Abstract
Two human monoclonal antibodies (MAbs) (2F5 and 4E10) against the human immunodeficiency virus type 1 (HIV-1) envelope g41 cluster II membrane proximal external region (MPER) broadly neutralize HIV-1 primary isolates. However, these antibody specificities are rare, are not induced by Env immunization or HIV-1 infection, and are polyspecific and also react with lipids such as cardiolipin or phosphatidylserine. To probe MPER anti-gp41 antibodies that are produced in HIV-1 infection, we have made two novel murine MAbs, 5A9 and 13H11, against HIV-1 gp41 envelope that partially cross-blocked 2F5 MAb binding to Env but did not neutralize HIV-1 primary isolates or bind host lipids. Competitive inhibition assays using labeled 13H11 MAb and HIV-1-positive patient plasma samples demonstrated that cluster II 13H11-blocking plasma antibodies were made in 83% of chronically HIV-1 infected patients and were acquired between 5 to 10 weeks after acute HIV-1 infection. Both the mouse 13H11 MAb and the three prototypic cluster II human MAbs (98-6, 126-6, and 167-D) blocked 2F5 binding to gp41 epitopes to variable degrees; the combination of 98-6 and 13H11 completely blocked 2F5 binding. These data provide support for the hypothesis that in some patients, B cells make nonneutralizing cluster II antibodies that may mask or otherwise down-modulate B-cell responses to immunogenic regions of gp41 that could be recognized by B cells capable of producing antibodies like 2F5.
Design of immunogens capable of inducing broadly reactive neutralizing antibodies for human immunodeficiency virus type 1 (HIV-1) is a primary goal for HIV-1 vaccine development. Several rare broadly neutralizing human monoclonal antibodies (MAbs) reactive with membrane proximal external region (MPER) epitopes of the HIV-1 envelope gp41 regions have been isolated, including 2F5 (42, 47), 4E10 (7, 10, 50), and Z13 (56). Although these human MPER MAbs show considerable breadth of neutralization, anti-MPER neutralizing antibodies are not readily induced by Env immunization in animals or humans (8, 23, 37).
Hypotheses for the inability of HIV-1 gp160 envelope to induce broadly neutralizing antibodies include diversion of the B-cell immune response by nonneutralizing immunodominant Env epitope on HIV-1 virions (9, 17, 28), thermodynamic barriers to neutralizing antibody binding to Env (30), competition with nonfunctional forms of soluble Env immunogens for B-cell pools (40, 46), and control of broadly reactive neutralizing antibody producing B cells by tolerance mechanisms (2, 24, 25).
HIV-1 envelope gp41 antibody specificities have been divided into two clusters (18, 55). Cluster I antibodies are nonneutralizing and react with the immunodominant region of gp41 (amino acids [aa] 579 to 613). Cluster II antibodies react with MPER gp41 aa 644 to 667 and are either nonneutralizing (18) or neutralizing (i.e., the rare 2F5, 4E10, and Z13 MAbs) (10, 42, 47, 50, 56). HIV-1-infected patients have been reported to make nonneutralizing antibodies to cluster I and II regions of gp41 (3, 18, 19, 29, 36, 41, 43, 55); antibody titers to cluster I epitopes are higher than antibody levels to cluster II (19).
While 2F5 is a cluster II MAb, the relationship of the reactivity of this antibody to other gp41 cluster II antibodies has not been probed, nor have their kinetics of antibody binding been studied. To determine the repertoire of antibodies made to the HIV-1 gp41 envelope MPER, we have generated novel murine MPER MAbs as probes for human studies and to characterize MPER antibody specificities that arise during acute and chronic HIV-1 infection.
In this study, we have characterized the specificity and binding kinetics of novel nonneutralizing cluster II gp41 antibodies relative to the neutralizing cluster II MAb, 2F5. We show that some species of nonneutralizing cluster II antibodies in HIV-1-infected patients have the potential to mask MPER region gp41 epitopes.
MATERIALS AND METHODS
Peptides.
Peptides were synthesized (PrimmBiotech Inc., Cambridge, MA) and purified by reverse-phase high-performance liquid chromatography. The following peptides were used in this study: biotinylated versions of HIV-1 gp41 MPER peptides SP62 (GGG-QQEKNEQELLELDKWASLWN) and 8926 (GGG-EQELLELDKWASLWN), the full-length HR-2 peptide DP178 (YTSLIHSLIEESQNQQEKNEQELLELDKWASLWNF), and control peptides with scrambled sequences (8926 scrambled, GGG-WLKLNLSWEQLEAED; SP62 scrambled, GGG-NKEQDQAEESLQLWEKLNWL).
Mouse immunizations and production of murine anti-gp41 MPER MAbs.
BALB/c mice were immunized four times intramuscularly with 25 μg of the group M shortened consensus (CON-S) gp140 CFI [with the cleavage (C) site, the fusion (F) peptide, and immunodominant (I) region] envelope oligomer (34) formulated in either CpG-containing oligodeoxynucleotides (CpG ODNs) (27) in an oil-in-water emulsion (Emulsigen; MVP Laboratories, Inc., Omaha, NE) or in Ribi monophosphoryl lipid A-trehalose dicorynomycolate (MPL-TDM) adjuvant (Sigma, St. Louis, MO). Four days after the last immunization, a hybridoma fusion was performed using P3X63/Ag8 murine myeloma cells. From 1,536 wells seeded, two positive clones (13HII and 5A9) were identified that reacted strongly with the HIV-1 gp41 peptide SP62 (QQEKNEQELLELDKWASLWN). Another HIV-1 gp41 MPER peptide used in MAb characterization was 4E10P (SLWNWFNITNWLWYIK). Both 13H11 and 5A9 MAbs were immunoglobulin G2a(κ) [IgG2a(κ)].
Recombinant envelopes.
Group M consensus envelope CON-S gp140 CFI and JRFL gp140 CF oligomer Env proteins were produced, quality controlled, and stored for use as previously described (34).
Direct ELISA.
Assays for MAb binding to HIV-1 Envs and Env peptides were performed using standard enzyme-linked immunosorbent assays (ELISAs). Briefly, high-binding 96-well microtiter plates (Costar 3369; Corning, NY) were coated with Env protein or peptide in carbonate-bicarbonate (CBC) buffer, pH 9.6, at 0.2 μg/well and incubated overnight at 4°C. Plates were blocked with phosphate-buffered saline (PBS) containing 4% (wt/vol) whey protein, 15% normal goat serum, 0.5% Tween 20, and 0.05% sodium azide for 2 h at room temperature. All antibodies were diluted in the same blocking buffer. Secondary antibody was goat anti-mouse IgG (heavy- and light-chain)-specific alkaline phosphatase (1:3,000 dilution) (catalogue A3562; Sigma, St. Louis, MO). The substrate was 2 mM MgCl2, 1 mg/ml p-NPP [4-nitrophenyl phosphate di(2-amino-2-ethyl-1,3-propanediol) salt], and CBC, pH 9.6. Plates were read in an ELISA reader at 405 nm. For epitope mapping of 2F5, 4E10, 13H11, and 5A9 MAb binding sites, 15-mer (overlapping 11 residues) HIV-1 gp41 peptides derived from HIV-1 MN or HIV-1 consensus B (CON-B) Env sequences were obtained from the National Institute of Allergy and Infectious Diseases (NIAID) AIDS Reagent Repository (NIAID, NIH, Bethesda, MD) and used.
Competitive inhibition ELISAs.
Ninety-six-well plates (Costar 3369) were coated with 0.2 μg/well JRFL protein and blocked with assay diluent (PBS containing 4% [wt/vol] whey protein, 15% normal goat serum, 0.5% Tween 20, and 0.05% sodium azide). Each assay step was conducted in assay diluent (except substrate step) and incubated for 1 h (2F5 assay at room temperature; 13H11 assay at 37°) followed by washing with PBS-0.1% Tween 20. Serum was diluted 1:50 and incubated in triplicate wells. In addition, a titration of unlabeled 25F or 13H11 was included as an inhibition standard to generate a standard curve to assess inhibition from unknown serum samples and in some cases to calculate microgram equivalents of 13H11- or 2F5-like antibody in the plasma of HIV-1-infected patients with biotinylated 2F5- or 13H11-blocking activity. Biotinylated 2F5 or 13H11 was added at the 50% effective concentration determined by a direct binding curve (biotinylated-MAb versus JRFL protein). Inhibition of biotin-MAb binding was detected with streptavidin-alkaline phosphatase at 1:1,000 (Promega V5591), followed by incubation with alkaline phosphatase substrate (2 mM MgCl2, 1 mg/ml p-NPP, CBC, pH 9.6). Plates were read with a plate reader at 405 nm. For triplicate wells, background values were subtracted, and the results were averaged. Percent inhibition was calculated as follows: [100 − (mean of triplicate wells/mean of no-inhibition control)] × 100. MAb equivalents were calculated by entering the mean optical density (OD) of unknown serum samples into a fourth-order polynomial equation of the inhibition standard curve.
SPR assays.
