Abstract
Gut-associated lymphoid tissue (GALT) is an early target for human immunodeficiency virus type 1 (HIV-1) infection and is a site for severe CD4+ T-cell depletion. HIV-associated enteropathy is well-documented in chronic HIV-1 infection. However, the initial host responses to HIV infection in GALT and the early molecular correlates of HIV enteropathogenesis have not been characterized during primary HIV infection. In this study, we provide evidence of viral replication in GALT resident CD4+ T cells and macrophages in primary-stage patients and identify early patterns of host mucosal responses and changes in the molecular microenvironment through gene expression profiling. High levels of viral replication in GALT and marked CD4+ T-cell depletion correlated with decreased expression levels of genes regulating epithelial barrier maintenance and digestive/metabolic functions. These changes coincided with a marked increase in the transcription of immune activation-, inflammation-, and apoptosis-associated genes. Our findings indicate that HIV-induced pathogenesis in GALT emerges at both the molecular and cellular levels prior to seroconversion in primary HIV infection, potentially setting the stage for disease progression by impairing the ability to control viral replication and repair and regenerate intestinal mucosal tissues.
The primary stage of human immunodeficiency virus (HIV) infection is characterized by “flu-like” symptoms and peak viremia, followed by the establishment of a viral “set point,” marking entry into the chronic “asymptomatic” stage. Multiple lines of evidence suggest that the efficacy of host response in these critical early weeks of infection may have a significant impact on the course of disease progression (1, 2, 20, 21). Although HIV-associated clinical pathologies, such as peripheral CD4+ T-cell depletion, chronic inflammation, and immunosuppression, are typically well established in the chronic stage of infection, it is logical to predict that many of the molecular events that define and induce pathogenesis and underlie progression to AIDS may be initiated at earlier time points.
Gut-associated lymphoid tissue (GALT) is known to be an early mucosal target and a persistent viral reservoir as well as a site of severe CD4+ T-cell depletion in HIV-infected humans and simian immunodeficiency virus (SIV)-infected nonhuman primates (3, 6, 8, 10). Multiple studies have demonstrated that the majority of CD4+ T cells in GALT are depleted in primary HIV and SIV infections and are not restored in the absence of antiretroviral therapy (3, 8, 10). CD4+ T-cell depletion coincides with an increase in CD8+ T-cell prevalence, disrupting mucosal T-cell homeostasis and establishing an imbalance in host immune response due to the loss of T-helper cell function. In the SIV model, nutrient malabsorption and a loss of intestinal growth factor expression have also been shown to coincide with early CD4+ T-cell loss in the primary stage of infection (8, 12). The characteristics of early enteropathologic events and the timing of their emergence during HIV infection, however, remain largely unknown.
To gain insight into the mechanisms of enteropathy and the level of deterioration of the GALT microenvironment in primary HIV infection, we evaluated viral replication, T-cell homeostasis, and the regulation of host gene expression in jejunal biopsy samples collected from patients at 4 to 8 weeks following HIV infection. We report evidence of enteropathogenesis in all patients, highlighted by high levels of viral replication in intestinal CD4+ T cells and macrophages, severe CD4+ T-cell depletion, decreased expression of genes regulating epithelial barrier maintenance and digestive/metabolic functions and induction of broad-range innate and adaptive host responses. Strong up regulation of epithelial maintenance-associated gene expression was linked with a more dampened host response and lower viral loads. These findings suggest that the onset of enteropathogenesis occurs early in primary HIV infection and that the severity of enteropathy may be inextricably linked to incomplete viral suppression and the subsequent course of disease progression.
MATERIALS AND METHODS
Patients and patient samples.
Four highly active antiretroviral therapy (HAART)-naive patients in the primary stage of HIV infection (4 to 8 weeks postinfection) and 10 HIV-seronegative individuals between the ages of 23 and 52 years were enrolled at the CARES Center (Sacramento, CA) and through referring physicians (Table 1). Three of four HIV-infected patients (patients E116, E134, and E56) were HIV seronegative at the time of sampling. The fourth patient (E154) had the first positive antibody test 2 days prior to sample date. Peripheral blood samples and jejunal biopsy samples obtained by upper endoscopy were collected from patients under light anesthesia as described previously (10). Six pinch biopsy samples were flash frozen using liquid nitrogen and stored at −80° (until used for microarray analysis), and six biopsy samples were collected in complete medium for flow cytometric analysis as described below. Retrospective data from four patients in the chronic stage of infection and four long-term, HIV-infected nonprogressors (LTNP) classified as elite controllers in previous studies (25) were also used in comparative gene expression analyses. Written informed consent was obtained from the participants for this Institutional Review Board (IRB)-approved study (IRB no. 200311088-7).
TABLE 1.
Study group characteristicsa
| Study group | Patient ID | Age (yr) | CDC classification | Duration of infection (wk) | Peripheral CD4+ T-cell count |
|---|---|---|---|---|---|
| Primary HIV infection | E116 | 34 | A1 | 6 | 1,000 |
| E134 | 35 | A1 | 4 | 694 | |
| E56 | 33 | A1 | 4 | 696 | |
| E154 | 32 | A1 | 8 | 915 | |
| Uninfected controls | N8 | 22 | NA | NA | 1,046 |
| N12 | 22 | NA | NA | 1,236 | |
| N15 | 52 | NA | NA | ND | |
| N34 | 35 | NA | NA | 1,046 | |
| N38 | 48 | NA | NA | 632 | |
| N41 | 43 | NA | NA | 1,203 | |
| N65 | 37 | NA | NA | 634 | |
| N151 | 44 | NA | NA | 899 | |
| N153 | 50 | NA | NA | 406 | |
| N188 | 28 | NA | NA | 620 |
ID, patient identification number; NA, not applicable.
