Abstract
Norovirus is now recognized as the leading cause of nonbacterial acute gastroenteritis in adults, causing numerous outbreaks worldwide. We have developed two novel light-upon-extension (LUX) real-time PCR assays for detection and quantification of norovirus genogroups I and II. The LUX system uses a fluorophore attached to one primer having a self-quenching hairpin structure, making it cost-effective and specific. The assays were evaluated against clinical stool specimens (n = 103) from Sweden and Nicaragua and compared to established methods. The norovirus assay detected more positive stool specimens (47/103) than conventional PCR (39/103) and corresponded to a TaqMan real-time PCR, with the exception of one specimen. Furthermore, the assays correctly identified all (n = 11) coded control specimens in a reference panel containing various genogroups and genotypes. Both LUX real-time PCR assays had a wide dynamic range, detecting from ≤101 to 107 genes per reaction, resulting in a theoretical lower limit of ≤∼20 000 viruses per gram of stool. No cross-reactivity was noticed with specimens containing other enteric viruses, and by using melting curve analysis we could differentiate between norovirus genogroups I and II.
Norovirus (NV) is now recognized as the leading cause of nonbacterial acute gastroenteritis in adults, causing numerous outbreaks worldwide (3, 6, 8, 14). NVs constitute a genetically diverse group of viruses and can be further subdivided into five genogroups, where genogroup I (GI), with 8 genotypes (GI-1 to GI-8), and genogroup II (GII), with 17 genotypes (GII-1 to GII-17), contain most of the strains that infect humans (23). In recent years, real-time PCR has emerged as a highly reproducible and sensitive method for detection and diagnosis of NVs, proving to be more sensitive than previously used methods such as electron microscopy or conventional PCR (2, 4, 20). Due to the high sequence diversity of human NV, optimization of primers and/or probes is crucial for broad detection (3, 9, 23). There are various real-time PCR assays reported for detection of NV, including TaqMan assays (7, 10, 11, 20, 21) and assays based on the SYBR green chemistry (18, 19). Many of the recently developed assays target the ORF1-ORF2 junction downstream region B, which possibly is the most conserved part of the NV genome (11, 13).
The light-upon-extension (LUX) technique applied in this work uses a fluorophore attached near the 3′ end of one of the primers, constructed to form a hairpin loop and thus having fluorescence-quenching capability. When the primer becomes incorporated into the double-stranded PCR product, the fluorophore is dequenched, resulting in an increase of the fluorescent signal (15). This technique simplifies PCR kinetics, and the hairpin oligonucleotides prevent primer-dimer formation and mispriming (16). A further advantage is that the incorporation of a fluorophore enables the use of melting curve analysis. This system offers high sensitivity and specificity without the use of a probe or a quencher molecule.
The aim of this study was to apply LUX real-time PCR assays for detection and quantification of NV GI and GII. To our knowledge this is the first time an assay of this type has been developed for enteric viruses. The assay was evaluated with clinical specimens from different origins and compared to immunological methods and other PCR-based assays. In addition, melting curve analysis was performed, allowing simultaneous detection and differentiation between different NV genogroups.
MATERIALS AND METHODS
Sampling of clinical specimens.
Stool specimens were received from the Microbiological Laboratory at Ryhov County Hospital in Jönköping, Sweden (n = 61), and from the Department of Microbiology at the University of León, Nicaragua (n = 42). These specimens were randomly chosen from children and adults with acute gastroenteritis being screened for NV infection.
Furthermore, a reference panel of 15 stool specimens from the Swedish Institute for Infectious Disease Control in Stockholm, Sweden, was used for external validation of the NV diagnosis. The panel consisted of eight NV GII-positive specimens (four GII-4, one GII-6, one GII-7, one GII-13, and one GII-15), three NV GI-positive specimens (one GI-6, one GI-10, and one GI-14), two sapovirus-positive specimens, and two negative specimens. The genotyping of the material in the reference panel was based on a method described by Kageyama et al. (12).
RNA extraction.
Stool suspensions (10% [vol/vol] phosphate-buffered saline [pH 7.0]) were first clarified by centrifugation at 4,000 × g for 3 min. Viral RNA was then extracted from 140 μl of the stool suspension using the QIAamp viral RNA minikit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. RNA was eluted with 60 μl RNase-free water containing 0.04% sodium azide (AVE buffer; Qiagen, Hilden, Germany) and stored at −80°C. RNA extraction from stool specimens in Jönköping was performed using the EZ1 robot (Qiagen, Hilden, Germany) and stored at −80°C.
RT-PCR.