Surface plasmon resonance (SPR) binding assays were performed on a BIAcore 3000 instrument (Piscattaway, NJ) maintained at 20°C. Biotinylated versions of HIV-1 gp41 MPER peptides SP62 and 8926 or the full-length HR-2 peptide, DP178, and control peptides with scrambled sequences (8926 scrambled and SP62 scrambled) were individually anchored on a BIAcore SA sensor chip as described previously (1, 2). Each peptide was injected until 100 to 150 response units (RU) of binding to streptavidin was observed. Specific binding responses of MAb binding were obtained following subtraction of nonspecific binding on the scrambled 2F5 peptide surface. Rate constants were measured using the bivalent analyte model (to account for the avidity of bivalent Ig molecules) and global curve fitting to binding curves obtained from MAb titrations, which ranged from 0.01 to 119 nM and 6.0 to 1,400 nM for MAbs 2F5 and 4E10, respectively. MAbs were injected at 30 μl/min for 2 to 6 min, and glycine-HCl, pH 2.0, and surfactant P20 (0.01%) were used as the regeneration buffer. For cross-blocking studies, either MAb 2F5 or 13H11 was first injected to saturation, and then after a brief period of buffer flow, 13H11 or 2F5 was injected over 2F5 peptide surfaces. The steady-state binding RU of MAb 2F5 or 13H11 binding was compared to that in the presence of blocking MAb.
Patients studied.
Three groups of patients were studied. First, plasma samples from the Duke University Center for AIDS Research Tissue Repository that were obtained from 75 chronically HIV-1-infected patients (with CD4 counts >400/1 ml and no antiretroviral treatment) were studied. Second, plasma samples from six patients from Trinidad with acute HIV-1 subtype B infections were studied (13). Third, plasma samples from the Center for the AIDS Program of Research in South Africa (CAPRISA) cohort (Durban, South Africa) that were obtained from 14 subtype C acutely HIV-1-infected patients were studied (33). Studies were approved by the Duke Institutional Review Board.
Neutralization assays.
Neutralization assays were performed based on reductions in luciferase (Luc) reporter gene expression after a single round of virus infection with HIV-1 envelope-pseudotyped HIV-1 viruses including subtype B isolates B.QH0692, B.SF162, B.JRFL, and B.MN and subtype C isolate C.TV-1 in TZM-bl cells (34). Absorption of human serum with peptides was performed to determine the contribution of either V3 or MPER region antibodies to serum neutralization of the HIV-1 SF162.LS pseudovirus using methods described previously (34). Briefly, human plasma samples were diluted a minimum of twofold in PBS to optimize peptide blocking and to yield approximately equal concentrations of neutralizing antibodies in each sample based on measured neutralizing antibody titers. Separate portions of each diluted serum sample were incubated for 1 h with either the V3 peptide CON-B V3 (TRPNNNTRKSIHIGPGRAFYTTGEIIGDIRQAH; derived from the subtype B consensus envelope), the control peptide CON-B V3 scrambled (ITIDNIGHHNRITFRAANTRPISGQRGEYPGKT), or the HIV-1 Env MPER peptides SP62 or SP62 scrambled. Each of the above peptides was used at a final saturating concentration of 50 μg/ml. As an additional control, a portion of each diluted serum sample was incubated with a volume of PBS equal to the volume of peptide added to the other samples. Adsorbed and nonadsorbed samples were then assayed at multiple threefold dilutions for neutralizing activity against B.SF162. For assay of plasma for 2F- and 4E10-specific neutralizing antibodies, HIV-2 pseudoviruses expressing HIV-1 2F5 or 4E10 epitopes were used as described previously (20).
Flow cytometry of HIV-1-infected cells.
H9 T cells chronically infected with HIV-1 MN were propagated using standard tissue culture techniques. For flow cytometry experiments, the cells were harvested and resuspended in 5% goat serum in RPMI medium Cells (2 × 106 cells/tube) were stained with an equal volume of primary antibody to achieve a final saturating concentration of 10 μg/ml for the human MAbs F39F, 2F5, 4E10, 98-6, 126-6, and 167-D and 20 μg/ml for the murine MAbs 5A9 and 13H11. The primary antibodies were incubated at 37°C for 60 min and then washed three times with 5% goat serum in RPMI medium. Secondary antibodies (goat anti-human IgG [heavy and light chains] for the human MAbs and goat anti-mouse IgG [heavy and light chains]) (KPL, Inc., Gaithersburg, MD) were added at optimal concentrations and incubated for 30 min at 37C, followed by three washes. The cells were then resuspended in 2% methanol-free formaldehyde (Polysciences, Inc., Warrington, PA) in PBS and stored at 4°C prior to analysis. Cells were acquired using a Becton Dickinson fluorescence-activated cell sorter LSR II (BD Biosciences, San Jose, CA), and the data were analyzed using FlowJo (Tree Star, Inc, Ashland, OR). Negative controls, anti-respiratory syncytium virus antibody palivizumab (Medimmune Inc., Gaithersburg, MD) for the human MAbs and P3X63/Ag8 murine myeloma protein for the murine MAbs assayed, run concurrently, showed no increased fluorescence over unstained control cells.
Statistics.
A two-tailed Student's t test was used for determination of the significance of antibody levels induced in mice.
RESULTS
Production of anti-MPER MAbs from mice immunized with gp140 Env oligomers in CpG ODNs and Emulsigen.
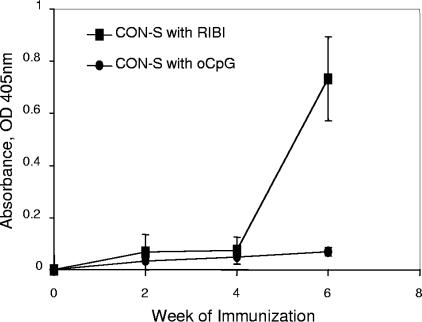
Initial studies of immunization of BALB/c mice with HIV-1 gp140 Env demonstrated no induction of MPER antibodies by HIV-1 gp140 Env formulated in Ribi MPL-TDM adjuvant (Fig. 1). It has previously been shown that autoantibodies can be induced in BALB/c mice using Toll-like receptor 9 (TLR9) agonists in oil adjuvants (51). We next immunized BALB/c mice with the HIV-1 CON-S gp140 CFI group M consensus Env formulated in B oligodinucleotides (CpG ODNs) in Emulsigen in an effort to similarly induce MPER antibody specificities that are under immunoregulatory control (24, 25).
FIG. 1.
Induction of antibodies to the HIV-1 envelope MPER 2F5 epitope in mice. Mice were immunized with HIV-1 Env CON-S formulated in either Ribi or CpG ODN (oCpG) on weeks 0, 2, and 4. Serum samples were collected as indicated and assayed against HIV-1 Env 2F5 epitope peptide in ELISA. Mean values of the absorbance at 405 nm of serum (1:25 dilution) binding to 2F5 epitope peptides are plotted in the y axis (n = 5).
Whereas the group M consensus Env gp140 oligomers in either Ribi or CpG ODN adjuvant induced the same level of anti-Env binding antibodies to CON-S and JRFL Envs, only Env formulated in CpG ODNs induced antibodies to the 2F5 epitope peptide, SP62 (Fig. 1). No antibodies to the 4E10 gp41 epitope peptide, 4E10P, were induced by Env in either adjuvant (data not shown). To determine if HIV-1 Env formulated in CpG ODNs was indeed able to induce autoantibodies known to be under immunoregulatory control, we determined the level of anti-cardiolipin (CL) antibody levels in mice immunized with Env plus Ribi and in mice immunized with Env plus CpG ODNs, and found anti-CL antibodies only in serum of BALB/c mice immunized with Env plus CpG ODNs (ODs at 405 nm [OD405s] after CpG ODN and Env versus OD after Ribi and Env were 0.864 and 0.093, respectively; P < 0.001).
To capture Env-induced anti-MPER epitope MAbs, splenocytes from mice immunized with Env plus CpG ODNs (27) were fused, and hybridomas were screened for reactivity with the MPER peptide (SP62) and also with the HIV-1 gp140 Env oligomers, CON-S and JRFL Envs. From this fusion, two MAbs were isolated, 13H11 and 5A9, that reacted strongly with the SP62 MPER peptide.
Comparison of binding properties of MAbs 2F5, 13H11, and 5A9.
