Abstract
Ten yeast bloodstream isolates identified as Candida parapsilosis by conventional methods grew as turquoise blue colonies on Chromagar media. Subsequent sequence analysis showed that these isolates were the species Lodderomyces elongisporus. To our knowledge, this is the first published report of L. elongisporus as a cause of human disease.
Candida parapsilosis is the third leading cause of Candida bloodstream infections in North America, the second leading cause of Candida bloodstream infections in Europe, and the second leading cause of candidemia in children (14, 15). This important pathogen can be found in a wide variety of niches and can cause life-threatening nosocomial infections. Recent studies have shown that some clinical isolates identified as C. parapsilosis are in fact isolates of the closely related species Candida orthopsilosis and Candida metapsilosis (8, 11, 19). Although comprising only about 10% of the total number of C. parapsilosis isolates (S. Lockhart, S. A. Messer, M. A. Pfaller, and S. Diekema, unpublished data), these other species may inhabit important niches in certain populations.
Molecular and biochemical investigations of other Candida species have also identified new species that had previously been identified as more common species, such as Candida dubliniensis/Candida albicans (18), Candida nivariensis/Candida glabrata (1), and Coccidioides posadasii/Coccidioides immitis (4). In our characterization of C. parapsilosis isolates from a worldwide collection, approximately 2% of the isolates were further examined because of their unique color on chromogenic media and because BanI-digested SADH fragment amplification screening did not reveal them to be C. parapsilosis, C. orthopsilosis, or C. metapsilosis (19).
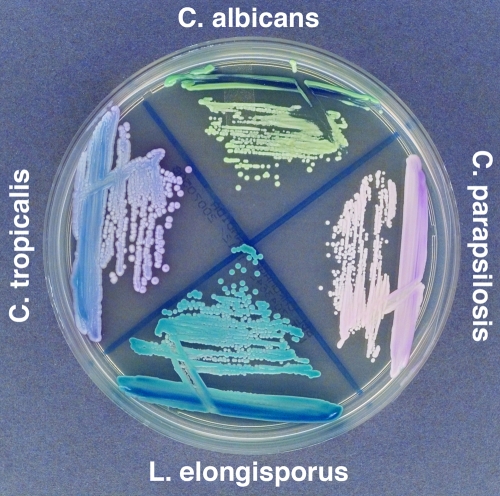
All yeast isolates submitted to the University of Iowa as part of the ARTEMIS antifungal surveillance program were identified using the Vitek yeast identification system (bioMerieux, Durham, NC) and plated on BBL Chromagar Candida medium (Becton Dickinson and Company, Sparks, MD). A number of isolates that were identified as C. parapsilosis by the Vitek system were noted to have a distinct turquoise color on Chromagar Candida medium rather than the pink/lavender color that is typical for C. parapsilosis (Fig. 1). These isolates were further evaluated using the API 20C identification kit (bioMerieux, Durham, NC) and again were biochemically identified as C. parapsilosis (Table 1).
FIG. 1.
Isolates were grown on BBL Chromagar Candida medium (Becton Dickinson and Company, Sparks, MD). L. elongisporus strain ATCC 22688 and L. elongisporus strain ATCC 11503 were the same color as isolate 1, shown here (labeled L. elongisporus).
TABLE 1.
Biochemical assimilation of our Lodderomyces isolates by use of the API20 C panel
| Strain(s) | Growth ina:
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glu | Gly | 2Kg | Ara | Xyl | Ado | Xlt | Gal | Ino | Sor | Mdg | Nag | Cel | Lac | Mal | Sac | Tre | Mlz | Raf | |
| L. elongisporus ATCC 22688 | + | + | + | − | − | + | + | + | − | + | + | + | − | − | + | + | + | + | − |
| L. elongisporus ATCC 11503 | + | + | + | − | − | + | − | + | − | + | + | + | − | − | + | + | − | + | − |
| Strains 1 to 10 | + | + | + | − | − | + | − | + | − | + | + | + | − | − | + | + | − | + | − |
+, growth; −, no growth. Glu, glucose; Gly, glycerol; 2Kg, calcium 2-keto-gluconate; Ara, arabinose; Xyl, xylose; Ado, adonitol; Xlt, xylitol; Gal, galactose; Ino, inositol; Sor, sorbitol; Mdg, methyl-α-d-glucopyranoside; Nag, N-acetyl-glucosamine; Cel, cellobiose; Lac, lactose; Mal, maltose; Sac, sucrose; Tre, trehalose; Mlz, melezitose; Raf, raffinose.