Immunohistochemistry.
Immunohistochemical (IHC) analysis was performed to detect and localize HIV-infected cells in gut biopsy samples as well as to visualize CD3+ T lymphocytes and macrophages. Colocalization of CD3 and HIV gag p24 signal was interpreted as an infected CD4+ T cell, and colocalization of p24 with HAM56 was interpreted as an infected macrophage. Jejunal biopsy samples were embedded in OCT (Triangle Biomedical Sciences, Durham, NC) at the time of sample collection and flash frozen. Tissue sections (5 μm) were fixed in Streck fixative (Streck Laboratories, La Vista, NE) for 10 min and 90% acetone-methanol fixative for a further 10 min. Following protease treatment, we blocked nonspecific binding by incubating the slides with 10% donkey serum (Jackson ImmunoResearch, West Grove, PA) and 1% FC blocker (Miltenyi, Auburn, CA). Sections were incubated with polyclonal anti-CD3, anti-p24, and anti-HAM56 antibodies (Dako, Carpentaria, CA), for 12 h at 4°C, followed by incubation with anti-mouse immunoglobulin G fluorescein isothiocyanate, anti-mouse immunoglobulin M Cy5, and anti-rabbit Cy3 (Jackson ImmunoResearch, West Grove, PA) for 1 h. Sections were mounted using Slow Fade with DAPI (4′,6′-diamidino-2-phenylindole) (Invitrogen, Carlsbad, CA), and images were captured by confocal laser microscopy using LSM 5 and PASCAL software (Zeiss, NY).
The expression levels of CCL5, caspase 3, and epithelial antigen were also determined by IHC analysis. Tissue sections (6 μm thick) were incubated with anti-hCCL5 antibody (R&D Systems, Minneapolis, MN) or anti-active caspase 3 antibody (BD Biosciences Pharmingen) and anti-human epithelial antigen clone Ber-EP4 antibody (DakoCytomation) at a 1:100 dilution. Combined immunohistochemistry was performed using anti-caspase 3 and anti-Ber-EP4 antibodies to localize and identify the epithelial cells undergoing apoptosis. Following three washes, appropriate fluorescence-tagged antibodies were then used at 1:200 and counterstained with DAPI (Molecular Probes) and viewed at ×40 magnification under a confocal microscope.
Cell isolation and flow cytometry.
Intestinal biopsy samples were processed as previously described (10). Briefly, tissue samples were incubated in RPMI 1640 (Gibco/Invitrogen, Carlsbad, CA) and collagenase (Sigma-Aldrich, St Louis, MO) at 37°C, rapidly shaken for 45 min and then subjected to Percoll (Sigma-Aldrich, St. Louis, MO) density gradient centrifugation to enrich for T cells and eliminate tissue debris. Lymphocytes were then stained with anti-CD3FITC (BD), anti-CD4TC (Invitrogen, Carlsbad, CA), and anti-CD8APC (Invitrogen, Carlsbad, CA) and scanned on a Becton Dickinson FACSCalibur flow cytometer. A minimum of 100,000 events were collected. Flow cytometry data were analyzed using Becton Dickinson CellQuest software.
Microarray analysis.
Intestinal mucosal gene expression was investigated by oligonucleotide microarray analysis as previously described (7, 25). Briefly, total RNA from jejunal biopsy samples was extracted using the RNeasy kit (QIAGEN, Valencia, CA) and cDNA was synthesized (Superscript Choice system; Gibco Life Technologies, Rockville, MD) utilizing an oligo(dT24) primer. Biotinylated cRNA was synthesized using a BioArray HighYield RNA transcription labeling kit (ENZO Diagnostics, Farmingdale, NY) and purified through RNeasy nucleic acid columns. The quality of the cRNA was evaluated by hybridization to Test3 GeneChips (Affymetrix, Santa Clara, CA), and only samples whose 3′/5′ ratios were less than 3 were utilized for subsequent hybridization to HuGene U95-AV2 GeneChips (Affymetrix). Following scanning, the fluorescence data were initially processed through the GeneChip operating system (version 1.1; Affymetrix). Background correction, normalization, generation of expression values, and statistical analysis of differential gene expression were performed using dChip analysis software (DNA-Chip analyzer [dChip], version 1.3; Harvard University). Genes differentially expressed (≥1.5-fold above or below the mean baseline from healthy, uninfected controls; P ≤ 0.05) in GALT in the majority of patients tested were hierarchically clustered to identify patterns of up and down regulation in response to HIV infection and HAART. The prevalence of enriched biological functions within up and down regulated genes was analyzed (EASE, version 1.0; NIAID) to determine the processes that were statistically overrepresented in the microarray data set (5).
Real-time PCR analysis.