Briefly, 28 μl of extracted RNA was mixed with 50 pmol of Pd(N)6 (random hexamer) primer (GE-Healthcare, Uppsala, Sweden), denatured at 97°C for 5 min, and quickly chilled on ice for 2 min, followed by addition of one Illustra Ready-To-Go reverse transcriptase PCR (RT-PCR) bead (GE-Healthcare, Uppsala, Sweden) and RNase-free water to a final volume of 50 μl. The RT reaction was then carried out for 60 min at 42°C to produce the cDNA, later used for real-time PCR, and stored at −20°C.
Primer design.
Alignments of 28 representative NV GI sequences and 70 NV GII sequences were performed using ClustalW. The reference strain for NV GI, used for querying the database, was strain Norwalk/68/US (accession no. M87661), and that for NV GII was strain Lordsdale/93/UK (accession no. X86557).
LUX primers were manually designed, and their properties were calculated with the software OligoAnalyzer 3.0 (Integrated DNA Technologies, Coralville, IA) and Oligo Property Calculator 3.1 (Northwestern University, Chicago, IL). We aimed for all primer pairs to have an optimal annealing temperature of 55°C. Investigation of primer cross-specificity was done using the BLAST software (1).
Cloning of viral gene fragments used as reference DNA in the real-time PCR assay.
A 597-bp fragment, spanning nucleotides 5075 to 5671 of the NV GI genome, was amplified by PCR from a clinical GI-4 strain, using the primers G1FFc and G1SKR (12). A 1,188-bp fragment spanning nucleotides 4495 to 5683 of the NV GII genome was amplified from a clinical GII-4 strain using the NI and Mon383 primers (5, 17).
The fragments were cloned into a pPCR-Script Amp SK(+) vector and transformed into XL10-Gold Kan ultracompetent cells using the Stratagene PCR-Script Amp cloning kit (Stratagene, La Jolla, CA) according to the manufacturer's instructions. After overnight incubation, plasmid DNA was extracted and purified using Qiagen plasmid miniprep kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Concentrations were determined using the NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE).
Optimization of PCR assay conditions and evaluation of PCR primers using conventional PCR.
Designed PCR primers without fluorophores were optimized using conventional PCR with cDNA from patient material from both Sweden and Nicaragua which had previously been diagnosed as NV GI or NV GII positive. Different annealing temperatures were tested in order to optimize the reaction. For the optimization, a final volume of 25 μl of reaction mixture containing 2.5 μl cDNA from the RT reaction, 2.5 μl of 10× PCR buffer, 1 μl of MgCl2 (50 mM), 2 μl of deoxynucleoside triphosphates (2.5 mM), 0.5 μl of each primer (10 pmol μl−1) (Table 1), 0.125 μl of Taq polymerase (5 U μl−1) (Invitrogen, Carlsbad, CA), and 15.875 μl of RNase-free water was used, and the reaction was carried out on a PCT-100 programmable thermal controller (MJ Research, Waltham, MA.). The reaction mixture was preheated at 95°C for 5 min, followed by 35 thermal cycles of 15 s at 94°C, 30 s at 55°C, and 30 s at 72°C and finally an extension step at 72°C for 10 min. The PCR products were analyzed on a 2% agarose gel and visualized by ethidium bromide staining.
TABLE 1.
Primers used for evaluation of the assay conditions with conventional PCR on clinical specimens
| Group and primera | Sequence (5′→3′)b | Positionc | Reference |
|---|---|---|---|
| GI | |||
| NVG1f1b | CGY TGG ATG CGN TTC CAT GA | 5311-5330 | This study |
| NVG1f2 | GAT CGC RAT CTC YTG CCC G | 5350-5368 | This study |
| NVG1rlux | gatga GTC CTT AGA CGC CAT CA TC | 5397-5379 | This study |
| GII | |||
| NVG2flux1 | garaa ATG TTY AGR TGG ATG AGR TTY TC | 5012-5033 | This study |
| NVG2flux2 | agatt GGG AGG GCG ATC GCA A TCT | 5049-5067 | This study |
| COG2R | TCG ACG CCA TCT TCA TTC ACA | 5100-5081 | 11 |
Norovirus LUX primers for ORF1-ORF2 junction amplification. The primer pairs selected for the LUX real-time PCR assays are shown in bold.
The bases in lowercase at the 5′ ends of the primers correspond to additional bases added to create a hairpin loop. The bases in bold at the 3′ ends of the LUX primers are labeled with the fluorophore 6-carboxyfluorescein.
Positions for GI correspond to GenBank accession no. M87661 (Norwalk 68, complete genome); those for GII correspond to GenBank accession no. X86557 (Lordsdale, complete genome).
LUX real-time PCR assay.
