Abstract
Nocardia cyriacigeorgica is recognized as an emerging pathogen in many parts of the world. We present the first case description of invasive N. cyriacigeorgica pulmonary infection in the United States identified to the species level by 16S rRNA and hsp65 sequence analysis. A subsequent retrospective molecular screening of recent Nocardia clinical isolates at our New York City medical center yielded an additional six N. cyriacigeorgica isolates. Because routine laboratory algorithms for the phenotypic identification of Nocardia species are limited in practice, the true prevalence of N. cyriacigeorgica infections may be greater than currently appreciated. Indeed, we present evidence confirming that N. cyriacigeorgica is coincident with the unofficial species designation Nocardia asteroides complex antimicrobial susceptibility pattern type VI and distinct from the N. asteroides sensu stricto strain ATCC 19247T. As nocardial species identity can predict antimicrobial susceptibility and guide clinical management, we offer simplified phenotypic and molecular protocols to assist the identification of N. cyriacigeorgica.
Nocardiae are aerobic actinomycetes ubiquitously found in soil and aquatic habitats. Nocardia are beaded, branching gram-positive rods that are partially acid-fast and are generally slow growing (5). Approximately 50 Nocardia species have been described to date, about 30 of which are known to cause human disease (5). Infections due to Nocardia spp. are generally acquired through inhalation or percutaneous inoculation from environmental sources. Nosocomial transmission has also been reported (12, 16, 36). The individuals mainly affected are immunocompromised (5, 21), although cutaneous infections may also be seen in immunocompetent hosts (5). Mortality appears to correlate with the causative species and the site of infection and can be as high as 50% in patients with disseminated disease (15, 21, 30).
Laboratory identification of nocardiae and their differentiation from nontuberculous mycobacteria can be challenging and time-consuming using routine culture and chemotaxonomic methods. Indeed, most Nocardia isolates fail to react in many of the standard biochemical reactions utilized in clinical microbiology laboratories, thus oftentimes rendering it extremely difficult to make a precise species-level determination. To address this problem, Wallace et al. reported on species-indicative differences in antimicrobial susceptibility patterns that could be used to segregate many of the strains previously identified as Nocardia asteroides or as a species of the former N. asteroides complex (34). Included in these six designations are the Nocardia nova complex (type II), the highly antimicrobial-resistant Nocardia transvalensis complex (type IV), and Nocardia farcinica (type V), as well as the unnamed species with drug pattern type VI (reviewed in reference 37). Unfortunately, this methodology based on antimicrobial susceptibility patterns is not well suited for the routine clinical microbiology laboratory as it is technically demanding, time-consuming, labor-intensive, and slow to produce results, and it does not always provide a definitive identification because patterns can be shared between species (34). Notably, the N. asteroides sensu stricto species (represented by strain ATCC 19247T) has not been isolated clinically, nor is it included in any drug pattern group (5). More recently, Nocardia species identification and taxonomy have been improved by molecular methods such as 16S rRNA and hsp65 gene sequence analysis (5). In general, these procedures are rapid, accurate, and reproducible and can better discriminate among strains of actinomycetes than is possible with phenotypic methods (7). However, there have been few reports on the application of these methodologies in clinical microbiology laboratories for the diagnosis of Nocardia infections (4, 10, 23, 31).
Recent reviews of collections of Nocardia clinical isolates by genotypic methods have found that a significant proportion, originally identified by routine phenotypic and chemotaxonomic methods, belong to a new species, Nocardia cyriacigeorgica (18, 27, 29, 35). This species was first described in 2001 (38), and strains of N. cyriacigeorgica have since been recovered as the etiologic agent of human infection in Western Europe, Greece, Turkey, Japan, Thailand, and Canada (1, 3, 6, 7, 11, 17, 18, 22, 27, 35, 39). Most cases of infection have occurred in the context of human immunodeficiency virus-related or iatrogenic immune suppression. However, only a few complete clinical histories of N. cyriacigeorgica infection have been published to date (1, 3, 11, 22).
At present, N. cyriacigeorgica per se has not been described as a cause of infection in the United States. However, a growing consensus of opinion holds that the species N. cyriacigeorgica is coincident with strains previously classified as having a type VI drug pattern (5, 11). In addition to sulfonamide susceptibility, type VI strains are generally susceptible to broad-spectrum cephalosporins, amikacin, imipenem, and linezolid but resistant to penicillins, clarithromycin, and ciprofloxacin. The type VI antibiogram is similar to that of most reported N. cyriacigeorgica strains with the exception of a few strains of N. cyriacigeorgica that have been reported to be susceptible to ciprofloxacin (3, 39). N. cyriacigeorgica and type VI strains also share identical partial 16S rRNA gene sequences (5). Most importantly, type VI strains appear to account for up to 35% of Nocardia strains recovered from patients in the southern United States (34). The species N. cyriacigeorgica may therefore be a significant unrecognized cause of disease in the United States.
In this study, we report on the application of molecular methods to identify N. cyriacigeorgica as the causative agent of a case of atypical pneumonia in a heart transplant recipient. We also describe the recent prevalence and basic clinical histories of infection by this species at a New York City medical center. We make note of N. cyriacigeorgica intraspecies genotypic and phenotypic variability as well as provide key characteristics to guide N. cyriacigeorgica species-level identification and differentiation from other Nocardia species. Evidence is provided to support the recognition of type VI strains in the United States as N. cyriacigeorgica and the distinction of N. cyriacigeorgica from the representative laboratory strain of N. asteroides (ATCC 19247T).
The case.
The index patient was a diabetic 55-year-old Caucasian male heart transplant recipient who presented with a several-day history of fatigue, productive cough, fever (38.3°C), and abdominal pain. At the time of presentation, the patient's immunosuppressive regimen to prevent transplant rejection consisted of cyclosporine, mycophenolate-mofetil, and prednisone. The initial clinical laboratory work-up demonstrated leukocytosis with neutrophilia, lymphopenia, an accelerated erythrocyte sedimentation rate, and an elevated lactate dehydrogenase level (822 U/liter). Imaging studies revealed nodular opacities in the right upper and lower lobes and a dense consolidation in the left lower lobe (Fig. 1A and B). Antimicrobial prophylaxis at admission consisted of atovaquone, valganciclovir, and nystatin mouthwash. Sputum and blood cultures, as well as serum cryptococcal and Aspergillus galactomannan antigen tests, were negative. The differential diagnosis included atypical/fungal pneumonia and posttransplant lymphoproliferative disorder. Empirical antimicrobial therapy with ceftriaxone, azithromycin, and voriconazole was initiated. Over the following week the patient displayed increasingly productive cough, intermittent fever (up to 40°C), and persistence of radiological findings.