Large-subunit rRNA gene sequencing has recently been shown to be an accurate method for species identification of clinical yeast isolates (12). We amplified a large portion of the 18S and 26S rRNA gene, including the D1/D2 region, of seven isolates of our unknown species using primers ITS1 (5′TCCGTAGGTGAACCTGCGG3′) and 26SR (5′GTTCGATTAGTCTTTCGCCCCTAT3′). Upon alignment using the ClustalW alignment software (2), all seven isolates were found to have identical 26S rRNA gene sequences over 992 base pairs. BlastN searches using the GenBank nucleotide database (http://www.ncbi.nlm.nih.gov/BLAST/) revealed 100% matches to submitted and published gene sequences of the variable region of the large ribosomal subunit from L. elongisporus (Table 2) (9, 10, 17). Identical sequence matches were also made for an SADH-like gene between the sequence of strain NRRL YB-4239 (Lodderomyces elongisporus Sequencing Project, the Broad Institute of Harvard and MIT [http://www.broad.mit.edu]), our isolates 1 to 4, and L. elongisporus strain ATCC 22688. On cornmeal agar, the isolates formed abundant short pseudohyphae that were indistinguishable from those of C. parapsilosis. However, when the isolates were inoculated on Saccharomyces sporulation medium, single large round ascospores were produced by most of the cells after 12 to 14 days.
TABLE 2.
Sequence similarity of the partial 26S rRNA genes between various related species and isolate 1
| Species | Gene sequence length (nt) | % Similarity to isolate 1 |
|---|---|---|
| C. metapsilosis | 662 | 97 |
| C. orthopsilosis | 662 | 97 |
| C. parapsilosis | 662 | 97 |
| C. albicans | 661 | 93 |
| L. elongisporus | 662 | 100 |
The gene sequence length for isolate 1 is 662 nt.
All 10 of the isolates were isolated from patients with bloodstream infections, and all but 1 were reported to be nosocomial. Table 3 gives the origin and dates of isolation of our L. elongisporus isolates. Eight of the 10 isolates originated from a single hospital in Mexico, while the other 2 were from Asia. The isolates were distributed equally between men and women, but there was a preponderance of isolates from patients above the age of 40 years (80%). Only one of the patients died, but it was not clear whether the death was due to the L. elongisporus infection or other underlying causes. Random amplified polymorphic DNA analysis revealed two very distinct patterns for the isolates from the hospital in Mexico (data not shown). When also considering the temporal spread of the isolates, we did not believe that these isolates were the cause of a single sustained outbreak within that hospital, but we could not rule out the possibility that L. elongisporus had a nosocomial foothold within that hospital.
TABLE 3.
L. elongisporus isolates identified in this study
| Isolate | Origin | Culture date | Patient age (yr) | Patient sexa |
|---|---|---|---|---|
| 1 | Malaysia | May 2005 | >79 | M |
| 2 | Mexico | January 2005 | 70-79 | M |
| 3 | Mexico | February 2005 | <1 | F |
| 4 | Mexico | February 2005 | 40-49 | F |
| 5 | Mexico | February 2005 | >79 | F |
| 6 | Mexico | April 2005 | 60-69 | F |
| 7 | Mexico | October 2005 | 50-59 | F |
| 8 | Mexico | November 2005 | 40-49 | M |
| 9 | Mexico | September 2006 | 30-39 | F |
| 10 | China | July 2006 | 60-69 | M |
M, male; F, female.
Antifungal susceptibility testing was performed on 9 of the 10 L. elongisporus isolates by broth microdilution with fluconazole (range, 0.12 to 128 μg/ml), caspofungin (range, 0.007 to 8 μg/ml), anidulafungin (range, 0.007 to 8 μg/ml), and micafungin (range, 0.007 to 8 μg/ml) and by Etest for amphotericin B (13a; Table 4). There are no established breakpoint values for L. elongisporus. However, the MICs of our isolates are well below the normally achieved plasma levels for these drugs.
TABLE 4.
