Abstract
Lentivectors stimulate potent immune responses to antigen transgenes and are being developed as novel genetic vaccines. To improve safety while retaining efficacy, we constructed a lentivector in which transgene expression was restricted to antigen-presenting cells using the mouse dectin-2 gene promoter. This lentivector expressed a green fluorescent protein (GFP) transgene in mouse bone marrow-derived dendritic cell cultures and in human skin-derived Langerhans and dermal dendritic cells. In mice GFP expression was detected in splenic dectin-2+ cells after intravenous injection and in CD11c+ dendritic cells in the draining lymph node after subcutaneous injection. A dectin-2 lentivector encoding the human melanoma antigen NY-ESO-1 primed an NY-ESO-1-specific CD8+ T-cell response in HLA-A2 transgenic mice and stimulated a CD4+ T-cell response to a newly identified NY-ESO-1 epitope presented by H2 I-Ab. As immunization with the optimal dose of the dectin-2 lentivector was similar to that stimulated by a lentivector containing a strong constitutive viral promoter, targeting antigen expression to dendritic cells can provide a safe and effective vaccine.
Dectin-2 is a C-type lectin-like receptor encoded in the natural killer complex of C-type lectin genes (4, 17). Dectin-2 is expressed on cells of the myeloid lineage (41); it recognizes high-mannose structures (28) and acts as a pattern recognition receptor for fungi (37). Injection of soluble dectin-2 can block UV radiation-induced tolerance, perhaps by interaction of dectin-2 with a ligand on T cells (3).
A 3.2-kb promoter fragment from the dectin-2 gene has been used to drive a luciferase reporter system in transgenic mice (8). The highest luciferase expression was observed in skin; within the epidermis expression was restricted to the potent antigen-presenting cells, Langerhans cells (LC) (8). Considerably lower levels of luciferase were detected in splenic dendritic cells (DC) and peritoneal macrophages (8). Activation of DC and macrophages increased luciferase expression to 20% of that seen in LC (7). There is some discrepancy between promoter activity and expression of murine dectin-2, detected using a monoclonal antibody. The endogenous protein was found in LC and some tissue macrophages and DC; the highest levels were observed on activated, inflammatory monocytes (41).
There is currently much interest in the development of immunization strategies that target antigens to antigen-presenting cells in vivo to improve vaccine efficacy (reviewed in reference 40). Because of its tissue distribution, dectin-2 has been considered a potential molecular target on antigen-presenting cells. Subcutaneous injection of a dectin-2 antibody results in the labeling of a subset of CD11c+ DC in the draining lymph node (LN) (9). Injection of an antigen coupled to the same antibody with poly(I·C) as an adjuvant stimulates a potent CD8+ T-cell response, equivalent to that obtained with an antibody directed against the classic DC targeting molecule CD205 (9). The 3.2-kb dectin-2 gene promoter fragment has also been used to drive green fluorescent protein (GFP) expression in a DNA vaccine construct. After delivery to mouse skin by gene gun, expression of GFP was detected in LC in the skin and in DC in the draining LN; antibody and T-cell responses to GFP were stimulated (31).
We along with others have shown that recombinant viruses based on human immunodeficiency virus type 1 ([HIV-1] lentivectors), engineered to encode antigens, stimulate potent T-cell immunity (15, 16, 23, 34, 36). He and colleagues demonstrated that lentivectors transduce skin DC after cutaneous injection; these cells then migrate to the LN and can stimulate CD8+ T cells ex vivo (23). However, this study did not monitor transduction of other cells by the lentivector. To investigate whether antigen expression in antigen-presenting cells is sufficient for stimulation of an immune response, we constructed a lentivector with antigen gene expression restricted to these cells using the 3.2-kb dectin-2 promoter fragment. After injection of a lentivector with a constitutive promoter (spleen focus-forming virus [SFFV] long terminal repeat) driving GFP expression, GFP+ keratinocytes, DC, T cells, and B cells were detected. However, the dectin-2 promoter lentivector restricted GFP expression to CD11c+ DC after subcutaneous injection. Dectin-2 and SFFV lentivectors encoding the human melanoma antigen NY-ESO-1 stimulated similar CD8+ and CD4+ T-cell responses in HLA-A2 transgenic mice. These data demonstrate that antigen expression in DC is responsible for the potent immune response stimulated by lentivectors. Restricting transcriptional activity to nondividing antigen-presenting cells reduces the potential risk of cell transformation following lentivector integration.
MATERIALS AND METHODS
Lentiviral vector production.
The HIV-1-based vector pHRSIN-CSGW was kindly provided by A. Thrasher and is described in Demaison et al. (14). This vector contains the SFFV-driven GFP expression cassette (yielding the SFFV-GFP lentivector). We then subcloned the NY-ESO-1 tumor antigen into the BamHI-NotI site to replace GFP (yielding SFFV-ESO). For the dectin-2-driven constructs, we subcloned the dectin-2 gene promoter (3.2-kb mouse dectin-2 gene promoter in a pG3L-basic plasmid) into the EcoRI-BamHI site to replace SFFV (yielding the DEC-GFP lentivector). We again subcloned the NY-ESO-1 tumor antigen into the BamHI-NotI site to replace GFP (yielding DEC-ESO). To obtain virus, 293T cells were cotransfected with the vector plasmid, alongside the packaging (pCMV8.91, where CMV is cytomegalovirus) and envelope—either pMDG or phCMV-envFriend (gift from T. Heidmann, Institut Gustave Roussy, Villejuif, France)—plasmids as previously described (43). Viral supernatants were concentrated by ultracentrifugation. Vector titers were determined by infecting 293T cells and performing TaqMan PCR, giving the number of copies of the transgene integrated per cell. Primer and probe sequences (primers, TGTGTGCCCGTCTGTTGTGT and GAGTCCTGCGTCGAGAGAGC; probe, CAGTGGCGCCCGAACAGGGA) specific to the HIV-1 packaging signal (ψ) were used. Titers of GFP vectors were also determined by fluorescence-activated cell sorting (FACS). The concentrations of Friend-pseudotyped virus and vesicular stomatitis virus protein G (VSV-G) used in comparisons were also determined by measuring the amount of HIV reverse transcriptase using an enzyme-linked immunosorbent assay kit (Roche).
Cell cultures and lentiviral infections.
293T cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen) plus 10% heat-inactivated fetal calf serum (FCS; PAA Laboratories) at 37°C and 10% CO2. NIH 3T3 cells were cultured in RPMI 1640 medium (Invitrogen) with 10% FCS and maintained at 37°C and 5% CO2. For lentiviral infection 293T and NIH 3T3 cells were plated at 2 × 105 cells/well in 24-well plates and infected with virus for 18 h. GFP expression was detected 3 days later by cytometric fluorescence (FACS) analysis.
Murine DC were obtained from bone marrow by culturing cells in Iscove's modified Dulbecco's medium with 10% FCS and 40 ng/ml of recombinant mouse granulocyte-macrophage colony-stimulating factor (GM-CSF; Peprotech), as previously described (26). Immature DC were harvested on day 5 of culture, replated at 5 × 105 cells/well in 24-well plates and infected with lentivirus at a multiplicity of infection (MOI) of 10 for 18 h. GFP expression was detected 6 days later by FACS.
