Abstract
Given the increasing prevalence of cryptococcosis caused by Cryptococcus gattii (serotypes B and C) strains, there is a need for rapid and reliable tests that discriminate C. gattii from Cryptococcus neoformans (serotypes A, D, and AD). Seventy-two C. neoformans strains, sixty-seven C. gattii strains, and five Candida albicans strains were analyzed for their ability to grow and produce pigment on minimal d-tryptophan d-proline (m-DTDP) medium, on yeast carbon base d-tryptophan d-proline (YCB-DTDP) medium, and on fructose d-tryptophan glycine (m-FDTG) medium. Of the C. gattii and C. neoformans isolates, 94% and 0% grew on m-DTDP agar, respectively, and 98% and 0% grew in YCB-DTDP medium, respectively. C. gattii produced large amounts of brown intracellular pigment(s) on m-DTDP agar and smaller amounts of yellow-brown (amber) extracellular pigment(s). C. albicans grew on both media and produced a pink photoactivated pigment on m-DTDP agar. C. gattii produced large amounts of brown intracellular pigments on the differential medium m-FDTG, whereas C. neoformans produced smaller amounts of the brown pigments and C. albicans produced a pink pigment. The pigments produced by C. gattii from d-tryptophan were distinct and were not related to melanin formation from 3,4-dihydroxyphenylalanine. Thin-layer chromatography of the methanol-extracted C. gattii cells detected four different pigments, including brown (two types), yellow, and pink-purple compounds. We conclude that tryptophan-derived pigments are not melanins and that growth on m-DTDP or YCB-DTDP agar can be used to rapidly differentiate C. gattii from C. neoformans.
Cryptococcus neoformans was classically subdivided into the three varieties: C. neoformans var. gattii (serotypes B and C), C. neoformans var. neoformans (serotype D), C. neoformans var. grubii (serotype A) (17), and C. neoformans (serotype AD). However, recent evidence suggests that the genetic separation between the varieties is sufficient to split these three varieties into two species, Cryptococcus gattii and C. neoformans (27). C. neoformans is found worldwide, whereas C. gattii is usually found in tropical and subtropical climates. C. neoformans has long been associated with disease in immunocompromised patients, and more recently multiple cases of C. gattii were also detected in immunocompromised patients (11, 29, 33, 34, 52). Recent outbreaks of C. gattii on Vancouver Island in Canada (21, 23) and the isolation of this yeast from additional geographic locations (11, 29, 30) indicate that it is emerging as a primary human pathogen. Cases of C. gattii caused by the Vancouver strain are now occurring in the United States (54). Given that there are clinical differences in cryptococcosis caused by the two cryptococcal species, there is a need for rapid diagnostic tests that can distinguish these isolates. The classical methodology for distinguishing between serotypes is based on serological reagents. Serotyping was usually accomplished by factor serum agglutination using the Crypto-Check kit (Iatron, Inc., Tokyo, Japan), but these reagents are no longer made. Furthermore, this method is expensive compared to the traditional medium-based diagnostic assays for the separation of the strains into varieties. Various methods have been reported for separating C. gattii from C. neoformans, including use of canavanine-glycine-bromthymolblue agar (CGB) (25), glycine-cycloheximide-phenol red agar (48), and creatinine-dextrose bromthymol blue (24) agar. The CGB agar was reported to give fewer false-positive and false-negative results than the others. However, it is noteworthy that these assays each contained inhibitors of growth, such as l-canavanine or cycloheximide, to reduce the likelihood of false-positive results (25, 48).
Various studies revealed that C. gattii could assimilate d-proline (2, 3, 13, 31) and d-tryptophan (2, 3, 35) whereas C. neoformans, including serotype AD, did not assimilate these amino acids. Additionally, only C. gattii assimilated l-malic, fumaric, and succinic acids (4). C. gattii produced a brown diffusible pigment when d-tryptophan was assimilated (35). 3-Hydroxyanthranilic acid (42), a tryptophan metabolite, was previously detected in the supernatant of C. neoformans grown cells. Anthranilic acid, a precursor/metabolite of tryptophan, was metabolized to an intracellular pigment by C. neoformans (10).
Pigment production was reported for the Candida spp. growing in l- or dl-tryptophan, with approximately one-half of the medically important species producing brown pigments and the other half, including Candida albicans, producing, a pink light-catalyzed pigment (7). Some time ago, Chaskes and Tyndall (10) reported that some strains of Cryptococcus neoformans produced brown cell-associated intracellular pigments while others produced pink extracellular pigments from l- or dl-tryptophan. The C. neoformans isolates used in that study were not classified by serotype, and consequently no association between serotypes or varieties of C. neoformans and pigment production was made. Additionally, various other Cryptococcus species produced brown or pink pigments when cultured on l- or dl-tryptophan (10). Our goal in the current study was to develop a new diagnostic medium which allowed the growth of C. gattii but prevented the growth of C. neoformans. A second goal of the study was to characterize the pigments produced by the two Cryptococcus spp. and C. albicans from d-tryptophan and to investigate their relationship to melanin-type pigments.
MATERIALS AND METHODS
Cultures.
Sixty-seven C. gattii strains were obtained from Thomas Mitchell (Durham, NC), June Kwon-Chung (Bethesda, MD), and Uma Banerjee (New Delhi, India). These strains included both clinical and environmental isolates. The C. gattii strains included NIH isolates 34, 191, 198, and 444. The lab stock of 33 isolates of C. neoformans var. grubii consisted of 28 clinical isolates that were obtained from cryptococcal meningitis patients from New York City hospitals (53). Additionally, MY2061 (serotype A) was obtained from Merck and Company (Whitehouse Station, New Jersey) and H99 (serotype A) was obtained from the New York State Herbarium, Albany, NY. Three clinical isolates of C. neoformans var. grubii were obtained from Uma Banerjee. The lab stock of 25 clinical strains of C. neoformans var. neoformans was obtained from Cryptococcal meningitis patients from New York City hospitals (53), or from Laurie Watt (bioMerieux, Marcy l'Etoile, France). Strain 24067 (serotype D) was obtained from the American Type Culture Collection (Manassas, VA). The lab stock of 14 isolates of serotype AD was obtained from Mary Brandt, Centers for Disease Control and Prevention, Atlanta, GA). The congenic serotype D strains, 2ETU (laccase deletion) and 2ETU-C (complemented strain), have been described previously (47). C. albicans (BSMY 212) was provided by David Goldman, Albert Einstein College of Medicine, New York, NY, and four additional strains were obtained from Jean Pollack, New York University Medical Center, New York, NY.
Inoculum.
Two- to 5-day-old yeast cells from Sabouraud dextrose agar plates were transferred to each quad plate using a 10-μl inoculating loop. The inoculums were applied to achieve confluent growth on the quad plates. Five strains of each yeast, C. neoformans and C. gattii, were used to determine the optimum concentration of each ingredient needed to achieve rapid growth and pigment production using minimal d-tryptophan d-proline (m-DTDP), yeast carbon base d-tryptophan d-proline (YCB-DTDP), and minimal fructose d-tryptophan glycine (m-FDTG) agars. Final testing employed 67 strains of C. gattii, 72 strains of C. neoformans, and 5 strains of C. albicans.
Growth and pigment production for C. gattii on m-DTDP agar.
d-tryptophan (2 g/liter) was tested as the sole nitrogen source. d-Tryptophan and d-proline were tested in the following combinations: 0.5 g/liter d-tryptophan and 2 g/liter d-proline; 2 g/liter d-tryptophan and 2 g/liter d-proline; 2 g/liter d-tryptophan and 0.5 g/liter d-proline. The following carbon sources were tested at 5, 10, 20, 40 or 80 g/liter: glucose, sucrose, fructose, maltose, galactose, mannose, xylose, citric acid, and succinic acid. Media with pHs of 5.35 and 7.35 were evaluated. The following salt levels were tested: 4, 2, or 1 g/liter KH2PO4 and 2.5, 1, and 0.5 g/liter MgSO4·7H2O. The following incubation temperatures were tested: 25°C, 30°C, and 37°C.
Preparation of selective/differential media (m-DTDP agar).
Solutions with 2× chemicals were prepared in 500 ml distilled water (dH2O)-40 g glucose or 40 g fructose (Fisher Scientific, Pittsburg PA), 2 g d-tryptophan (Sigma, St. Louis, MO), 0.5 g d-proline (Sigma, St.Louis, MO), 4 g KH2PO4, 2.5 g MgSO4·7H2O, and 5 ml of 1 mg/ml thiamine hydrochloride (Fisher Scientific, Pittsburg, PA). The 2× chemical solutions were adjusted to a pH of 5.35 and filter sterilized. A volume of 500 ml of 3% agar solution was autoclaved and immediately added to the 2× chemical solution. The medium was then poured into quad petri plates (Becton-Dickinson, Franklin Lakes, NJ). Broth shake cultures were prepared as needed.