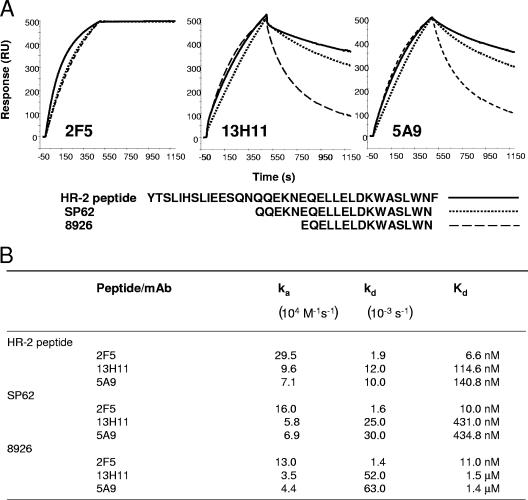
First, we used SPR to study the binding kinetics of 2F5, 13H11, and 5A9 MAbs to gp41 peptides (Fig. 2). We found that MAbs 5A9 and 13H11 bound in SPR assays to the 2F5 nominal extended epitope peptide, 8926 (45), but bound with about 40 to 50 times faster dissociation rate constants (kd; off-rate) compared to 2F5 MAb binding to the same peptide (Fig. 2B). The length of the HR-2 peptide studied had no effect on MAb 2F5 binding, as 2F5 bound to the full HR-2 peptide (YTSLIHSLIEESQNQQEKNEQELLELDKWASLWNF) with similar Kds (binding dissociation constants) and dissociation rate constants as to the shorter 20-mer, SP62, and the 15-mer peptide, 8926. However, the lengths of these same peptides had dramatic effects on the dissociation rate constants of 13H11 and 5A9 MAbs. The dissociation rate constants for both antibodies when binding to the 20-mer SP62 peptide were compared to binding to the 35-mer peptide of the entire HR-2 region (Fig. 2B). The dissociation rate constant was threefold greater for 13H11 and 5A9 when assayed on the 15-mer 8926, again confirming the conformational nature of the 13H11 and 5A9 epitopes. The overall binding Kd of 13H11 and 5A9 to the 15-mer 8926 was 2 logs lower than the binding of 2F5 to the same peptide (Fig. 2B).
FIG. 2.
(A) Binding of MAbs 2F5, 13H11, and 5A9 to HIV-1 MPER peptides. Biotinylated full-length HR-2 peptide, SP62, or 8926 was immobilized at 200 to 300 RU on a streptavidin (SA) sensor chip. Biotin-DP107 was used as a control to subtract nonspecific binding. Representative overlay of specific (control subtracted) binding curves show binding of each of the MAbs to HR-2 (solid line), SP62 (dotted line), or 8926 (broken line) peptides. (B) Kd and rate constants of each of the indicated MAbs binding to HIV-1 MPER peptides. Rate constants were calculated using the bivalent analyte model to account for the avidity of the bivalent MAb. ka, association constant.
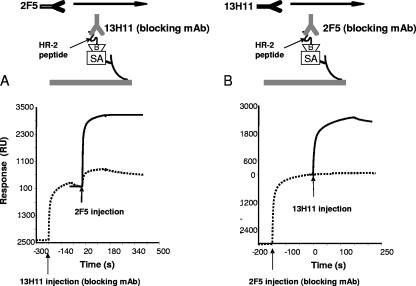
Next, we determined if 13H11 and 5A9 MAbs could cross-block the binding of 2F5 MAb to gp41 MPER epitopes. Both 2F5 and 13H11 (or 5A9 [data not shown]) could cross-block each other (Fig. 3). When saturating amounts of 2F5 MAb were first bound, the subsequent binding of 13H11 MAb was completely blocked (Fig. 3B). Due to its faster dissociation rate, only partial but significant blocking of 2F5 MAb binding was observed when 13H11 (or 5A9) was injected prior to 2F5 binding (Fig. 3A). Taken together, these results suggested that the 13H11 and 5A9 MAb binding determinants were spatially close to the 2F5 binding epitope.
FIG. 3.
Cross-blocking of 2F5 and 13H11 MAb binding to HR-2 peptide. Biotin-HR-2 (about 200 RU) peptide was anchored on a streptavidin chip (Biacore SA chip) and either blocking antibody 13H11 (A) or 2F5 (B) was injected until the peptide surface was near saturation. Following the blocking step, either 2F5 (A) or 13H11 (B) was injected, and binding was monitored for MAb blocking. Binding of each MAb in the absence of blocking antibody (solid line) or in the presence of blocking antibody (dotted line) is shown. Arrows indicate the injection point for each MAb.
Comparison of 5A9 and 13H11 MAbs with previously reported HIV-1 gp41 cluster II MAbs.
Xu et al. (55) previously defined cluster I gp41 antibodies as those that bind to gp160 aa 579 to 604 and cluster II antibodies as those that bind to aa 644 to 663. Antibodies 98-6, 126-6, 126-50, and 167-7 are prototype cluster II MAbs (18, 55). It was important to determine the ability of these human cluster II MAbs to block 2F5 and 13H11 MAbs and also to be reciprocally blocked in order to define the ability of 13H11 MAb to represent other cluster II MAbs.
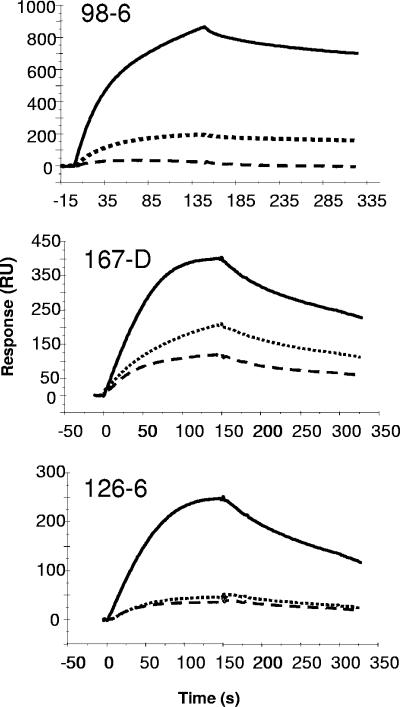
Cluster II MAbs were similar to 13H11 and 5A9 MAbs in that all three cluster II MAbs bound strongly to the full-length DP178 peptide and bound poorly to the shorter SP62 and 8926 peptides (Fig. 4). Among the three cluster II MAbs, 98-6 bound more strongly to DP178 with a slower dissociation rate, while MAb 167-D and 126-6 bound to DP178 with faster dissociation rates (Fig. 4). Binding of all three cluster II MAbs to HR-2 peptide was blocked by MAb 2F5 (Table 1). While 2F5 blocking of all cluster II MAbs was observed, blocking of 2F5 binding by prototype cluster II MAbs was weaker (<50%) than observed for 13H11 (Table 1). Among the three prototype cluster II MAbs, the strongest 2F5 blocking was observed with MAb 98-6. The inability of MAbs 126-6 and 167-D to effectively block binding of 2F5 MAb was due to either their fast dissociation kinetics (Fig. 4) or the distance of their epitope from the 2F5 epitope.
FIG. 4.
Binding of human cluster II MAbs to MPER peptides. Binding of Cluster II MAbs to HR-2 peptide was monitored on a streptavidin chip loaded with HR-2 peptides as described above in the legend of Fig. 2. An overlay of binding of each MAb to HR-2 peptide (DP178; solid line), SP62 (dotted line), and 8926 (dashed line) is shown.
TABLE 1.
Binding cross-blocking activity of anti-gp41 cluster II MAbsa
| Cluster II MAb | Blocking MAb | % Blocking |
|---|---|---|
| 98-6 | 2F5 | 75 |
| 167-D | 2F5 | 90 |
| 126-6 | 2F5 | 70 |
| 13H11 | 2F5 | 90 |
| 2F5 | 13H11 | 65 |
| 2F5 | 98-6 | 35 |
| 2F5 | 167-D | 25 |
| 2F5 | 126-6 | 20 |
| 2F5 | 13H11 + 98-6 | 100 |
| 13H11 | 5A9 | 85 |
| 13H11 | 98-6 | 14 |
| 13H11 | 126-6 | 20 |
| 13H11 | 167-D | 22 |
| 5A9 | 13H11 | 80 |
| 98-6 | 13H11 | 20 |
| 126-6 | 13H11 | 80 |
| 167-D | 13H11 | 78 |
Each blocking MAb was prebound to an SPR sensor surface immobilized with a gp41 HR-2 peptide as described in the legend of Fig. 3. The percent blocking was calculated by comparing binding of each cluster II MAb with and without the indicated blocking antibody. The data are representative of at least two independent experiments.
Next, we determined the ability of prototype cluster II MAbs and 13H11 MAbs to reciprocally block each other in SPR gp41 epitope binding assays. We found that MAbs 98-6 and 13H11 only minimally blocked their binding to gp41 MPER peptide SP62. In contrast, the two murine MPER MAbs 13H11 and 5A9 completely blocked each other's binding (data not shown). MAb 13H11 also blocked the binding of cluster II MAbs, 126-6 and 167-D (Table 1), while both cluster II MAbs, 126-6 and 167-D, partially blocked 13H11 binding to MPER peptides by ∼20% (Table 1). Thus, MAbs 13H11 and 5A9 bind closely to the 2F5 nominal peptide epitope and to the epitope of the prototypic cluster II MAbs analyzed.