Human skin was obtained from mammoplasty patients with informed consent and local ethical approval from the Northumberland and North Tyneside Research Ethics Committee. Skin was cut with a skin graft knife at 250 μm and incubated with 1 mg/ml dispase for 60 min at 37°C. Epidermal and dermal sheets were separated and incubated for a further 60 h in RPMI medium with 10% FCS and 500 U/ml GM-CSF (Sagramostim clinical grade; Berlex). Migratory LC and dermal DC (dDC) were harvested from the fluid phase. Typically these preparations contain 60 to 80% LC or dDC. LC or dDC (2 × 105) were infected with lentivirus at an MOI of 60 in a minimum volume (100 to 200 μl) for 60 min at 37°C. Fresh medium containing 500 U/ml GM-CSF was added, and the cells were transferred to a 48-well plate. A further 200 μl of medium was added on day 2, and 200 μl was replaced again on day 4. Cells were analyzed on day 6 postinfection after staining with anti-human HLA-DR-phycoerythrin (PE)-labeled antibody (BD Pharmingen) on a FACSCalibur instrument using FlowJo (TreeStar).
To examine the GFP expression in murine skin ex vivo, ear skin was obtained from C57BL/6 mice, and the epidermal sheet was separated. The epidermal sheet was placed in a culture dish and incubated for 8 h with virus solution (5 × 105 IU). Culture medium was then added, and infected tissue was incubated for 2 days. The epidermal sheet was examined for GFP expression using confocal microscopy.
In vivo transduction experiments.
Eight-week-old C57BL/6 mice (Harlan, United Kingdom) received phosphate-buffered saline (PBS) or 3 × 108 IU of SFFV-GFP or DEC-GFP lentivector by injection in the tail vein (intravenous) or by subcutaneous injection (footpad and base of tail). Analysis of GFP expression was performed 10 days later in spleen cells from mice injected intravenously and 5 days later in cells from the draining LNs from mice injected subcutaneously. Cell suspensions were obtained after incubation with collagenase-D (Worthington). CD11c-positive- and CD11c-negative-enriched cell fractions from spleen or LNs were obtained using CD11c magnetic cell sorting beads (Miltenyi Biotech), according to manufacturer's instructions. Cells from both fractions were stained with fluorescent antibodies specific to surface markers for phenotypic characterization of GFP-positive cells by FACS. Antibodies used were CD11c-PE, I-Ab-biotin, CD3-biotin, CD19-PE (eBioscience), CD8α-allophycocyanin (APC), DEC205-biotin, (BD Bioscience), and dectin-2-biotin (kind gift from Philip Taylor, School of Medicine, Cardiff, United Kingdom [3]). Streptavidin-conjugated APC or peridinin chlorophyll protein (eBioscience) was used as a secondary reagent for biotin-conjugated antibodies. Cells (106/sample) were stained for 30 min on ice, washed, and analyzed on a FACS LSR instrument using CellQuest software (BD Bioscience). At least 150,000 events/sample were acquired.
Immunizations.
Adult transgenic mice expressing the α1 and α2 domains of the human HLA-A2 molecule (HHD) crossed onto the C57BL/6 background were obtained from F. Lemonier (18) and bred at our animal facilities.
Mice were immunized with SFFV-ESO, DEC-ESO, or DEC-GFP lentivectors (see legend to Fig. 4 for doses), and control mice received PBS, by either intravenous or subcutaneous injection. In some experiments mice received a boost immunization of recombinant vaccinia virus expressing NY-ESO-1 (ESO-vaccinia at 2 × 106 PFU/mouse, intravenously) 3 weeks after first immunization.
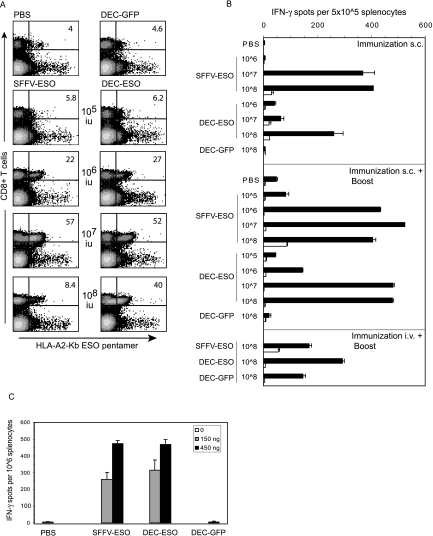
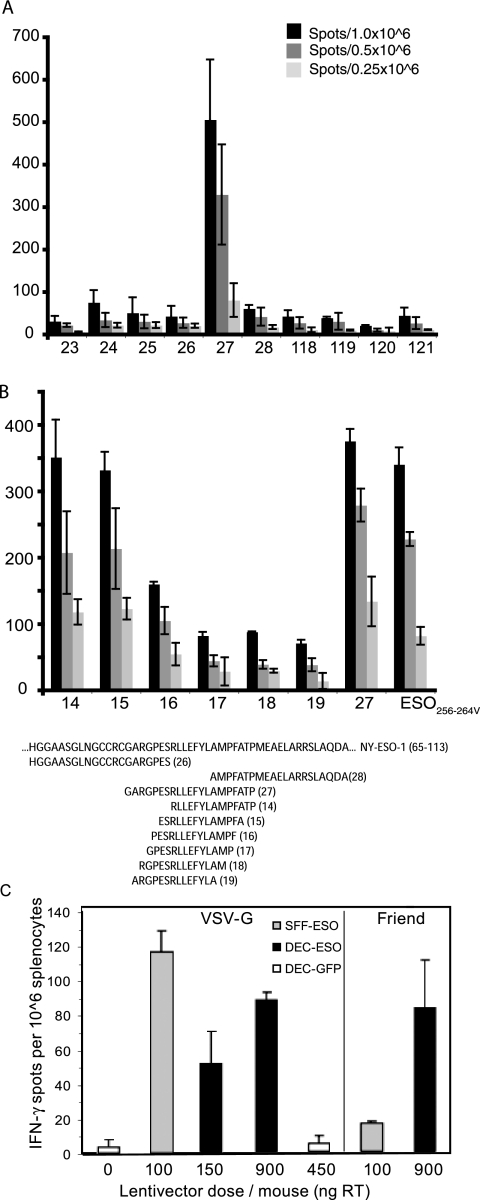
FIG. 4.
