F. L. Schuster, R. Sriram, and G. S. Visvesvara. 2006. Acanthamoeba encephalitis in patient with systemic lupus, India. Emerg. Infect. Dis. 12984-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singhal, T., A. Bajpai, V. Kalra, S. K. Kabra, J. C. Samantaray, G. Satpathy, and A. K. Gupta. 2001. Successful treatment of Acanthamoeba meningitis with combination oral antimicrobials. Pediatr. Infect. Dis. J. 20623-627. [DOI] [PubMed] [Google Scholar]
- 13.Sison, J. P., C. A. Kemper, M. Loveless, D. McShane, G. S. Visvesvara, and S. C. Deresinski. 1995. Disseminated Acanthamoeba infection in patients with AIDS: case reports and review. Clin. Infect. Dis. 201207-1216. [DOI] [PubMed] [Google Scholar]
- 14.Steinberg, J. P., R. L. Galindo, E. S. Kraus, and K. G. Ghanem. 2002. Disseminated acanthamebiasis in a renal transplant recipient with osteomyelitis and cutaneous lesions: case report and literature review. Clin. Infect. Dis. 35e43-e49. [DOI] [PubMed] [Google Scholar]
- 15.Thomas, P. A., and T. Kuriakose. 1990. Rapid detection of Acanthamoeba cysts in corneal scrapings by lactophenol cotton blue staining. Arch. Ophthalmol. 108168. [DOI] [PubMed] [Google Scholar]
- 16.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 254876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Velho, V., G. K. Sharma, and D. A. Palande. 2003. Cerebrospinal acanthamebic granulomas. Case report. J. Neurosurg. 99572-574. [DOI] [PubMed] [Google Scholar]
- 18.Walochnik, J., M. Duchene, K. Seifert, A. Obwaller, T. Hottkowitz, G. Wiedermann, A. Eibl, and H. Aspock. 2002. Cytotoxic activities of alkylphosphocholines against clinical isolates of Acanthamoeba spp. Antimicrob. Agents Chemother. 46695-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walochnik, J., A. Hassl, K. Simon, G. Benyr, and H. Aspock. 1999. Isolation and identification by partial sequencing of the 18S ribosomal gene of free-living amoebae from necrotic tissue of Basilliscus plumifrons (Sauria: Iguanidae). Parasitol. Res. 85601-603. [DOI] [PubMed] [Google Scholar]
- 20.Walochnik, J., A. Obwaller, E. M. Haller-Schober, and H. Aspock. 2001. Anti-Acanthamoeba IgG, IgM, and IgA immunoreactivities in correlation to strain pathogenicity. Parasitol. Res. 87651-656. [DOI] [PubMed] [Google Scholar]