We used the following protocol for both assays. Four microliters of cDNA from the RT-PCR was added to a reaction mixture consisting of 10 μl Platinum quantitative PCR SuperMix-UDG (Invitrogen, Carlsbad, CA), 0.04 μl ROX reference dye (Invitrogen, Carlsbad, CA), 0.4 μl LUX primer (10 pmol μl−1) (Table 1), 0.4 μl unlabeled primer (10 pmol μl−1) (Table 1), and 5.16 μl RNase-free water to a total volume of 20 μl. The real-time PCRs were performed on a 7500 Fast real-time PCR system (Applied Biosystems, Foster City, CA). An initial uracil DNA glycosylase contamination protection step at 50°C for 2 min was followed by 2 min at 95°C and then 40 cycles of 15 s at 95°C, 30 s at 55°C, and 30 s at 72°C. Melting curve analysis was performed immediately after PCR completion by heating at 95°C for 15s, followed by cooling to 60°C for 1 min and subsequent heating to 95°C at 1.5°C 0.8/min−1 with continuous fluorescence recording. Melting temperatures were determined for all samples using the Sequence Detection software version 1.3.1 (Applied Biosystems, Foster City, CA) and visualized by plotting the negative derivatives against temperature.
Multiplex LUX real-time PCR using melting curve analysis.
We developed a duplex real-time PCR assay using the primers for NV GI and NV GII detection on clinical specimens (8 NV GI, 13 NV GII, 9 negative specimens, and 1 specimen with a mix of NV GI and NV GII). The protocol was as described above with the addition of equal amounts of primers and the removal of a corresponding volume of water.
Validation of LUX real-time PCR assays.
We compared our LUX real-time PCR to the TaqMan-based real-time PCR method described by Kageyama et al. (11), which was used with minor modifications at Ryhov County Hospital in Jönköping for routine analysis of NV. The Swedish Institute for Infectious Disease Control has reported that this TaqMan based real-time PCR had 100% correct results with specimens sent for external quality assurance. Furthermore, we compared our LUX real-time PCR to a PCR-based method using degenerate primers described by Zintz et al. (24). For the Nicaraguan specimens, the LUX real-time PCR was also compared to a commercial enzyme-linked immunosorbent assay (ELISA) kit, IDEIA Norovirus (reference no. K6044) from DakoCytomation, Copenhagen, Denmark. Moreover, the assays were investigated for cross-reactivity using specimens positive for rotavirus, sapovirus, astrovirus, adenovirus, and feline calicivirus.
LUX real-time PCR properties and quantification of virus concentrations.
Using a serial dilution of reference DNA, we determined the detection limits, efficiency, and dynamic range of the real-time PCR assay. We estimated the number of viruses in the starting material, measured as genome equivalents based on the assumption of a 100% yield. At Ryhov Hospital, a different protocol for RNA extraction from fecal specimens was used; hence, we show only genes per PCR in the tables concerning the Swedish specimens.
Nucleotide sequencing.
Nucleotide sequencing was performed by Macrogen Inc. (Seoul, Korea) on the reference DNAs for NV GI and NV GII in order to verify them. The sequencing reaction was based on BigDye chemistry, using M13 forward and reverse primers as sequencing primers.
Statistical analysis.
Statistical analysis of variance of melting temperature and standard curve means was performed using the t test with the SPSS 14.0 package (SPSS Inc., Chicago, IL).
RESULTS
Design and evaluation of PCR primers.
A GenBank search, followed by alignment using ClustalW software, revealed a highly conserved region in the ORF1-ORF2 junction for both NV GI and NV GII (data not shown). We designed LUX primers targeting these regions and designed all primers with an annealing temperature of ∼55°C. All primers designed were able to detect clinical specimens containing NV GI (n = 7) or NV GII (n = 15) using conventional PCR as determined by analyzing PCR products by agarose gel electrophoresis (data not shown). Finally, one primer pair for each group of virus was selected to be used in the LUX real-time PCR assays (Table 1).
LUX real-time PCR properties.