NY-ESO-specific CD8+ T-cell responses after immunization with lentivectors. (A and B) HHD mice were immunized subcutaneously or intravenously with PBS, DEC-GFP, SFFV-ESO, or DEC-ESO VSV-G lentivectors. Lentivector doses per mouse are indicated. Some mice received an intravenous boost with ESO-vaccinia virus. (A) CD8+ T-cell response measured by pentamers (day 8 after boost) in the blood of mice that were immunized subcutaneously and boosted. Numbers indicate the percentages of T cells responding to the HLA-A201Kb-ESO-157 pentamers within the CD8+ population. Data are from one mouse out of two per group. (B) T cells were tested in an ELISPOT IFN-γ assay 12 days after immunization or boost. Spleen cells were pooled (n = 2/group), and incubated in duplicates, either with the HLA-A021-restricted ESO-1157-165 peptide (black bars) or medium only (white bars). Standard error bars represent the mean count from duplicate wells. One representative experiment out of three is shown. s.c., subcutaneous; i.v., intravenous. (C) HHD mice were immunized subcutaneously with Friend-pseudotyped lentivectors carrying the ESO peptide, and spleen cells were tested in an ELISPOT IFN-γ assay 12 days later. Spleen cells were pooled (n = 3/group), and incubated with the HLA-A021-restricted ESO-1157-165 peptide. Values are the means ± standard error of duplicate wells after subtraction of background values of cells incubated with medium only.
To identify the major histocompatibility complex class II (MHC-II) H-2b-restricted NY-ESO-1 epitope, recognized by CD4+ T cells, HHD mice were immunized twice, 14 days apart, with a DNA vaccine encoding full-length NY-ESO-1.
Pentamer staining.
Blood samples were collected 8 days after boost, and red blood cells were lysed with red blood cell lysis buffer (Gentra Systems). Cells were washed and incubated along with APC-conjugated HLA-A201-Kb-ESO-157 pentamers (ProImmune, United Kingdom) for 10 min, at room temperature. After washes cells were incubated with anti-mouse CD8-fluorescein isothiocyanate for 20 min on ice and then washed. Samples were analyzed by flow cytometry (150,000 events/sample were acquired) on a FACS LSR instrument using Cell-Quest software (BD Bioscience).
ELISPOT assays.
Gamma interferon (IFN-γ) production by spleen cells was measured 12 days after the first immunization or boost. Enzyme-linked immunospot (ELISPOT) plates (Millipore) were coated with anti-IFN-γ antibody (BD Pharmingen) overnight at 4°C. Serial dilutions of total spleen cells (pool of 2 to 3 mice/group) in RPMI 1640 medium supplemented with 5% heat-inactivated FCS were incubated in duplicates, in either the presence of the HLA-A*0201-restricted ESO peptide comprised of residues 157 to 165 (ESO-1157-165; SLLMWITQV; ProImmune) or medium. To measure the class II response to NY-ESO-1, cells were incubated with the H-2b-restricted ESO-186-99 peptide (RLLEFYLAMPFATP; ProImmune). Cells were cultured for 20 h, and the assay was developed following the manufacturer's instructions. Spots were counted using AID ELISPOT counter and software (Germany).
RESULTS
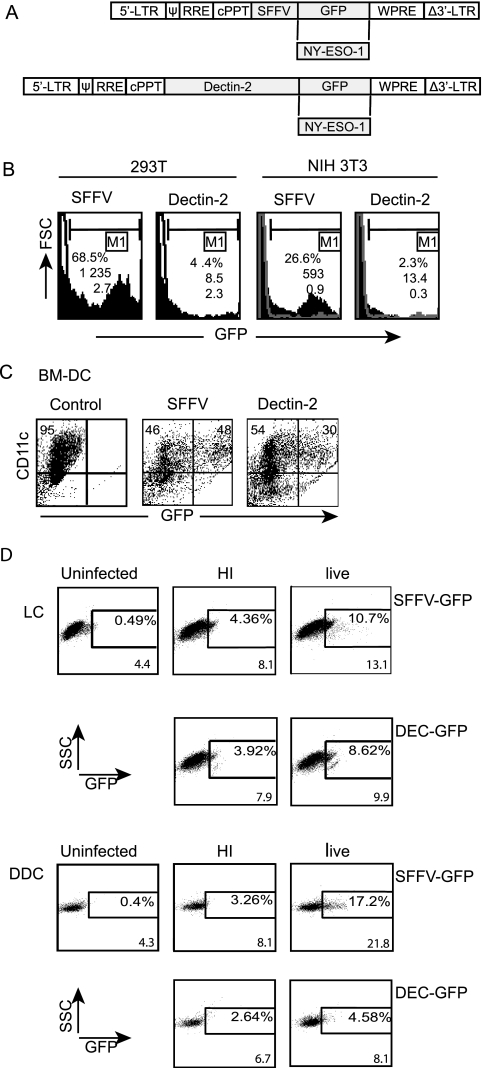
In order to target lentivector gene expression to antigen-presenting cells, we replaced the SFFV promoter in the lentivector CSGW (14) with a 3.2-kb promoter fragment from the mouse dectin-2 gene (8) (Fig. 1A). Lentivectors with the SFFV or dectin-2 promoter driving GFP expression were then used to infect mouse (NIH 3T3) or human (293T) cell lines. While integration of both lentivector genomes was detected, the dectin-2 gene promoter gave very little GFP expression (Fig. 1B). However, the dectin-2 gene promoter expressed GFP in mouse bone marrow-derived DC (Fig. 1C) at a high level; the mean fluorescence intensity (MFI) of the infected cells was 54% of that obtained with the strong SFFV viral promoter. The lentivector with the mouse dectin-2 gene promoter also expressed GFP in human skin-derived LC, and to a lesser extent in human skin-derived dDC (Fig. 1D). The GFP expression detected after transduction with vector that was heat treated to inactivate reverse transcriptase must be due to expression from the vector genomic RNA in the absence of reverse transcription, as reported for retroviral vectors (19). GFP protein uptake by the cells cannot explain this fluorescence as there was no GFP in the dectin-2 vector preparation since the promoter is inactive in 293T cells (Fig. 1B). This expression of GFP driven by the dectin-2 gene promoter in LC is in agreement with the observation that human dectin-2 mRNA is detected in LC (20).
FIG. 1.
Lentiviral vectors, transgene expression in vitro. (A) Lentiviral vector (pHRSINCSGW) expressing GFP under the SFFV promoter was used as a control. Experimental vectors were made by replacing SFFV with the dectin-2 gene promoter and GFP with NY-ESO-1 antigen. cPPT, central polypurine tract; WPRE, woodchuck hepatitis virus posttranscriptional regulatory element; LTR, long terminal repeat; RRE, Rev response element. (B) Human 293T cells and murine NIH 3T3 cells were transduced (MOI of 1) with SFFV-GFP or DEC-GFP lentivectors, and GFP expression was analyzed by FACS 72 h postinfection (solid profiles); nontransduced cells are shown as empty profiles. Numbers indicate percentage of transduced cells, MFI values, and the number of copies of transgene per cell (as determined by TaqMan PCR). (C) Bone marrow-derived DC (BM-DC) were transduced with SFFV-GFP or DEC-GFP lentivectors (MOI of 10), stained with anti-CD11c-PE antibody on day 6 postinfection, and then analyzed for GFP expression by FACS. Cell surface staining shows that 95% of the cell population was CD11c+. Numbers in the upper right quadrants indicate the percentage of GFP-expressing cells among the CD11c+ cells, with MFI values for GFP as 4,161 and 2,256 for the SFFV and dectin-2 promoters, respectively. This is a representative result out of four experiments performed. (D) Lentiviral transduction of primary human LC and dDC. Both cell populations were characterized by surface staining with cell-specific makers. The LC were CD45+ DR+ CD1a+ Langerin+ CD14− and the dDCs were CD45+ DR+ CD1alow CD14− (not shown). Only DR staining is shown for the sake of simplicity. Cells were transduced with SFFV-GFP or DEC-GFP lentivectors (MOI of 60), and GFP expression was detected by FACS after gating on forward-scatter high HLA-DR+ cells in the preparation. Comparison of uninfected controls and cells exposed to heat-inactivated virus (HI) and live virus are shown. Numbers indicate percentages of transduced cells and MFI values. This experiment was performed 3 times with similar results. FSC, forward scatter; SSC, side scatter.