Since the blocking of 2F5 binding by MAb 13H11 and the prototype cluster II MAb 98.6 was only partial, we next tested if these two nonneutralizing cluster II MAbs could act in synergy to completely block the binding of 2F5. When both 13H11 and 98.6 were prebound to the HIV-1 gp41 full-length HR-2 peptide, the binding of 2F5 to the HR-2 peptide was now completely blocked (Table 1). Thus, the blocking effect of MAbs 13H11 and 98.6 was additive. However, in pseudovirus neutralization assays in which MAb 2F5 was strongly neutralizing, the combination of MAbs 13H11 and 98-6 did not neutralize SF162 or BG1168 clade B pseudoviruses (data not shown). Thus, a combination of antibodies that completely blocked the binding of MAb 2F5 to its nominal gp41 Env epitope was not sufficient to neutralize HIV-1 in pseudovirus infection assays.
Ability of cluster II MAbs to bind to HIV-1-infected T cells.
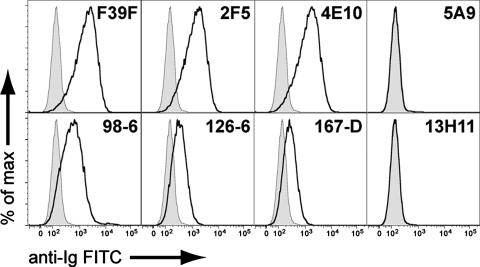
As shown in Fig. 5, MAbs directed to the V3 and MPER regions of HIV-1 Env can bind to the surface of HIV-1-infected cells. Control MAbs (the human anti-respiratory syncytial virus MAb palivizumab and murine MAb P3X63/Ag8) gave curves coincident with unstained cells. The anti-HIV-1 gp120 V3 loop MAbs F39F (human) and 7B9 (murine [not shown]) both reacted strongly with infected (Fig. 5) but not uninfected (data not shown) H9 cells. The human MPER MAbs 2F5 and 4E10 both showed strong binding to HIV-1-positive infected cells, as did the nonneutralizing human cluster II MAbs 98-6, 126-6, and 167-D. In contrast, neither of the murine cluster II MAbs 5A9 and 13H11 showed any binding to HIV-1 MN-infected H9 T cells (Fig. 5). Similarly, we found that a threefold greater amount of 13H11 or 5A9 MAb did not significantly block the neutralizing activity of MAb 2F5 when tested against the HIV-1 SF162 pseudovirus (data not shown).
FIG. 5.
Reactivity of HIV-1.Env gp41 cluster II MAbs with the surface of HIV-1-infected T cells. Shown are histograms of viable H9 HIV-1-infected T cells reacted with the indicated MAbs in indirect immunofluorescence assays. In all panels, unstained cells are shown by a shaded curve, and antibody-labeled cells are shown by a black line. Control antibodies (not shown) gave curves overlapping with the unstained cells. The anti-gp120 V3 loop antibody F39F shows a positive signal as do the MPER antibodies 2F5 and 4E10. The cluster II MAbs 98-6, 126-6, and 167-D also reacted with HIV-1-infected T cells. The MAbs 5A9 and 13H11 show no binding to HIV-1 infected T cells. FITC, fluorescein isothiocyanate.
HIV-1-infected patients make anti-MPER antibodies that block the binding of cluster II 13H11 and 2F5 MAbs to clade B HIV-1 envelope gp140.
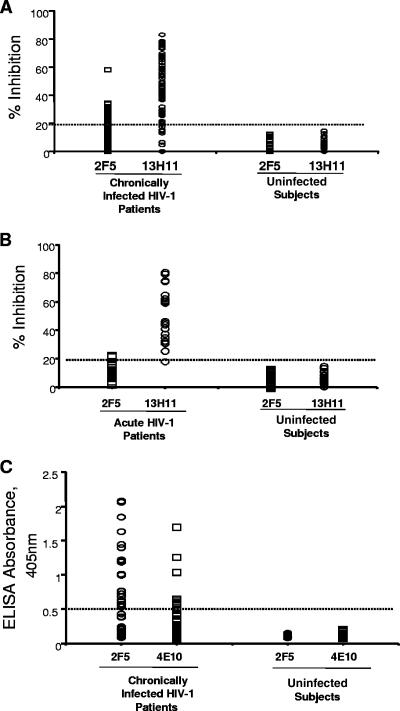
Since MAb 13H11 blocked the binding of 2F5 and the cluster II MAbs, 126-6 and 167-D, we next used MAbs 2F5 and 13H11 as binding MAbs in competitive inhibition assays with plasma from HIV-1-infected patients to investigate whether cluster II antibodies were present in the plasma of chronic HIV-1-infected patients that could block the binding of 13H11 or 2F5 to HIV-1 gp41. We found that whereas 20 of 75 (27%) patients had levels of cluster II antibodies that, at a 1:50 dilution of plasma, blocked binding of biotinylated MAb 2F5 to the clade B Env JRFL gp140, 62 of 75 (83%) of plasma samples from chronically HIV-1-infected patients had cluster II antibodies that blocked the binding of biotinylated MAb 13H11 under the same conditions (Fig. 6A). In comparison, 61/75 (81%) of patient plasma samples contained antibodies that bound to CON-B V3 Env peptide in direct binding ELISA.
FIG. 6.
(A) Ability of plasma from 75 chronically HIV-1-positive patients (CD4 counts of >400; no antiretroviral therapy) and HIV-1-negative control plasma from 25 healthy donors to inhibit either the biotinylated MAb 2F5 or MAb 13H11. Twenty of 50 plasma samples from the chronically HIV-1-positive patients inhibited MAb 2F5 binding at ≥20% levels, while 62 of 75 plasma samples from the chronically HIV-1-positive patients inhibited MAb 13H11 binding at ≥20% levels. No significant inhibition of either MAb 2F5 or 13H11 binding by HIV-1-negative control plasma (n = 50) was observed. (B) Ability of plasma from 20 acutely HIV-1-infected patients and HIV-1-negative control plasma from 26 healthy donors to inhibit either the biotinylated MAb 2F5 or MAb 13H11. Two of 20 plasma samples from the acutely HIV-1-infected patients inhibited MAb 2F5 binding at ≥20% levels, while 19 of 20 plasma samples from the acutely HIV-1-infected patients inhibited MAb 13H11 binding at ≥20% levels. No significant inhibition of either MAb 2F5 or 13H11 binding by HIV-1-negative control plasma (n = 26) was observed. (C) Ability of plasma from 75 chronically HIV-1-positive patients (CD4 counts of >400; no antiretroviral therapy) to bind either the 2F5 epitope peptide SP62 or the 4E10 epitope peptide 4E10P in direct ELISA. A total of 23% of plasma samples from chronically HIV-1-positive patients were reactive with SP62 2F5 peptide while 8% were reactive with the 4E10 gp41 epitope peptide. Plasma samples were considered positive in peptide reactivity if the OD value was ≥0.500.
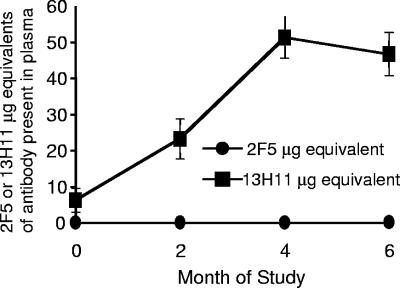
Next, to determine when in the course of HIV-1 infection anti-MPER antibodies arose, we tested 6 subtype B and 14 subtype C acutely HIV-1-infected patients during the first 6 months to 1 year of infection for development of 2F5-blocking or 13H11-blocking plasma antibodies. We found that 13/14 (93%) of subtype C and 6/6 (100%) of patients with acute clade B HIV-1 infections had blocking antibodies (>20% inhibition) for 13H11 MAb binding to HIV-1 Env gp140 6 months after acute HIV-1 infection (Fig. 6B). HIV-1 gp41 cluster II 13H11-blocking plasma antibodies began to rise 5 weeks after enrollment in patients with acute HIV-1 clade C (Fig. 7) and clade B (not shown) infection.
FIG. 7.
Antibody responses to HIV-1 Env MPER epitopes in acutely HIV-1-infected patients. Serial plasma samples were collected from 12 individuals from the clade C CAPRISA cohort from the initial study point through approximately 70 weeks, as shown on the x axis, and assayed for their ability to block the binding of biotinylated MAbs 2F5 or 13H11 to HIV-1 JRFL gp140 Env in ELISA. Mean values (n = 12) of microgram equivalents of either 2F5 or 13H11 antibody in plasma are shown on the y axis.
As mentioned, there were low levels of plasma MAb 2F5-blocking activity in the plasma of 20 of 75 chronic HIV-1-positive patients (Fig. 6A), and one patient plasma sample (200313) blocked 2F5 MAb binding to Env by 58% (Fig. 6A). We suspected that these levels of 2F5 blocking activity in HIV-positive patient plasma samples represented blocking of 2F5 MAb binding by nonneutralizing cluster II antibodies and were possibly similar to the cluster II antibody species blocking 13H11 MAb binding to gp140. If this were the case, the patient plasma sample that contained 13H11 and 2F5 blocking antibodies should contain no 2F5-like neutralizing antibodies. Therefore, we next tested patient plasma samples to determine if plasma 2F5 or 13H11 MAb-blocking antibodies had neutralizing activity.