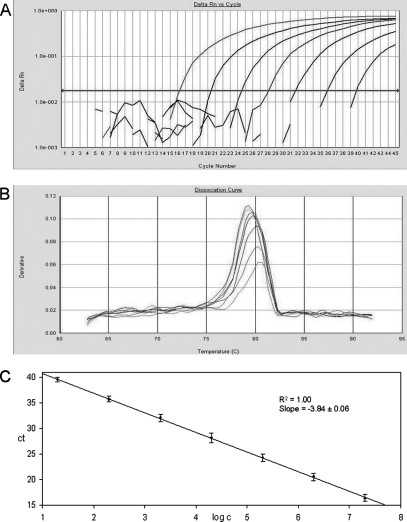
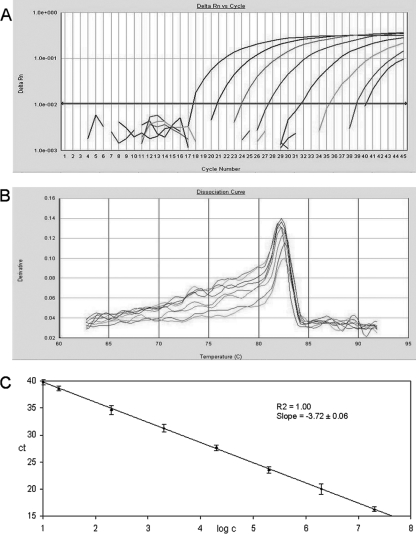
The fluorescence signals from the reference DNAs for NV GI and NV GII and their corresponding standard curves and melting curves are shown in Fig. 1 and 2. The lower quantification limit, estimated by using a dilution series of reference DNA, was 10 gene copies per PCR for the NV GII assay and 20 gene copies per PCR for the NV GI assay. The assays were able to detect ≤10 gene copies in 47 out of 50 and 22 out of 27 reference samples investigated for NV GII and NV GI, respectively. At fewer than 10 copies, however, reliable quantification was not possible due to lower linearity of the standard curve. Ten gene copies per PCR is equivalent to ∼20,000 genes per gram of stool. The upper quantification limit was estimated to ∼3.8 × 1010 gene equivalents per gram of stool for both assays (2 × 107 gene copies per PCR). Cross-reactivity between the NV GI and NV GII LUX real-time PCR assays was not observed using the reference DNA. All assays were further evaluated with clinical specimens positive for NV GI, NV GII, rotavirus, sapovirus, adenovirus, astrovirus, and feline calicivirus, and no cross-reactivity was observed (data not shown).
FIG. 1.
Detection and quantification of NV genogroup I using the LUX real-time PCR assay. The plasmid standards were diluted in 1:10 steps from 2 × 107 to 2 × 101. (A) Amplification plot of fluorescence intensities (ΔRn) versus the PCR cycle numbers. (B) First-derivative dissociation curves with melting temperatures of the amplicons. (C) Relationship of known numbers of plasmid standards to the threshold cycle (CT), with standard derivations calculated from three independent real-time PCR experiments.
FIG. 2.
Detection and quantification of NV genogroup II using the LUX real-time PCR assay. The plasmid standards were diluted in 1:10 steps from 2 × 107 to 2 × 101, followed by a dilution step of 1:2 to 1 × 101. (A) Amplification plot of fluorescence intensities (ΔRn) versus the PCR cycle numbers. (B) First-derivative dissociation curves with melting temperatures of the amplicons. (C) Relationship of known numbers of plasmid standards to the threshold cycle (CT), with standard deviations calculated from three independent real-time PCR experiments.
Detection and quantification of NV in clinical specimens.
We found a 100% correlation between our LUX-based method and the TaqMan-based method with the Swedish specimens (n = 61). Both these assays detected the same 18 positive specimens, 15 NV GII and 3 NV GI (Tables 2 and 3). The PCR-based method described by Zintz et al. (24) detected 15 of 18 positive specimens, and 46 specimens were detected as negative. For the Nicaraguan specimens, the LUX real-time PCR assay identified NV in 29 of 42 specimens (22 NV GII, 6 NV GI, and 1 mixed infection containing NV GII and NV GI) (Tables 3 and 4). Our results corresponded to those of the TaqMan assay for all but one specimen, which was positive in the TaqMan assay and negative in the LUX assay and also the other methods. The conventional PCR method detected a total of 25 positive specimens, and the ELISA detected 24. However, one sample detected by the conventional PCR and not detected by the other methods was demonstrated to be a sapovirus-positive specimen by using sapovirus specific primers SR80 and JV33 (22) (data not shown). Of the reference panel consisting of 15 stool specimens, the LUX real-time PCR detected all 11 positive and genotyped specimens.
TABLE 2.