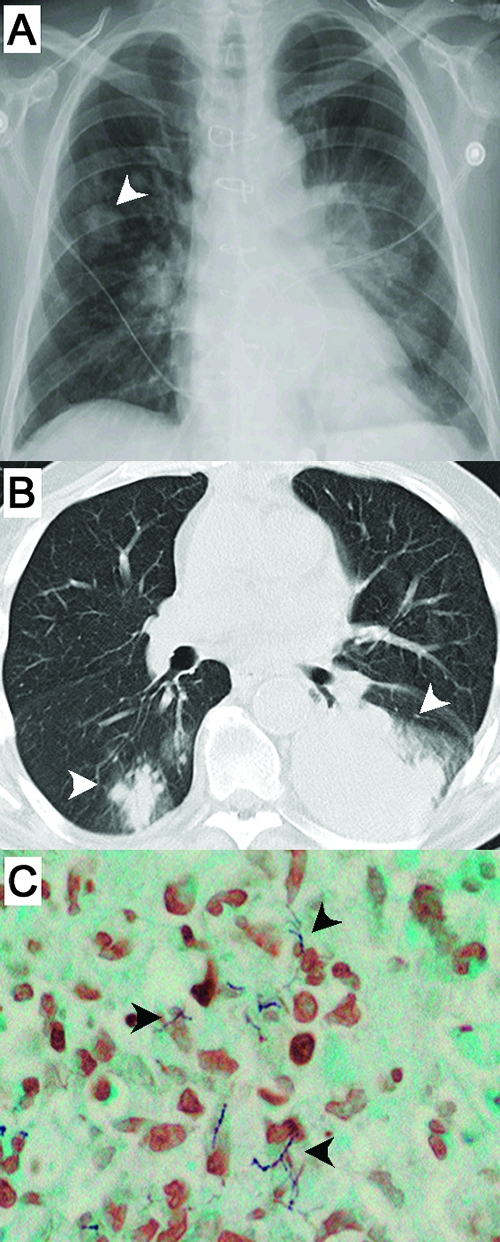
FIG. 1.
Radiological and histopathological findings from the index patient. Posterior-anterior chest X ray (A) and chest computerized tomography (B) show nodular opacities in the right lower lobe and dense consolidation in the left lower lobe (arrowheads) in addition to other small irregular opacities in the right upper lobe and elsewhere. The chest computerized tomography was performed 6 days and the chest X ray 2 days before the first positive sputum specimen was obtained. (C) A computerized tomography-guided biopsy from the left lung obtained 4 days later showed gram-positive, branching, beaded filaments and an acute inflammatory infiltrate (original magnification, ×400).
From day 9 of admission, branching gram-positive, beaded, partially acid-fast filaments were identified by direct microscopic analysis of sputum, bronchoalveolar lavage, and lung biopsy samples. Histopathological examination showed necrotizing inflammation and similar gram-positive, beaded microorganisms with branching filaments (Fig. 1C). Heavy growth of a chalky white microorganism was apparent following 1 day of incubation on several solid growth media (eight separate patient specimens were culture positive). Small white colonies were clearly visible after 2 days of incubation following subculture. Routine phenotypic test results were characteristic of N. asteroides complex (partially acid-fast, resistant to lysozyme, and negative for the decomposition of casein, tyrosine, and xanthine, as well as negative for starch utilization), and concurrent 16S rRNA and hsp65 gene sequencing identified the bacterium as N. cyriacigeorgica. From these results the patient's antimicrobial regimen was adjusted to trimethoprim-sulfamethoxazole (TMP-SMX) and imipenem-cilastatin. Specific antimicrobial therapy resulted in the rapid alleviation of symptoms and improvement of radiological findings. Antimicrobial susceptibility testing on a representative isolate showed an N. asteroides drug type VI pattern (34): susceptible to amikacin, cefotaxime, ceftriaxone, gentamicin, imipenem, linezolid, meropenem, minocycline, SMX, sulfisoxazole, and tobramycin but resistant to augmentin, ciprofloxacin, clarithromycin, gatifloxacin, and kanamycin. The patient was later discharged, receiving intravenous ceftriaxone and vancomycin for 2 weeks. Oral TMP-SMX was administered for an additional 6 months. The patient recovered and remained relapse-free after 15 months. This case presentation is consistent with previous reports noting the heightened risk of serious nocardial infections in transplant patients receiving immunosuppressive therapy (14, 21, 26).
MATERIALS AND METHODS
Nocardial phenotype-based identification.
Initial specimen handling and the identification of microorganisms as members of the genus Nocardia followed standard laboratory protocols (20, 37). Additional biochemical testing (metabolism of acetamide, glucose, esculin, maltose, rhamnose, trehalose, and urea [Becton Dickinson, Sparks, MD]; arylsulfatase activity [Remel, Lenexa, KS]; pyrrolidonyl arylamidase [PYR], and α-glucosidase activity [API Coryne system; bioMérieux, France]) was performed as per the manufacturers' recommendations, with the exception that tests requiring an overnight incubation were read after 48 to 72 h to compensate for the slower growth rate of Nocardia. Suspensions for inoculation of the API Coryne strips were vortexed with silicate beads to achieve homogeneous mixtures. Testing for growth at 45°C after 3 days was performed on Sabouraud (SAB) dextrose agar (37). Over the time period from 2000 to 2006, there were 37 patients diagnosed with nocardiosis at our institution. From these patients, 27 isolates were available for retrospective analysis.
Antimicrobial susceptibility and synergy testing.
For a subset of the isolates, initial antimicrobial susceptibility testing was done by disk diffusion and/or broth microdilution methodologies at the Mycobacterial/Nocardia Research Laboratory, University of Texas Health Center. Additional antimicrobial susceptibility and synergy testing on 15-cm Mueller-Hinton agar plates was performed as described previously (2). Briefly, disks containing amikacin (30 μg), imipenem (10 μg), and meropenem (10 μg) (Becton Dickinson) were placed at an adequate distance apart and incubated for 10 days. For synergy testing, discs were spaced 3.5 cm apart. Zones of inhibition were recorded at 24-h intervals for 4 days. Etests (AB Biodisk, Sweden) for the carbapenems were read at 24-h intervals for 4 days.
16S rRNA and hsp65 PCR and sequence analysis.
