Abstract
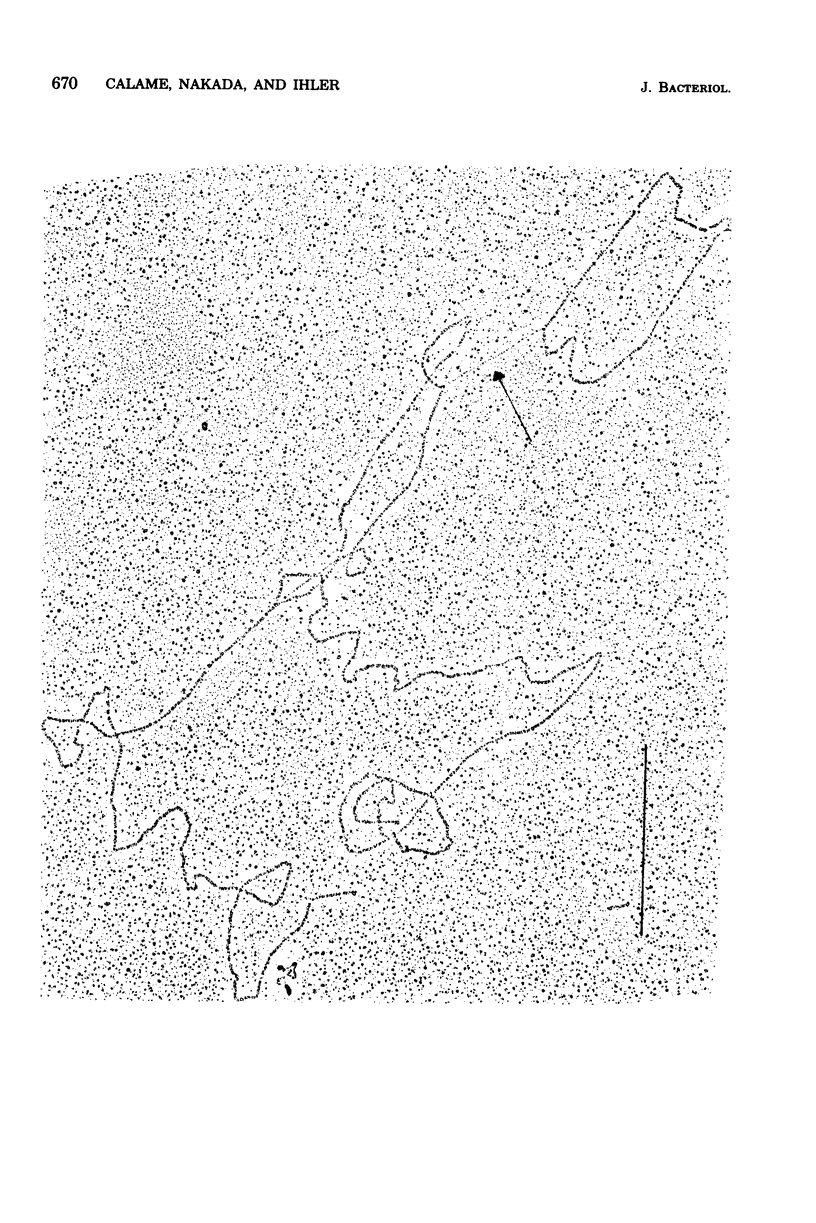
Eight ribosome-binding sites were located on the single-stranded Tn10 DNA loop which was formed after denaturation of lambda phage DNA containing the Tn10 transposon sequence. Ribosomes were bound only to the Tn10 loop contained on the R strand of lambda DNA but not to that on the L strand, suggesting that one of the two strands of Tn10 DNA is selectively transcribed. Six of the eight ribosome binding sites were located in one-half of the DNA loop. The maximum sizes of potential polypeptides were calculated for these genes to range between 9,500 and 84,000 daltons.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calame K., Ihler G. Physical location of ribosome binding sites on lambda DNA. J Mol Biol. 1977 Nov;116(4):841–853. doi: 10.1016/0022-2836(77)90274-1. [DOI] [PubMed] [Google Scholar]
- Calame K., Ihler G. Visualization of ribosome--single-stranded DNA complexes in the electron microscope. Biochemistry. 1977 Mar 8;16(5):964–971. doi: 10.1021/bi00624a024. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C. Revised interpretation of the origin of the pSC101 plasmid. J Bacteriol. 1977 Nov;132(2):734–737. doi: 10.1128/jb.132.2.734-737.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., Bedinger P., Champoux J. J., Falkow S. Deletions affecting the transposition of an antibiotic resistance gene. Proc Natl Acad Sci U S A. 1977 Feb;74(2):702–706. doi: 10.1073/pnas.74.2.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihler G., Nakada D. Selective binding of ribosomes to initiation sites on single-stranded DNA from bacterial viruses. Nature. 1970 Oct 17;228(5268):239–242. doi: 10.1038/228239a0. [DOI] [PubMed] [Google Scholar]
- Ihler G., Rupp W. D. Strand-specific transfer of donor DNA during conjugation in E. coli. Proc Natl Acad Sci U S A. 1969 May;63(1):138–143. doi: 10.1073/pnas.63.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N. Translocatable elements in procaryotes. Cell. 1977 May;11(1):11–23. doi: 10.1016/0092-8674(77)90313-0. [DOI] [PubMed] [Google Scholar]
- Lee F., Yanofsky C. Transcription termination at the trp operon attenuators of Escherichia coli and Salmonella typhimurium: RNA secondary structure and regulation of termination. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4365–4369. doi: 10.1073/pnas.74.10.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S. B., McMurry L. Detection of an inducible membrane protein associated with R-factor-mediated tetracycline resistance. Biochem Biophys Res Commun. 1974 Feb 27;56(4):1060–1068. doi: 10.1016/s0006-291x(74)80296-2. [DOI] [PubMed] [Google Scholar]
- Platt T., Squires C., Yanofsky C. Ribosome-protected regions in the leader-trpE sequence of Escherichia coli tryptophan operon messenger RNA. J Mol Biol. 1976 May 15;103(2):411–420. doi: 10.1016/0022-2836(76)90320-x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Air G. M., Barrell B. G., Brown N. L., Coulson A. R., Fiddes C. A., Hutchison C. A., Slocombe P. M., Smith M. Nucleotide sequence of bacteriophage phi X174 DNA. Nature. 1977 Feb 24;265(5596):687–695. doi: 10.1038/265687a0. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Cohen S. N., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. II. Structure of drug resistance (R) factors and F factors. J Mol Biol. 1973 Apr 5;75(2):235–255. doi: 10.1016/0022-2836(73)90018-1. [DOI] [PubMed] [Google Scholar]
- Tait R. C., Boyer H. W. On the nature of tetracycline resistance controlled by the plasmid pSC101. Cell. 1978 Jan;13(1):73–81. doi: 10.1016/0092-8674(78)90139-3. [DOI] [PubMed] [Google Scholar]
- Yang H. L., Zubay G., Levy S. B. Synthesis of an R plasmid protein associated with tetracycline resistance is negatively regulated. Proc Natl Acad Sci U S A. 1976 May;73(5):1509–1512. doi: 10.1073/pnas.73.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]