Detection and quantification of NV GI and NV GII in LUX real-time PCR assays on 61 clinical specimens from Sweden
| Samplea | LUX real-time PCR resultb
|
Conventional PCR resultc | TaqMan real-time PCR resultd | |
|---|---|---|---|---|
| Group | Quantification (genes/PCR−1) | |||
| 1 | GI | 3E+03 | ++ | GI |
| 2 | GI | 8E+02 | − | GI |
| 3 | GII | 2E+03 | +++ | GII |
| 4 | GII | 4E+03 | + | GII |
| 5 | GII | 1E+05 | +++ | GII |
| 6 | GI | 3E+02 | + | GI |
| 7 | GII | 2E+04 | − | GII |
| 8 | GII | 2E+06 | +++ | GII |
| 9 | GII | 5E+03 | ++ | GII |
| 10 | GII | 6E+03 | ++ | GII |
| 11 | GII | 3E+01 | − | GII |
| 12 | GII | 4E+03 | ++ | GII |
| 13 | GII | 4E+04 | +++ | GII |
| 14 | GII | 1E+05 | +++ | GII |
| 15 | GII | 8E+02 | ++ | GII |
| 16 | GII | 4E+02 | + | GII |
| 17 | GII | 1E+05 | +++ | GII |
| 18 | GII | 8E+05 | +++ | GII |
TABLE 3.
Summary of norovirus detection in clinical stool specimens from Sweden and Nicaragua using different methods
| Location, assay, and specimen type (n) | No. of specimens detected by LUX real-time PCR for norovirus
|
||
|---|---|---|---|
| Positive
|
Negative | ||
| GI | GII | ||
| Sweden (61) | |||
| TaqMan real-time PCR assaya | |||
| Positive (18) | |||
| GI | 3 | 0 | 0 |
| GII | 0 | 15 | 0 |
| Negative (43) | 0 | 0 | 43 |
| Conventional PCRb | |||
| Positive (15) | 2 | 13 | 0 |
| Negative (46) | 1 | 2 | 43 |
| Nicaragua (42) | |||
| TaqMan real-time PCR assaya | |||
| Positive (30) | |||
| GI | 7d | 0 | 0 |
| GII | 0 | 23d | 1 |
| Negative (12) | 0 | 0 | 12 |
| Conventional PCRb | |||
| Positive (25) | 6d | 19d | 1e |
| Negative (17) | 1 | 4 | 12 |
| ELISAc | |||
| Positive (24) | 6a | 19d | 0 |
| Negative (18) | 1 | 4 | 13 |
TABLE 4.
Detection and quantification of norovirus GI and GII in LUX real-time PCR assays on 42 clinical specimens from Nicaragua
| Samplea | LUX real-time PCR resultb
|
Conventional PCR resultc | TaqMan real-time PCR resultd | ELISA resulte | |
|---|---|---|---|---|---|
| Group | Quantification (genes/g feces−1) | ||||
| 19 | GII | 7E+08 | + | GII | 0.323 |
| 20 | GII | 3E+06 | +++ | GII | 0.959 |
| 21 | GII | 3E+06 | + | GII | 0.229 |
| 22 | GII | 7E+07 | +++ | GII | 0.915 |
| 23 | GII | 2E+06 | + | GII | 0.567 |
| 24 | GII | 2E+07 | + | GII | |
| 25 | − | GII | |||
| 26 | GII | 3E+05 | − | GII | 0.834 |
| 27 | GII | 5E+06 | + | GII | 0.718 |
| 28 | GII | 1E+07 | + | GII | 1.086 |
| 29 | GII | 7E+04 | − | GII | |
| 30 | GII | 2E+06 | + | GII | 0.577 |
| 31 | GII | 1E+05 | − | GII | |
| 32 | GII | 6E+06 | + | GII | 0.322 |
| 33 | GI | 6E+07 | ++ | GI | 0.848 |
| 34 | GI | 3E+08 | +++ | GI | 0.571 |
| 35 | GII | 3E+08 | + | GII | 0.384 |
| 36 | GII | 4E+08 | + | GII | 0.563 |
| 37 | GII | 7E+06 | − | GII | |
| 38 | GII | 3E+09 | +++ | GII | 0.923 |
| 39 | GI | 1E+08 | ++ | GI | 1.304 |
| 40 | GI | 9E+07 | +++ | GI | 0.614 |
| 41 | GII | 2E+08 | ++ | GII | 0.232 |
| 42 | GII | 3E+08 | + | GII | 0.236 |
| 43 | GII | 4E+07 | + | GII | 0.769 |
| 44 | GI | 2E+07 | +++ | GI | 0.771 |
| 45 | GI+GII | 9E+04; 4E+09 | +++ | GI+GII | 1.531 |
| 46 | GII | 7E+08 | +++ | GII | 2.000 |
| 47 | GI | 5E+06 | − | GI | |
| 48 | ++ | ||||
| 49 | GII | 1E+09 | +++ | GII | 0.691 |
Only specimens that were positive in at least one of the methods are listed.
From this study.
From reference 24. The band intensities of the PCR products of expected size visualized on agarose gels are indicated as high (+++), medium (++), or low (+).