Samples from pure subcultures of various nocardial strains were thermolysed (80°C for 30 min), and these served as the source of DNA template for PCR. The nearly complete length of the 16S rRNA gene (∼1,512 bp) of each test isolate was targeted for PCR amplification using the following universal bacterial primers: 16SF, 5′-AGAGTTTGATCMTGGCTCAG-3′ (Escherichia coli positions 8 to 27 [GenBank nucleotide accession number J01859]); 16SR, 5′-TAAGGAGGTGATCCARCCGCA-3′ (E. coli positions 1541 to 1522). Each PCR was prepared with 25 μl of PCR Master Mix (Roche Diagnostics, Indianapolis, IN), 18 μl of water, 2.5 μl of dimethyl sulfoxide, 1 μl of each primer at 20 μM, and 2.5 μl of thermolysate. Each PCR amplification was performed in an MJ Research PTC-200 Peltier Thermal Cycler (Bio-Rad, Hercules, CA) using the following program: an initial denaturation step of 5 min at 94°C followed by 45 cycles of 1 min at 94°C, 4 min at 60°C, and 1 min at 72°C, ending with a final elongation step for 10 min at 72°C. PCR products and a 100-bp DNA ladder (Invitrogen, Carlsbad, CA) were visualized by 2% agarose gel electrophoresis and ethidium bromide staining. Images were captured using a Gel Doc XR (Bio-Rad) digital image capture system and Quality One 1-D Analysis software, version 4.6.0 (Bio-Rad). The hsp65 gene (441 bp) was amplified in the same manner using primers hsp65F (5′-ACCAACGAYGGTGTBTCCAT-3′) and hsp65R (5′-CTTGTCGAASCGCATRCCCT-3′). These primers were adapted from oligonucleotides previously described for the amplification of hsp65 in mycobacteria (32).
For 16S rRNA sequencing, additional universal bacterial primers that anneal to sites internal to the target 16S rRNA amplicon were utilized: 16SiF2, 5′-GTGCCAGCAGCCGCGGTAATAC-3′ (E. coli positions 514 to 535); 16SiF3, 5′-GGTTAAGTCCCGYAACGAGCG-3′ (E. coli positions 1087 to 1107); and 16SiR, 5′-GGACTACCAGGGTATCKTAAT-3′ (E. coli positions 805 to 786). The hsp65 amplification primers were used for sequencing. Direct sequencing of PCR fragments was performed at the Columbia University DNA Sequencing Lab (https://www.dnasequencing.hs.columbia.edu) using a BigDye Terminator kit (PE Applied Biosystems) and an ABI 3700 DNA sequencer. The Lasergene program (DNASTAR Inc., Madison, WI) was used to analyze the derived sequence data. By this protocol, complete coverage of the 16S rRNA and hsp65 amplicon sequences was acquired for each bacterial strain with one or more sections of overlap. Consensus 16S rRNA and hsp65 sequences for each strain were constructed based upon alignments of the sequence data and a careful examination of each electropherogram trace representation of the data. This strategy was necessary in order to reconcile any ambiguous bases or conflicting base assignments and to look for consistent double peaks, which would indicate the presence of one or more 16S rRNA alleles. To identify each microbe to the species level, sequencing data were queried against previously submitted sequences in the GenBank database (http://www.ncbi.nlm.nih.gov) using the BLASTN program. A similarity of >99.5% to the closest relative 16S rRNA sequences was used as the criterion for identification.
Nucleotide sequence accession numbers.
N. cyriacigeorgica sequences derived in this study were submitted to the GenBank under the accession numbers EF127493.1, EF127494.1, EF127495.1, EF127496.1, EF127497.1, EF127498.2, EF127499.1, and EF127500.1 for the 16S rRNA gene and EF127503.1, EF127504.1, EF127505.1, EF127506.1, EF127509.1, EF127510.1, EF127511.1, and EF127512.1 for hsp65.
ATCC accession numbers.
One to two strains of each genotype and sequence subtype variant were submitted to the ATCC as follows: genotype I strain 02-50508 (accession number ATCC BAA-1517), genotype II strains 00-12607 and 05-49102 (ATCC BAA-1519 and ATCC BAA-1516, respectively), and genotype III strain 06-51518 (ATCC BAA-1518).
RESULTS
16S rRNA and hsp65 gene sequence analysis of index case isolates.
A total of eight isolates were obtained from the index patient, and the respective 16S rRNA and hsp65 gene sequences from each were identical. An ambiguous nucleotide (C/T) was present at position 448 of the consensus 16S rRNA gene sequence of each strain (see Table 2). This indicates that N. cyriacigeorgica may possess more than one copy of this gene as it has been reported for other nocardiae (5, 8). Comparison of the 16S rRNA and hsp65 gene sequences from each isolate with GenBank deposits by BLASTN analysis revealed N. cyriacigeorgica strain DSM 44484T to be the best match for each sequence (>99.5%). Based upon these data, we believed the true identity of the infecting microorganism to be N. cyriacigeorgica. Isolate 06-51518 originated from a lung biopsy and was used as the index patient's representative strain.
TABLE 2.
SNPs in 16S rRNA and hsp65 genes of N. cyriacigeorgicaa
| Strain | Genotypeb | SNP at the indicated position
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 16S rRNA
|
hsp65
|
|||||||||||||||||
| 448 | 1427 | 1480 | 216 | 249 | 279 | 282 | 285 | 342 | 345 | 408 | 435 | 450 | 453 | 460 | 546 | 561 | ||
| ATCC 14759T | I | C | T | A | G | C | T | G | T | T | C | G | T | G | C | G | C | C |
| DSM 44484Tc | I | T | G | |||||||||||||||
| 02-50508 | I | G | ||||||||||||||||
| 00-12607 | II | C | G | C | C | G | A | C | C | G | G | |||||||
| 00-7841 | II | C | G | C | C | G | A | C | C | G | G | |||||||
| 03-23155 | II | C | G | C | C | G | A | C | C | G | G | |||||||
| 05-49102 | II | C | G | C | C | C | G | A | C | C | G | G | ||||||
| 03-70718 | III | G | C | G | Ce | G | ||||||||||||
| 06-51518d | III | Y | G | C | G | Ce | G | |||||||||||
Nucleotide position numbers for the 16S rRNA gene are according to E. coli (GenBank accession number J01859). Nucleotide position numbers for hsp65 are according to N. farcinica strain IFM 10152 (GenBank accession number NC_006361).
Genotype assignments are based on SNPs in 16S rRNA positions 1427 (29) and 1480.
DSM 44484T sequences from the GenBank database, accession numbers AF430027 (16S rRNA) and AY756522 (hsp65).
Isolate from the index patient.
Nonsynonymous SNP.
Molecular-based retrospective review of clinical isolates.
To estimate the prevalence of N. cyriacigeorgica infections at our institution, we screened a collection of 27 Nocardia patient isolates acquired over a 6-year period (2000 to 2006) by sequence analysis of the 16S rRNA and hsp65 genes. Overall, six strains were found to be N. cyriacigeorgica (26% of patients, including the index). These strains were originally identified either as Nocardia sp., N. asteroides complex, or N. asteroides complex drug pattern type VI (Table 1), as per the standard diagnostic work-up and accepted taxonomy at the time of isolation. Each was a confirmed pathogen by clinical chart review. The 16S rRNA and hsp65 sequences from these strains were, however, clearly distinct from the analogous sequences available in GenBank for the N. asteroides sensu stricto species (represented by the laboratory strain ATCC 19247T). The N. cyriacigeorgica strains were isolated from respiratory samples (n = 4), a pharyngeal abscess (n = 1), and an orbital abscess (n = 1). Similar to prior reports on infections by this species, the six patients exhibited a spectrum of immunocompromising conditions and/or pulmonary disorders that included AIDS, corticosteroid treatment, chronic granulomatous disease (CGD), chronic obstructive pulmonary disease, and interstitial lung disease (Table 1). All but two patients were known to be on corticosteroid treatment. Notably, whereas multiple infections by this species have been described in kidney transplant patients (26, 39), we now report the first two cases of N. cyriacigeorgica infection in heart transplant patients.