MICs of five antifungal agents for study isolatesa
| Isolate | MIC (μg/ml)
|
||||
|---|---|---|---|---|---|
| Fluconazole | Amphotericin | Caspofungin | Anidulafungin | Micafungin | |
| 1 | 0.12 | 0.75 | 0.015 | 0.03 | 0.015 |
| 2 | 0.25 | 0.75 | 0.03 | 0.03 | 0.015 |
| 3 | 0.25 | 0.75 | 0.03 | 0.12 | 0.015 |
| 4 | 0.25 | 0.50 | 0.03 | 0.12 | 0.03 |
| 5 | 0.25 | 0.375 | 0.03 | 0.12 | 0.015 |
| 6 | 0.25 | 0.375 | 0.03 | 0.015 | 0.015 |
| 7 | 0.25 | 0.75 | 0.03 | 0.015 | 0.015 |
| 8 | 0.25 | 0.5 | 0.03 | 0.015 | 0.015 |
| 9 | 0.25 | 0.375 | 0.03 | 0.015 | 0.015 |
Fluconazole, caspofungin, anidulafungin, and micafungin were tested according to the CLSI M27-A2 broth microdilution method (13a); the fluconazole MIC endpoint was read at 48 h, and echinocandin MIC endpoints were read as prominent inhibition of growth at 24 h (13b). Amphotericin B MICs were determined by the Etest method (AB Biodisk, Solna, Sweden).
To our knowledge, L. elongisporus has never been reported as a cause of human infection and yet we have isolated it as a cause of bloodstream infections in 10 patients. Its physiological similarity to C. parapsilosis may have allowed this species to remain undetected in clinical samples since its discovery and description (16, 20), and in fact two typing systems identified our isolates as C. parapsilosis. This is the third recent yeast species found to be closely related to, and clinically impersonating, C. parapsilosis.
Aside from phylogenetic studies, there is very little published information about L. elongisporus. The ATCC collection hints at a worldwide distribution for this yeast, with isolates from South Africa, Finland, The Netherlands, and the United States. However, our clinical samples came from Asia and a single center in Mexico despite a survey of 542 C. parapsilosis isolates from 25 countries on five continents. Our current collection of L. elongisporus isolates is limited to nosocomial bloodstream pathogens from older patients. We are currently prospectively analyzing a worldwide collection of C. parapsilosis isolates for the presence of L. elongisporus.
L. elongisporus was previously believed to be the teleomorph of C. parapsilosis (6), but recent small-subunit rRNA gene sequencing data have shown that it is a closely related but distinct species (7). In a large multigenic sequence analysis study of Candida and related species, L. elongisporus falls within the clade of pathogenic species containing C. parapsilosis, Candida tropicalis, C. albicans, and C. dubliniensis (3). It is the only species in that clade which forms ascospores.
Molecular identification of fungal isolates is becoming an important diagnostic tool (5, 12, 13). There have been a number of recent cases where two species are so similar phenotypically that only molecular techniques can distinguish them (1, 4, 11, 19). It is quite possible that there are a number of new yeast species in clinical material that cannot be distinguished phenotypically from a more common species. As molecular identification and genotyping become more common in clinical practice, we may find more examples of masquerading species. In the 1990s, C. parapsilosis was a single species of yeast; now it is a four-species complex.
Acknowledgments
This study was supported in part by research grants from Pfizer and Merck.
We thank Linda Boyken, Shailesh Tendolkar, Jennifer Kroeger, and Richard Hollis for their contributions to this work.
Footnotes
Published ahead of print on 24 October 2007.
REFERENCES
- 1.Alcoba-Flórez, J., S. Méndez-Álvarez, J. Cano, J. Guarro, E. Pérez-Roth, and M. del Pilar Arévalo. 2005. Phenotypic and molecular characterization of Candida nivariensis sp. nov., a possible new opportunistic fungus. J. Clin. Microbiol. 434107-4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chenna, R., H. Sugawara, T. Koike, R. Lopez, T. J. Gibson, D. G. Higgins, and J. D. Thompson. 2003. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 313497-3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diezmann, S., C. J. Cox, G. Schönian, R. J. Vilgalys, and T. G. Mitchell. 2004. Phylogeny and evolution of medical species of Candida and related taxa: a multigenic analysis. J. Clin. Microbiol. 425624-5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher, M. C., G. L. Koenig, T. J. White, and J. W. Taylor. 2002. Molecular and phenotypic description of Coccidioides posadasii sp. nov., previously recognized as the non-California population of Coccidioides immitis. Mycologia 9473-84. [PubMed] [Google Scholar]