Modified from reference 11.
DAKO 6044. The absorbance levels of the ELISA are indicated; the cutoff value is 0.150.
Multiplex real-time PCR for detection and differentiation of NV GI and NV GII.
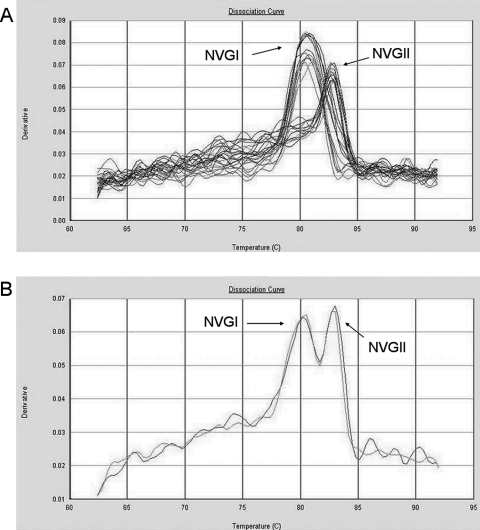
For each PCR product a specific melting temperature interval was determined. The melting temperature range for NV GI amplicons (n = 16) was 78.9 to 81.6 (99% confidence interval, 79.8 to 80.7), and for NV GII amplicons (n = 38) it was 81.9 to 83.8 (99% confidence interval, 82.8 to 83.2). We were able to simultaneously detect and distinguish between NV GI- and NV GII-positive specimens (Fig. 3A) and mixed infections (Fig. 3B), using a duplex assay containing primers for both NV GI and NV GII.
FIG. 3.
First-derivative dissociation curves. (A) Eight NV GI-positive samples, 10 NV GII-positive samples and 8 negative samples run in triplicates in a duplex real-time PCR assay consisting of both NV GI- and NV GII-specific primer pairs. (B) A patient sample with a mixed infection with NV GI and NV GII, run in duplicate in a duplex real-time PCR assay with both NV GI and NV GII primer pairs.
DISCUSSION
We have established a novel, sensitive, and specific LUX real-time PCR assay, which is able to broadly detect and quantify NV GI and GII. Using specimens from both Sweden and Nicaragua, we have shown that the assays can be applied successfully to specimens from different geographic regions. By the use of melting curve analysis, we not only can ensure the specificity but we also can distinguish between the two types of viruses, due to the differences in melting temperatures in multiplex real-time PCRs.
We observed a 99% correlation between the NV LUX real-time PCR assay and the TaqMan real-time PCR assay. One specimen was detected only with the TaqMan real-time PCR. This is possibly due to low quality of RNA because of long storage in the freezer before analysis with the LUX real-time PCR. Also, this sample has not been confirmed with any other method, so the possibility of a false positive cannot be excluded. Furthermore, the NV LUX real-time PCR was also examined against coded specimens containing various genogroups and genotypes and proved to be 100% correct. One reason why the conventional PCR method failed to detect some specimens may be that the concentrations of viruses were too low. Another reason may be that the sites targeted with the conventional PCR primers are less conserved, since the concentrations of virus in some of the undetected specimens were high (Tables 2 and 4). The primers used in the method of Zintz et al. (24) target the RNA polymerase gene, which has been shown to be less conserved than the ORF1-ORF2 junction targeted with our assays (11, 23), and the primers also cross-react with sapovirus, producing amplicons of very similar size (24).
The two LUX real-time PCR assays were shown to be more sensitive than the immunological assays and conventional PCR methods used in this study (Table 3). The short amplicons in the real-time PCR assays used in this study will likely result in more efficient amplification and higher sensitivity.
Our two assays can be used for quantification of the viral load in clinical specimens. The lowest quantification limits were estimated to be ∼20,000 (NV GII) and ∼40,000 (NV GI) gene equivalents per gram of stool, and the two assays demonstrate a broad dynamic range of >107 genome equivalents with a r2 value of 1.00. Using known copy numbers of reference DNA, we could detect down to ∼3 genome equivalents per PCR (∼6,000 gene equivalents per gram of stool), but this low concentration results in lower r2 values. Kageyama et al. (11) report a detection limit of ∼20,000 gene equivalents per gram of stool for NV GI and NV GII, using plasmid standards, and Pang et al. (18) report a detection limit of 25,000 copies per gram of stool for NV GII and 2.5 × 107 copies per gram of stool for NV GI with RNA transcripts.