TABLE 1.
Patient characteristics and initial identification of N. cyriacigeorgica isolates, years 2000 to 2006
| Age of patient (yrs) | Sex | PMHa | Diagnosis | Sample sourceb | Treatmentc | Outcomed | Isolate | Initial IDe |
|---|---|---|---|---|---|---|---|---|
| 56f | M | HTX | Pneumonia | Sputum, BAL, lung biopsy | TMP-SMX/IPM | Recovered | 06-51518 | N. cyriacigeorgica |
| 59 | M | AIDS | Respiratory distress | Sputum | Not available | LTFU | 05-49102 | N. asteroides type VI |
| 27g | M | CGD | Lung abscess | Lung biopsy | TMP-SMX/MER, then IPM/LZD | Recovered | 03-70718 | N. asteroides type VI |
| 71 | F | RA, ILD, DM | Pneumonia | Sputum | TMP-SMX/IPM/AMK | LTFU | 03-23155 | N. asteroides complex |
| 73 | M | HTX, COPD | Pneumonia | Tracheal aspirate | TMP-SMX/TOB | Recovered | 02-50508 | N. asteroides complex |
| 19g | M | CGD | Pharyngeal abscess | Biopsy | I&D + TMP-SMX/IPM | Recovered | 00-12607 | Nocardia sp. |
| 59 | F | Sarcoidosis, DM | Orbital abscess | Biopsy | I&D | LTFU | 00-7841 | Nocardia sp. |
PMH, past medical history; HTX, heart transplant; RA, rheumatoid arthritis; ILD, interstitial lung disease; DM, diabetes mellitus type II; COPD, chronic obstructive pulmonary disease.
BAL, bronchoalveolar lavage fluid.
TMP-SMX, trimethoprim-sulfamethoxazole; IPM, imipenem; LZD, linezolid; AMK, amikacin; TOB, tobramycin; MER, meropenem; I&D, incision and drainage.
LTFU, lost to follow-up.
Initial ID, original identity after molecular and/or phenotypic testing.
Index patient.
Siblings.
We also reexamined the 16S rRNA and hsp65 gene sequences from the N. asteroides drug pattern VI type strain ATCC 14759T. As others have indicated (5), it also had a comparably high index of similarity (>99.5%) to both our patient strains in these genes, as well as other sequences from N. cyriacigeorgica clinical isolates and the type strain DSM 44484T (Table 2). Hence, these data supported previous suggestions that Nocardia isolates with a type VI drug pattern in the United States are actually N. cyriacigeorgica (5).
Genotypic segregation of N. cyriacigeorgica.
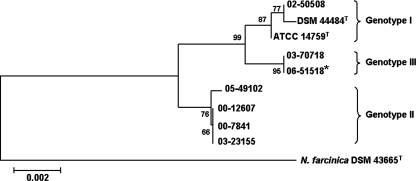
In comparing the 16S rRNA and hsp65 sequences of the N. cyriacigeorgica patient isolates, we noted intraspecies microheterogeneity (Table 2). Previously, Roth et al. distinguished two genotypes of N. cyriacigeorgica based upon a single-nucleotide polymorphism (SNP) at position 1427 of the 16S rRNA gene (29). According to this classification scheme, N. cyriacigeorgica strain DSM 44484T, the drug pattern VI type strain, and three out of our seven clinical strains belong to genotype I, while four belonged to genotype II. We identified an additional SNP at position 1480 that segregated genotype I, thus allowing us to designate a new genotype III represented by two clinical isolates, one of which is the N. cyriacigeorgica strain from the index patient. The N. cyriacigeorgica hsp65 sequences were relatively more polymorphic than the 16S rRNA sequences (28) but cosegregated by 16S rRNA genotype (Table 2). The 16S rRNA and hsp65 sequences from each respective strain were then concatenated and subjected to phylogenetic analysis. The resulting tree is shown in Fig. 2 and clearly illustrates the three clades.
FIG. 2.
Unrooted phylogenetic tree based on the concatenated 16S rRNA and hsp65 gene sequences (1,872 bp in total). Sequences were aligned using Molecular Evolutionary Genetics Analysis software, version 3.1, by ClustalW, and a phylogenetic tree was constructed using the neighbor-joining method and tested for robustness by bootstrapping with 2,000 replicates. N. farcinica strain DSM 43665T was used as the outgroup. The reference bar marks a 0.2% estimated sequence variance. An asterisk denotes the isolate from the index patient.
Interestingly, two of the patients with N. cyriacigeorgica infection were brothers, 5 years apart in age, and both suffered from CGD. However, a common source of infection is unlikely as the strains (00-12607 and 03-70718) were of different genotypes (II and III, respectively) and isolated 3 years apart. CGD is a known risk factor for infections with Nocardia spp. (5).
Phenotypic analysis of N. cyriacigeorgica strains.
Because many clinical microbiology laboratories do not have the resources to perform gene sequence analysis, we reevaluated and summarized the phenotypic and biochemical profiles of N. cyriacigeorgica. Each of the N. cyriacigeorgica clinical strains (n = 7), as well as the drug pattern VI type strain and the N. asteroides ATCC 19247T strain, were evaluated in parallel. The type strains of additional clinically relevant Nocardia spp. were included to act as controls.