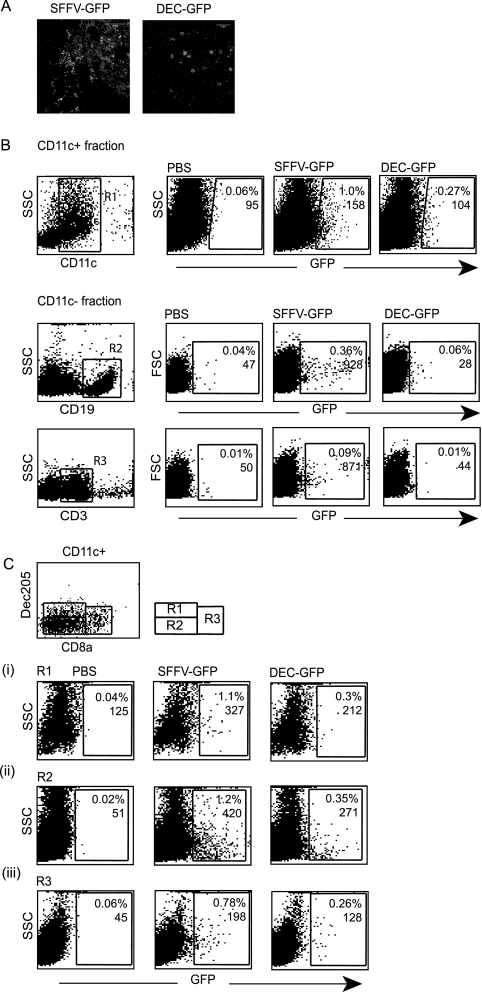
We next examined cell specificity of the dectin-2 gene promoter in vivo. Following subcutaneous injection a small number of epidermal cells around the injection site are exposed to the lentivector. To examine promoter specificity we therefore infected murine skin ex vivo, using separated epidermal sheets. Figure 2A shows that SFFV lentivector expressed GFP in most cells within the sheet, including keratinocytes, whereas the dectin-2 promoter expression was restricted to a very small number of cells that we hypothesize are antigen-presenting cells. The lentivectors were then injected subcutaneously, and transduction of cells in the draining lymph nodes was analyzed. Both vectors transduced CD11c+ cells (Fig. 2B). The SFFV lentivector but not the dectin-2 lentivector also transduced B cells and some T cells in the CD11c− fraction (Fig. 2B). We then analyzed which cells within the CD11c+ population were transduced by the two lentivectors, based on DEC205 and CD8α expression. Figure 2C shows that both vectors transduce the DEC205+ CD8α− population (Fig. 2C, region R1), which are skin-derived DC including LC and dDC (23). Both vectors also transduce the DEC205− CD8α− population and the CD8α+ population (Fig. 2C, regions R2 and R3), which are DC subsets resident in the LNs (23). This suggests that, after subcutaneous injection, the lentivectors transduce some skin-derived DC, either within the skin or after they have migrated to the LN. The lentivectors also traffic to the LN where they infect LN-resident cells; the dectin-2 gene promoter restricts GFP expression to CD11c+ DC.
FIG. 2.
Transgene expression following subcutaneous injection. (A). The epidermal sheet obtained from mouse ear skin was treated in vitro with lentivectors, and skin was analyzed for GFP expression 48 h later. (B). Transgene expression in LN. Mice were injected subcutaneously with PBS (control) or SFFV-GFP or DEC-GFP lentivector. Five days later, LNs were collected, cells were pooled (n = 3 mice/group), and CD11c+- and CD11c−-enriched cell fractions were obtained using CD11c microbeads. Cells from each fraction were analyzed for GFP expression by FACS after staining with specific antibodies. The CD11c+ fraction was stained with anti-CD11c antibody and region R1 was established. GFP+ cells in the CD11c fraction were then analyzed within the R1 region. Cells from the CD11c− fraction were stained for B- and T-cell markers (anti-CD19 or anti-CD3 antibodies, respectively), and analysis of GFP+ cells in each group was performed after gating in the corresponding population. (C). LN DC were costained with anti-CD11c, anti-DEC205, and anti-CD8α antibodies, and three DC subsets were identified, as shown in the diagram. Analysis of GFP expression within each region was then performed. Numbers indicate the percentages of transduced cells and the MFI values. At least 30,000 events per sample were analyzed. This experiment was repeated twice with similar results. SSC, side scatter.
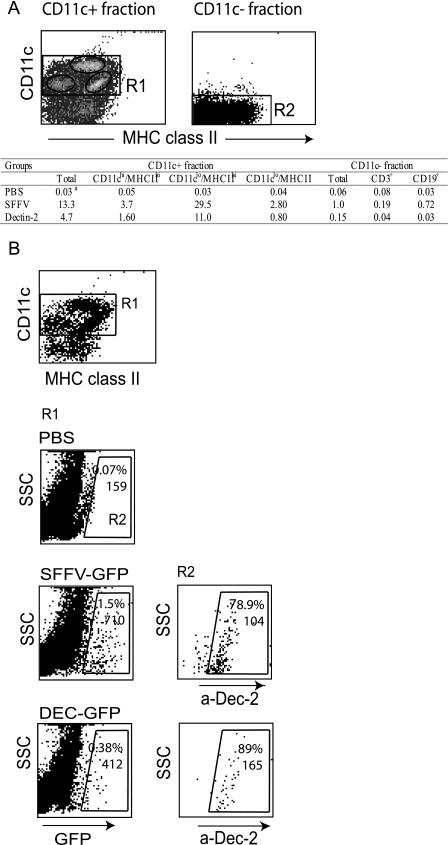
To further examine the cell specificity of the dectin-2 gene promoter in vivo, we also analyzed spleen cells for GFP expression after lentivector injection. We enriched DC from spleen on the basis of CD11c expression and found that GFP expression from the dectin-2 lentivector was restricted to CD11c+-enriched cells (>75% CD11c+) (Fig. 3A). The SFFV lentivector expressed GFP in both CD11c+ and CD11c− (>96% CD11c−) cell populations, although a higher percentage of CD11c+ cells were GFP positive (Fig. 3A). Interestingly, both vectors mainly expressed GFP in the dectin-2-positive subset of CD11c+ cells, which constitutes only 7% of the CD11c+ cells, with the dectin-2 lentivector expressing GFP essentially only in cells that expressed endogenous dectin-2 (Fig. 3B). This suggests that this dectin-2+ subset may preferentially interact with blood-borne viruses. Figure 3A shows that within the CD11c+-enriched fraction, both lentivectors expressed GFP in the CD11chi/MHC-IIhi subset (myeloid DC), in the CD11clo/MHC-IIhi subset (also F4/80+, macrophages [data not shown]) and in the CD11clo/MHC-IIlo subset (plasmacytoid DC and possibly DC precursors [32]). Within the CD11c− fraction the SFFV lentivector transduced mainly B cells, as previously reported (36, 42) (Fig. 3A).