Preparation of selective medium (YCB-DTDP).
Solutions with 2× chemicals were prepared in 500 ml dH2O-11.75 g yeast carbon base (Sigma), 10 g glucose, 2 g d-tryptophan, and 0.5 g d-proline. The agar was prepared by dissolving the yeast carbon base in 500 ml dH2O. Some heating (without boiling) may be required to get all the components in solution. The remaining chemicals were added. After cooling, the pH was adjusted to 5.35 and sterilized by filtration. A volume of 500 ml of a 3% agar solution was autoclaved and immediately added to the 2× chemical solution. The medium was then poured into quad petri plates.
Preparation of differential medium (m-FDTG).
Solutions with 2× chemicals prepared in 500 ml dH2O by mixing 40 g fructose, 2 g d-tryptophan, 0.5 g glycine, 4 g KH2PO4, 2.5 g MgSO4·7H2O, and 5 ml of 1 mg/ml thiamine. The 2× chemicals were adjusted to pH 5.35 and sterilized by filtration. A volume of 500 ml of a 3% agar solution was autoclaved and immediately added to the 2× chemical solution. The medium was poured into quad petri plates.
Preparation of 3,4-dihydroxyphenylalanine (DOPA) medium for in vitro melanization assay.
The chemically defined medium contained 3 g dextrose, 0.975 g glycine, 4 g KH2PO4, 2.5 g MgSO4, 3 μM thiamine, and 197 mg either l-, dl-, or d-DOPA dissolved in 1 liter distilled water.
Criteria for a positive test result on selective agar media (m-DTDP and YCB-DTDP).
Confluent growth was recorded as a positive test result. The rare formation of one or two single colonies after 3 to 7 days’ growth on a quad plate was recorded as a negative test result. The appearance of petite colonies was also recorded as a negative test result. C. neoformans and serotype AD occasionally formed petite colonies which failed to grow upon transfer to new quad plates (negative results).
Fluorescence of d-tryptophan- or DOPA-grown cells under a Woods lamp.
The natural fluorescence of C. neoformans, C. gattii, and C. albicans cells growing in m-DTDP, m-FDTG, or YCB-DTDP agar quad plates was studied using a Woods lamp at 365 and 254 nm.
Natural fluorescence microscopy of C. gattii, C. neoformans, and C. albicans cells from d-tryptophan or DOPA medium.
Fluorescence of the yeast cells was studied in broth shake cultures using m-DTDP, m-FDTG, YCB-DTDP, or DOPA medium. Yeast cells were removed from the shake cultures (30°C) every 2 days and examined for fluorescence using an Olympus AX 10 microscope (Olympus America, Melville, NY). The microscope was equipped with a 100× numerical aperture and standard fluorescein isothiocyanate (excitation, 480 nm; emission, 535 nm), DAPI (excitation, 360 nm; emission, 420 nm), and rhodamine (excitation, 535 nm; emission, 610 nm) mode and filters.
Detection of indole and aromatic compounds.
Salkowski reagent was prepared by adding 300 ml of 36 N sulfuric acid to 500 ml distilled water. Fifteen milliliters of 0.5 M FeCl3 was added to the diluted sulfuric acid. The modified Salkowski reagent contained 0.5 M ZnCl2 or MnCl2·4H2O instead of 0.5 M FeCl3·H2O. The Kovacs reagent consisted of 5 g of p-dimethylaminobenzaldehyde in 75 ml of amyl alcohol and 25 ml of 12 N HCl. Indole derivatives and/or tryptophan metabolites were detected by adding the reagents to broth supernatants. The Kovacs reagent detects indole and indole-like compounds, whereas the Salkowski reagent has a broader spectrum and reacts with many more aromatic compounds that contain tryptophan and indole derivatives. One milliliter of supernatant containing m-DTDP-cultured, m-FDTG-cultured, or l-, dl-, or d-DOPA-cultured C. gattii, C. neoformans, or C. albicans strains was tested with 9 ml of the various Salkowski reagents. One-half milliliter of the Kovacs reagent was added to 3 ml of the various supernatants.
Extraction of brown pigments and fluorescent compounds.
A volume of 50 ml of 1- to 3-week-old yeast cells that were grown in m-DTDP broth was collected by centrifugation at 3,000 × g for 60 min. Yeast cells were washed once in 0.85% saline and were centrifuged at 3,000 × g. The pellet was extracted twice with 5 ml 100% methanol. The methanol extract was centrifuged at 10,000 × g for 5 min. The soluble extract was concentrated by simple overnight evaporation in a petri plate. The concentrate was dissolved in 1 or 2 ml 100% methanol. The pigments and fluorescent compounds could also be extracted with ethanol or n-butanol.
Thin-layer chromatography (TLC).
The 100%-methanol extract was spotted on TLC Silica gel 60 plates (catalog no. 10028 [Selecto Scientific, Atlanta, GA] or catalogue no. 5748/7 [Merck K GaA, Darmstadt, Germany]). Chloroform, acetone, acetonitrile, xylene, and methanol were tested as solvents.
Isolation of d-Tryptophan particles.
C. gattii cells were grown in m-DTDP broth for 3 weeks. Yeast cells were then centrifuged for 60 min at 3,000 × g. The cells were washed in 0.85% saline (three to five times) until the supernatants were clear. The procedure of Wang et al. (56) that was used to make melanin particles (“ghosts”) was adapted to C. gattii cells which formed brown pigments in d-tryptophan (m-DTDP) broth.
Spectroscopy.
Sabouraud dextrose-grown 2-week-old C. gattii (NIH 34 and 444), C. neoformans (H99, 2ETU, and 2ETU-C), and C. albicans (BSMY 212) cells (negative control) and cells grown in d- or l-DOPA or d- or l-tryptophan were suspended in water at a concentration of approximately 5 × 107 yeast cells per ml. Approximately 500 μl of suspensions was pipetted into 4-mm quartz electron paramagnetic resonance (EPR) tubes (Wilmad LabGlass, Buena, NJ) and slowly frozen in liquid nitrogen. EPR spectra were obtained with a Varian E112X-Band model spectrometer (Varian Medical Systems, Palo Alta, CA). In addition, the pigments produced from d- and l-tryptophan by C. gattii (NIH 444) were extracted with 100% methanol. The methanol-soluble pigments and the remnants of the extracted cells were also frozen in liquid nitrogen, and the EPR spectra were obtained. The parameters for EPR were as follows: modulation amplitude, 1.6 G; center field, 3,250.0 G; sweep width, 80.0 G; microwave frequency, 9.107 GHz; microwave power, 5.00 mW; and temperature, 77 K. Samples that resulted in weak EPR signals were scanned nine times, and the averaged signal was recorded. The relative strength of each EPR signal was determined by the amplitude distance of each EPR signal.
Zeta potential measurements.
The zeta potential was determined for C. gattii cells that were cultured on Sabouraud dextrose, d- or l-DOPA, or d- or l-tryptophan broth. The zeta potential, or surface charge, of the particles was determined by applying an electric field to the particles in suspension and determining the direction and velocity of the particle movement by measuring light scattering of a laser beam passed through the sample. Samples were prepared at 107 cells per ml in 10 mM KCl. For each experiment, each sample was measured three times with 10 readings per measurement. Measurements more than three standard errors from the mean were thrown out. Values represent an average of all readings.
Rapid dl-DOPA melanin test.
The procedure of Chaskes et al. (6) was used to perform a rapid melanin test. Briefly, an inoculating loop (10 μl) transferred 2 loopfuls of Sabouraud dextrose-grown C. gattii cells to a starvation phosphate buffer (pH 7) agar medium. After the transferred cells were starved for 24 h at 25°C, the following substrates at 0.3% concentrations were directly added to the C. gattii cells on the quad petrie plates: dl-, l-, or d-DOPA, dopamine, 4-hydroxymetanilamide, 2,5-diaminobenzenesulfonic acid, or d- or l-tryptophan). The plates were observed every 30 min for pigment production.
Monochromator determination of required wavelengths for pink pigment production by C. albicans.