Neutralizing activity of cluster II MPER MAbs 13H11, 5A9, and 2F5 and patient plasma samples that blocked the binding of 2F5 and 13H11 MAbs to gp140.
First, we tested MAbs 2F5, 13H11, and 5A9 for the ability to neutralize HIV-1 isolates B.SF162, B.BG1168, B.JRFL, B.MN, and C.TV-1 in pseudovirus infectivity assays. Whereas 2F5 neutralized each isolate in 50% inhibitory concentrations ranging from 14 μg/ml (C.TV-1) to 0.05 μg/ml (B.MN), neither 13H11 nor 5A9 MAbs neutralized any of the primary isolate Env pseudoviruses at concentrations as high as 100 μg/ml (data not shown). Next, we tested plasma samples from six chronically HIV-1-infected patients that each blocked the binding of 13H11 to Env gp140 by >50%; we also tested the one subtype B plasma (200313) that blocked MAb 2F5 and 13H11 binding to gp140 by >50% for absorption of neutralizing activity against the B.SF162 HIV-1 pseudovirus by either CON-B V3 (as a positive control) or MPER peptide (SP62) (Table 2).
TABLE 2.
Inability of membrane proximal peptides to absorb neutralizing antibodies against SF162 in the Env pseudovirus assaya
| Patient no. | Blocking assay (% absorbed)b
|
Neutralizing activity of CON-B V3 peptides
|
Neutralizing activity of SP62 peptides
|
Neutralizing activity of CON-B V3 and SP62
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 13H11 MAb to Env | 2F5 MAb to Env | Scr CON-B V3 Ab titer | CON-B V3 Ab titer | % Absorbed | Scr SP62 Ab titer | SP62 Ab titer | % Absorbed | CON-B V3 Ab titer | CON-B V3+SP62 Ab titer | % Absorbed | |
| 220216 | 75 | 34 | 4,506 | 5,056 | 0 | 7,852 | 5,149 | 34 | 5,056 | 3,599 | 29 |
| 200225 | 76 | 29 | 1,583 | 596 | 62 | 3,210 | 2,178 | 32 | 596 | 608 | 0 |
| 200235 | 83 | 30 | 4,421 | 2,229 | 50 | 6,745 | 4,692 | 30 | 2,229 | 2,981 | 0 |
| 200279 | 77 | 19 | 1,255 | 940 | 25 | 1,907 | 1,579 | 17 | 940 | 1,899 | 0 |
| 200292 | 77 | 9 | 3,054 | 1,774 | 42 | 3,740 | 4,306 | 0 | 1,774 | 3,159 | 0 |
| 200296 | 78 | 14 | 1,677 | 794 | 53 | 2,525 | 2,137 | 15 | 794 | 1,074 | 0 |
| 200313 | 78 | 58 | 1,468 | 888 | 40 | 2,587 | 2,377 | 8 | 888 | 1,352 | 0 |
| Mean | 39 | 19 | 4 | ||||||||
Neutralization assays were performed as described using the SF162.LS pseudovirus. Peptide absorptions were performed as described in Materials and Methods. The 2F5 MAb neutralized SF162.LS at a 50% inhibitory concentration of 0.15 μg/ml and a 90% inhibitory concentration of 3.3 μg/ml. The 13H11 MAb did not neutralize SF162.LS at a concentration of 100 μg/ml. SP62 is the MPER peptide QQEKNEQELLELDKWASLWN. Scr, scrambled; Ab, antibody.
Blocking was determined at a 1:50 dilution of plasma.
While the CON-B V3 peptide absorbed neutralizing activity in 6 of 7 plasma samples (mean percent absorption in the six positive plasmas, 45%), the MPER peptide SP62 absorbed a mean of only 19% of neutralizing activity. To further evaluate the significance of this low level of MPER peptide absorption of plasma neutralizing activity, we next compared plasmas absorbed with CON-B V3 peptide alone with plasma absorbed with a combination of the V3 and MPER peptides (i.e., to determine if the MPER peptide could absorb neutralization activity over and above that absorbed by V3 peptide) and found that only one of seven plasma samples had even a minimal level of neutralizing activity removed by MPER peptide after V3 peptide absorption (29% absorption by MPER peptide in patient no. 200216) (Table 2).
To further evaluate for the presence of 2F5- or 4E10-like neutralizing activity in the seven plasma samples identified in Table 2, we tested these plasmas in a HIV-2/HIV-1 chronic pseudovirus neutralization assay (20) and found that none of the plasmas had 2F5 or 4E10 epitope-specific anti-MPER neutralizing antibodies (data not shown). Moreover, neither the 5A9 nor the 13H11 MAbs had neutralizing activity for the 2F5 or the 4E10 epitope when assayed against HIV-2/HIV-1 chimeric pseudoviruses (data not shown). To confirm the rarity of the 2F5 specificity of neutralizing antibodies, we tested all 75 plasma samples shown in Fig. 7A from chronically infected HIV-1 patients in HIV-2/HIV-1 2F5 epitope chimera pseudovirus assays (20), and none had 2F5 epitope-specific neutralizing antibodies (data not shown).
Thus, while there may be low levels of neutralizing activity to MPER epitopes in select patient plasma samples (such as seen in patient 200216), the majority of HIV-1-positive patients tested that had 2F5 or 13H11 antibody plasma blocking activity had nonneutralizing cluster II-like anti-MPER activity and did not have 2F5-like neutralizing antibodies.
As shown in Fig. 4, MAbs 13H11 and 2F5 cross-blocked each other when binding to MPER Env peptide epitopes. However, the dissociation rates for MAbs 13H11 and 5A9 for gp41 peptides were much greater than for 2F5 (Fig. 3). This disparity in dissociation rates likely explains why plasma MPER antibodies blocked biotinylated 13H11 MAb binding to JRFL gp140 better than the binding of biotinylated 2F5 MAb to JRFL gp140 in a competitive inhibition ELISA. To directly compare 2F5 and 13H11 binding to the recombinant JRFL gp140 Env protein oligomer (the Env oligomer used in the 2F5 and 13H11 competitive inhibition assay) (34), we assayed the two MAbs in direct binding ELISAs with JRFL gp140 coated on ELISA plates. We found the relative affinity of 2F5 to be approximately 40-fold higher than that of 13H11 (50% effective concentrations of 2F5 and 13H11 were 0.65 and 24.2 nM, respectively), thus additionally clarifying why, although the cluster II MAbs 13H11 and 98.6 do cross-block MAb 2F5 in SPR assays, it is more difficult for competitive ELISA to detect plasma MPER antibody to block the binding of 2F5 to JRFL Env gp41. For technical reasons, we were unable to assay the ability of 13H11-like antibodies from patient plasma samples to block the binding of MAb 2F5 in SPR assays.
It was of interest that, although most HIV-1-positive patients do not have plasma 2F5- or 4E10-like neutralizing antibodies, ∼30% had antibodies to gp41 MPER peptides containing the 2F5 (SP62) or 4E10 (4E10P) peptide epitopes, as assayed in ELISA direct binding assays (Fig. 6C). Taken together, these data demonstrate a spectrum of nonneutralizing cluster II antibodies in HIV-1 infection.
DISCUSSION
In this study, we have probed the specificities of MPER antibodies in human plasma using novel murine cluster II gp41 MAbs generated from mice immunized with HIV-1 gp140 Env protein. We have shown that the majority of chronically HIV-1-infected patients have gp41 cluster II antibodies that arise early in clade B and clade C infections between 5 to 10 weeks after transmission and that species of nonneutralizing cluster II antibodies can also partially block 2F5 MAb binding to Env gp41 epitopes.
We have provided the following data that argue for the idea that nonneutralizing antibodies modulate the binding of neutralizing 2F5-like antibodies: (i) we show binding data that both 13H11 and the other nonneutralizing human cluster II MAbs (98-6, 167-D, and 126-6) partially block binding of 2F5 to gp41 epitopes and that these three human MAbs also bind to HIV-1-infected cells; and (ii) some patient sera can indeed block the binding of 2F5 in our competitive inhibition assay (Fig. 6A), with one serum sample inhibiting 60% of 2F5 binding. Although not all patient sera blocked the binding of 2F5 MAb to oligomeric Env (Fig. 6A), these data clearly showed that some patients have detectable nonneutralizing 2F5 epitope-blocking antibodies. Taken together, these data support the hypothesis that the lack of 2F5-like neutralizing antibodies in chronic and acutely infected patients could, in part, involve nonneutralizing cluster II antibodies that either mask immunogenic epitopes or otherwise inhibit the induction of neutralizing antibodies.