FIG. 3.
Transgene expression in spleen following intravenous injection. Mice were injected intravenously with PBS (control) or SFFV-GFP or DEC-GFP lentivector. Spleen cells were collected and pooled (10 days postinfection; n = 2 mice/group), and CD11c+ cells were isolated using CD11c microbeads. Transgene expression in both CD11c+- and CD11c−-enriched cell fractions was analyzed by FACS after costaining for cell surface-specific markers as indicated. The CD11c+ fraction contains ≥75% CD11c+ cells, the CD11c− fraction is ≥96% CD11c−. The table shows the percentage of GFP+ cells in three subpopulations of cells within the CD11c+ cell fraction, according to their CD11c and MHC-II expression levels (as defined in panel A) and within the CD11C− fraction after costaining with anti-CD19 and anti-CD3 antibodies. The CD11clo/MHC-IIhi population includes autofluorescent and F4/80+ cells, consistent with the description for spleen macrophages. (B) Surface expression of dectin-2 protein in GFP+ cells in the CD11c+ fraction. Cells were stained with CD11c, MHC-II, and dectin-2 antibodies, and region R1 was established according to CD11c and MHC-II+ staining. GFP+ cells within R1 were then gated (R2), and the percentage of dectin-2-expressing cells in R2 (i.e., GFP+) was determined. Numbers represent the percentage of positive cells for the marker expressed in the x axis and the MFI value. Overall, dectin-2+ cells in the CD11c+ fraction in all three groups corresponded to ≤7% of the CD11c+ population. The data represent one out of five experiments performed with similar results. SSC, side scatter.
To examine whether antigen expression in dectin-2+ cells could induce an immune response, we constructed lentivectors in which the SFFV promoter or the dectin-2 promoter drove expression of the human tumor antigen NY-ESO-1 (11). NY-ESO-1 is a promising cancer vaccine antigen; it is expressed in many human tumors and elicits potent antitumor immune responses in preclinical and clinical trials (reviewed in reference 21). We previously reported that the SFFV lentivector is effective in priming a CD8+ T-cell response to the NY-ESO-1157-165 presented on HLA-A2 in HHD transgenic mice (34). We therefore injected various doses of either NY-ESO-1 lentivector or a control lentivector with the dectin-2 gene promoter driving GFP expression subcutaneously in HHD mice and then boosted the response by intravenous injection of vaccinia virus expressing NY-ESO-1. To ensure optimal binding of mouse CD8 molecules to HLA-A2 molecules and staining of high- and low-affinity T cells, the frequency of NY-ESO-1157-165-specific CD8+ T cells was measured using an HLA-A2Kb pentamer (12, 13). Figure 4A shows that the two NY-ESO-1 lentivectors stimulated expansion of NY-ESO-1157-165-specific CD8+ T cells in peripheral blood in a similar manner. Optimal expansion occurred after injection of 107 infectious units followed by boosting with vaccinia virus expression NY-ESO-1.
We also examined the number of T cells within spleens from the immunized mice that released IFN-γ in response to the NY-ESO-1157-165 peptide in an ex vivo ELISPOT assay. Figure 4B again shows a similar response to the two NY-ESO-1 lentivectors. At the highest dose of 108 infectious units, there was a decrease in NY-ESO-1157-165-specific CD8+ T cells detected by pentamer in peripheral blood but not in the NY-ESO-1157-165-specific CD8+ T cells detected by ELISPOT in spleen. Subcutaneous injection was more effective in the induction of NY-ESO-1157-165-specific CD8+ T cells detected by ELISPOT in spleen than intravenous injection (Fig. 4B) and in the induction of NY-ESO-1157-165-specific CD8+ T cells detected by pentamer in peripheral blood (data not shown). We also detected significant expansion of NY-ESO-1157-165-specific CD8+ T cells in spleen following a single subcutaneous lentivector immunization (Fig. 4B). A 1 log higher dose of lentivector was needed to induce equivalent responses to those seen in the prime/boost protocol (Fig. 4B). Again, both promoters were effective though maximal response to the SFFV lentivector was achieved at a lower dose.
We recently reported that preparations of lentivectors pseudotyped with VSV-G can contain envelope structures contaminated with plasmid DNA (35). These contaminants cause type 1 IFN release from plasmacytoid DC in a Toll-like receptor 9-dependent manner (35). To examine whether this response was involved in lentivector vaccination, we also immunized HHD mice subcutaneously with SSFV and dectin-2 lentivectors pseudotyped with a gammaretroviral envelope that does not cause type 1 IFN release from plasmacytoid DC (35). For these experiments viruses were quantitated by reverse transcriptase measurement to avoid problems with the different infectivity levels of the envelopes on the cells used in the TaqMan PCR assay. For VSV-G-pseudotyped lentivector, 150 ng of reverse transcriptase was equivalent to approximately 5 × 107 TaqMan infectious units. Figure 4C shows that pseudotyping the SFFV or dectin-2 lentivectors with a Friend gammaretroviral envelope did not affect their ability to induce NY-ESO-1157-165-specific CD8+ T cells, demonstrating that VSV-G/plasmid contaminants are not involved in the response to lentivectors following subcutaneous immunization.
In order to monitor the CD4+ T-cell response to NY-ESO-1 vaccination in HHD mice, an epitope of NY-ESO-1 presented by H2 I-Ab was identified. The region of NY-ESO-1 containing an epitope recognized by CD4+ T cells was initially mapped on the basis of the help it provided in raising an immune response to the HLA-A2-restricted epitope (ESO-1157-165) within the same gene. Immunization of HLA-A2-expressing mice with incremental deletions of the NY-ESO-1 gene, which contained the A2 epitope, identified a region of the gene encoding amino acids 32 to 100 which provides help to the CD8+ HLA*02-restricted response (data not shown). 21-mer peptides spanning this region were then screened in an IFN-γ ELISPOT recall assay using splenocytes from mice immunized with plasmid DNA encoding the full-length NY-ESO-1 gene. The peptide spanning amino acids 79 to 99 was the only one to be recognized in this assay (Fig. 5A, peptide 27) and depletion of CD4+ cells abolished this recognition (data not shown). A series of overlapping 14-mer peptides focused the identification of the helper epitope further to amino acids 86 to 89 (Fig. 5B, peptide 14). Subcutaneous injection of both the SFFV lentivector and the dectin-2 lentivector also stimulated expansion of cells in spleen that recognized the C57BL/6 CD4+ T-cell epitope NY-ESO-186-99 in an ex vivo ELISPOT assay (Fig. 5C). This response was observed with either the VSV-G envelope or a Friend gammaretroviral envelope (Fig. 5C). The maximal response to the SFFV lentivector was again achieved at a lower dose (Fig. 5C). Thus, restricting NY-ESO-1 expression to antigen-presenting cells does not impair its ability to stimulate a CD4+ T-cell response, which will be critical for effective antitumor immunity.