A Till-Photonics Polychrome II illumination and control unit, along with the necessary auxiliary equipment (Till-Photonics, Eugene, OR), was used to select and focus a specific wavelength of light on a petri plate that contained C. albicans growing on m-DTDP or m-FDTG agar. The petri plates inoculated with C. albicans were exposed to 16 h of light during the stationary phase of growth (5 to 10 days). The experiments were conducted in a dark room where the only source of light was the Polychrome illumination unit. The covered petri plates were exposed to selected wavelengths of light which ranged from 340 nm to 700 nm. The wavelength increment was 10 nm. Wavelength experiments were also conducted with a Woods lamp (365 nm or 254 nm). A standard fluorescent lamp was tested with the lamp placed 3 to 6 in. above the m-DTDP or m-FDTG plates. The plates were exposed to fluorescent light for at least 48 h.
RESULTS
Determination of optimum conditions for growth and pigment production for C. gattii on m-DTDP agar. (i) d-Tryptophan and d-proline variations.
d-Tryptophan did not support growth when used as the sole nitrogen source. A weight ratio of 1 to 4 d-tryptophan/d-proline supported excellent growth but produced only light pigmentation. In contrast, a weight ratio of 4 to 1 for d-tryptophan/d-proline supported excellent growth and strong dark pigmentation.
(ii) Carbon source variations.
Fructose, glucose, sucrose, galactose, and xylose all supported both growth and strong pigment production by nearly all C. gattii strains. Pigmentation was not apparent by day one of growth but was usually noticeable between days two and five. Pigmentation intensity increased gradually with time, and the development of maximum brown color usually required 2 or 3 weeks. Intense pigment production required sugar concentrations of at least 20 g/liter, with optimal levels requiring 40 to 80 g/liter. Maltose and mannose supported growth, but pigment production was slightly less intense. Citric acid and succinic acid (10 to 40 g/liter) supported neither growth nor pigmentation. Fructose or glucose at 40 g/liter was selected as the carbohydrate to be used in m-DTDP agar.
(iii) pH, salt, and temperature variations.
We observed little difference in the growth or pigmentation of C. gattii strains in the pH range 5.35 to 7.35. Consequently, pH 5.35 was selected as the working pH. Since we did not observe a significant effect of the salt concentration on either growth or pigment production, we selected 4 g/liter KH2PO4 and 2.5 g/liter MgSO4·7H2O, given that this was used in the prior studies of pigment induction by tryptophan (7, 10). However, temperature affected pigmentation significantly, such that coloration was less intense at 37°C than at either 25 or 30°C. Hence, 30°C was selected as the initial incubation temperature. After day 3, plates were incubated at room temperature to prevent excessive dehydration.
Selective/differential agar. (i) Growth and pigmentation on m-DTDP agar.
Ninety-four percent (63/67) of C. gattii strains grew on m-DTDP agar, whereas none (0/72) of the C. neoformans strains grew. Figure 1 shows typical results observed on m-DTDP agar. The results for serotype AD strains (not shown) were identical to those for C. neoformans. Growth of the C. gattii strains (60/67) was usually evident between days 1 and 3. Only a few strains of C. gattii (3/67) required 4 or 5 days to achieve confluent growth. Pigmentation continued to develop for several weeks. Each C. gattii strain that grew produced at least some pigment, with approximately 10% of the C. gattii strains producing small amounts of brown pigment as measured by visual inspection. Growth was comparable in agar containing glucose or fructose, but pigmentation was usually greater with fructose as the carbon source (not shown). C. albicans (5/5) strains produced a pink, light-catalyzed water soluble pigment (not shown). A small amount of a brown intracellular pigment was also produced after exposure to light.
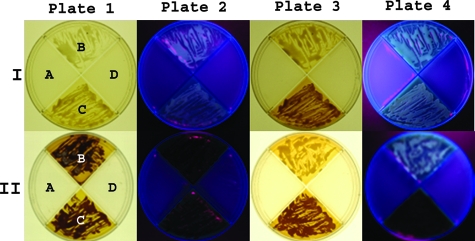
FIG. 1.
Growth and fluorescence of C. gattii on m-DTDP agar. Row I, plate 1 shows typical growth and a small amount of pigmentation of C. gattii on m-DTDP agar (B and C) on day 2, whereas C. neoformans (A and D) fail to grow. Plate 2 illustrates weak fluorescence (365 nm) on day 2. Plate 3 shows the beginning of strong pigmentation on day 3 for C. gattii (B and C). Plate 4 illustrates the fluorescence observed on day 3 under a Woods lamp (365 nm.). Row II, plate 1 shows typical growth and dark-brown pigmentation of C. gattii on m-DTDP agar (B and C) after 5 to 7 days. In contrast, C. neoformans (A and D) cannot use the d-amino acids and fails to grow. Plate 2 illustrates the absence of fluorescence of C. gattii after the formation of the dark-brown pigments (masks fluorescence). Plates 3 and 4 show the less-typical pattern observed for C. gattii, quad B (top), which produced smaller amounts of the brown pigments with the fluorescence intensity remaining strong when illuminated with a Woods lamp (365 nm).
Shaking versus stationary cultures using m-DTDP media.
C. gattii cells grown in m-DTDP agar or broth medium manifested pigmentation by day 2 to 5. A gradient phenomenon was often observed after shake cultures were centrifuged (3,000 × g for 60 min) after 5 to 7 days of growth. Underneath the supernatant was a gradient of brown-pigmented yeast cells. The cells at the top of the pellet were the darkest, and the cells at the bottom of the pellet contained the smallest amount of pigment. The supernatant contained some debris and including some water-insoluble pigment that was probably secreted by the yeast cells. The filtered supernatant was an amber-brown color. A gradient or sectoring was not observed for agar-grown cells. Shake cultures are not recommended for C. albicans since the surface area is decreased in comparison to petri plates and light activation is more difficult.
(ii) Selective YCB-DTDP agar.
This selective agar was excellent for differentiating C. gattii from C. neoformans. However, pigmentation and fluorescence were minimal on YCB-DTDP agar. When strains of C. gattii and C. neoformans were compared on YCB-DTDP agar, 98% (66/67) of the C. gattii isolates grew whereas none of the C. neoformans isolates grew (0/72). Light pigmentation was evident for the yeast cells on this agar, and although pigmentation increased with time, overall pigment production was less intense than that observed with m-DTDP. The YCB-DTDP agar contained only 20 g/liter glucose, since higher concentrations resulted in an increased precipitate in the medium. High heat cannot be applied to dissolve the precipitate since tryptophan is heat sensitive. Fluorescence of C. gattii on YCB-DTDP agar (not shown) under a Woods lamp (365 nm) was less intense than the fluorescence observed with m-DTDP agar. C. albicans grew well but produced just a trace of the pink pigment after exposure to light. YCB-DTDP is recommended only to separate C. gattii from C. neoformans.
(iii) Differential m-FDTG agar.
Figure 2 illustrates a dramatic difference in the amount of pigment produced by the varieties. C. neoformans and serotype AD (not shown) produced smaller amounts of pigment than C. gattii. This medium required 7 to 10 days to differentiate the varieties. C. albicans produced a pink light-catalyzed pigment on m-FDTG media. Pink pigment production by C. albicans was equally intense for both m-FDTG and m-DTDP agars. The C. albicans cells exhibited faint fluorescence under a Woods lamp (365 nm) (not shown).
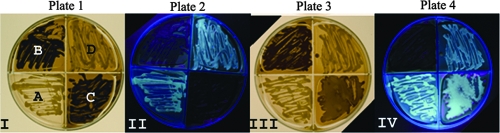
FIG. 2.
Growth and fluorescence of C. gattii and C. neoformans on m-FDTG agar. Plate 1 shows the typical growth and brown pigmentation on m-FDTG agar of C. gattii (B and C) and neoformans (A and D) after 5 to 10 days. Substituting glycine for d-proline resulted in the growth of C. neoformans, which produced light-brown pigments. Strong fluorescence was observed for the lighter-pigmented C. neoformans when it was illuminated with light of 365 nm. In contrast, the fluorescence disappeared once C. gattii produced large amounts of the brown pigment(s). Plate 3 (less typical than plate 1) shows C. gattii (C) producing a moderate amount of brown pigment which is not able to mask the fluorescence at 365 nm (plate 4).
(iv) DOPA agar.
C. gattii, C. neoformans, and C. albicans were not fluorescent under a Woods lamp.
Natural fluorescent microscopy of yeast cells from d-tryptophan media. (i) m-DTDP.