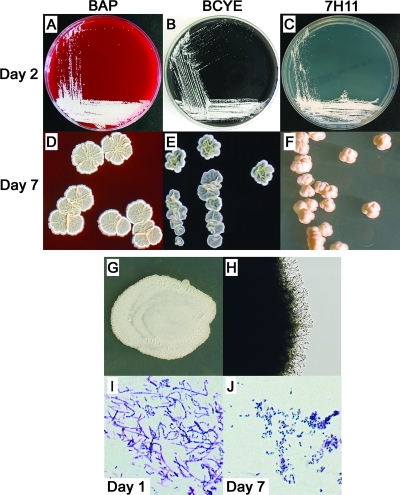
All N. cyriacigeorgica strains (which we henceforth consider to include the drug pattern VI type strain) grew clearly visible individual colonies after 1 to 2 days of incubation on Columbia 5% sheep's blood agar plates (BAP), buffered charcoal yeast extract (BCYE) agar, and Middlebrook 7H11 agar (Fig. 3A to C), as well as SAB agar (data not shown). After 3 to 4 days of incubation, the colony morphologies of all N. cyriacigeorgica strains began to vary by medium. By day 7, individual colonies on BAP were white to pale yellow, chalky, flat to mildly ruffled, and approximately 3 to 8 mm in diameter. Colonies on BCYE agar by day 7 appeared similar but had a highly ruffled texture, and the colony edges curled up from the agar. In contrast, day 7 colonies on 7H11 agar formed discrete cream-colored bodies that grew up from the agar with a central indentation. An isolate from the index patient grown on BAP, BCYE agar, and 7H11 agar, respectively, is shown in Fig. 3D to F. When a heavy inoculum from liquid culture was plated on SAB agar and examined after 4 days, the resulting mega-colonies of N. cyriacigeorgica were a chalky white to pale yellow, flat, and relatively smooth on their surfaces (Fig. 3G). Microscopically, colonies showed delicate aerial hyphae with multiple branch points (Fig. 3H). The Gram stain appearances from SAB cultures showed gram-positive, beaded, branching filaments after 2 days of growth that fragmented into single, coccobacillary forms by day 7 (Fig. 3I and J). After an extended period of incubation, all N. cyriacigeorgica strains produced an earthy odor that is typical of Nocardia. The microscopic and morphological features of the N. asteroides laboratory strain ATCC 19247T are illustrated for counterpoint comparison in Fig. 4.
FIG. 3.
Unique macroscopic and microscopic features distinguish N. cyriacigeorgica. (A to F) Growth of N. cyriacigeorgica strain 06-51518 at 48 h (day 2) and day 7 on Columbia 5% sheep's BAP BCYE agar, and Middlebrook 7H11 agar, as indicated. (G) Mega-colony of type VI strain ATCC 14759T after 4 days of incubation on SAB agar. (H) Microscopic view (original magnification, ×100) of the growing mega-colony edge of panel G. Gram stains of ATCC 14759T after growth for 24 h (I) and 7 days (J) on SAB agar (original magnification, ×400) are shown.
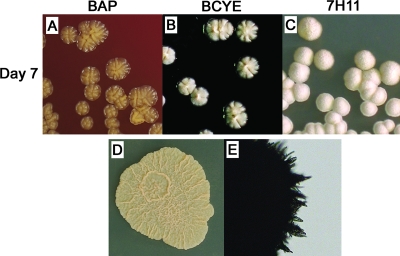
FIG. 4.
Unique macroscopic and microscopic features distinguish the N. asteroides laboratory type strain ATCC 19247T. Colony morphology varied with medium after 7 days of incubation at 35°C. (A) Individual colonies were shiny, honey-colored, slightly elevated, and growing to a peak with an eroded surface texture on BAP. (B) Colonies were similar but cream colored on BCYE agar. (C) Colonies were white and dome shaped, with a buff velvety surface on 7H11 agar. (D) Mega-colonies after 4 days of incubation on SAB agar were coral colored, flat, and moderately ruffled. (E) Microscopically, aerial hyphae protruding from these large colonies had a bundled, pointed-end appearance. Note that all N. cyriacigeorgica strains differed from the N. asteroides species type strain in each of the above descriptions (compare with Fig. 3) as well as by urease activity and the ability to grow at 45°C (Table 3).
All N. cyriacigeorgica strains also formed a cohesive group by chemotaxonomic testing. Each strain demonstrated the expected phenotype of an N. asteroides complex species: positive in the lysozyme resistance test and negative for starch utilization, as well as negative for casein, xanthine, and tyrosine decomposition. However, unlike the N. asteroides laboratory strain ATCC 19247T, all N. cyriacigeorgica strains were negative for urea hydrolysis and grew at 45°C. Further biochemical profiling was performed according to previously published phenotypic descriptions of N. cyriacigeorgica and type VI strains (5, 38). The results are provided in Table 3. In summary, growth at 45°C, esculin hydrolysis, and α-glucosidase activity were each positive for the N. cyriacigeorgica strains. The fermentation of glucose, maltose, rhamnose, and trehalose, as well as PYR and 14-day arylsulfatase activity was negative. The utilization of acetamide as the sole source of carbon, a test known to be variable in N. cyriacigeorgica (25), was positive only for strains 02-50508 and the drug pattern VI type strain. Interestingly, N. cyriacigeorgica strain DSM 44484T is also reportedly acetamide test positive and, along with the latter two strains, is included in the genotype I grouping by 16S rRNA (5, 38). A positive acetamide test may therefore distinguish this subgroup of N. cyriacigeorgica strains.
TABLE 3.
Comparative chemotaxonomic testing of N. cyriacigeorgicaa
| Strainb | Genotype | Biochemical test result
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ESC | URE | ACE | PYR | ARYL | α-GLU | GLU | MAL | RHA | TRE | Growth at 45°C | ||
| ATCC 14759T (drug pattern type VI) | I | + | − | + | − | − | + | − | − | − | − | + |
| N. cyriacigeorgica strains | ||||||||||||
| 02-50508 | I | + | − | + | − | − | + | − | − | − | − | + |
| 00-12607 | II | + | − | − | − | − | + | − | − | − | − | + |
| 00-7841 | II | + | − | − | − | − | + | − | − | − | − | + |
| 03-23155 | II | + | − | − | − | − | + | − | − | − | − | + |
| 05-49102 | II | + | − | − | − | − | + | − | − | − | − | + |
| 03-70718 | III | + | − | − | − | − | + | − | − | − | − | + |
| 06-51518c | III | + | − | − | − | − | + | − | − | − | − | + |
| N. asteroides ATCC 19247T | + | + | − | − | − | + | − | − | − | − | − | |
| N. farcinica ATCC 3318T | + | + | + | + | − | + | + | + | + | + | + | |
| N. nova ATCC 33726T | − | − | − | + | + | + | − | − | − | − | + | |
| N. paucivorans ATCC BAA-278T | − | − | − | − | − | − | − | − | − | − | + | |
ESC, esculin; URE, urease; ACE, acetamide; PYR, pyrrolidonyl arylamidase; ARYL, arylsulfatase; α−GLU, α-glucosidase; GLU, glucose; MAL, maltose; RHA, rhamnose; TRE, trehalose.
N. cyriacigeorgica patient isolates. Select additional Nocardia spp. were evaluated in parallel as controls, based upon the expected results of key biochemical tests.
Isolate from the index patient.
Overall, the combined results of the phenotypic and chemotaxonomic testing of the index case isolates (n = 8), the other clinical strains (n = 6), and the drug pattern VI type strain were in accordance with those previously reported for N. cyriacigeorgica. As such, these results confirmed the species assignments based on 16S rRNA and hsp65 sequences and supported prior suppositions that the drug pattern VI type strain is indeed N. cyriacigeorgica (5). Importantly, the N. cyriacigeorgica strains together possessed an overall unique pattern of morphological, microscopic, phenotypic, and biochemical test results compared to the various Nocardia type strains that were evaluated (Table 3 and data not shown), including the N. asteroides sensu stricto laboratory strain ATCC 19247T (Table 3 and Fig. 4).