To further quantitate differences in mRNA levels for selected genes between healthy controls and HIV-infected patients as well as HIV levels in tissue samples, primers and probes specific to the selected genes and to HIV gag, respectively, were designed and used in a quantitative reverse transcription-PCR (QRT-PCR). Probes were tagged with a fluorescent dye (6-carboxyfluorescein) at the 5′ end and a quencher dye at the 3′ end. The reaction was carried out using one tube of RT-PCR master mix (PE Applied Biosystems, CA) on the ABI Prism 7900 sequence detector (PE Applied Biosystems, CA). The data were analyzed with Sequence Detector software and quantitated using the relative computational method (15, 16). TaqMan real-time PCR validation of gene expression was performed using Assays on Demand systems (Applied Biosystems, CA). The level of gene transcription was normalized to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) and expressed as a relative difference.
RESULTS
Macrophages and CD4+ T cells are HIV targets in GALT during primary HIV infection.
Measurement of HIV loads in plasma samples by the B-DNA assay showed that viral loads ranged from 41,464 to >500,000 copies per milliliter of plasma. The plasma viral burden was higher in patients at 4 weeks post-HIV exposure than in patients at 6 weeks postinfection (Fig. 1A). These results were consistent with previous HIV studies of viral burden during primary HIV infection (10, 13). Three of the four patients (E116, E134, and E56) were seronegative at the time of enrollment and collection of the first biopsy samples. Sera from these three patients did not test positive for anti-HIV antibodies until 2 to 4 weeks following the first biopsy. The fourth patient first tested positive for anti-HIV antibodies 2 days prior to collection of the first biopsy sample.
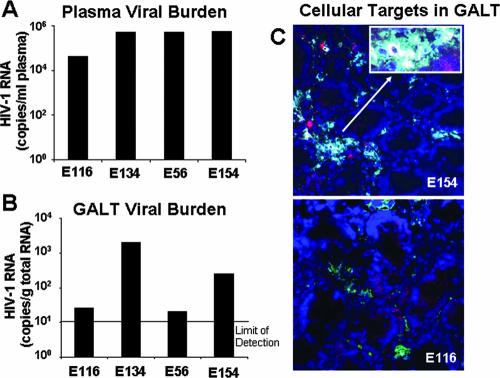
FIG. 1.
Quantitation of viral loads and localization of HIV-infected cells in GALT during primary-stage HIV infection. (A) Viral RNA levels, measured by B-DNA assay, ranged between 4 and 6 logs per ml of plasma. (B) At corresponding time points, viral burden was detected in GALT biopsy samples, varying between 1 and 4 log copies per μg of total RNA. (C) Viral p24 was localized to both lymphocytes and macrophages. CD3+ T cells appear red, macrophages pink, and HIV p24 yellow. Double staining in a p24-positive lymphocyte results in a green cell, while double staining in a p24-positive macrophage appears blue.
HIV RNA was detected in GALT in all four patients in the early stages of infection (Fig. 1B), demonstrating that dissemination and active replication of the virus were well established in the gastrointestinal mucosa in primary-stage infection. Patients E116 and E56 also displayed substantially lower tissue viral loads than did patients E134 and E154, suggesting variable degrees of viral replication in GALT in the first few weeks of infection. Intestinal mucosal viral loads in patients E134 and E154 were comparable to those measured in drug-naïve individuals during chronic HIV infection (25), indicating that substantial seeding of a viral reservoir in GALT had occurred within weeks of infection.
IHC analysis of HIV p24 expression was also performed to detect and localize HIV-infected cells with active viral replication in the intestinal mucosa (Fig. 1C). As previously reported (10, 11), we observed increased macrophage infiltration into the lamina propria. p24 staining was observed both in macrophages and in CD4+ T cells at levels that appeared to correlate with the RNA expression as measured by QRT-PCR analysis. Interestingly, infected cells were often observed in aggregates comprised of macrophages as well as lymphocytes.
Severe CD4+ depletion and disruption of mucosal T-cell homeostasis.
The clinical characteristics of the HIV-infected and uninfected cohorts participating in the study have been previously reported (10, 11, 25) and are summarized in Table 1. As expected, circulating CD4+ T-cell numbers were not substantially different from the levels observed for healthy uninfected controls (694 to 1,000 CD4+ T cells/μl of plasma compared to 406 to 1,236 cells/μl in HIV-negative control patients). However, CD8+ T-cell percentages exceeded circulating CD4+ T-cell percentages in all infected patients, except patient E116, at the first sample collection time point (Fig. 2A).
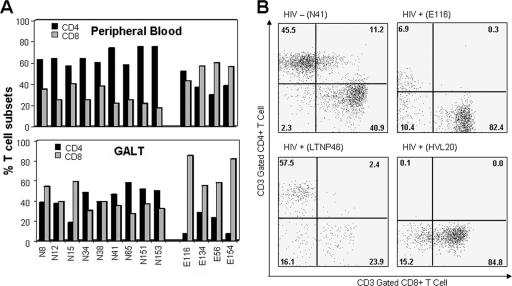
FIG. 2.
Disruption in T-cell homeostasis in GALT during primary-stage HIV infection. (A) CD4+ T-cell percentages in GALT were markedly decreased in all four primary-stage patients, with the largest depletion occurring in patients at 6 and 8 weeks postinfection. (B) The vast majority of CD4+ depletion in GALT appears to occur early in the primary stage, leading to the disruption of T-cell homeostasis, similarly to observations in chronic-stage patients but in striking contrast to LTNP patients, who maintain natural control over viral replication and disease progression.