The ability of pigmented and nonpigmented cells to fluoresce was evaluated under various wavelengths (Fig. 3). Nonpigmented cells from early m-DTDP broth cultures (1 to 2 days) demonstrated no fluorescence. Cellular fluorescence was always linked to pigment production and correlated with pigment intensity, such that dark pigmentation was associated with intense fluorescence. These results should not be interpreted to mean that the pigments are fluorescent compounds. TLC results (see Fig. 5) revealed that a pink-violet water-insoluble pigment lacked fluorescence. Some of the brown pigment(s) can be removed from the cells via repeated washing with water or phosphate-buffered saline, leaving cells that still contain fluorescent compounds. Three common fluorescent patterns observed included a peripheral pattern with most of the light intensity confined to the cell wall (Fig. 3B), a weaker peripheral pattern with most of the light intensity also confined to the cell wall (not shown), and a cytoplasmic pattern whereby internal structures such as vacuoles fluoresced brightly (Fig. 3D and E). A portion of the brown pigments (Fig. 3C) produced by C. gattii was observed in the vacuoles/interior of the yeast cell, and the pigment location was not restricted to the cell wall. C. albicans cells were also fluorescent after the formation of the pink pigment, and results were very similar to those previously described for l- and dl-tryptophan (7).
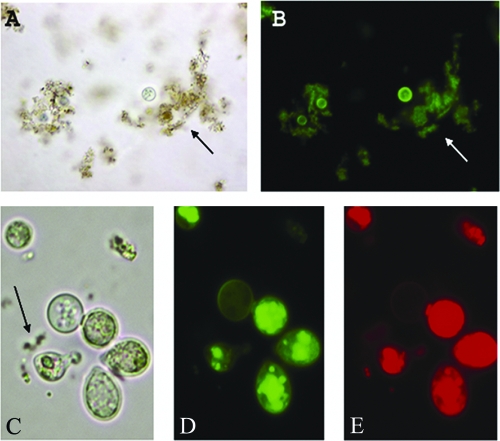
FIG. 3.
Fluorescent microscopy of C. gattii. C. gattii cells and debris of 10-day-old m-DTDP shake cultures viewed in visible (A) or fluorescent (B) illumination, demonstrating fluorescence in the yeast cell wall. The debris (arrow) is thought to be either secreted pigment or extracellularly synthesized pigment that is not water soluble. Strong fluorescence in the cell wall was a commonly observed pattern (B). (C) gattii cells from a 10-day-old m-DTDP shake culture illustrated strong cytoplasmic fluorescence that was concentrated in distinct foci, shown in panels C, D, and E. Quenching of the fluorescence was not observed for B, D, or E. The arrow (C) points to water-insoluble pigment in the medium. The magnification was ×1,000 for panels A and B, and the photographs (C, D, and E) were enlarged to ×2,000 in order to better observe the location of the brown pigments.
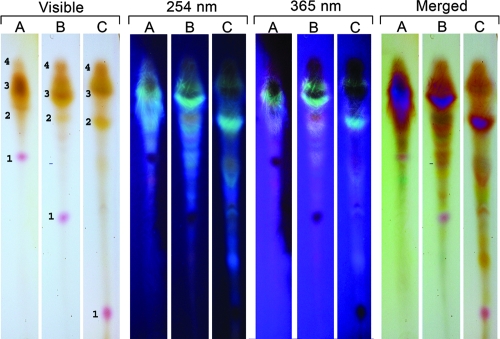
FIG. 5.
TLC separation of pigments and fluorescent compounds extracted from C. gattii. TLC plates illustrating partial separation of pigments and fluorescent compounds extracted with methanol from 2-week-old C. gattii cells grown on m-DTDP medium. The solvent in row A is 100% methanol, that in row B is 90% methanol, and that in row C is 75% methanol. The merged columns are a composite of the visible/254 nm columns. The results indicate the presence of at least four pigments, including a pink/purple pigment (1), a yellow pigment (2), and at least two brown pigments (3 and 4). The Rf values of the pink/purple pigment (visible) are sensitive to changes in the methanol concentration.
(ii) YCB-DTDP.
Fluorescence microscopy analysis of C. gattii and C. albicans from YCB-DTDP broth (not shown) showed very weak fluorescence in comparison to the fluorescence observed in m-DTDP broth (Fig. 3).
(iii) m-FDTG.
C. gattii grown in m-FDTG broth (results not shown) exhibited fluorescence similar to that of the C. gattii cells grown in m-DTDP broth. Cellular fluorescence was more intense for C. gattii than for C. neoformans after growth in m-FDTG broth. Candida albicans cells were also fluorescent after the formation of the pink pigment, and results were very similar to those described from l- and dl-tryptophan (7).
(iv) DOPA.
C. gattii, C. neoformans, and C. albicans grown in DOPA containing broths were negative for fluorescence.
Detection of aromatic compounds in supernatants. (i) m-DTDP.
Supernatants from cryptococcal cultures demonstrate coloration, and pigmented cells extracted with methanol were fluorescent (Fig. 4) The noninoculated filtered m-DTDP media failed to react with acids, and colored compounds were not produced. Concentrated sulfuric acid was not used since it reacted with the noninoculated m-DTDP media to form a dark-brown color. Instead, diluted sulfuric acid (37.5%) was utilized since no reaction was observed with the original media or with the supernatants of m-DTDP. Addition of the Salkowski reagent in 37.5% sulfuric acid with ferric chloride changed the color of the supernatant to a brilliant purple. Substituting zinc chloride or manganese chloride for the ferric chloride yielded a pink/fuchsia color which usually changed to purple upon standing for several hours. The pink/fuchsia and purple colors indicate that d-tryptophan was metabolized to various aromatic compounds. The addition of acetic acid, bleach, or the Kovacs reagent to culture supernatants did not change their color. The supernatants of C. albicans failed to react with the Salkowski reagent.
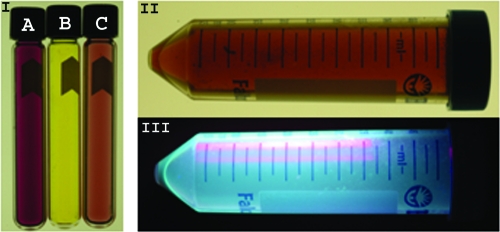
FIG. 4.
Supernatants from cryptococcal cultures demonstrate coloration, and pigmented cells extracted with methanol were fluorescent. Tube B (section I) is the amber color of the filtered supernatant from m-DTDP grown C. gattii cultures. Tube A (section I) shows the pink/fuchsia color resulting from addition of the Salkowski reagent to the supernatant. Tube C (section I) shows the lighter pink color resulting from addition of hydrochloric acid, nitric acid, or phosphoric acid to the supernatant. The methanol-extracted brown pigments from m-DTDP cells are shown in section II. The methanol-extracted pigments and chemical compounds were highly fluorescent at 365 nm (section III).
(ii) YCB-DTDP.
The Salkowski reagent failed to react with the supernatants of C. gattii or C. albicans.
(iii) m-FDTG.
The Salkowski reagent also strongly reacted with the m-FDTG supernatants of both C. gattii and C. neoformans (purple color). Supernatants of C. albicans failed to react with the Salkowski reagent.
(iv) l-, dl-, and d-DOPA.
The Salkowski reagent failed to react with the DOPA supernatants of both C. gattii and C. neoformans.
Extraction of brown pigments and fluorescent compounds.
Methanol, ethanol, or n-butanol extracted the pigments and fluorescent compounds from C. gattii cells that were grown on m-DTDP broth. The brown pigments and fluorescent compounds could not be extracted from the C. gattii cells with 100% chloroform, acetone, xylene, or acetonitrile. Figure 4, section II, shows the brown pigments extracted with methanol. The brown pigments masked fluorescence at 365 nm in water-based medium. However, the fluorescence returned once the yeast cells were extracted with alcohol (Fig. 4, section III). Similar results were obtained for m-FDTG-grown C. gattii cultures.
TLC partial separation of pigments and fluorescent compounds.
The methanol-extracted intracellular pigments and fluorescent compounds from C. gattii cultured on m-DTDP media migrated from the origin when the solvent system was at least 75% methanol (Fig. 5). At least four pigments (a pink/purple, two browns, and a yellow) were observed under visible light, and multiple fluorescent compounds were detected under UV light (254 or 365 nm). Similar results were obtained from C. gattii strains cultured on m-FDTG media. The TLC separation was more complete and distinct when the solvent was 75% or 90% methanol. In contrast, 100% methanol was less satisfactory. The pigments and fluorescent compounds remained at the TLC origin when the solvent system was chloroform, acetone, xylene, or 50% methanol. The resolution and separation of the pigments were unacceptable when acetonitrile was used as the solvent. These results strongly suggest that the pigments and fluorescent compounds are not lipid like. Methanol-extracted pigments and fluorescent compounds from C. neoformans (m-FDTG media) could also be separated by TLC (not shown).