Antimicrobial susceptibility and synergy testing.
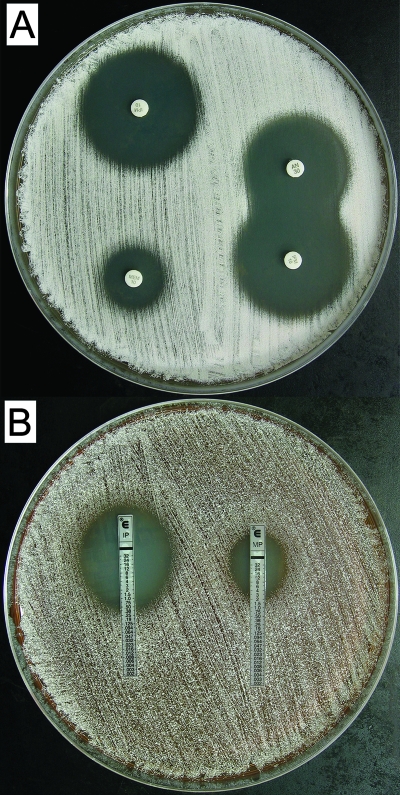
Amikacin is the most commonly used aminoglycoside to treat severe nocardial infections in the United States and is often combined with a carbapenem such as imipenem (5). Susceptibility of our N. cyriacigeorgica strains (n = 8) to select antimicrobials was assessed by the disk diffusion method. The zones of absolute inhibition ranged from 19 to 23 mm for amikacin, 26 to 32 mm for imipenem, and 11 to 19 mm for meropenem (Fig. 5A). This is consistent with the reported susceptibility of N. cyriacigeorgica and type VI strains (6). To confirm the observed higher susceptibility to imipenem than to meropenem, strains 06-51518 and the drug pattern VI type strain were also tested by Etest. MICs for strains 06-51518 and the drug pattern VI type strain were 0.5 and 0.75 μg/ml for imipenem and 4 and 3 μg/ml for meropenem, respectively (Fig. 5B). Imipenem, therefore, appeared to be the more effective carbapenem (by five- to sixfold) against N. cyriacigeorgica by in vitro testing.
FIG. 5.
Differential carbapenem susceptibility of N. cyriacigeorgica and synergistic activity of amikacin and imipenem. (A) Antimicrobial susceptibility and synergy were evaluated for amikacin (upper right), imipenem (upper left/lower right), and meropenem (lower left) by disk diffusion. (B) MICs of imipenem (left) and meropenem (right) were determined by Etest. Results for type VI strain ATCC 14759T are shown and are representative of all N. cyriacigeorgica clinical isolates. N. cyriacigeorgica strains also produced a brown pigment when grown on Mueller-Hinton agar that was clearly visible on the plate reverse (not shown). Note that, based upon preliminary testing, disks were spaced at a nonstandard 3.5-cm distance apart in order to best illustrate synergy between amikacin and imipenem.
Synergistic effects of aminoglycoside and carbapenem antimicrobials on growth inhibition of Nocardia have been reported (13, 19). Based upon the potent in vitro activity of imipenem, we evaluated for synergy between amikacin and imipenem against N. cyriacigeorgica by disk diffusion. Indeed, a modestly expanded area of clearance where the zones of inhibition meet for each disk was observed, suggesting the existence of a synergistic effect between these antimicrobials (Fig. 5A). These results therefore provide supporting in vitro evidence to explain the underlying basis of combination therapy with amikacin and imipenem as an antimicrobial regimen for serious N. cyriacigeorgica infections.
DISCUSSION
The incidence of nocardial infections is believed to be on the rise in the United States as a result of a growing immunocompromised population and improved methods for pathogen isolation and molecular identification (5, 38). The number of recognized Nocardia species causing infections is also increasing (5, 38). These factors have combined to place ever greater demands on clinical microbiology laboratories to accurately identify Nocardia clinical isolates to the species level. Indeed, accurate and timely identification of nocardiae is important because the pathogenic potential between species varies and because the species identity provides a critical guide for physicians in the choice of targeted therapy owing to species-specific differences in resistance patterns to key antimicrobial agents (15, 21, 30, 33).
Historically, infections with Nocardia were associated with very high mortality rates. Treatment of serious nocardial infections with sulfonamides has greatly decreased mortality, and TMP-SMX is the current basis for antimicrobial treatment of nocardiosis in the United States. However, combination treatment is preferred, especially in severe, disseminated, and central nervous system infections (5). Amikacin plus a beta-lactam (ceftriaxone or imipenem) are typically added to TMP-SMX to ensure the susceptibility of all Nocardia spp. to at least two antimicrobials. Because of its distinct and favorable antimicrobial susceptibility pattern, the specific identification of N. cyriacigeorgica may improve clinical management. Our index case suffered from a life-threatening pneumonia due to infection with N. cyriacigeorgica, was treated with TMP-SMX and imipenem, and recovered. Previously, patients with N. cyriacigeorgica infection have done well when treated with a variety of antimicrobial regimens (1, 3, 11), although treatment failures have also been noted (22). In this study, we present data to indicate that N. cyriacigeorgica may be more susceptible to imipenem than meropenem and that amikacin and imipenem appear to work synergistically in vitro against N. cyriacigeorgica. However, an optimal management protocol for nocardiosis has not been defined, and guidelines for specific treatment by species are needed.
N. cyriacigeorgica is an emerging pathogenic entity (as defined in reference 24) that we found to be the cause of infection in seven cases at our New York City medical center, thus representing the first such cases officially reported in the United States. These strains were identified by molecular means and confirmed by phenotypic and biochemical testing. Whether previously isolated type VI strains in the United States are truly synonymous with N. cyriacigeorgica remains to be definitively proven. However, by nearly all phenotypic, chemotaxonomic, and genotypic measures that we evaluated, the type VI strain ATCC 14759T matched the clinical isolates of N. cyriacigeorgica tested in this study. Similarly, the strain from the index case, as well as strains 03-70718 and 05-49102 (the only others so evaluated), each displayed a type VI antimicrobial susceptibility pattern. Therefore, the evidence supports the idea that the species N. cyriacigeorgica and type VI are overlapping. This is an important point to clarify because it would mean that, at least in the southern United States, N. cyriacigeorgica is the most important cause of nocardiosis (5). The fact that N. cyriacigeorgica has been identified in Canada (11) and now in the northeastern United States with this report suggests that N. cyriacigeorgica might actually be distributed across all of North America, in addition to Europe and Asia (1, 3, 7, 11, 17, 18, 22, 27, 35). Clearly, N. cyriacigeorgica is a species that requires closer attention, and clinical microbiology laboratories need to adapt laboratory protocols for its specific identification.