In contrast to circulating CD4+ T cells, mucosal CD4+ T-cell levels were severely reduced in all four HIV-infected patients (Fig. 2A). CD4+ T-cell depletion in GALT was greater at 6 to 8 weeks postinfection (patients E116 and E154) than at 4 weeks postinfection (patients E134 and E56). These data support previous reports of severe gastrointestinal CD4+ T-cell depletion in HIV patients in primary (10) and chronic (25) stages of infection and stand in contrast to results for LTNP patients (Fig. 2B), who maintain CD4+ T cells in GALT and natural control over disease progression. This was validated by IHC of tissue sections for the presence of CD4+ T cells as previously published (10, 25). As observed in previous studies, mucosal CD4+ T-cell depletion coincided with a dramatic increase in the prevalence of CD8+ T cells.
Primary HIV infection shifts the physiological balance of host mucosal gene transcription.
To gain insights into the characteristics of early host responses in GALT during primary HIV infection and the overall impact of elevated viral replication on gastrointestinal processes, we evaluated global gene expression profiles in jejunal mucosal biopsy samples by DNA microarray analysis. Alterations in mucosal gene transcription were determined in each primary-stage patient (E116, E134, and E154) in comparison to mean baseline transcription levels in six healthy uninfected controls (N4, N8, N12, N34, N38, and N41). Hierarchical clustering of genes whose expression was significantly altered due to HIV infection resulted in the identification of distinct patterns of up and down regulated transcription (Fig. 3, left panel). The functional attributes of genes within up and down regulated subclusters were then further evaluated to determine the most prominent molecular pathways/physiologic processes impacted by HIV infection.
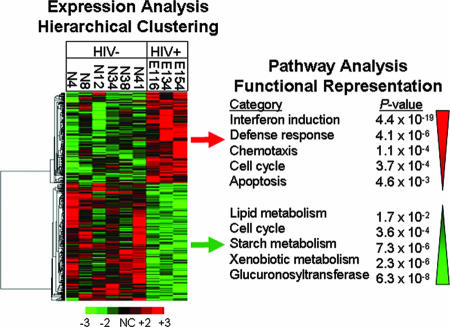
FIG. 3.
Modulation of host gene expression in GALT during primary HIV infection. Genes whose transcription was altered by HIV infection in the intestinal mucosa were identified through microarray analysis of jejunal biopsy samples and subjected to hierarchical clustering to identify common patterns of up and down regulation. Pathways statistically overrepresented were identified through functional analysis of the genes in each subcluster. Up regulated genes were predominated by defense response factors, cell cycle mediators, and apoptosis regulators, while genes associated with a broad range of metabolic pathways and tissue regeneration were down modulated, highlighting a pathogenic shift in the normal physiological balance within the GALT microenvironment.
The up regulated subcluster was statistically overrepresented with genes involved in interferon induction, defense responses, chemotaxis, cell cycle regulation, and apoptosis (Fig. 3, right panel), while the down regulated cluster was enriched with genes associated with lipid, starch, and xenobiotic metabolism, cell cycle regulation, and glucuronosyltransferase activity. Overrepresentation of cell cycle-associated genes in both the up and down regulated gene lists was not surprising, given the complexity of cell types comprising the GALT biopsy samples, and may be indicative of increased proliferation in some cells (e.g., CD8+ T cells) and repressed expansion in others. Interestingly, the pathways that were up regulated in primary HIV-1 infection were similar to those pathways up regulated in chronic-stage patients with high viral loads but not up regulated in LTNP patients (25). In contrast, genes associated with metabolism were found to be down regulated in all patient groups, including LTNP patients (25). Collectively, the broad range of genes and pathways modulated suggested that fundamental changes in the molecular microenvironment of the intestinal mucosa were well established within the first few weeks of infection.
Link between local viral replication and increased expression of immune activation and inflammation-associated genes in GALT.
Patients with primary HIV infections demonstrated an induction of robust changes in gene expression associated with both innate and adaptive immune responses in GALT (Fig. 4G). In accordance with mucosal and peripheral blood viral loads, the level of transcription of innate antimicrobial factors Cig-5, Mx-1, APOBEC, and 2′-5′-oligoadenylate synthetase was greater in patients E134 and E154 than in patient E116. Patients E134 and E154 also displayed up regulation of numerous interferon-induced and cytotoxicity-associated genes that were largely normalized in patient E116. The divergence in expression profiles between patient E116 and patients E134 and E154 extended to genes involved in chemotaxis, including genes encoding molecules known to enhance HIV replicative capacity (RANTES and CCR5) and increase cellular infiltration (IP-10 and CD18). Supportive evidence for changes in inflammation-associated gene expression was provided by IHC analysis, as evidenced by increased expression of CCL5/RANTES at the protein level in crypt areas of the jejunum during primary infection (Fig. 4E and F). These data suggest that the onset of elevated immune activation and inflammation occurred very rapidly in the intestinal mucosal microenvironment in response to initial viral replication.
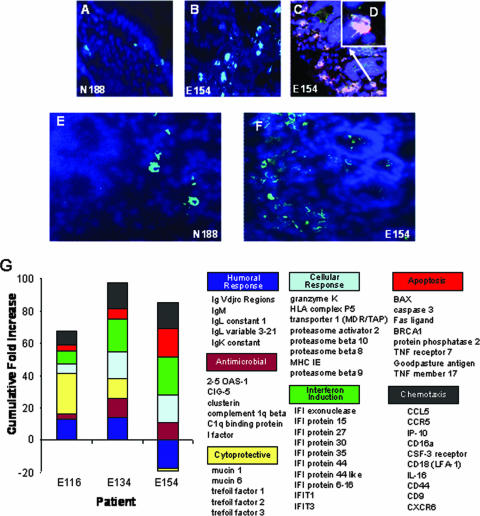
FIG. 4.