Acid resistance of d-tryptophan pigments.
Given that melanins are acid resistant and can be isolated from melanized cells by digestion in strong acids, we treated pigmented cells with acid. In contrast to l-DOPA-derived melanin particles (“ghosts”), no tryptophan particles were recovered from the brown-pigmented C. gattii grown on m-DTDP media. Therefore, the tryptophan-grown C. gattii cells were not acid resistant, suggesting that these pigments are not melanins (56).
Spectroscopy.
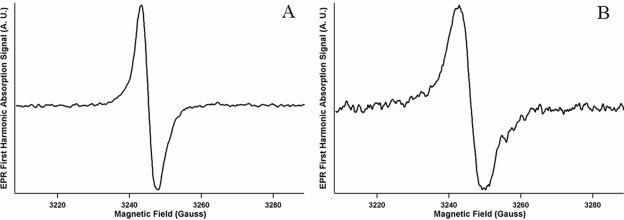
One of the important characteristics of melanin is the presence of a stable free-radical population that produces distinctive signals by EPR spectroscopy (16). The inability to detect an EPR signal over the noise level (referred to here as “negative EPR results”) for C. gattii (NIH 34 and 444), C. neoformans (H99, 2ETU, and 2ETU-C), and C. albicans (BSMY 212) occurred after growth in Sabouraud dextrose broth (control). Positive EPR results were obtained for C. gattii cells that produced melanin in d- or l-DOPA medium. C. gattii cells that produced pigments derived from d- or l-tryptophan (m-DTDP or m-FTDP) also gave a positive signal. The C. gattii (NIH 444) EPR spectrum for the DOPA-derived pigments (melanin) was distinct and clearly different from the EPR spectrum derived with the tryptophan pigments (Fig. 6). The g value is defined as the EPR spectroscopic splitting constant proportional to the ratio of EPR frequency to resonance field. Although all spectra exhibit similar g values for their zero crossing points (e.g., 2.0036 for NIH 444 with d-tryptophan and 2.0042 for NIH 444 with d-DOPA), the spectra derived from cells grown in tryptophan medium exhibited first harmonic peak-to-peak linewidths that were significantly broader (approximately 7 G) than for those grown in DOPA media (approximately 4 G). The EPR spectra from d- and l-DOPA were similar to each other in linewidth and g values, as were the EPR spectra from d- and l-tryptophan. Methanol-soluble pigments extracted from d- and l-tryptophan cells and the remnants of the extracted cells from C. gattii (NIH 444) gave negative EPR signals.
FIG. 6.
EPR spectra for C. gattii (NIH 444) pigments derived from d-DOPA (A) or d-tryptophan (B). The d-tryptophan EPR spectrum contained a distinctly broader peak-to-peak linewidth (∼7 G) than the d-DOPA spectra (∼4 G). Instrumental parameters are as follows: modulation amplitude, 1.6 G; microwave frequency, 9.104 GHz; microwave power, 0.50 mW; and temperature, 77K; number of scans averaged, nine.
A strong positive EPR signal was obtained after C. neoformans (H99) produced melanin from d-or l-DOPA. A weaker EPR signal was recorded for the d-tryptophan-derived pigments of C. neoformans (H99). The EPR signal for the C. neoformans laccase mutant 2ETU was negative with d- or l-DOPA. However, the EPR signal for the laccase mutant was strongly positive for l-tryptophan and weakly positive for d-tryptophan. The positive d- and l-tryptophan EPR signal for the laccase mutant (2ETU) indicates that the melanin and tryptophan pigments are two distinct and independent systems. The complemented strain 2ETU-C gave a weakly positive EPR signal for l- and d-DOPA and d-tryptophan. Negative EPR signals were recorded for C. albicans on all substrates.
Zeta potential measurements.
A consistent negative surface charge for C. gattii cells grown in Sabouraud dextrose broth (nonpigmented), d- or l-DOPA (pigmented), or d- and l-tryptophan (pigmented) broths was observed. The negative charge ranged from −22.82 to −34.22 mV. There were no significant differences in the zeta potential between the pigments produced from DOPA and those from tryptophan (data not shown). Likewise, significant differences in the zeta potential were not observed when C. neoformans produced pigments from DOPA or from tryptophan. The negative charge ranged from −18.53 to −42.21 mV. The negative surface charge on C. albicans (−12.73 to −20.72 mv) was substantially smaller than that for the two Cryptococcus spp. The strength of the negative charge did not correlate to the intensity of the EPR signal.
Differences between pigmentation from dl-, d-, and l-DOPA and d-tryptophan.
Pigmented cells grown on dl-, d-, or l-DOPA and d-tryptophan manifested numerous differences. Figure 4 illustrates the color changes to the d-tryptophan supernatants that occured when the Salkowski and acid reagents (HCl, HNO3, or H3PO4) were added. In contrast, color changes were not observed when the Salkowski and/or acid reagent was added to the d-, dl-, or l-DOPA supernatant. Rapid melanization of C. gattii strains from d-, dl-, or l-DOPA often occurred within 1 h when cells were deprived of all nutrients for 24 h. This rapid melanization was also observed for dopamine, 4-hydroxymetanilamide, and 2,5-diaminobenzenesulfonic acid. In contrast, cells incubated with l- or d-tryptophan failed to produce pigment between 1 h and 5 days of incubation. Table 1 compares and contrasts features of C. gattii pigmentation in medium containing DOPA or tryptophan. Major differences included the observation that the pigment derived from d-tryptophan was alcohol soluble whereas the melanin pigment formed from DOPA was insoluble in alcohol. Melanin production was optimal when the carbohydrate source was restricted, whereas d-tryptophan-derived pigment production required a high carbohydrate concentration. The location of melanin was restricted to the cell wall, whereas the tryptophan-derived pigments were not restricted to the cell wall.
TABLE 1.
Comparison of C. gattii pigmentation using d-tryptophan or d-, dl-, or l-DOPA
| Characteristic | Occurrence with medium
|
Reference(s) | |
|---|---|---|---|
| d-Tryptophan | d-, dl-, or l-DOPA and related substrates | ||
| Pigment extractable with alcohol | Yes | No | 8, 9 |
| Pigment location restricted to cell wall | No | Yes | 56 |
| Particle formation after acid treatment | No | Yes | 56 |
| Pigmentation decreased in the presence of high glucose, 20-80 g/liter | No | Yes | 6, 41, 43, 44 |
| Salkowski reagent turns supernatant pink, fuchsia, or purple | Yes | Noa | |
| Substrate must contain hydroxyl or amino groups on the aromatic ring | No | Yes | 9, 14, 15, 18-20, 26, 44, 46, 55 |
| C. gattii produces greater quantities of pigment than C. neoformansb | Yes | No | 15, 18, 19, 41, 45 |
| Yeast cells fluorescent under Woods lamp (365 nm) | Yes | Noa | |
| Yeast cells fluorescent with FITC filterc | Yes | Noa | |
| Pigmentation positive in starved cells | No | Yes | 6, 44 |
| Positive and unique EPR spectra | Yes | Yesa | 15 |
| Negative surface charge | Yes | Yes | 38 |
| Pigmentation decreased at 37°C | Yes | Yes | 22 |
| Pigments produced by C. albicans | Yes | No | 8,9 |
| Yeast cells form firm pellet at 3,000 × g after 10 min | No | Yesa | |
| Top portion of yeast pellet has greater color intensity than the bottom | Yes | Noa | |
Results for DOPA are found in the text.
Results with m-FDTG agar (tryptophan agar).
FITC, fluorescein isothiocyanate.
Wavelength dependence of pink pigment production by C. albicans.
C. albicans strains remained colorless when cultured for at least 10 days on either m-DTDP or m-FDTG agar under normal laboratory lighting. A fluorescent or UV light must be placed approximately 6 in. above plates to catalyze pink pigment formation. Since the Polychrome II illuminator does not generate heat, it was placed a few centimeters above the m-DTDP or m-FDGT agar plates. We studied the ability of light ranging in wavelength from 340 nm to 490 nm to photoactivate the pink pigment. Figure 7 shows typical pink pigment formation using the Polychrome II illuminator (420 nm for 16 h). Wavelengths above 490 nm did not induce pink pigment production by C. albicans. Photoactivated pink pigment production was strongest at 340 nm (lowest wavelength of the Polychrome II illuminator) and weakest at 490 nm. A Woods lamp set at either 365 nm or 254 nm also photoactivated the pink pigment. The data indicate that the pink pigment can be induced by wavelengths from 254 nm to 490 nm. A standard fluorescent light also was able to photoactivate the pigment.