Molecular techniques offer several advantages over traditional phenotypic methods of Nocardia spp. identification and have the potential to greatly improve patient diagnosis. To date, molecular techniques have provided the most definitive means of identifying N. cyriacigeorgica. As we noted significant microheterogeneity in the targets of sequencing, sequence data enabled strain differentiation and so may also have the potential for use as a first-line screen in source investigations of suspected nosocomial transmissions.
In this study, we also evaluated the chemotaxonomic properties of molecularly validated isolates of N. cyriacigeorgica. All strains were distinguished from the N. asteroides laboratory strain ATCC 19247T and the type strains of other clinically relevant Nocardia spp. by a combination of their relatively rapid growth rate, differential agar type-specific colonial appearance, and the microscopic appearance of their aerial hyphae, as well as their unique phenotypic and biochemical test profiles. More strains need to be evaluated before firm conclusions can be drawn, but the pattern of outcomes in this set of tests might serve as an aid to identify N. cyriacigeorgica when molecular testing is not available.
Lastly, the differentiation of N. cyriacigeorgica from the N. asteroides laboratory strain ATCC 19247T requires the evaluation of tests, such as for urease activity, which may not be considered informative in the context of the Nocardia. As a consequence, many clinical isolates of N. cyriacigeorgica are likely classified and reported as N. asteroides, which would explain why the species has remained underdiagnosed as a cause of infection. In contrast, our data illustrate several genotypic, phenotypic, and biochemical differences between the species. In fact, the N. asteroides sensu stricto species does not represent any of the common taxa associated with clinical nocardiosis or drug pattern group, and so its widely utilized representative strain ATCC 19247T serves as a poor comparator for clinical microbiology laboratories (5, 25). Perhaps a more appropriate representative Nocardia laboratory strain, possibly an N. cyriacigeorgica strain, should be designated. Indeed, the taxonomic nomenclature of the clinically important Nocardia is better clarified now that the former N. asteroides complex has been resolved into separate species that are clearly distinguished from the noninfectious N. asteroides sensu stricto species. It is hoped that with increased awareness of N. cyriacigeorgica as a distinct pathogenic entity and with improved diagnosis, an increased understanding of its biology, epidemiology, and optimal course of treatment for infection will follow.
ADDENDUM
Subsequent to the preparation of our manuscript, Conville and Witebsky (9) published on the comparative DNA-DNA hybridization results for N. cyriacigeorgica DSM44484T and drug pattern type VI strain ATCC 14759T. Their data confirmed that these two strains are of the same species, consistent with the data presented in our current study.
Acknowledgments
We thank John M. Austin for assistance with interpretation of radiological studies, Richard Wallace and Barbara A. Brown-Elliott for initial antimicrobial testing, and Steven Spitalnik for support and encouragement.
Footnotes
Published ahead of print on 14 November 2007.
REFERENCES
- 1.Alp, E., O. Yildiz, B. Aygen, B. Sumerkan, I. Sari, K. Koc, A. Couble, F. Laurent, P. Boiron, and M. Doganay. 2006. Disseminated nocardiosis due to unusual species: two case reports. Scand. J. Infect. Dis. 38545-548. [DOI] [PubMed] [Google Scholar]
- 2.Ambaye, A., P. C. Kohner, P. C. Wollan, K. L. Roberts, G. D. Roberts, and F. R. Cockerill III. 1997. Comparison of agar dilution, broth microdilution, disk diffusion, E-test, and BACTEC radiometric methods for antimicrobial susceptibility testing of clinical isolates of the Nocardia asteroides complex. J. Clin. Microbiol. 35847-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnaud, G., C. Deschamps, V. Manceron, E. Mortier, F. Laurent, F. Bert, P. Boiron, P. Vinceneux, and C. Branger. 2005. Brain abscess caused by Nocardia cyriacigeorgica in a patient with human immunodeficiency virus infection. J. Clin. Microbiol. 434895-4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bocchino, M., M. G. Paglia, A. Marruchella, S. Contini, A. Festa, and C. Saltini. 30 June 2006. Molecular diagnosis of fatal Nocardia farcinica pneumonia in an HIV-negative patient. Respiration. [Epub ahead of print.] doi: 10.1159/000094390. [DOI] [PubMed]
- 5.Brown-Elliott, B. A., J. M. Brown, P. S. Conville, and R. J. Wallace, Jr. 2006. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin. Microbiol. Rev. 19259-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cercenado, E., M. Marin, M. Sanchez-Martinez, O. Cuevas, J. Martinez-Alarcon, and E. Bouza. 2007. In vitro activities of tigecycline and eight other antimicrobials against different Nocardia species identified by molecular methods. Antimicrob. Agents Chemother. 511102-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cloud, J. L., P. S. Conville, A. Croft, D. Harmsen, F. G. Witebsky, and K. C. Carroll. 2004. Evaluation of partial 16S ribosomal DNA sequencing for identification of Nocardia species by using the MicroSeq 500 system with an expanded database. J. Clin. Microbiol. 42578-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conville, P. S., and F. G. Witebsky. 2007. Analysis of multiple differing copies of the 16S rRNA gene in five clinical isolates and three type strains of Nocardia species, and implications for species assignment. J. Clin. Microbiol. 451146-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conville, P. S., and F. G. Witebsky. 2007. Organisms designated as Nocardia asteroides drug pattern type VI are members of the species Nocardia cyriacigeorgica. J. Clin. Microbiol. 452257-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Couble, A., V. Rodriguez-Nava, M. P. de Montclos, P. Boiron, and F. Laurent. 2005. Direct detection of Nocardia spp. in clinical samples by a rapid molecular method. J. Clin. Microbiol. 431921-1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elsayed, S., A. Kealey, C. S. Coffin, R. Read, D. Megran, and K. Zhang. 2006. Nocardia cyriacigeorgica septicemia. J. Clin. Microbiol. 44280-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Exmelin, L., B. Malbruny, M. Vergnaud, F. Prosvost, P. Boiron, and C. Morel. 1996. Molecular study of nosocomial nocardiosis outbreak involving heart transplant recipients. J. Clin. Microbiol. 341014-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gombert, M. E., and T. M. Aulicino. 1983. Synergism of imipenem and amikacin in combination with other antibiotics against Nocardia asteroides. Antimicrob. Agents Chemother. 24810-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haddad, F., S. A. Hunt, M. Perlroth, H. Valantine, R. Doyle, and J. Montoya. 2007. Pulmonary nocardiosis in a heart transplant patient: case report and review of the literature. J. Heart Lung Transplant. 2693-97. [DOI] [PubMed] [Google Scholar]