(A to F) Immunohistochemistry-based detection of RANTES and activated caspase 3 in GALT. Increased levels of caspase 3 were observed in GALT during primary HIV infection (B) compared to an uninfected healthy control (A). Caspase 3 (dark pink) signal also overlapped substantially with the epithelial marker Ber EP4 (green), appearing as a light pink stain (×40 magnification in panel C and ×100 magnification in panel D). RANTES expression was also increased (light blue punctuate stain) in primary-stage infections (F) compared to that in uninfected controls (E). (G) Gene expression profile of host immune responses in GALT during primary HIV infection. n-fold increases over uninfected controls in the expression of genes controlling various host response pathways in GALT during primary-stage infection were determined for patients E116, E134, and E154. A broad range of antiviral, cytotoxic, antimicrobial, and humoral response mediators were significantly up regulated. Stronger levels of interferon induction and cytoxic responses correlated with high levels of local viral replication (E154), while lower viral burden was associated with dampened immune responses and increased transcription of cytoprotective genes (E116).
Imbalance between intestinal epithelial barrier turnover and renewal during primary HIV infection.
Elevated levels of caspase 3 protein expression were observed during primary HIV infection (Fig. 4A and B) and supported microarray data on increased apoptosis-related gene expression (Fig. 3 and 4G). Combined IHC analysis revealed increased levels of apoptosis in the intestinal epithelial cells during primary HIV infection (Fig. 4C and D). These results suggest an increased turnover of intestinal epithelial cells very early during HIV infection, possibly leading to an impaired intestinal mucosal epithelial barrier.
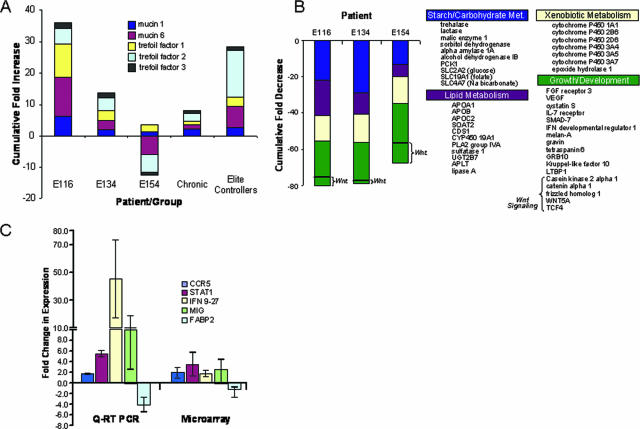
Given the divergence in immune response-associated gene expression between patient E116 and patients E134 and E154, it was noteworthy that patient E116 also displayed large increases in genes with intestinal mucosal protective and regenerative functions, such as trefoil factors 1, 2, and 3 and mucins 1 and 6. We further determined that these gene products were also highly up regulated in the therapy-naïve elite controllers within our HIV patient cohort, while strikingly reduced in therapy-naïve patients with chronic progressive HIV infection (Fig. 5A). These data indicate that a strong compensatory induction of mucosal barrier protective machinery and a dampened immune response may be associated with reduced enteropathy and greater control of disease progression.
FIG. 5.
(A) Suppression of HIV replication is linked to induction of genes with cytoprotective and regenerative function. Genes encoding mucins and trefoil factors were substantially up regulated in the primary-stage HIV patient that displayed the lowest viral burden (E116). The cumulative level of increase was similar to those observed for elite long-term HIV controllers and in contrast to those observed for chronic-stage patients as well as E134 and E154, suggesting a potential link between primary-stage control of enteropathy and effective suppression of disease progression. (B) Repressed expression of metabolic factors, growth mediators, and cellular differentiation markers in primary-stage HIV patients. mRNA levels for a broad range of genes controlling lipid, carbohydrate, and xenobiotic metabolism, organogenesis, and cellular differentiation were repressed in essentially all primary-stage HIV patients tested, indicating that impairment of tissue growth and regeneration may have contributed to the early development of enteropathy. Patient E154, with elevated mucosal viral loads, showed the lowest expression of genes involved in epithelial growth and differentiation (e.g., Wnt pathway genes). (C) RT-PCR-based analysis of immune activation and lipid metabolism-associated gene expression. TaqMan validation of microarray results demonstrated similar patterns of up regulation of immune activation-associated genes CCR5, STAT1, IFN9-27, and MIG and a down regulation of lipid metabolism-associated transcription (FABP2). Error bars indicate standard deviations.
Microarray data showed that the genes involved in epithelial differentiation pathways (casein kinase 2A1, Wnt 5A, frizzled homolog 1, catenin alpha 1, transforming growth factor [TGF] beta binding protein, and SMAD-7) were down modulated in patients during primary HIV infection (Fig. 5A). Interestingly, patient E154 displayed the strongest down regulated profile in this functional category, highlighted by more substantial losses of Wnt-associated transcription. Our data on increased apoptosis of epithelial cells, combined with decreased expression of epithelial differentiation, suggest that the impairment of epithelial barrier renewal was an early event during primary HIV infection.
Decreased tissue regeneration and metabolism-associated transcription in patients with elevated immune responses in GALT.