FIG. 5.
CD4+ response to NY-ESO-1 in HLA-A2 transgenic mice. (A) Splenocytes from HHD mice immunized with a DNA vaccine encoding full-length NY-ESO-1 were screened in an ELISPOT IFN-γ recall assay with overlapping 21-mer peptides. Values are the number of spots per the indicated number of splenocytes. (B) Splenocytes from HHD mice immunized with a DNA vaccine encoding full-length NY-ESO-1 were screened in an ELISPOT IFN-γ recall assay with overlapping 14-mer peptides. Numbers along the x axis represent the peptides, whose sequences are given below the graph. Numbers along the x axis represent the peptides; the sequences of critical peptides are below the graph. (C) HHD mice were immunized subcutaneously with PBS or the indicated dose of VSV-G or Friend-pseudoptyped lentivectors, and 12 days later cells were tested in an ELISPOT IFN-γ assay. Spleen cells were pooled (n = 2/group) and incubated in duplicate in either the presence or absence of the ESO-186-99 peptide (peptide 14). Values are the means ± standard error of duplicate wells after subtraction of background values of cells incubated with medium only.
DISCUSSION
Our results show that a lentivector that restricts antigen expression to the dectin-2+ subset of cells is effective in the induction of cellular immunity. Immunization at lower lentivector doses was obtained with the viral SFFV promoter, probably because it drives a higher level of gene expression, as we observed with GFP lentivectors. Dectin-2 lentivector injection in the tail vein results in the transduction of macrophages and DC in spleen, whereas subcutaneous injection results in transduction of CD11c+ DC. Subcutaneous injection results in more effective immunization. The same dectin-2 gene promoter has also been used in gene gun-mediated delivery of plasmid DNA, and in this case it also induced cellular immune responses (31). A similar result was obtained when the DC-specific fascin promoter was used in gene gun-mediated immunization (39). In this case the authors showed that the fascin promoter induced a Th1 immune response, whereas a constitutive CMV promoter led to a Th2 response (39). However, the CD11c promoter was ineffective compared to the CMV promoter or the keratinocyte-specific K14 promoter in another gene gun vaccination study (24). Perhaps the efficacy of a particular promoter depends on its precise pattern or level of expression. The selection of the most effective vector for human immunization will probably require direct comparison of promoters in phase I clinical trials.
Our results show that lentivector injection subcutaneously transduces both skin-derived DC and also LN-resident DC. A previous report has shown that cell-sorted skin-derived DC form the most potent antigen-presenting subset after cutaneous lentivector immunization (23). This is in contrast to results with other viral vectors or leishmania infection, where LN-resident DC are critical for priming CD8+ T cells (1, 5, 6, 25, 38). Transduction of the LN-resident DC by lentivectors may depend on trafficking of the virus to the LN by skin-derived DC, as has been demonstrated for herpes simplex virus (2).
A number of studies have shown that direct lentivector immunization is particularly effective in the induction of antitumor immunity (10, 15, 23, 27, 36). Therefore, we propose that this approach should be tested in phase I clinical trials for the treatment of appropriate patients, such as those with metastatic melanoma. This approach is more practical for treatment of large numbers of patients than the costly and time-consuming ex vivo expansion of autologous DC. Insertional mutagenesis is probably the main risk in using an integrating vector, such as a lentivector. When the related retroviral vectors were used to transduce hematopoietic stem cells for the treatment of X-linked severe combined immunodeficiency, three patients developed leukemia following retroviral activation of the LMO2 oncogene (22). In another clinical trial for X-linked chronic granulomatous disease clonal expansion of cells with a retroviral insertion in the Evi1 locus was reported (33).
The enhancer within the retroviral long terminal repeat is likely responsible for activation of adjacent cellular genes in these clinical trials. However, in a cell culture test system, Modlich and colleagues have demonstrated that a retroviral vector with enhancer-deleted long terminal repeats and the SFFV as a strong internal promoter can cause Evi1 up-regulation (29). While retroviral vectors are oncogenic in a mouse model, lentivectors with an internal phosphoglycerate kinase promoter are not, within the sensitivity of the assay, but this obviously does not extend to other lentivectors or higher doses (30). It will therefore be advantageous in future clinical trials to identify promoters that are active only in nondividing, terminally differentiated cells. In this respect the use of a DC-specific promoter in lentivector vaccination trials may be useful. We have shown here that the mouse dectin-2 gene promoter is active in human LC and skin DC, and this is a therefore a good candidate for inclusion in a clinical vaccine. Its oncogenicity will clearly need to be tested in preclinical models before this step. However, the fact that it is effective at relatively low lentivector doses in the mouse model is encouraging as limiting vector dose is one strategy to reduce the risk of oncogenesis.
Our experiments provide some new information on the role of dectin-2 itself. Strikingly, intravenous injection of lentivectors, even those with the nonspecific SFFV promoter, results in preferential transduction of the dectin-2+ subset of cells in spleen. Whether this is a direct effect of dectin-2 interaction with high-mannose sugars present on the viral envelope, more efficient access of lentivector to these cells, or a higher postentry permissivity of this subset to lentivector remains to be determined. The experiments do, however, demonstrate that the dectin-2+ subset of cells can act as potent antigen-presenting cells.
Acknowledgments
We thank Clare Bennett for advice and antibodies for staining of LN cells and Phillip Taylor for the anti-dectin-2 antibody.
This study was supported by Cancer Research UK (M.C.) (C484/A4566) and by Cancer Research UK (C399/A2291) and the United Kingdom Medical Research Council (V.C.).
Footnotes
Published ahead of print on 24 October 2007.