FIG. 7.
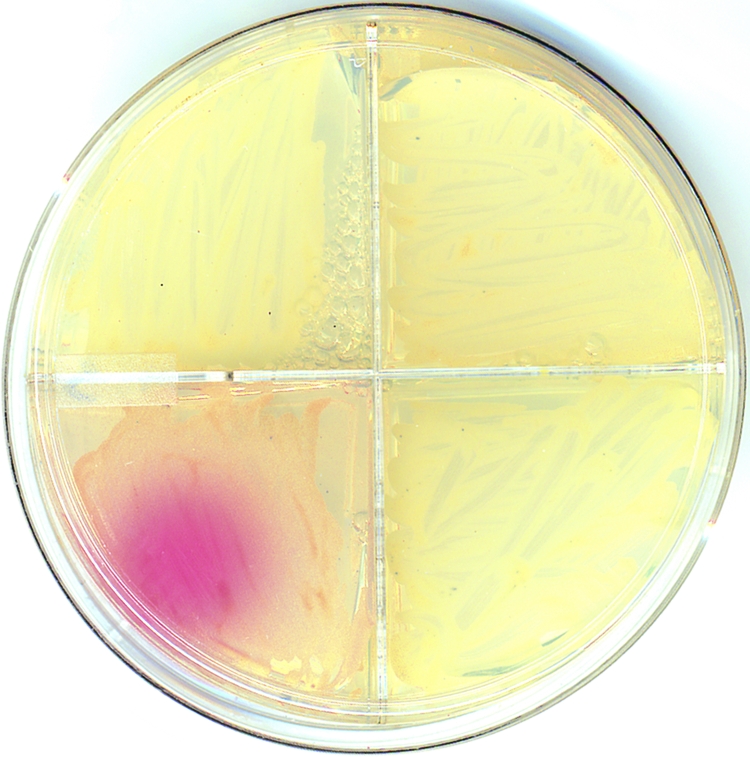
Pigmentation of C. albicans after exposure with monochromatic light. After 1 week of growth on m-DTDP, C. albicans was exposed to monochromatic light (420 nm) for 16 h. The lower left quadrant shows typical results using the Polychrome II illumination unit. The pink pigment was photoactivated between 340 nm and 490 nm.
DISCUSSION
Studies indicate that the accuracy of correct identification of the C. gattii strains using d-proline assimilation tests (2, 3, 13, 31) or d-tryptophan assimilation tests (2, 3, 35) was favorable with the CGB (25) method. The assimilation assays are based on the fact that only the C. gattii strains utilized these d-amino acids as the sole nitrogen source. In some studies the accuracy of these tests approaches 99% but the assay was relatively slow because the formulation used low glucose concentrations (1 to 2%). The current study suggests that it is feasible to combine d-tryptophan and d-proline into a single medium to separate C. gattii from C. neoformans. The use of m-DTDP or YCB-DTDP increased the carbohydrate source from 2 to 4%, such that most C. gattii strains achieved growth in 1 to 3 days. The current limitation for growth is the nitrogen source. We suggest using YCB-DTDP agar or m-DTDP agar, since the accuracy is similar to those of previously published methods and test results are available in a rapid fashion. Furthermore, we recommend keeping the cultures for 5 to 10 days if the goal is to observe maximum pigmentation of the C. gattii isolates. Ninety-four percent of the C. gattii strains grew on m-DTDP agar, whereas ninety-eight percent grew on the YCB-DTDP agar. The four strains that failed to grow on the minimal agar may require a specific vitamin/coenzyme or cofactor that was present in the assimilation base but not in m-DTDP agar. It must be emphasized that a positive test for the identification of a strain as C. gattii is growth in this medium irrespective of pigmentation.
Chaskes and Tyndall described production of two pigments (a water-soluble pink pigment and a water-insoluble brown pigment) when C. neoformans was cultured on l- or dl-tryptophan agar (10). The serotypes used in that study were not known. Mukamurangwa et al. (35) subsequently reported that C. gattii strains produced a brown diffusible pigment from d-tryptophan. We now report production of both intracellular brown pigments and an extracellular brown/amber pigment from d-tryptophan. Therefore, we deduce that the water-soluble pink pigment (10) is produced by C. neoformans from only l-tryptophan. The pink pigment was not produced by C. gattii or C. neoformans on any of the d-tryptophan agars employed in the current study. In contrast, C. albicans was able to produce a pink water-soluble pigment from d-tryptophan, the stereoisomer of l-tryptophan. The pink pigments produced by C. albicans from d- and l-tryptophan are probably identical, since both require photoactivation and both are pH indicators. The pink color intensifies with acid, begins to fade at pH 5.6, and completely disappears at pH 6.5. Pink pigment production was more rapid with l-tryptophan (7) than with d-tryptophan. Several other Candida species can also produce the pink pigment from d-tryptophan (Chaskes and Casadevall, unpublished results).
The pigments produced from d-tryptophan by C. gattii are not melanins. In contrast to melanins, which are insoluble in common organic solvents (8, 9), d-tryptophan-derived pigments were extractable with various alcohols. Melanin formation occurs in the cell wall (56), whereas the pigments formed from d-tryptophan are not confined to the cell wall and most of the pigment appears to be localized within the interior of the yeast cell. Furthermore, the pigment derived from d-tryptophan was not acid resistant, and no particles were recovered from pigmented cells that were treated with hot acids. In contrast, the cell wall melanin is acid resistant, and melanin “ghost” particles are readily recovered following hot acid treatment. A low glucose concentration of 0.3 to 0.5% is usually required for intense melanin production (9, 41, 43), whereas pigment production from d-tryptophan was enhanced when yeast cultures were grown at higher glucose concentrations (2 to 8%). The supernatant of m-DTDP tested positive for aromatic derivatives with Salkowski reagent that contained iron, zinc, or manganese. Concentrated hydrochloric, nitric, or phosphoric acid also reacted with the supernatants. The Kovacs reagent, which specifically detects indole and indole-like compounds, was negative. The Salkowski reagent detects a wider range of aromatic derivatives. A positive Salkowski test is indicated by the development of a strong fuchsia color. Salkowski-positive supernatants have not been reported for C. neoformans or C. gattii cultures that produce melanin.
Additionally, d-tryptophan does not contain hydroxyl or amino groups on the phenyl ring (8, 9, 14, 15, 18-20, 44, 46, 55) that can be oxidized to melanin by the usual l-DOPA melanogenesis pathway found in C. neoformans (41, 45). d-DOPA can also serve as a substrate, and melanization occurred equally well with both enantiomers (15). Both laccase and the pigment melanin are associated with the virulence of C. neoformans (39, 40, 47, 58). Kwon-Chung et al. (26) also reported that many indole compounds that have amino or hydroxyl groups on the phenyl ring were converted to a melanin-like pigment by C. neoformans.
In this study, we noted dramatic differences in the quantity of pigment produced from d-tryptophan on m-FDTG agar, with C. gattii producing much larger quantities than C. neoformans. In contrast, both C. gattii and C. neoformans are good melanin producers from DOPA. Even though many substrates have been tested, there are no reports of C. gattii consistently producing greater amounts of melanin than C. neoformans. Furthermore, melanin is rapidly produced within 5 min to 4 h from l-, dl-, or d-DOPA by C. gattii cells that are deprived of nutrients for 24 h. In contrast, l-, dl-, or d-tryptophan did not induce pigment production when the cells were starved.
Melanin (16, 38, 49) and the pigments derived from d-tryptophan both have similar but distinctive positive magnetic properties (EPR) and similar negative zeta potentials. However, the d-tryptophan-derived pigments are acid soluble and hence do not meet the classical definition of melanins. Pigmentation is decreased for both systems when the yeast cells are incubated at 37°C. Finally, the Candida genus metabolizes dl-, l- (7), or d-tryptophan (current study) to pigmented products but cannot convert DOPA to melanin (8, 9). This study concludes that the melanin pigment and the tryptophan-derived pigments are the products of two separate and distinctive systems.