It was of interest that the cluster II MPER antibodies were induced in mice with a TLR9 agonist regimen associated with breaking tolerance to autoantigens (46). We have previously shown that the 2F5 and 4E10 MAbs are polyspecific anti-CL antibodies (24, 25), with binding characteristics of well-characterized anti-CL autoantibodies such as IS4 and IS6 (2). The inability of gp140 Env to induce neutralizing 2F5-like or 4E10-like antibodies in mice could be due to lack of a native gp41 prefusion or intermediate conformation of the MPER (28), lack of a lipid component of the MPER antigen (21, 42), inability of mice to be able to make an anti-HIV-1 antibody such as 2F5 with a long CDR3 region, or inability of the TLR9 agonist in oil to reverse the type of B-cell tolerance mechanisms controlling of 2F5 and 4E10 antibody-producing B-cell clones. In particular, 4E10 is a high-affinity anti-CL phosphatidylserine MAb (2, 6, 24, 49). Li et al. have shown that anti-phosphatidylserine-reactive B-cell clones are deleted in bone marrow due to receptor editing (32). Meffre et al. have similarly shown that most human antibodies with long hydrophobic CDR3 regions such as 2F5 and 4E10 are usually deleted in bone marrow (38). We have not been able to induce anti-4E10 epitope-specific antibodies with any immunogen or adjuvant formulation, and our current hypothesis remains that antibodies against this MPER epitope will require strategies that interfere with B-cell selection mechanisms to induce these types of antibodies. In contrast, it has been possible to induce nonneutralizing antibodies that bind near the 2F5 epitope, raising the hope that multiple mechanisms may regulate the production of antibodies to the 2F5 MPER neutralizing region, including, but not limited to, B-cell tolerance mechanisms (2, 24, 25).
The most common example of IgG-mediated suppression of antibody responses is the use of passive IgG to prevent anti-erythrocyte antibody production (4, 26). This strategy has been used since the 1960s to prevent anti-rhesus (Rh) antibody production in Rh-negative women carrying Rh-positive babies (4, 12). Two different mechanisms exist to explain how IgG exerts suppression of antibody responses (26). First, as discussed above, IgG may mask antigenic epitopes and thus prevent specific B cells from recognizing antigen, i.e., the ELDKWAS epitope may be occluded by nonneutralizing cluster II antibodies which thus prevents presentation of the correct epitope to reactive B cells. In this scenario, nonneutralizing cluster II antibodies may bind nonnative gp41 forms (36). Thus, B cells capable of producing neutralizing cluster II antibody specificities would be prevented from responding to the epitope on the native HIV-1 Env trimer by antibodies made against nonnative Env forms (36). It is important to note that the low-avidity nonneutralizing antibodies like the murine 13H11 MAb that did not bind to HIV-infected T cells would largely bind to nonnative gp41 Env forms and, therefore, would be least effective in epitope masking unless the stimulating antigen was shed. However, the type of human cluster II antibodies, represented by MAbs 98-6, 126-6, and 167-D, that did bind to HIV-1-infected cells (Fig. 5) and bind to conformational gp41 epitopes (14; also S. M. Alam and B. Haynes, unpublished data) could potentially block the binding of neutralizing anti-gp41 MAbs to native gp41 epitopes. Thus, the nonneutralizing antibody responses observed in chronic HIV infection could potentially inhibit the relatively weaker neutralizing responses against Env gp41. This notion is consistent with studies on anti-hapten antibodies that show that lower valency nonstimulatory epitopes, when used at higher doses, can inhibit the response to higher-avidity multivalent epitopes in vitro and in vivo (15, 16).
Second, antigen-antibody complexes may cross-link B cells that express inhibitory Fc receptor IIB receptors (FcRIIBs) and by stimulation of inhibitory FcRIIBs inhibit antigen-specific B-cell activation (26). In the case of inhibition of 2F5-like antibodies mediated by nonneutralizing cluster II gp41 MAbs, these cluster II antibodies may bind to FcRIIB on MPER-specific B cells and inhibit the production of 2F5-like antibodies. D. Montefiori et al. (unpublished observations) have recently shown that of a number of human monoclonal antibodies tested, only 2F5 and 4E10 MAbs had the capacity to bind to the inhibitory FcRIIB molecule, thus giving credence to the notion that some antibody types may down-modulate their continued production by inhibitory FcR binding. Against this notion of feedback by nonneutralizing MPER antibodies inhibiting the production of neutralizing MPER antibodies is the observation that a subset of patients (17%) have minimal or no 13H11-blocking cluster II antibodies yet do not make 2F5-like broadly neutralizing antibodies. Thus, it remains to be directly proven if low-affinity cluster II antibodies can play a significant role in inhibiting the induction of 2F5-like antibodies by masking MPER neutralizing epitopes on Env forms or otherwise downregulating MPER-responsive B cells.
We found only one patient that had high levels (>50%) of 2F5 blocking plasma antibodies (no. 200313) (Table 2). However, this patient's plasma did not neutralize the HIV-1/HIV-2 chimera containing the HIV-1 nominal 2F5 epitope. Therefore, if anti-MPER neutralizing activity is present in select plasma, it is weak and not directed to the ELDKWAS 2F5 nominal gp41 epitope. Our data are most consistent with the notion of other investigators that only very rare patients have 2F5-like anti-MPER neutralizing antibodies and that most anti-MPER antibodies in both patients and immunized animals are nonneutralizing (9, 19-25). If a large of number of patients can be found with 2F5 blocking antibodies, it would be of interest to perform a statistical analysis of the presence of such blocking antibodies with peptide binding and neutralizing antibody levels.
The concept of nonneutralizing immunodominant B-cell epitopes on HIV-1 Env has been reported, and several strategies have been suggested to divert immune responses from nonneutralizing determinants to neutralizing determinants (14, 17, 44). For example, adding g lycos ylation sites to the V3 loop redirected B-cell antibody responses to the Env V1 region (17). Thus, an effective immunogen may be required that expresses only gp41 membrane proximal neutralization determinants but does not bind nonneutralizing gp41 cluster II MAbs.
Finally, it is important to carefully determine if there is any anti-HIV-1 activity of 13H11-like cluster II gp41 antibodies in humans because similar gp41 antibodies, when expressed intracellularly or on the surface of CD4+ CCR5+ T cells, can protect cells from HIV-1 infection (31). Cluster II anti-gp41 antibodies that are nonneutralizing in standard HIV-1 neutralization assays have also been associated with antibody-dependent cellular cytotoxicity activity of HIV-1-infected cells (52) or neutralize HIV-1 only in peripheral blood mononuclear cell assays (11). Of the gp41 cluster II MAbs studied, it is of interest that only MAb 98.6 had any neutralizing activity in the peripheral blood mononuclear cell-based neutralizing assays (14; also S. M. Alam and B. F. Haynes, unpublished data). We found that nonneutralizing gp41 cluster II antibodies arise only between 5 to 10 weeks (i.e., late) after acute HIV-1 infection. Autologous neutralizing antibodies arise even later after acute HIV-1 infection (48, 54). Recent studies have shown that a large percentage of the total-body CD4+ T-cell pool is destroyed within the first 20 days of HIV-1 infection (5, 22, 35, 39, 53). Thus, any successful preventive HIV-1 vaccine will need to prime for antiviral protective antibodies to be made in the first hours to days following HIV-1 transmission. Therefore, if antibody-dependent cellular cytotoxicity-mediating or -neutralizing cluster II gp41 antibodies could be induced very early within the first days of HIV-1 infection, these antibodies might have some salutary effect on HIV-1 replication.
In summary, we have defined species of nonneutralizing anti-gp41 antibodies that may have the potential to mask epitopes of N-terminal membrane-proximal region forms of HIV-1 gp41 to which neutralizing antibody 2F5 also binds. This hypothesis that nonneutralizing cluster II antibodies may inhibit MPER neutralizing antibody induction can be tested by design of gp41 MPER epitope immunogens that bind MAb 2F5 but do not bind nonneutralizing cluster II MAbs to directly determine if these immunogens are able to induce 2F5-like neutralizing antibodies.
Acknowledgments
We thank Kim R. McClammy for expert secretarial assistance. We thank Carolyn Williamson and Koleka Milsana, cochairs of the CAPRISA Acute Infection Study protocol, and the CHAVI Clinical Study Site investigators for discussions.
This work was supported by the Center for HIV/AIDS Vaccine Immunology (CHAVI) grant U01 AI0678501, the Duke Center for AIDS Research AI 067854-02, and a Collaboration for AIDS Vaccine Development Center grant from the Bill and Melinda Gates Foundation. Support for the CAPRISA Cohort was from a U.S. NIAID grant (U19AI51794); support from the Trinidad Cohort was from an NIH PO1 grant (AI40237). Human cluster II MAbs were produced with the support of the Viral Immunology Core of the New York University Center for AIDS Research (AI27742).
Footnotes
Published ahead of print on 17 October 2007.