- 15.Husain, S., K. McCurry, J. Dauber, N. Singh, and S. Kusne. 2002. Nocardia infection in lung transplant recipients. J. Heart Lung Transplant. 21354-359. [DOI] [PubMed] [Google Scholar]
- 16.Kachi, S., M. Okazaki, H. Takeda, H. Igarashi, O. Kobayashi, H. Watanabe, K. Nakata, S. Kawai, M. Aoshima, T. Watanabe, and H. Goto. 2006. Outbreak of Nocardia farcinica infection with the same pattern in randomly amplified polymorphic DNA analysis. J. Hosp. Infect. 62502-506. [DOI] [PubMed] [Google Scholar]
- 17.Kageyama, A., Y. Hoshino, K. Yazawa, N. Poonwan, N. Takeshita, S. Maki, and Y. Mikami. 2005. Nocardia cyriacigeorgica is a significant pathogen responsible for nocardiosis in Japan and Thailand. Mycopathologia 16015-19. [DOI] [PubMed] [Google Scholar]
- 18.Kageyama, A., K. Yazawa, J. Ishikawa, K. Hotta, K. Nishimura, and Y. Mikami. 2004. Nocardial infections in Japan from 1992 to 2001, including the first report of infection by Nocardia transvalensis. Eur. J. Epidemiol. 19383-389. [DOI] [PubMed] [Google Scholar]
- 19.Kanemitsu, K., H. Kunishima, T. Saga, H. Harigae, S. Ishikawa, H. Takemura, and M. Kaku. 2003. Efficacy of amikacin combinations for nocardiosis. Tohoku J. Exp. Med. 201157-163. [DOI] [PubMed] [Google Scholar]
- 20.Land, G. A. 1992. Identification of the aerobic actinomycetes, p. 4.0.1-4.1.9. In H. D. Isenberg (ed.), Clinical microbiology procedures handbook, vol. 1. American Society for Microbiology, Washington, DC. [Google Scholar]
- 21.Lederman, E. R., and N. F. Crum. 2004. A case series and focused review of nocardiosis: clinical and microbiologic aspects. Medicine 83300-313. [DOI] [PubMed] [Google Scholar]
- 22.Maraki, S., E. Panagiotaki, D. Tsopanidis, E. Scoulica, N. M. Miari, K. Hainis, G. Dotis, I. Katsoula, and Y. Tselentis. 2006. Nocardia cyriacigeorgica pleural empyema in an immunocompromised patient. Diagn. Microbiol. Infect. Dis. 56333-335. [DOI] [PubMed] [Google Scholar]
- 23.Marchandin, H., A. Eden, H. Jean-Pierre, J. Reynes, E. Jumas-Bilak, P. Boiron, and F. Laurent. 2006. Molecular diagnosis of culture-negative cerebral nocardiosis due to Nocardia abscessus. Diagn. Microbiol. Infect. Dis. 55237-240. [DOI] [PubMed] [Google Scholar]
- 24.Millar, B. C., and J. E. Moore. 2006. Emerging pathogens in infectious diseases: definitions, causes and trends. Rev. Med. Microbiol. 17101-106. [Google Scholar]
- 25.Patel, J. B., R. J. Wallace, Jr., B. A. Brown-Elliott, T. Taylor, C. Imperatrice, D. G. Leonard, R. W. Wilson, L. Mann, K. C. Jost, and I. Nachamkin. 2004. Sequence-based identification of aerobic actinomycetes. J. Clin. Microbiol. 422530-2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ponticelli, C., and M. R. Campise. 2005. Neurological complications in kidney transplant recipients. J. Nephrol. 18521-528. [PubMed] [Google Scholar]
- 27.Poonwan, N., N. Mekha, K. Yazawa, S. Thunyaharn, A. Yamanaka, and Y. Mikami. 2005. Characterization of clinical isolates of pathogenic Nocardia strains and related actinomycetes in Thailand from 1996 to 2003. Mycopathologia 159361-368. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez-Nava, V., A. Couble, G. Devulder, J. P. Flandrois, P. Boiron, and F. Laurent. 2006. Use of PCR-restriction enzyme pattern analysis and sequencing database for hsp65 gene-based identification of Nocardia species. J. Clin. Microbiol. 44536-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roth, A., S. Andrees, R. M. Kroppenstedt, D. Harmsen, and H. Mauch. 2003. Phylogeny of the genus Nocardia based on reassessed 16S rRNA gene sequences reveals underspeciation and division of strains classified as Nocardia asteroides into three established species and two unnamed taxons. J. Clin. Microbiol. 41851-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saubolle, M. A., and D. Sussland. 2003. Nocardiosis: review of clinical and laboratory experience. J. Clin. Microbiol. 414497-4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tatti, K. M., W. J. Shieh, S. Phillips, M. Augenbraun, C. Rao, and S. R. Zaki. 2006. Molecular diagnosis of Nocardia farcinica from a cerebral abscess. Hum. Pathol. 371117-1121. [DOI] [PubMed] [Google Scholar]
- 32.Telenti, A., F. Marchesi, M. Balz, F. Bally, E. C. Bottger, and T. Bodmer. 1993. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J. Clin. Microbiol. 31175-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torres, O. H., P. Domingo, R. Pericas, P. Boiron, J. A. Montiel, and G. Vazquez. 2000. Infection caused by Nocardia farcinica: case report and review. Eur. J. Clin. Microbiol. Infect. Dis. 19205-212. [DOI] [PubMed] [Google Scholar]
- 34.Wallace, R. J., Jr., L. C. Steele, G. Sumter, and J. M. Smith. 1988. Antimicrobial susceptibility patterns of Nocardia asteroides. Antimicrob. Agents Chemother. 321776-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wauters, G., V. Avesani, J. Charlier, M. Janssens, M. Vaneechoutte, and M. Delmee. 2005. Distribution of Nocardia species in clinical samples and their routine rapid identification in the laboratory. J. Clin. Microbiol. 432624-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wenger, P. N., J. M. Brown, M. M. McNeil, and W. R. Jarvis. 1998. Nocardia farcinica sternotomy site infections in patients following open heart surgery. J. Infect. Dis. 1781539-1543. [DOI] [PubMed] [Google Scholar]
- 37.Winn W. C., S. D. Allen, W. M. Janda, E. W. Koneman, G. W. Procop, P. C. Schreckenberger, and G. L. Woods (ed.). 2005. Koneman's color atlas and textbook of diagnostic microbiology, 6th ed., vol. 1, p.858-876. Lippincott Williams and Wilkins, Philadelphia, PA.
- 38.Yassin, A. F., F. A. Rainey, and U. Steiner. 2001. Nocardia cyriacigeorgici sp. nov. Int. J. Syst. Evol. Microbiol. 511419-1423. [DOI] [PubMed] [Google Scholar]
- 39.Yildiz, O., E. Alp, B. Tokgoz, B. Tucer, B. Aygen, B. Sumerkan, A. Couble, P. Boiron, and M. Doganay. 2005. Nocardiosis in a teaching hospital in the Central Anatolia region of Turkey: treatment and outcome. Clin. Microbiol. Infect. 11495-499. [DOI] [PubMed] [Google Scholar]