Young C. gattii cultures that were grown on m-DTDP or m-FDTG agar exhibited fluorescence under a Woods lamp (365 nm). The fluorescence was later masked by the production of the dark-brown pigments. In contrast, the fluorescence of C. neoformans on m-FDTG agar remained intense, since this yeast produces smaller quantities of the brown pigments. Similar observations were reported by Slots and Reynolds with Prevotella melaninogenic (51). Blood agar colonies of Prevotella melaninogenica produce a salmon, orange, or pink fluorescence after 48 h when illuminated with the long wavelength of a woods lamp. After the colonies darken to a brown color, the fluorescence was a vivid red. The red fluorescence disappeared after the colonies become black. Suspending the black bacterial cells in methanol resulted in a return of fluorescence. We also observed the return of fluorescence when the brown-pigmented cells produced by C. gattii were suspended in methanol. The fluorescence of Prevotella intermedius and Porphyromonas asaccharolytica also decreases with the formation of black pigment (32, 36).
The low density of the pigmented C. gattii cells that were grown in d-tryptophan broth is evident, since after centrifugation at 3,000 × g for 60 min, the darker-pigmented cells were located in the pellicle and the top layer of the pellet whereas the lightest pigmented C. gattii cells were confined to the bottom layer of the pellet. The most likely explanation is that the intensely pigmented cells are less dense than the lighter-pigmented cells. Tryptophan is the most hydrophobic of all amino acids and has been detected along with indole derivatives in many types of cell membranes (5, 12, 28, 37, 50, 57). Hence, one possible explanation for the lower density of the pigmented C. gattii cells is a higher lipid concentration. Balish and Svihla (1) reported that C. albicans possessed a higher-than-usual lipid content when cultured on tryptophan. However, the pigments and fluorescent compounds produced from d-tryptophan in the current study are not lipid like, since they are insoluble in lipid solvents such as chloroform and acetone.
In summary, we show that C. gattii, C. neoformans, and C. albicans metabolize and produce pigments from d-tryptophan. The current study concludes that C. gattii can be rapidly separated from C. neoformans, since only the former can grow and produce pigments on d-tryptophan and d-proline agar. The pigments produced by C. gattii from d-tryptophan are distinct and separate from the melanin pigment produced from DOPA. C. neoformans produces smaller amounts of pigment than C. gattii when glycine is substituted for d-proline (m-FDTG agar). Finally, C. albicans produces a water-soluble pink light-catalyzed pigment from d-tryptophan. We believe this assay can be readily adapted for discriminating C. gattii from C. neoformans strains in clinical laboratories.
Acknowledgments
Funding for this project was provided by NIH awards R21GM071421 and AI052733-03.
We thank Yan Deng (Albert Einstein College of Medicine Analytical Imaging Facility) for the quad plate photography. We thank Sarah Gross, Carla Martin, Matthew Bahamonde, David Goldman, Helene Eisenman, and Josh Noshanchuk for helping with the layout of the pictures and reviewing the manuscript. We thank Robert Singer and Shailesh M. Shenoy for loaning the Till-Photonics Polychrome II equipment.
Footnotes
Published ahead of print on 7 November 2007.
REFERENCES
- 1.Balish, E., and G. Svihla. 1968. Ultraviolet microscopy of purines and amino acids in the vacuole of Candida albicans. J. Bacteriol. 96259-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baro, T., J. M. Torres-Rodriguez, M. H. De Mendoza, Y. Morera, and C. Alia. 1998. First identification of autochthonous Cryptococcus neoformans var. gattii isolated from goats with predominantly severe pulmonary disease in Spain. J. Clin. Microbiol. 36458-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baro, T., J. M. Torres-Rodriguez, Y. Morera, C. Alia, O. Lopez, and R. Mendez. 1999. Serotyping of Cryptococcus neoformans isolates from clinical and environmental sources in Spain. J. Clin. Microbiol. 371170-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett, J. E., K. J. Kwon-Chung, and T. S. Theodore. 1978. Biochemical differences between serotypes of Cryptococcus neoformans. Sabouraudia 16167-174. [PubMed] [Google Scholar]
- 5.Black, K. M., I. Clark-Lewis, and C. J. Wallace. 2001. Conserved tryptophan in cytochrome c: importance of the unique side-chain features of the indole moiety. Biochem. J. 359715-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaskes, S., S. C. Edberg, and J. M. Singer. 1981. A DL-DOPA drop test for the identification of Cryptococcus neoformans. Mycopathologia 74143-148. [DOI] [PubMed] [Google Scholar]
- 7.Chaskes, S., and A. W. Phillips. 1974. Pigmentation and autofluorescence of Candida species after growth on tryptophan media. Can. J. Microbiol. 20595-603. [DOI] [PubMed] [Google Scholar]
- 8.Chaskes, S., and R. L. Tyndall. 1978. Pigment production by Cryptococcus neoformans and other Cryptococcus species from aminophenols and diaminobenzenes. J. Clin. Microbiol. 7146-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaskes, S., and R. L. Tyndall. 1975. Pigment production by Cryptococcus neoformans from para- and ortho-diphenols: effect of the nitrogen source. J. Clin. Microbiol. 1509-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaskes, S., and R. L. Tyndall. 1978. Pigmentation and autofluorescence of Cryptococcus species after growth on tryptophan and anthranilic acid media. Mycopathologia 64105-112. [DOI] [PubMed] [Google Scholar]
- 11.Chaturvedi, S., M. Dyavaiah, R. A. Larsen, and V. Chaturvedi. 2005. Cryptococcus gattii in AIDS patients, southern California. Emerg. Infect. Dis. 111686-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Planque, M. R., B. B. Bonev, J. A. Demmers, D. V. Greathouse, R. E. Koeppe II, F. Separovic, A. Watts, and J. A. Killian. 2003. Interfacial anchor properties of tryptophan residues in transmembrane peptides can dominate over hydrophobic matching effects in peptide-lipid interactions. Biochemistry 425341-5348. [DOI] [PubMed] [Google Scholar]
- 13.Dufait, R., R. Velho, and C. De Vroey. 1987. Rapid identification of the two varieties of Cryptococcus neoformans by D-proline assimilation. Mykosen 30483. [PubMed] [Google Scholar]
- 14.Edberg, S. C., S. J. Chaskes, E. Alture-Werber, and J. M. Singer. 1980. Esculin-based medium for isolation and identification of Cryptococcus neoformans. J. Clin. Microbiol. 12332-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenman, H. C., M. Mues, S. Weber, S. Frases, S. Chaskes, G. Gerfen, and A. Casadevall. 2007. Cryptococcus neoformans laccase catalyzes melanin synthesis from both D- and L-DOPA. Microbiol. 1533954-3962. [DOI] [PubMed] [Google Scholar]
- 16.Enochs, W. S., M. J. Nilges, and H. M. Swartz. 1993. A standard test for the identification and characterization of melanins using electron paramagnetic resonance (EPR) spectroscopy. Pigment Cell Res. 691-99. [DOI] [PubMed] [Google Scholar]
- 17.Franzot, S. P., I. F. Salkin, and A. Casadevall. 1999. Cryptococcus neoformans var. grubii: separate varietal status for Cryptococcus neoformans serotype A isolates. J. Clin. Microbiol. 37838-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frases, S., S. Chaskes, E. Dadachova, and A. Casadevall. 2006. Induction by Klebsiella aerogenes of a melanin-like pigment in Cryptococcus neoformans. Appl. Environ. Microbiol. 721542-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frases, S., A. Salazar, E. Dadachova, and A. Casadevall. 2007. Cryptococcus neoformans can utilize the bacterial melanin precursor homogentisic acid for fungal melanogenesis. Appl. Environ. Microbiol. 73615-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia-Rivera, J., H. C. Eisenman, J. D. Nosanchuk, P. Aisen, O. Zaragoza, T. Moadel, E. Dadachova, and A. Casadevall. 2005. Comparative analysis of Cryptococcus neoformans acid-resistant particles generated from pigmented cells grown in different laccase substrates. Fungal Genet. Biol. 42989-998. [DOI] [PubMed] [Google Scholar]
- 21.Hoang, L. M., J. A. Maguire, P. Doyle, M. Fyfe, and D. L. Roscoe. 2004. Cryptococcus neoformans infections at Vancouver Hospital and Health Sciences Centre (1997-2002): epidemiology, microbiology and histopathology. J. Med. Microbiol. 53935-940. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda, R., T. Shinoda, T. Morita, and E. S. Jacobson. 1993. Characterization of a phenol oxidase from Cryptococcus neoformans var. neoformans. Microbiol. Immunol. 37759-764. [DOI] [PubMed] [Google Scholar]