REFERENCES
- 1.Allan, R. S., C. M. Smith, G. T. Belz, A. L. van Lint, L. M. Wakim, W. R. Heath, and F. R. Carbone. 2003. Epidermal viral immunity induced by CD8α+ dendritic cells but not by Langerhans cells. Science 3011925-1928. [DOI] [PubMed] [Google Scholar]
- 2.Allan, R. S., J. Waithman, S. Bedoui, C. M. Jones, J. A. Villadangos, Y. Zhan, A. M. Lew, K. Shortman, W. R. Heath, and F. R. Carbone. 2006. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity 25153-162. [DOI] [PubMed] [Google Scholar]
- 3.Aragane, Y., A. Maeda, A. Schwarz, T. Tezuka, K. Ariizumi, and T. Schwarz. 2003. Involvement of dectin-2 in ultraviolet radiation-induced tolerance. J. Immunol. 1713801-3807. [DOI] [PubMed] [Google Scholar]
- 4.Ariizumi, K., G. L. Shen, S. Shikano, R. Ritter, 3rd, P. Zukas, D. Edelbaum, A. Morita, and A. Takashima. 2000. Cloning of a second dendritic cell-associated C-type lectin (dectin-2) and its alternatively spliced isoforms. J. Biol. Chem. 27511957-11963. [DOI] [PubMed] [Google Scholar]
- 5.Belz, G. T., K. Shortman, M. J. Bevan, and W. R. Heath. 2005. CD8α+ dendritic cells selectively present MHC class I-restricted noncytolytic viral and intracellular bacterial antigens in vivo. J. Immunol. 175196-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belz, G. T., C. M. Smith, D. Eichner, K. Shortman, G. Karupiah, F. R. Carbone, and W. R. Heath. 2004. Cutting edge: conventional CD8α+ dendritic cells are generally involved in priming CTL immunity to viruses. J. Immunol. 1721996-2000. [DOI] [PubMed] [Google Scholar]
- 7.Bonkobara, M., T. Yudate, H. Yagihara, T. Washizu, P. D. Cruz, Jr., and K. Ariizumi. 2004. Transcriptional regulation of dectin-2 promoter in transgenic mouse. J. Vet. Med. Sci. 661483-1489. [DOI] [PubMed] [Google Scholar]
- 8.Bonkobara, M., P. K. Zukas, S. Shikano, S. Nakamura, P. D. Cruz, Jr., and K. Ariizumi. 2001. Epidermal Langerhans cell-targeted gene expression by a dectin-2 promoter. J. Immunol. 1676893-6900. [DOI] [PubMed] [Google Scholar]
- 9.Carter, R. W., C. Thompson, D. M. Reid, S. Y. Wong, and D. F. Tough. 2006. Induction of CD8+ T cell responses through targeting of antigen to dectin-2. Cell Immunol. 23987-91. [DOI] [PubMed] [Google Scholar]
- 10.Chapatte, L., S. Colombetti, J. C. Cerottini, and F. Levy. 2006. Efficient induction of tumor antigen-specific CD8+ memory T cells by recombinant lentivectors. Cancer Res. 661155-1160. [DOI] [PubMed] [Google Scholar]
- 11.Chen, Y. T., M. J. Scanlan, U. Sahin, O. Tureci, A. O. Gure, S. Tsang, B. Williamson, E. Stockert, M. Pfreundschuh, and L. J. Old. 1997. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc. Natl. Acad. Sci. USA 941914-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi, E. M., J. L. Chen, L. Wooldridge, M. Salio, A. Lissina, N. Lissin, I. F. Hermans, J. D. Silk, F. Mirza, M. J. Palmowski, P. R. Dunbar, B. K. Jakobsen, A. K. Sewell, and V. Cerundolo. 2003. High avidity antigen-specific CTL identified by CD8-independent tetramer staining. J. Immunol. 1715116-5123. [DOI] [PubMed] [Google Scholar]
- 13.Choi, E. M., M. Palmowski, J. Chen, and V. Cerundolo. 2002. The use of chimeric A2K(b) tetramers to monitor HLA A2 immune responses in HLA A2 transgenic mice. J. Immunol. Methods 26835-41. [DOI] [PubMed] [Google Scholar]
- 14.Demaison, C., K. Parsley, G. Brouns, M. Scherr, K. Battmer, C. Kinnon, M. Grez, and A. J. Thrasher. 2002. High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency [correction of imunodeficiency] virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum. Gene Ther. 13803-813. [DOI] [PubMed] [Google Scholar]
- 15.Dullaers, M., S. Van Meirvenne, C. Heirman, L. Straetman, A. Bonehill, J. L. Aerts, K. Thielemans, and K. Breckpot. 2006. Induction of effective therapeutic antitumor immunity by direct in vivo administration of lentiviral vectors. Gene Ther. 13630-640. [DOI] [PubMed] [Google Scholar]
- 16.Esslinger, C., L. Chapatte, D. Finke, I. Miconnet, P. Guillaume, F. Levy, and H. R. MacDonald. 2003. In vivo administration of a lentiviral vaccine targets DCs and induces efficient CD8+ T cell responses. J. Clin. Investig. 1111673-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandes, M. J., A. A. Finnegan, L. D. Siracusa, C. Brenner, N. N. Iscove, and B. Calabretta. 1999. Characterization of a novel receptor that maps near the natural killer gene complex: demonstration of carbohydrate binding and expression in hematopoietic cells. Cancer Res. 592709-2717. [PubMed] [Google Scholar]
- 18.Firat, H., F. Garcia-Pons, S. Tourdot, S. Pascolo, A. Scardino, Z. Garcia, M. L. Michel, R. W. Jack, G. Jung, K. Kosmatopoulos, L. Mateo, A. Suhrbier, F. A. Lemonnier, and P. Langlade-Demoyen. 1999. H-2 class I knockout, HLA-A2.1-transgenic mice: a versatile animal model for preclinical evaluation of antitumor immunotherapeutic strategies. Eur. J. Immunol. 293112-3121. [DOI] [PubMed] [Google Scholar]
- 19.Galla, M., E. Will, J. Kraunus, L. Chen, and C. Baum. 2004. Retroviral pseudotransduction for targeted cell manipulation. Mol. Cell 16309-315. [DOI] [PubMed] [Google Scholar]
- 20.Gavino, A. C., J. S. Chung, K. Sato, K. Ariizumi, and P. D. Cruz, Jr. 2005. Identification and expression profiling of a human C-type lectin, structurally homologous to mouse dectin-2. Exp. Dermatol. 14281-288. [DOI] [PubMed] [Google Scholar]
- 21.Gnjatic, S., H. Nishikawa, A. A. Jungbluth, A. O. Gure, G. Ritter, E. Jager, A. Knuth, Y. T. Chen, and L. J. Old. 2006. N.Y.-ESO-1: review of an immunogenic tumor antigen. Adv. Cancer Res. 951-30. [DOI] [PubMed] [Google Scholar]
- 22.Hacein-Bey-Abina, S., C. Von Kalle, M. Schmidt, M. P. McCormack, N. Wulffraat, P. Leboulch, A. Lim, C. S. Osborne, R. Pawliuk, E. Morillon, R. Sorensen, A. Forster, P. Fraser, J. I. Cohen, G. de Saint Basile, I. Alexander, U. Wintergerst, T. Frebourg, A. Aurias, D. Stoppa-Lyonnet, S. Romana, I. Radford-Weiss, F. Gross, F. Valensi, E. Delabesse, E. Macintyre, F. Sigaux, J. Soulier, L. E. Leiva, M. Wissler, C. Prinz, T. H. Rabbitts, F. Le Deist, A. Fischer, and M. Cavazzana-Calvo. 2003. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 302415-419. [DOI] [PubMed] [Google Scholar]