Moreover, the assays developed in this study can be used in duplex reactions for simultaneous detection and differentiation of the two genogroups. The amplicons show a nonoverlapping interval of melting temperatures, and it is therefore possible to distinguish the genogroups from each other. The conserved nature of this part of the NV genome makes it plausible to assume that the melting temperature of different amplicons will not differ much from the intervals described. We have confirmed this by doing an in silico analysis of the melting temperatures of amplicons from different genotypes and variants using sequence data found in GenBank (data not shown). We have demonstrated that a duplex system, consisting of primers targeting NV GI and NV GII, can correctly detect and distinguish between NV GI and NV GII infections (Fig. 3A). This duplex system was also used to detect mixed NV genogroup infections in a patient (Fig. 3B). A multiplex system with several LUX primer pairs, without loss of efficiency, has also been described elsewhere (15). Richards et al., (19) used a similar approach; the degenerate primers Mon432 and Mon434 for detection of NV GI and primers Mon431 and Mon434 for detection of NV GII were combined in one reaction in a SYBR green-based real-time RT-PCR assay. The resulting melting curves could not, however, distinguish between the two genogroups. To our knowledge, this is the first real-time PCR assay described that is able to simultaneously detect and differentiate between NV GI- and NV GII-positive specimens based on melting curve analysis. None of the simplex or duplex assays have yielded unspecific fluorescent signals or unspecific melting curves; primer-dimer formation occurred rarely, in those cases yielding an easily distinguishable melting curve with a peak at ∼74°C.
The LUX system can be used on most real-time PCR platforms, and it is simple and cost-effective, since it does not use probes or various fluorophores. There is no need for post-PCR processing, which reduces the time and possibility of contamination. Furthermore, the use of a random hexamer primer in the RT reaction allows a single protocol for the analysis of multiple enteric viruses, which may be differentiated from each other using melting curve analysis.
Acknowledgments
This study was supported by the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (grant 245-2004-1821) and by the Swedish Research Council (grant 10392).
We thank Felix Espinoza, Margarita Panagua, and Andreas Matussek for kindly providing patient material. Thanks also to Stefan B̄orjesson for fruitful discussions.
Footnotes
Published ahead of print on 24 October 2007.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215403-410. [DOI] [PubMed] [Google Scholar]
- 2.Atmar, R. L., and M. K. Estes. 2001. Diagnosis of noncultivatable gastroenteritis viruses, the human caliciviruses. Clin. Microbiol. Rev. 1415-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fankhauser, R. L., S. S. Monroe, J. S. Noel, C. D. Humphrey, J. S. Bresee, U. D. Parashar, T. Ando, and R. I. Glass. 2002. Epidemiologic and molecular trends of “Norwalk-like viruses” associated with outbreaks of gastroenteritis in the United States. J. Infect. Dis. 1861-7. [DOI] [PubMed] [Google Scholar]
- 4.Glass, R. I., J. Noel, T. Ando, R. Fankhauser, G. Belliot, A. Mounts, U. D. Parashar, J. S. Bresee, and S. S. Monroe. 2000. The epidemiology of enteric caliciviruses from humans: a reassessment using new diagnostics. J. Infect. Dis. 181(Suppl. 2)S254-S261. [DOI] [PubMed] [Google Scholar]
- 5.Green, J., C. I. Gallimore, J. P. Norcott, D. Lewis, and D. W. Brown. 1995. Broadly reactive reverse transcriptase polymerase chain reaction for the diagnosis of SRSV-associated gastroenteritis. J. Med. Virol. 47392-398. [DOI] [PubMed] [Google Scholar]
- 6.Hedlund, K. O., E. Rubilar-Abreu, and L. Svensson. 2000. Epidemiology of calicivirus infections in Sweden, 1994-1998. J. Infect. Dis. 181(Suppl. 2)S275-S280. [DOI] [PubMed] [Google Scholar]
- 7.Hohne, M., and E. Schreier. 2004. Detection and characterization of norovirus outbreaks in Germany: application of a one-tube RT-PCR using a fluorogenic real-time detection system. J. Med. Virol. 72312-319. [DOI] [PubMed] [Google Scholar]
- 8.Inouye, S., K. Yamashita, S. Yamadera, M. Yoshikawa, N. Kato, and N. Okabe. 2000. Surveillance of viral gastroenteritis in Japan: pediatric cases and outbreak incidents. J. Infect. Dis. 181(Suppl. 2)S270-S274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang, X., P. W. Huang, W. M. Zhong, T. Farkas, D. W. Cubitt, and D. O. Matson. 1999. Design and evaluation of a primer pair that detects both Norwalk- and Sapporo-like caliciviruses by RT-PCR. J. Virol. Methods 83145-154. [DOI] [PubMed] [Google Scholar]