REFERENCES
- 1.Alam, S. M., C. A. Paleos, H-X. Liao, R. M. Scearce, J. Robinson, and B. F. Haynes. 2004. An inducible HIV-1 gp41 HR-2 peptide binding site on HIV-1 envelope gp120. AIDS Res. Hum. Retrovir. 20836-845. [DOI] [PubMed] [Google Scholar]
- 2.Alam, S. M., M. McAdam, D. Boren, M. Rak, R. M. Scearce, F. Gao, Z. T. Camacho, D. Gewirth, G. Kelsoe, P. Chen, and B. F. Haynes. 2007. The role of antibody polyspecificity and lipid reactivity in binding of broadly neutralizing anti-HIV-1 envelope human monoclonal antibodies 2F5 and 4E10 to gp41 membrane proximal envelope epitopes. J. Immunol. 1784424-4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barin, F., M. F. McLane, J. S. Allan, T. H. Lee, J. E. Groopman, and M. Essex. 1985. Virus envelope protein of HTLV-III represents major target antigen for antibodies in AIDS patients. Science 2281094-1096. [DOI] [PubMed] [Google Scholar]
- 4.Bowman, J. M. 1988. The prevention of Rh immunization. Transfus. Med. Rev. 2129-150. [DOI] [PubMed] [Google Scholar]
- 5.Brenchley, J. M., T. W. Schacker, L. E. Ruff, D. A. Price, J. H. Taylor, G. J. Beilman, P. L. Nguyen, A. Khoruts, M. Larson, A. T. Haase, and D. C. Douek. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, B. K., N. Karasavvas, Z. Beck, G. R. Matyas, D. L. Birx, V. R. Polonis, and C. R. Alving. 2007. Monoclonal antibodies to phosphatidylinositol phosphate neutralize human immunodeficiency virus type 1: role of phosphate-binding subsites. J. Virol. 812087-2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchacher, A., R. Predl, K. Strutzenberger, W. Steinfellner, A. Trkola, M. Purtscher, G. Gruber, C. Tauer, F. Steindl, and A. Jungbauer. 1994. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res. Hum. Retrovir. 10359-369. [DOI] [PubMed] [Google Scholar]
- 8.Burton, D. R., R. L. Stanfield, and I. A. Wilson. 2005. Antibody vs. HIV in a clash of evolutionary titans. Proc. Natl. Acad. Sci. USA 10214943-14948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calarota, S., M. Jansson, M. Levi, K. Broliden, O. Libonatti, H. Wigzell, and B. Wahren. 1996. Immunodominant glycoprotein 41 epitope identified by seroreactivity in HIV type 1-infected individuals. AIDS Res. Hum. Retrovir. 12705-713. [DOI] [PubMed] [Google Scholar]
- 10.Cardoso, R. M., M. B. Zwick, R. L. Stanfield, R. Kunert, J. M. Binley, H. Katinger, D. R. Burton, and I. A. Wilson. 2005. Broadly neutralizing anti-HIV antibody 4E10 recognizes a helical conformation of a highly conserved fusion-associated motif in gp41. Immunity 22163-173. [DOI] [PubMed] [Google Scholar]
- 11.Choudhry, V., M. Y. Zhang, I. Harris, I. A. Sidorov, B. Vu, A. S. Dimitrov, T. fouts, and D. S. Dimitrov. 2006. Increased efficacy of HIV-1 neutralization by antibodies at low CCR5 surface concentration. Biochem. Biophys. Res. Commun. 3481107-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarke, C. A., W. T. A. Donohoe, J. C. Woodrow, R. Finn, J. R. Krevans, W. Kulke, D. Lehane, and P. M. Sheppard. 1963. Further experimental studies on the prevention of Rh haemolytic disease. Br. Med. J. 1979-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cleghorn, F. R., N. Jack, J. K. Carr, J. Edwards, B. Mahabir, A. Sill, C. B. McDanal, S. M. Connolly, D. Goodman, R. Q. Bennetts, T. R. O'Brien, K. J. Weinhold, C. Bartholomew, W. A. Blattner, and M. L. Greenberg. 2000. A distinctive clade B HIV type 1 is heterosexually transmitted in Trinidad and Tobago. Proc. Natl. Acad. Sci. USA 9710523-10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cleveland, S. M., E. Buratti, T. D. Jones, P. North, F. Baralle, L. McLain, T. McInerney, Z. Durrani, and N. J. Dimmock. 2000. Immunogenic and anitgenic dominance of a neutralizing epitope over a highly conserved neutralizing epitope in the gp41 envelope glycoprotein of human immunodeficiency virus type 1: its deletion leads to a strong neutralizing response. Virology 26666-78. [DOI] [PubMed] [Google Scholar]
- 15.Dintzis, R., Z. M. H. Middleton, and H. M. Dintzis. 1983. Studies on the immunogenicity and tolerogenicity of T-independent antigens. J. Immunol. 1312196-2203. [PubMed] [Google Scholar]
- 16.Dintzis, R. Z., M. Okajima, M. H. Middleton, and H. M. Dintzis. 1990. Inhibition of antibody formation by receptor crosslinking: the molecular characteristics of inhibitory haptenated polymers. Eur. J. Immunol. 20229-232. [DOI] [PubMed] [Google Scholar]
- 17.Garrity, R. R., G. Rimmelzwaan, A. Minassian, W. P. Tsai, G. Lin, J. J. de Jong, J. Goudsmit, and P. L. Nara. 1997. Refocusing neutralizing antibody response by targeted dampening of an immunodominant epitope. J. Immunol. 159279-289. [PubMed] [Google Scholar]
- 18.Gorny, M. K., and S. Zolla-Pazner. 2000. Recognition of human monoclonal antibodies of free and complexed peptides representing the prefusogenic and fusogenic forms of human immunodeficiency virus type I gp41. J. Virol. 746186-6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goudsmit, J., R. H. Meloen, and R. Brasseur. 1990. Map of sequential B cell epitopes of the HIV-1 transmembrane protein using human antibodies as probe. Intervirology 31327-338. [DOI] [PubMed] [Google Scholar]
- 20.Gray, E. S., P. L. Moore, I. A. Choge, F. Bibollet-Ruche, H. Li, N. Leseka, F. Treurnicht, K. Milsana, G. M. Shaw, S. S. Abdool Karim, C. Williamson, and L. Morris. 4 April 2007. Neutralizing antibody responses in acute human immunodeficiency virus type 1 subtype C infection. J. Virol. doi: 10.1128/JVI.00239-07. (Subsequently published, J. Virol. 81:6187-6196, 2007.) [DOI] [PMC free article] [PubMed]
- 21.Grundner, C., T. Mirzabekov, J. Sodroski, and R. Wyatt. 2002. Solid-phase proteoliposomes containing human immunodeficiency virus envelope glycoproteins. J. Virol. 763511-3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guadalupe, M., E. Reay, S. Sankaran, T. Prindiville, J. Flamm, A. McNeil, and S. Dandekar. 2003. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 7711708-11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haynes, B. F., and D. C. Montefiori. 2006. Aiming to induce broadly reactive neutralizing antibody responses with HIV-1 vaccine candidates. Expert Rev. Vaccines 5347-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haynes, B. F., J. Fleming, E. W. St Clair, H Katinger, G. Stiegler, R. Kunert, J. Robinson, R. M. Scearce, K. Plonk, H. F. Staats, T. L. Ortel, H. X. Liao, and S. M. Alam. 2005. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science 3081906-1908. [DOI] [PubMed] [Google Scholar]
- 25.Haynes, B. F., M. A. Moody, L. Verkoczy, G. Kelsoe, and S. M. Alam. 2005. Antibody polyspecificity and neutralization of HIV-1: a hypothesis. Hum. Antibodies 1459-67. [PMC free article] [PubMed] [Google Scholar]
- 26.Hjelm, F., F. Carlsson, A. Getahun, and B. Heyman. 2006. Antibody-mediated regulation of the immune response. Scand. J. Immunol. 64177-184. [DOI] [PubMed] [Google Scholar]
- 27.Ioannou, X. P., S. M. Gomis, B. Karvonen, R. Hecker, L. A. Babiuk, and S. van Drunen Littel-van den Hurk. 2002. CpG-containing oligodeoxynucleotides, in combination with conventional adjuvants, enhance the magnitude and change the bias of the immune responses to a herpesvirus glycoprotein. Vaccine 21127-137. [DOI] [PubMed] [Google Scholar]
- 28.Kim, M., Z. Qiao, J. Yu, D. Montefiori, and E. L. Reinherz. 9 October 2006. Immunogenicity of recombinant human immunodeficiency virus type 1-like particles expressing gp41 derivatives in a pre-fusion state. Vaccine 255102-5114. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klasse, P. J., R. Pipkorn, and J. Blomberg. 1988. Presence of antibodies to a putatively immunosuppressive part of human immunodeficiency virus (HIV) envelope glycoprotein gp41 is strongly associated with health among HIV-positive subjects. Proc. Natl. Acad. Sci. USA 855225-5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwong, P. D., M. L. Doyle, DJ Casper, C. Cicala, S. A. Leavitt S. Majeed, T. D. Steenbeke, M. Venturi, I. Chaiken, M. Fung, H. Katinge, P. W. Parren, J. Robinson, D. Van Ryk, L. Wang, D. R. Burton, E. Freire, R. Wyatt, J. Sodroski, W. A. Hendrickson, and J. Arthos. 2002. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature 420678-682. [DOI] [PubMed] [Google Scholar]