In contrast to increased transcription of genes involved in host immune response and apoptosis, a broad range of metabolism- and growth-associated genes were down regulated in GALT during primary HIV infection (Fig. 5B). A similar loss in the expression of this set of genes has been reported for patients with chronic HIV infections (25), suggesting that the profile represents a defined molecular signature for enteropathy in GALT. Lipid, carbohydrate, and xenobiotic metabolism-linked transcription were all similarly repressed. It is also noteworthy that down regulation of xenobiotic metabolic factors included several cytochrome P450 family members involved in detoxification and drug metabolism, notably CPY2J and CYP3A, which are believed to play critical roles in metabolizing many of the HIV-targeted drugs utilized in HAART. These data are supported by similar findings during primary infection in the SIV model (7) and indicate that immunodeficiency virus-induced enteropathy is characterized by an early impairment of enterocyte function.
The broad range and magnitude of loss in transcription of genes controlling metabolic function prompted us to further examine the gene array data for the regulation of genes controlling mucosal growth, maintenance, and repair (Fig. 5A and B). We found a consistent pattern of repression of multiple growth factors (vascular endothelial growth factor, hepatocyte growth factor-like, and Kruppel-like factor 10) and their receptors (fibroblast growth factor receptor 3) as well as of genes involved in epithelial differentiation pathways. Also noteworthy was the observed down modulation of interleukin-7 receptor expression in all infected patients, suggesting that severe CD4+ T-cell depletion may lead to the disruption in interleukin-7 signaling that is required for maintenance of naïve and memory T-cell populations. Collectively, these data provided a comprehensive molecular profile, depicting a disruption of regenerative capacity that contributes to an early loss of intestinal epithelial barrier and mucosal functions in primary HIV infection.
To provide supportive evidence for the alterations in the expression of selected genes indicated by the microarray analysis, we compared mRNA levels between infected patients and uninfected controls by quantitative real-time PCR (Fig. 5C). The real-time PCR results confirmed the microarray findings and further supported our conclusions that high levels of immune activation (STAT-1, CCR5, IFN9-27, and MIG) and impairment of metabolic (FABP2) functions were contributing factors in the early manifestation of HIV-associated enteropathogenesis.
DISCUSSION
Historically, HIV infection was initially labeled as a “wasting disease” due to the common presentation of severe gastrointestinal complications and digestive dysfunction (9, 23, 26). Over the last decade, multiple groups have reported GALT to be an early target of HIV and a site of severe CD4+ T-cell depletion (10, 20). Recently, it has been shown that unlike the blood compartment, there is a substantial delay in the restoration of CD4+ T cells in intestinal mucosa following initiation of HAART (11). Additional studies have established that in chronic HIV infection, immune activation, inflammatory responses, and apoptosis dominate the molecular environment in GALT (17, 25). Collectively, these data suggest that deterioration of the intestinal mucosa may initiate rapidly following infection and progress at a rate potentially insurmountable by the body's normal repair and maintenance mechanisms. Our current investigation of primary HIV infection supports this hypothesis and provides the first comprehensive evidence that HIV-induced enteropathy is well established within the first few weeks of infection, potentially even prior to seroconversion.
As expected and reported previously, patients with primary HIV infection in our study showed no appreciable drop in peripheral CD4+ T-cell numbers. However, each patient displayed a marked decline in CD4+ T-cell percentages in GALT (Fig. 2) accompanied by a significant increase in CD8+ T-cell prevalence. The severity of CD4+ T-cell depletion in GALT has been attributed in previous reports to massive localized infection of target cells as well as bystander death resulting from apoptosis or necrosis (18). We were able to readily detect HIV RNA in the lamina propria of the intestinal mucosa in all patients by both immunohistochemistry and quantitative real-time PCR (Fig. 1), indicating that local viral replication was ongoing in primary infection. HIV gag p24 was observed in both CD4+ T cells and macrophages, highlighting the role of GALT as a viral reservoir and suggesting that a large proportion of lymphocyte depletion could result directly from infection.
CD8+ T cells that infiltrate an HIV-infected, CD4+ T-cell-depleted, GALT microenvironment may be in a state of constant activation due the presence of a large antigen stimulus (10, 28). The loss of T-helper cell function, however, suggests that a large proportion of host response may not be HIV antigen directed. Thus, the balance of host response to primary-stage HIV infection appears to be dominated by a large nonspecific cytotoxic response and increased production of proinflammatory chemo- and cytokines that also serve to damage the epithelial barrier (22, 24). Our current study indicates that patients with better viral suppression in GALT in primary infection display higher up regulation of trefoil factors and mucins that function to protect and maintain the epithelial barrier (Fig. 5A). Importantly, this phenotype is also observed in HIV-infected patients that are elite controllers (LTNP) of viral replication and disease progression in the absence of HAART, but it is not observed in patients with chronic progressive HIV infections (Fig. 5A). Moreover, a similar expression profile was identified for CD24 (data not shown), which is known as a multipotent stem cell biomarker that has recently been identified in epithelial lineages (14, 19). Taken together, these data suggest that an early and sustained increase in intestinal epithelial repair and regeneration processes may be necessary to effectively suppress HIV-associated enteropathy, and by extension, disease progression.
Other, non-HIV-associated enteropathological conditions resulting in chronic digestive dysfunction, such as inflammatory bowel disease, have been shown to involve the disruption of epithelial maturation and turnover (29). Epithelial migration and differentiation involve complex orchestration between TGF beta and Wnt signaling pathways and coordinated interaction and dissociation of cells with the extracellular matrix (27, 29). Our investigation uncovered evidence of the dysregulation of Wnt and TGF beta signaling (Fig. 5B), providing an additional indication that the epithelial barrier is a rapid target of HIV-associated enteropathogenesis in primary HIV infection.