- 23.Kidd, S. E., F. Hagen, R. L. Tscharke, M. Huynh, K. H. Bartlett, M. Fyfe, L. Macdougall, T. Boekhout, K. J. Kwon-Chung, and W. Meyer. 2004. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada). Proc. Natl. Acad. Sci. USA 10117258-17263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwon-Chung, K. J., J. E. Bennett, and T. S. Theodore. 1978. Cryptococcus bacillisporus sp. nov: serotype B-C of Cryptococcus neoformans. Int. J. Syst. Bacteriol. 28616-620. [Google Scholar]
- 25.Kwon-Chung, K. J., I. Polacheck, and J. E. Bennett. 1982. Improved diagnostic medium for separation of Cryptococcus neoformans var. neoformans (serotypes A and D) and Cryptococcus neoformans var. gattii (serotypes B and C). J. Clin. Microbiol. 15535-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwon-Chung, K. J., W. K. Tom, and J. L. Costa. 1983. Utilization of indole compounds by Cryptococcus neoformans to produce a melanin-like pigment. J. Clin. Microbiol. 181419-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwon-Chung, K. J., and A. Varma. 2006. Do major species concepts support one, two or more species within Cryptococcus neoformans? FEMS Yeast Res. 6574-587. [DOI] [PubMed] [Google Scholar]
- 28.Ladokhin, A. S. 1999. Evaluation of lipid exposure of tryptophan residues in membrane peptides and proteins. Anal. Biochem. 27665-71. [DOI] [PubMed] [Google Scholar]
- 29.Litvintseva, A. P., R. Thakur, L. B. Reller, and T. G. Mitchell. 2005. Prevalence of clinical isolates of Cryptococcus gattii serotype C among patients with AIDS in sub-Saharan Africa. J. Infect. Dis. 192888-892. [DOI] [PubMed] [Google Scholar]
- 30.MacDougall, L., S. E. Kidd, E. Galanis, S. Mak, M. J. Leslie, P. R. Cieslak, J. W. Kronstad, M. G. Morshed, and K. H. Bartlett. 2007. Spread of Cryptococcus gattii in British Columbia, Canada, and detection in the Pacific Northwest, USA. Emerg. Infect. Dis. 1342-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez Machin, G., L. Barrial de la Rosa, M. Illnuit Zargozi, C. Valdez, C. Fernindez Andreu, M. Peruren Lancha, J. Polo, and D. Mendoza Llanes. 2004. Usefulness of D-proline in the differentiation of varieties Cryptococcus neoformans. Rev. Cubana Med. 177-79. [PubMed] [Google Scholar]
- 32.Mayrand, D., and S. C. Holt. 1988. Biology of asaccharolytic black-pigmented Bacteroides species. Microbiol. Rev. 52134-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCarthy, K. M., J. Morgan, K. A. Wannemuehler, S. A. Mirza, S. M. Gould, N. Mhlongo, P. Moeng, B. R. Maloba, H. H. Crew-Brown, M. E. Brandt, and R. A. Hajjeh for the Gauteng Cryptococcal Surveillance Initiative Group. 2006. Population-based surveillance for cryptococcosis in an antiretroviral-naïve South African province with a high HIV seroprevalence. AIDS 202199-2206. [DOI] [PubMed] [Google Scholar]
- 34.Morgan, J., K. M. McCarthy, S. Gould, K. Fan, B. Arthington-Skaggs, N. Iqbal, K. Stamey, R. A. Hajjeh, and M. E. Brandt for the Guateng Cryptococcal Surveillance Iniative Group. 2006. Cryptococcus gattii infection: characteristics and epidemiology of cases identified in a South African province with high HIV seroprevalence, 2002-2004. Clin. Infect. Dis. 431077-1080. [DOI] [PubMed] [Google Scholar]
- 35.Mukamurangwa, P., C. Raes-Wuytack, and C. De Vroey. 1995. Cryptococcus neoformans var. gattii can be separated from var. neoformans by its ability to assimilate D-tryptophan. J. Med. Vet. Mycol. 33419-420. [PubMed] [Google Scholar]
- 36.Myers, M. B., G. Cherry, B. B. Bornside, and G. H. Bornside. 1969. Ultaviolet red fluorescence of Bacteroides melaninogenicus. Appl. Microbiol. 17760-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Norman, K. E., and H. Nymeyer. 2006. Indole localization in lipid membranes revealed by molecular simulation. Biophys. J. 912046-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nosanchuk, J. D., and A. Casadevall. 1997. Cellular charge of Cryptococcus neoformans: contributions from the capsular polysaccharide, melanin, and monoclonal antibody binding. Infect. Immun. 651836-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nosanchuk, J. D., and A. Casadevall. 2003. The contribution of melanin to microbial pathogenesis. Cell Microbiol. 5203-223. [DOI] [PubMed] [Google Scholar]
- 40.Nosanchuk, J. D., J. Rudolph, A. L. Rosas, and A. Casadevall. 1999. Evidence that Cryptococcus neoformans is melanized in pigeon excreta: implications for pathogenesis. Infect. Immun. 675477-5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nurudeen, T. A., and D. G. Ahearn. 1979. Regulation of melanin production by Cryptococcus neoformans. J. Clin. Microbiol. 10724-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nyhus, K. J., A. T. Wilborn, and E. S. Jacobson. 1997. Ferric iron reduction by Cryptococcus neoformans. Infect. Immun. 65434-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paliwal, D. K., and H. S. Randhawa. 1978. Evaluation of a simplified Guizotia abyssinica seed medium for differentiation of Cryptococcus neoformans. J. Clin. Microbiol. 7346-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Polacheck, I., V. J. Hearing, and K. J. Kwon-Chung. 1982. Biochemical studies of phenoloxidase and utilization of catecholamines in Cryptococcus neoformans. J. Bacteriol. 1501212-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Polacheck, I., and K. J. Kwon-Chung. 1988. Melanogenesis in Cryptococcus neoformans. J. Gen. Microbiol. 1341037-1041. [DOI] [PubMed] [Google Scholar]
- 46.Polacheck, I., Y. Platt, and J. Aronovitch. 1990. Catecholamines and virulence of Cryptococcus neoformans. Infect. Immun. 582919-2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salas, S. D., J. E. Bennett, K. J. Kwon-Chung, J. R. Perfect, and P. R. Williamson. 1996. Effect of the laccase gene CNLAC1, on virulence of Cryptococcus neoformans. J. Exp. Med. 184377-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salkin, I. F., and N. J. Hurd. 1982. New medium for differentiation of Cryptococcus neoformans serotype pairs. J. Clin. Microbiol. 15169-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sarna, T., and P. M. Plonka. 2005. Biophysical studies on melanin: paramagnetic, ion-exchange and redox properties of melanin pigments and their photoreactivity, p. 125-146. In Biomedical EPR, part A: free radical, metals, medicine, and physiology, vol. 23. Springer, New York, NY. [Google Scholar]
- 50.Schiffer, M., C. H. Chang, and F. J. Stevens. 1992. The functions of tryptophan residues in membrane proteins. Protein Eng. 5213-214. [DOI] [PubMed] [Google Scholar]
- 51.Slots, J., and H. S. Reynolds. 1982. Long-wave UV light fluorescence for identification of black-pigmented Bacteroides spp. J. Clin. Microbiol. 161148-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sorrell, T. C. 2001. Cryptococcus neoformans variety gattii. Med. Mycol. 39155-168. [PubMed] [Google Scholar]
- 53.Steenbergen, J. N., and A. Casadevall. 2000. Prevalence of Cryptococcus neoformans var. neoformans (serotype D) and Cryptococcus neoformans var. grubii (serotype A) isolates in New York City. J. Clin. Microbiol. 381974-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Upton, A., J. A. Fraser, S. E. Kidd, C. Bretz, K. H. Bartlett, J. Heitman, and K. A. Marr. 2007. First contemporary case of human infection with Cryptococcus gattii in Puget Sound: evidence for spread of the Vancouver Island outbreak. J. Clin. Microbiol. 453086-3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang, H. S., R. T. Zeimis, and G. D. Roberts. 1977. Evaluation of a caffeic acid-ferric citrate test for rapid identification of Cryptococcus neoformans. J. Clin. Microbiol. 6445-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang, Y., P. Aisen, and A. Casadevall. 1996. Melanin, melanin “ghosts,” and melanin composition in Cryptococcus neoformans. Infect. Immun. 642420-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yau, W. M., W. C. Wimley, K. Gawrisch, and S. H. White. 1998. The preference of tryptophan for membrane interfaces. Biochemistry 3714713-14718. [DOI] [PubMed] [Google Scholar]
- 58.Zhu, X., and P. R. Williamson. 2004. Role of laccase in the biology and virulence of Cryptococcus neoformans. FEMS Yeast Res. 51-10. [DOI] [PubMed] [Google Scholar]