- 31.Lee, S. J., R. Arora, L. M. Bull, R. C. Arduino, L. Garza, J. Allan, J. T. Kimata, and P. Zhou. 2006. A nonneutralizing anti-HIV type 1 antibody turns into a broad neutralizing antibody when expressed on the surface of HIV type 1-susceptible cells. II. Inhibition of HIV type 1 captured and transferred by DC-SIGN. AIDS Res. Hum. Retrovir. 22874-883. [DOI] [PubMed] [Google Scholar]
- 32.Li, H., Y. Jiang, H. Cao, M. Radic, E. L. Prak, and M. Weigert. 2003. Regulation of anti-phosphatidylserine antibodies. Immunity 18185-192. [DOI] [PubMed] [Google Scholar]
- 33.Li, M., J. F. Salazar-Gonzalez, C. A. Derdeyn, L. Morris, C. Williamson, J. E. Robinson, J. M. Decker, Y. Li, M. G. Salazar, V. R. Polonis, K. Mlisana, S. A. Karim, K. Hong, K. M., Greene, M. Bilska, J. Zhou, S. Allen, E. Chomba, J. Mulenga, C. Vwalika, F. Gao, M. Zhang, B. T. Korber, E. Hunter, B. H. Hahn, and D. C. Montefiori. 2006. Genetic and neutralization properties of subtype C human immunodeficiency virus type 1 molecular env clones from acute and early heterosexually acquired infections in Southern Africa. J. Virol. 8011776-11790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liao, H.-X., L. L. Sutherland, S-M Xia, M. E. Brock, R. M. Scearce, S. Vanleeuwen, M-S. Alam, M. McAdams, E. A. Weaver, Z. T. Camacho, B-J. Ma, Y. Li, J. M. Decker, G. J. Nabel, D. C. Montefiori, B. H. Hahn, B. T. Korber, F. Gao, and B. F. Haynes. 2006. A group M consensus envelope glycoprotein induces antibodies that neutralize subsets of subtype B and C HIV-1 primary viruses. Virology 353268-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mattapallil, J. J., D. C. Douek, B. Hill, Y. Nishimura, M. Martin, and M. Roederer. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 4341093-1097. [DOI] [PubMed] [Google Scholar]
- 36.McGaughey, G. B., G. Barbato, E. Bianchi, R. M. Freidinger, V. M. Garsky, W. M. Hurni, J. G. Joyce, X. Liang, M. D. Miller, A. Pessi, J. W. Shiver, and M. J. Bogusky. 2004. Progress towards the development of a HIV-1 gp41-directed vaccine. Curr. HIV Res. 2193-204. [DOI] [PubMed] [Google Scholar]
- 37.McGaughey, G. B., M. Citron, R. C. Danziesen, R. M. Freidinger, V. M. Garsky, W. M. Hurni, J. G. Joyce, X. Liang, M. D. Miller, J. W. Shiver, and M. J. Bogusky. 2003. HIV-1 vaccine development: constrained peptide immunogens show improved binding to the anti-HIV-1 gp41 MAb. Biochemistry 423214-3223. [DOI] [PubMed] [Google Scholar]
- 38.Meffre, E., M. Milili, C. Blanco-Betancourt, H. Antunes, M. C. Nussenzweig, and C. Schiff. 2001. Immunoglobulin heavy chain expression shapes the B cell receptor repertoire in human B cell development. J. Clin. Investig. 108879-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehandru, S., M. A. Poles, K. Tenner-Racz, A. Horowitz, A. Hurley, C. Hogan, D Boden, P. Racz, and M. Markowitz. 2004. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 200761-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore, P. L., E. T. Crooks, L. Porter, P. Zhu, C. S. Cayanan, H. Grise, P. Corcoran, M. B. Zwick, M. Franti, L. Morris, K. H. Roux, D. R. Burton, and J. M. Binley. 2006. Nature of nonfunctional envelope proteins on the surface of human immunodeficiency virus type 1. J. Virol. 802515-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neurath, A. R., N, Strick, P. Taylor, P. Rubinstein, and C. E. Stevens. 1990. Search for epitope-specific antibody responses to the human immunodeficiency virus (HIV-1) envelope glycoproteins signifying resistance to disease development. AIDS Res. Hum. Retrovir. 61183-1192. [DOI] [PubMed] [Google Scholar]
- 42.Ofek, G., M. Tang, A. Sambor, H. Katinger, J. R. Mascola, R. Wyatt, and P. D. Kwong. 2004. Structure and mechanistic analysis of the anti-human immunodeficiency virus type 1 antibody 2F5 in complex with its gp41 epitope. J. Virol. 7810724-10737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Opalka, D., A. Pessi, E. Bianchi, G. Ciliberto, W. Schleif, M. McElhaugh, R. Danzeisen, R. Geleziunas, R. Miller, M. Eckert, D. M. Bramhill, J. Joyce, J. Cook, W. Magilton, J. Shiver, E. Emini, and M. T. Esser. 2004. Analysis of the HIV-1 gp41 specific immune response using a multiplexed antibody detection assay. J. Immunol. Methods 28749-65. [DOI] [PubMed] [Google Scholar]
- 44.Pantophlet, R., I. A. Wilson, and D. R. Burton. 2003. Hyperglycosylated mutants of human immunodeficiency virus (HIV) type 1 monomeric gp120 as novel antigens for HIV vaccine design. J. Virol. 775889-5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parker, C. E., L. J. Deterding, C. Hager-Braun, J. M. Binley, N. Schulke, H. Katinger, J. P. Moore, and K. B. Tomer. 2001. Fine definition of the epitope on the gp41 glycoprotein of human immunodeficiency virus type 1 for the neutralizing monoclonal antibody 2F5. J. Virol. 7510906-10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parren, P. W., D. R. Burton, and Q. J. Sattentau. 1997. HIV-1 antibody: debris or virion? Nat. Med. 3366-367. [DOI] [PubMed] [Google Scholar]
- 47.Purtscher, M., A. Trkola, G. Gruber, A. Buchacher, R. Predl, F. Steindl, C. Tauer, R. Berger, N. Barrett, and A. Jungbauer. 1994. A broadly neutralizing human monoclonal antibody against gp41 of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 101651-1658. [DOI] [PubMed] [Google Scholar]
- 48.Richman, D. D., T. Wrin, S. J. Little, and C. J. Petropoulos. 2003. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. USA. 1004144-4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanchez-Martinez, S., M. Lorizate, H. Katinger, R. Kunerte, and J. L. Nieva. 2006. Membrane association and epitope recognition by HIV-1 neutralizing anti-gp41 2F5 and 4E10 antibodies. AIDS Res. Hum. Retrovir. 22998-1006. [DOI] [PubMed] [Google Scholar]
- 50.Stiegler, G., R. Kunert, M. Purtscher, S. Wolbank, R. Voglauer, F. Steindl, and H. Katinger. 2001. A potent cross-clade neutralizing human monoclonal antibody against a novel epitope on gp41 of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 171757-1765. [DOI] [PubMed] [Google Scholar]
- 51.Tran, T. T., C. F. Reich III, M. Alam, and D. S. Pisetsky. 2003. Specificity and immunochemical properties of anti-DNA antibodies induced in normal mice by immunization with mammalian DNA with a CpG oligonucleotide as adjuvant. Clin. Immunol. 109278-287. [DOI] [PubMed] [Google Scholar]
- 52.Tyler, D. S., S. D. Stanley, S. Zolla-Pazner, M. K. Gorny, P. P. Shadduck, A. J. Langlois, T. J., Matthews, D. P. Bolognesi, T. J. Palker, and K. J. Weinhold. 1990. Identification of sites within gp41 that serve as targets for antibody-dependent cellular cytotoxicity by using human monoclonal antibodies. J. Immunol. 1453276-3282. [PubMed] [Google Scholar]
- 53.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280427-431. [DOI] [PubMed] [Google Scholar]
- 54.Wei, X., J. M. Decker, S. Wang, H. Hui, J. C. Kappes, X. Wu, J. F. Salazar-Gonzalez, M. G. Salazar, J. M. Kilby, M. S. Saag, N. L. Komarova, M. A. Nowak, B. H. Hahn, P. D. Kwong, and G. M. Shaw. 2003. Antibody neutralization and escape by HIV-1. Nature 422307-312. [DOI] [PubMed] [Google Scholar]
- 55.Xu, J. Y., M. K. Gorny, T. Palker, S. Karwowska, and S. Zolla-Pazner. 1991. Epitope mapping of two immunodominant domains of gp41, the transmembrane protein of human immunodeficiency virus type 1, using ten human monoclonal antibodies. J. Virol. 654832-4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zwick, M. B., A. F. Labrijn, M. Wang, C. Spenlehauer, E. O. Saphire, J. M. Binley, J. P. Moore, G. Stiegler, H. Katinger, D. R. Burton, and P. W. Parren. 2001. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J. Virol. 7510892-10905. [DOI] [PMC free article] [PubMed] [Google Scholar]