Whether extensive down regulation of genes controlling metabolic functions (lipid, carbohydrate, and xenobiotic) (Fig. 5B) resulted exclusively from a breach of the epithelial barrier or from multiple mechanisms remains unclear. Recent in vitro studies may suggest the latter, showing that the exposure of a Caco-2 cell line to HIV tat resulted in a loss of glucose uptake, potentially through the disruption of tubulin networks and missorting, as evidenced by the loss of Na-d-glucose symporter (SGLT-1) expression and activity (4). These data suggest that at least some of the disruption in metabolic and digestive functions could result from interaction with cell-free viral proteins. Our study suggests that the functional disruption may also be associated with increased epithelial apoptosis (Fig. 4). In summary, our findings suggest that enteropathic effects of HIV infection are observed very early in HIV infection and develop prior to the long-term inflammation and immune activation characteristic of HIV infection. Clearly, further in vivo and in vitro studies of epithelial cell differentiation and repair and maintenance of the epithelial barrier in the context of HIV infection will be necessary to fully understand the impact of enteropathy on disease progression and to develop novel strategies to repair the gut mucosal epithelium and restore immune integrity and function.
Acknowledgments
We thank the patients for their participation in the study, the nursing staff for their support, Monica Macal for her assistance coordinating analyses of archived data, and the School of Medicine Microarray Core Facility, the Lucy Whittier Molecular Core Facility, and the Optical Biology Core for technical support.
This study was supported by National Institutes of Health grants DK61297 and AI43274 and the California HIV AIDS research program grant CH05-D-606.
Footnotes
Published ahead of print on 24 October 2007.
REFERENCES
- 1.Andersson, J., S. Kinloch, A. Sonnerborg, J. Nilsson, T. E. Fehniger, A. L. Spetz, H. Behbahani, L. E. Goh, H. McDade, B. Gazzard, H. Stellbrink, D. Cooper, and L. Perrin. 2002. Low levels of perforin expression in CD8+ T lymphocyte granules in lymphoid tissue during acute human immunodeficiency virus type 1 infection. J. Infect. Dis. 1851355-1358. [DOI] [PubMed] [Google Scholar]
- 2.Blattner, W. A., K. A. Oursler, F. Cleghorn, M. Charurat, A. Sill, C. Bartholomew, N. Jack, T. O'Brien, J. Edwards, G. Tomaras, K. Weinhold, and M. Greenberg. 2004. Rapid clearance of virus after acute HIV infection: correlates of risk of AIDS. J. Infect. Dis. 1891793-1801. [DOI] [PubMed] [Google Scholar]
- 3.Brenchley, J. M., D. A. Price, and D. C. Douek. 2006. HIV disease: fallout from a mucosal catastrophe? Nat. Immunol. 7235-239. [DOI] [PubMed] [Google Scholar]
- 4.Canani, R. B., G. De Marco, A. Passariello, V. Buccigrossi, S. Ruotolo, I. Bracale, F. Porcaro, G. Bifulco, and A. Guarino. 2006. Inhibitory effect of HIV Tat protein on the sodium-d-glucose symporter of human intestinal epithelial cells. AIDS 205-10. [DOI] [PubMed] [Google Scholar]
- 5.Dennis, G., Jr., B. T. Sherman, D. A. Hosack, J. Yang, W. Gao, H. C. Lane, and R. A. Lempicki. 2003. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 4P3. [PubMed] [Google Scholar]
- 6.Douek, D. C., L. J. Picker, and R. A. Koup. 2003. T cell dynamics in HIV infection. Annu. Rev. Immunol. 21265-304. [DOI] [PubMed] [Google Scholar]
- 7.George, M. D., E. Reay, S. Sankaran, and S. Dandekar. 2005. Early antiretroviral therapy for simian immunodeficiency virus infection leads to mucosal CD4+ T-cell restoration and enhanced gene expression regulating mucosal repair and regeneration. J. Virol. 792709-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.George, M. D., S. Sankaran, E. Reay, A. C. Gelli, and S. Dandekar. 2003. High-throughput gene expression profiling indicates dysregulation of intestinal cell cycle mediators and growth factors during primary simian immunodeficiency virus infection. Virology 31284-94. [DOI] [PubMed] [Google Scholar]
- 9.Giovanni, B., C. Calabrese, R. Manfredi, A. M. Pisi, G. Di Febo, R. Hakim, G. Cenacchi, and G. Biasco. 2005. HIV enteropathy: undescribed ultrastructural changes of duodenal mucosa and their regression after triple antiviral therapy. A case report. Dig. Dis. Sci. 50617-622. [DOI] [PubMed] [Google Scholar]