- 5.Hall, L., S. Wohlfiel, and G. D. Roberts. 2003. Experience with the MicroSeq D2 large-subunit ribosomal DNA sequencing kit for identification of commonly encountered, clinically important yeast species. J. Clin. Microbiol. 415099-5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamajima, K., A. Nishikawa, T. Shinoda, and Y. Fukazawa. 1987. Deoxyribonucleic acid base composition and its homology between two forms of Candida parapsilosis and Lodderomyces elongisporus. J. Gen. Appl. Microbiol. 33299-302. [Google Scholar]
- 7.James, S. A., M. D. Collins, and I. N. Roberts. 1994. The genetic relationship of Lodderomyces elongisporus to other ascomycete yeast species as revealed by small subunit rRNA gene sequences. Lett. Appl. Microbiol. 19308-311. [DOI] [PubMed] [Google Scholar]
- 8.Kocsubé, S., M. Tóth, C. Vógvölgyi, I. Dóczi, M. Pesti, I. Pócsi, J. Szabó, and J. Varga. 2007. Occurrence and genetic variability of Candida parapsilosis sensu lato in Hungary. J. Med. Microbiol. 56190-195. [DOI] [PubMed] [Google Scholar]
- 9.Kurtzman, C. P., and C. J. Robnett. 1997. Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5′ end of the large-subunit (26S) ribosomal DNA gene. J. Clin. Microbiol. 351216-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurtzman, C. P., and C. J. Robnett. 1998. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie van Leeuwenhoek 73331-371. [DOI] [PubMed] [Google Scholar]
- 11.Lin, D., L. C. Wu, M. G. Rinaldi, and P. F. Lehmann. 1995. Three distinct genotypes within Candida parapsilosis from clinical sources. J. Clin. Microbiol. 331815-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linton, C. J., A. M. Borman, G. Cheung, A. D. Holmes, A. Szekely, M. D. Palmer, P. D. Bridge, C. K. Campbell, and E. M. Johnson. 2007. Molecular identification of unusual pathogenic yeast isolates by large ribosomal subunit gene sequencing: 2 years of experience at the United Kingdom Mycology Reference Laboratory. J. Clin. Microbiol. 451152-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leaw, S. N., H. C. Chang, H. F. Sun, R. Barton, J. P. Bouchara, and T. C. Chang. 2006. Identification of medically important yeast species by sequence analysis of the internal transcribed spacer regions. J. Clin. Microbiol. 44693-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth microdilution antifungal susceptibility testing of yeast, 2nd edition. Approved standard M27-A2. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 13b.Odds, Frank C., Mary Motyl, Roberto Andrade, Jacques Bille, Emilia Cantón, Manuel Cuenca-Estrella, Amanda Davidson, Christian Durussel, David Ellis, Elyse Foraker, Annette W. Fothergill, Mahmoud A. Ghannoum, Robert A. Giacobbe, Miguel Gobernado, Rosemary Handke, Michel Laverdière, Wendy Lee-Yang, William G. Merz, Luis Ostrosky-Zeichner, Javier Pemán, Sophia Perea, John R. Perfect, Michael A. Pfaller, Laurie Proia, John H. Rex, Michael G. Rinaldi, Juan-Luis Rodriguez-Tudela, Wiley A. Schell, Christine Shields, Deanna A. Sutton, Paul E. Verweij, and David W. Warnock. 2004. Interlaboratory comparision of results of susceptibility testing with caspofungin against Candida and Aspergillus species. J. Clin. Microbiol. 423475-3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfaller, M. A., L. Boyken, R. J. Hollis, S. A. Messer, S. Tendolkar, and D. J. Diekema. 2006. In vitro susceptibilities of Candida spp. to caspofungin: four years of global surveillance. J. Clin. Microbiol. 44760-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfaller, M. A., and D. J. Diekema. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20133-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Recca, J., and E. M. Mrak. 1952. Yeasts occurring in citrus products. Food Technol. 6450-454. [Google Scholar]
- 17.Rycovska, A., M. Valach, L. Tomaska, M. Bolotin-Fukuhara, and J. Nosek. 2004. Linear versus circular mitochondrial genomes: intraspecies variability of mitochondrial genome architecture in Candida parapsilosis. Microbiology 1501571-1580. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan, D. J., T. J. Westerneng, K. A. Haynes, D. E. Bennett, D. C. Coleman. 1995. Candida dubliniensis sp. nov.: phenotypic and molecular characterization of a novel species associated with oral candidosis in HIV-infected individuals. Microbiology 1411507-1521. [DOI] [PubMed] [Google Scholar]
- 19.Tavanti, A., A. D. Davidson, N. A. R. Gow, M. C. J. Maiden, and F. C. Odds. 2005. Candida orthopsilosis and Candida metapsilosis spp. nov. to replace Candida parapsilosis groups II and III. J. Clin. Microbiol. 43284-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Walt, J. P. 1966. Lodderomyces, a new genus of the Saccharomycetaceae. Antonie van Leeuwenhoek 321-5. [DOI] [PubMed] [Google Scholar]