- 23.He, Y., J. Zhang, C. Donahue, and L. D. Falo, Jr. 2006. Skin-derived dendritic cells induce potent CD8(+) T cell immunity in recombinant lentivector-mediated genetic immunization. Immunity 24643-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hon, H., A. Oran, T. Brocker, and J. Jacob. 2005. B lymphocytes participate in cross-presentation of antigen following gene gun vaccination. J. Immunol. 1745233-5242. [DOI] [PubMed] [Google Scholar]
- 25.Iezzi, G., A. Frohlich, B. Ernst, F. Ampenberger, S. Saeland, N. Glaichenhaus, and M. Kopf. 2006. Lymph node resident rather than skin-derived dendritic cells initiate specific T cell responses after Leishmania major infection. J. Immunol. 1771250-1256. [DOI] [PubMed] [Google Scholar]
- 26.Inaba, K., M. Inaba, M. Deguchi, K. Hagi, R. Yasumizu, S. Ikehara, S. Muramatsu, and R. M. Steinman. 1993. Granulocytes, macrophages, and dendritic cells arise from a common major histocompatibility complex class II-negative progenitor in mouse bone marrow. Proc. Natl. Acad. Sci. USA 903038-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim, J. H., N. Majumder, H. Lin, S. Watkins, L. D. Falo, Jr., and Z. You. 2005. Induction of therapeutic antitumor immunity by in vivo administration of a lentiviral vaccine. Hum. Gene Ther. 161255-1266. [DOI] [PubMed] [Google Scholar]
- 28.McGreal, E. P., M. Rosas, G. D. Brown, S. Zamze, S. Y. Wong, S. Gordon, L. Martinez-Pomares, and P. R. Taylor. 2006. The carbohydrate-recognition domain of dectin-2 is a C-type lectin with specificity for high mannose. Glycobiology 16422-430. [DOI] [PubMed] [Google Scholar]
- 29.Modlich, U., J. Bohne, M. Schmidt, C. von Kalle, S. Knoss, A. Schambach, and C. Baum. 2006. Cell-culture assays reveal the importance of retroviral vector design for insertional genotoxicity. Blood 1082545-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montini, E., D. Cesana, M. Schmidt, F. Sanvito, M. Ponzoni, C. Bartholomae, L. Sergi Sergi, F. Benedicenti, A. Ambrosi, C. Di Serio, C. Doglioni, C. von Kalle, and L. Naldini. 2006. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat. Biotechnol. 24687-696. [DOI] [PubMed] [Google Scholar]
- 31.Morita, A., K. Ariizumi, R. Ritter, 3rd, J. V. Jester, T. Kumamoto, S. A. Johnston, and A. Takashima. 2001. Development of a Langerhans cell-targeted gene therapy format using a dendritic cell-specific promoter. Gene Ther. 81729-1737. [DOI] [PubMed] [Google Scholar]
- 32.Naik, S. H., D. Metcalf, A. van Nieuwenhuijze, I. Wicks, L. Wu, M. O'Keeffe, and K. Shortman. 2006. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat. Immunol. 7663-671. [DOI] [PubMed] [Google Scholar]
- 33.Ott, M. G., M. Schmidt, K. Schwarzwaelder, S. Stein, U. Siler, U. Koehl, H. Glimm, K. Kuhlcke, A. Schilz, H. Kunkel, S. Naundorf, A. Brinkmann, A. Deichmann, M. Fischer, C. Ball, I. Pilz, C. Dunbar, Y. Du, N. A. Jenkins, N. G. Copeland, U. Luthi, M. Hassan, A. J. Thrasher, D. Hoelzer, C. von Kalle, R. Seger, and M. Grez. 2006. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat. Med. 12401-409. [DOI] [PubMed] [Google Scholar]
- 34.Palmowski, M. J., L. Lopes, Y. Ikeda, M. Salio, V. Cerundolo, and M. K. Collins. 2004. Intravenous injection of a lentiviral vector encoding N.Y.-ESO-1 induces an effective CTL response. J. Immunol. 1721582-1587. [DOI] [PubMed] [Google Scholar]
- 35.Pichlmair, A., S. S. Diebold, S. Gschmeissner, Y. Takeuchi, Y. Ikeda, M. K. Collins, and C. Reis e Sousa. 2007. Tubulovesicular structures within vesicular stomatitis virus G protein-pseudotyped lentiviral vector preparations carry DNA and stimulate antiviral responses via Toll-like receptor 9. J. Virol. 81539-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rowe, H. M., L. Lopes, Y. Ikeda, R. Bailey, I. Barde, M. Zenke, B. M. Chain, and M. K. Collins. 2006. Immunization with a lentiviral vector stimulates both CD4 and CD8 T cell responses to an ovalbumin transgene. Mol. Ther. 13310-319. [DOI] [PubMed] [Google Scholar]
- 37.Sato, K., X. L. Yang, T. Yudate, J. S. Chung, J. Wu, K. Luby-Phelps, R. P. Kimberly, D. Underhill, P. D. Cruz, Jr., and K. Ariizumi. 2006. Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor gamma chain to induce innate immune responses. J. Biol. Chem. 28138854-38866. [DOI] [PubMed] [Google Scholar]
- 38.Smith, C. M., G. T. Belz, N. S. Wilson, J. A. Villadangos, K. Shortman, F. R. Carbone, and W. R. Heath. 2003. Cutting edge: conventional CD8 alpha+ dendritic cells are preferentially involved in CTL priming after footpad infection with herpes simplex virus-1. J. Immunol. 1704437-4440. [DOI] [PubMed] [Google Scholar]
- 39.Sudowe, S., I. Ludwig-Portugall, E. Montermann, R. Ross, and A. B. Reske-Kunz. 2003. Transcriptional targeting of dendritic cells in gene gun-mediated DNA immunization favors the induction of type 1 immune responses. Mol. Ther. 8567-575. [DOI] [PubMed] [Google Scholar]
- 40.Tacken, P. J., R. Torensma, and C. G. Figdor. 2006. Targeting antigens to dendritic cells in vivo. Immunobiology 211599-608. [DOI] [PubMed] [Google Scholar]
- 41.Taylor, P. R., D. M. Reid, S. E. Heinsbroek, G. D. Brown, S. Gordon, and S. Y. Wong. 2005. Dectin-2 is predominantly myeloid restricted and exhibits unique activation-dependent expression on maturing inflammatory monocytes elicited in vivo. Eur. J. Immunol. 352163-2174. [DOI] [PubMed] [Google Scholar]
- 42.VandenDriessche, T., L. Thorrez, L. Naldini, A. Follenzi, L. Moons, Z. Berneman, D. Collen, and M. K. Chuah. 2002. Lentiviral vectors containing the human immunodeficiency virus type-1 central polypurine tract can efficiently transduce nondividing hepatocytes and antigen-presenting cells in vivo. Blood 100813-822. [DOI] [PubMed] [Google Scholar]
- 43.Zufferey, R., D. Nagy, R. J. Mandel, L. Naldini, and D. Trono. 1997. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat. Biotechnol. 15871-875. [DOI] [PubMed] [Google Scholar]