- 10.Guadalupe, M., E. Reay, S. Sankaran, T. Prindiville, J. Flamm, A. McNeil, and S. Dandekar. 2003. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 7711708-11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guadalupe, M., S. Sankaran, M. D. George, E. Reay, D. Verhoeven, B. L. Shacklett, J. Flamm, J. Wegelin, T. Prindiville, and S. Dandekar. 2006. Viral suppression and immune restoration in the gastrointestinal mucosa of human immunodeficiency virus type 1-infected patients initiating therapy during primary or chronic infection. J. Virol. 808236-8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heise, C., P. Vogel, C. J. Miller, C. H. Halsted, and S. Dandekar. 1993. Simian immunodeficiency virus infection of the gastrointestinal tract of rhesus macaques. Functional, pathological, and morphological changes. Am. J. Pathol. 1421759-1771. [PMC free article] [PubMed] [Google Scholar]
- 13.Kahn, J. O., and B. D. Walker. 1998. Acute human immunodeficiency virus type 1 infection. N. Engl. J. Med. 33933-39. [DOI] [PubMed] [Google Scholar]
- 14.Lawson, D. A., L. Xin, R. U. Lukacs, D. Cheng, and O. N. Witte. 2007. Isolation and functional characterization of murine prostate stem cells. Proc. Natl. Acad. Sci. USA 104181-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leutenegger, C. M., J. Higgins, T. B. Matthews, A. F. Tarantal, P. A. Luciw, N. C. Pedersen, and T. W. North. 2001. Real-time TaqMan PCR as a specific and more sensitive alternative to the branched-chain DNA assay for quantitation of simian immunodeficiency virus RNA. AIDS Res. Hum. Retrovir. 17243-251. [DOI] [PubMed] [Google Scholar]
- 16.Leutenegger, C. M., C. N. Mislin, B. Sigrist, M. U. Ehrengruber, R. Hofmann-Lehmann, and H. Lutz. 1999. Quantitative real-time PCR for the measurement of feline cytokine mRNA. Vet. Immunol. Immunopathol. 71291-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li, Q., T. Schacker, J. Carlis, G. Beilman, P. Nguyen, and A. T. Haase. 2004. Functional genomic analysis of the response of HIV-infected lymphatic tissue to antiretroviral therapy. J. Infect. Dis. 189572-582. [DOI] [PubMed] [Google Scholar]
- 18.Mattapallil, J. J., D. C. Douek, B. Hill, Y. Nishimura, M. Martin, and M. Roederer. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 4341093-1097. [DOI] [PubMed] [Google Scholar]
- 19.Matulka, L. A., A. A. Triplett, and K. U. Wagner. 2007. Parity-induced mammary epithelial cells are multipotent and express cell surface markers associated with stem cells. Dev. Biol. 30329-44. [DOI] [PubMed] [Google Scholar]
- 20.Mehandru, S., M. A. Poles, K. Tenner-Racz, A. Horowitz, A. Hurley, C. Hogan, D. Boden, P. Racz, and M. Markowitz. 2004. Primary HIV infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 200761-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nilsson, J., S. Kinloch-de-Loes, A. Granath, A. Sonnerborg, L. E. Goh, and J. Andersson. 2007. Early immune activation in gut-associated and peripheral lymphoid tissue during acute HIV infection. AIDS 21565-574. [DOI] [PubMed] [Google Scholar]
- 22.Puleston, J., M. Cooper, S. Murch, K. Bid, S. Makh, P. Ashwood, A. H. Bingham, H. Green, P. Moss, A. Dhillon, R. Morris, S. Strobel, R. Gelinas, R. E. Pounder, and A. Platt. 2005. A distinct subset of chemokines dominates the mucosal chemokine response in inflammatory bowel disease. Aliment. Pharmacol. Ther. 21109-120. [DOI] [PubMed] [Google Scholar]
- 23.Reeders, J. W., J. Yee, R. M. Gore, F. H. Miller, and A. J. Megibow. 2004. Gastrointestinal infection in the immunocompromised (AIDS) patient. Eur. Radiol. 14(Suppl. 3)E84-E102. [DOI] [PubMed] [Google Scholar]
- 24.Resnick, M. B., E. Sabo, P. A. Meitner, S. S. Kim, Y. Cho, H. K. Kim, R. Tavares, and S. F. Moss. 2006. Global analysis of the human gastric epithelial transcriptome altered by Helicobacter pylori eradication in vivo. Gut 551717-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sankaran, S., M. Guadalupe, E. Reay, M. D. George, J. Flamm, T. Prindiville, and S. Dandekar. 2005. Gut mucosal T cell responses and gene expression correlate with protection against disease in long-term HIV-infected nonprogressors. Proc. Natl. Acad. Sci. USA 1029860-9865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shenoy, S., S. Baliga, T. Kurnvilla, H. V. Prashanth, and R. M. Dominic. 2003. Opportunistic intestinal parasitic infections in human immunodeficiency virus infected patients in Mangalore, South India. Trop. Doct. 33250. [DOI] [PubMed] [Google Scholar]
- 27.Wittig, B. M., A. Stallmach, M. Zeitz, and U. Gunthert. 2002. Functional involvement of CD44 variant 7 in gut immune response. Pathobiology 70184-189. [DOI] [PubMed] [Google Scholar]
- 28.Wood, K. L., K. S. Knox, Y. Wang, R. B. Day, C. Schnizlein-Bick, and H. L. Twigg III. 2005. Apoptosis of CD57+ and CD57− lymphocytes in the lung and blood of HIV-infected subjects. Clin. Immunol. 117294-301. [DOI] [PubMed] [Google Scholar]
- 29.Zeitz, M., R. Ullrich, T. Schneider, H. L. Schieferdecker, and E. O. Riecken. 1994. Cell differentiation and proliferation in the gastrointestinal tract with respect to the local immune system. Ann. N. Y. Acad. Sci. 73375-86. [DOI] [PubMed] [Google Scholar]