- 10.Jothikumar, N., J. A. Lowther, K. Henshilwood, D. N. Lees, V. R. Hill, and J. Vinje. 2005. Rapid and sensitive detection of noroviruses by using TaqMan-based one-step reverse transcription-PCR assays and application to naturally contaminated shellfish samples. Appl. Environ. Microbiol. 711870-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kageyama, T., S. Kojima, M. Shinohara, K. Uchida, S. Fukushi, F. B. Hoshino, N. Takeda, and K. Katayama. 2003. Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J. Clin. Microbiol. 411548-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kageyama, T., M. Shinohara, K. Uchida, S. Fukushi, F. B. Hoshino, S. Kojima, R. Takai, T. Oka, N. Takeda, and K. Katayama. 2004. Coexistence of multiple genotypes, including newly identified genotypes, in outbreaks of gastroenteritis due to norovirus in Japan. J. Clin. Microbiol. 422988-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katayama, K., H. Shirato-Horikoshi, S. Kojima, T. Kageyama, T. Oka, F. Hoshino, S. Fukushi, M. Shinohara, K. Uchida, Y. Suzuki, T. Gojobori, and N. Takeda. 2002. Phylogenetic analysis of the complete genome of 18 Norwalk-like viruses. Virology 299225-239. [DOI] [PubMed] [Google Scholar]
- 14.Lopman, B. A., M. H. Reacher, Y. Van Duijnhoven, F. X. Hanon, D. Brown, and M. Koopmans. 2003. Viral gastroenteritis outbreaks in Europe, 1995-2000. Emerg. Infect. Dis. 990-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowe, B., H. A. Avila, F. R. Bloom, M. Gleeson, and W. Kusser. 2003. Quantitation of gene expression in neural precursors by reverse-transcription polymerase chain reaction using self-quenched, fluorogenic primers. Anal. Biochem. 31595-105. [DOI] [PubMed] [Google Scholar]
- 16.Nazarenko, I., B. Lowe, M. Darfler, P. Ikonomi, D. Schuster, and A. Rashtchian. 2002. Multiplex quantitative PCR using self-quenched primers labeled with a single fluorophore. Nucleic Acids Res. 30e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noel, J. S., T. Ando, J. P. Leite, K. Y. Green, K. E. Dingle, M. K. Estes, Y. Seto, S. S. Monroe, and R. I. Glass. 1997. Correlation of patient immune responses with genetically characterized small round-structured viruses involved in outbreaks of nonbacterial acute gastroenteritis in the United States, 1990 to 1995. J. Med. Virol. 53372-383. [DOI] [PubMed] [Google Scholar]
- 18.Pang, X., B. Lee, L. Chui, J. K. Preiksaitis, and S. S. Monroe. 2004. Evaluation and validation of real-time reverse transcription-PCR assay using the LightCycler system for detection and quantitation of norovirus. J. Clin. Microbiol. 424679-4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richards, G. P., M. A. Watson, R. L. Fankhauser, and S. S. Monroe. 2004. Genogroup I and II noroviruses detected in stool samples by real-time reverse transcription-PCR using highly degenerate universal primers. Appl. Environ. Microbiol. 707179-7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trujillo, A. A., K. A. McCaustland, D. P. Zheng, L. A. Hadley, G. Vaughn, S. M. Adams, T. Ando, R. I. Glass, and S. S. Monroe. 2006. Use of TaqMan real-time reverse transcription-PCR for rapid detection, quantification, and typing of norovirus. J. Clin. Microbiol. 441405-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vainio, K., and M. Myrmel. 2006. Molecular epidemiology of norovirus outbreaks in Norway during 2000 to 2005 and comparison of four norovirus real-time reverse transcriptase PCR assays. J. Clin. Microbiol. 443695-3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vinje, J., H. Deijl, R. van der Heide, D. Lewis, K. O. Hedlund, L. Svensson, and M. P. Koopmans. 2000. Molecular detection and epidemiology of Sapporo-like viruses. J. Clin. Microbiol. 38530-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng, D. P., T. Ando, R. L. Fankhauser, R. S. Beard, R. I. Glass, and S. S. Monroe. 2006. Norovirus classification and proposed strain nomenclature. Virology 346312-323. [DOI] [PubMed] [Google Scholar]
- 24.Zintz, C., K. Bok, E. Parada, M. Barnes-Eley, T. Berke, M. A. Staat, P. Azimi, X. Jiang, and D. O. Matson. 2005. Prevalence and genetic characterization of caliciviruses among children hospitalized for acute gastroenteritis in the United States. Infect. Genet. Evol. 5281-290. [DOI] [PubMed] [Google Scholar]