Abstract
Toll-like receptors (TLRs) and retinoic acid-inducible gene I-like helicases (RLHs) are two major machineries recognizing RNA virus infection of innate immune cells. Intracellular signaling for TLRs and RLHs is mediated by their cytoplasmic adaptors, i.e., MyD88 or TRIF and IPS-1, respectively. In the present study, we investigated the contributions of TLRs and RLHs to the cytotoxic T-lymphocyte (CTL) response by using lymphocytoid choriomeningitis virus (LCMV) as a model virus. The generation of virus-specific cytotoxic T lymphocytes was critically dependent on MyD88 but not on IPS-1. Type I interferons (IFNs) are known to be important for the development of the CTL response to LCMV infection. Serum levels of type I IFNs and proinflammatory cytokines were mainly dependent on the presence of MyD88, although IPS-1−/− mice showed a decrease in IFN-α levels but not in IFN-β and proinflammatory cytokine levels. Analysis of Ifna6+/GFP reporter mice revealed that plasmacytoid dendritic cells (DCs) are the major source of IFN-α in LCMV infection. MyD88−/− mice were highly susceptible to LCMV infection in vivo. These results suggest that recognition of LCMV by plasmacytoid DCs via TLRs is responsible for the production of type I IFNs in vivo. Furthermore, the activation of a MyD88-dependent innate mechanism induces a CTL response, which eventually leads to virus elimination.
Viral infections are initially recognized by the innate immune system, which eliminates invading viruses by itself and activates an antigen-specific acquired immune response (2, 4, 17). Type I interferons (IFNs) are produced by innate immune cells after virus infection and play a pivotal role in antiviral responses, including apoptosis of virus-infected cells, cellular resistance to viral infection, and activation of natural killer and T cells (15, 25, 34). The expression of type I IFN genes is controlled by intracellular signaling pathways triggered by recognition of viral components with innate pattern recognition receptors. Toll-like receptors (TLRs) and retinoic acid-inducible gene I (RIG-I)-like helicases (RLHs), also called RIG-I-like receptors, are two major receptor families responsible for initial viral recognition (20, 28).
TLRs are type I transmembrane receptors responsible for the recognition of microbial components. Among TLRs, TLR7 and TLR9 recognize single-stranded RNA and CpG motif-containing DNA, respectively, and play an important role in virus-induced type I IFN production by plasmacytoid dendritic cells (pDCs) (2, 20). MyD88, a cytoplasmic adaptor protein containing a Toll/interleukin-1 receptor (IL-1R) homology (TIR) domain, is essential for TLR/IL-1R signaling. Mice lacking MyD88 do not respond to various TLR ligands, except for TLR3, a receptor which utilizes TIR domain-containing adaptor-inducing IFN-β (TRIF) in its downstream pathway (2, 40). MyD88 forms a complex with IL-1R-associated kinase 1 (IRAK-1), IRAK-4, and IFN regulatory factor 7 (IRF-7) upon ligand stimulation. Phosphorylated IRF-7 finally translocates into the nucleus to upregulate the expression of a set of IFN-inducible genes (38).
RLHs, including RIG-I and melanoma differentiation-associated gene 5 (MDA5), are cytoplasmic proteins responsible for the recognition of viral double-stranded RNA (20, 41, 42). RIG-I and MDA5 are comprised of caspase recruitment domains (CARDs) and an RNA helicase domain. Studies using knockout mice revealed that RIG-I is responsible for the recognition of various RNA viruses, including vesicular stomatitis virus, influenza virus, Japanese encephalitis virus, and paramyxoviruses such as Newcastle disease virus and Sendai virus, whereas MDA5 detects viruses belonging to the picornavirus family, such as encephalomyocarditis virus (11, 18, 19). RIG-I and MDA5 detect double-stranded RNAs via the helicase domain and initiate downstream signaling cascades via the CARDs by associating with a CARD-containing signaling protein named IFN-β promoter stimulator 1 (IPS-1) (also known as MAVS, VISA, or CARDIF) (21, 27, 33, 39). IPS-1 signals through IκB kinase-related kinases, called IKK-i and TBK1, that phosphorylate IRF-3/7 to induce the expression of IFN-inducible genes (2). Cells deficient in IPS-1 fail to produce type I IFNs and NF-κB-dependent proinflammatory cytokines in response to infection with different families of RNA viruses recognized by RIG-I and MDA5 (24, 35). The RLH signaling pathway is critical for type I IFN production in various cell types, including conventional DCs (cDCs), fibroblasts, and macrophages, but not in pDCs (23, 24). Thus, both the TLR system and the RLH system participate in virus recognition as well as signal transduction leading to IFN induction. However, the exact mechanisms by which TLRs and RLHs are involved in the development of acquired immune responses have yet to be clarified.
Lymphocytoid choriomeningitis virus (LCMV) is an ambisense single-stranded RNA virus belonging to the family Arenaviridae. Numerous strains of this noncytolytic pathogen, such as WE and Armstrong 53b (ARM), cause acute infections in mice, whereas rapidly replicating immunosuppressive variants lead to virus persistence and a general immunosuppression (30). Nonimmunosuppressive LCMV infections result in a profound adaptive immune response that is highlighted by the generation of virus-specific CD4+ and CD8+ T lymphocytes. Activated T cells acquire effector functions, such as IFN-γ production and cytolytic activity, that are responsible for clearing the virus, usually within 7 to 15 days after infection, and eventually lead to a functional T-cell memory (32). Initiation of this specific T-lymphocyte induction is considered to rely on antigen-presenting cells, mainly CD8α+ DCs (3). After capturing the viral antigen, the DCs migrate to lymphoid organs, such as the spleen, where they activate naïve T cells (12). In this process, type I IFNs are believed to play a central role in controlling viral infections such as LCMV infection. They have been shown to act directly on T lymphocytes and to induce massive expansion of antigen-specific CD8+ T cells (1, 7, 22, 32).
Nevertheless, the source of type I IFN in response to LCMV remains controversial, and type I IFN-producing cells are not well characterized. pDCs have been shown to be a major source of type I IFNs in various murine virus infections (6, 8, 9). pDCs were also implicated as the source of IFN-α in the LCMV-infected spleen (29). Conversely, it was reported that production of type I IFNs in response to LCMV infection was not impaired in pDC-depleted mice, questioning the importance of pDCs in T-cell induction in response to LCMV (8). Furthermore, cDCs extracted from mice infected with LCMV were reported to produce high IFN-α levels (10).
In the present study, we investigated the involvement of TLRs and RLHs in the development of antigen-specific CD8+ T cells as well as in the production of type I IFNs in response to LCMV infection by using MyD88-deficient (MyD88−/−) and IPS-1−/− mice. The development of cytotoxic T lymphocytes (CTLs) was critically dependent on MyD88 but not on IPS-1. MyD88-deficient mice were revealed to be highly susceptible to LCMV infection. In contrast to previous reports, levels of IFN-α, IFN-β, and proinflammatory cytokines in sera were dependent on the presence of MyD88, whereas IPS-1 deficiency resulted in impaired IFN-α production but otherwise normal cytokine levels. pDCs were the major source of IFN-α in LCMV infection. These results suggest that LCMV activates pDCs via TLRs to produce type I IFNs in vivo and that the activation of an innate mechanism leads to the induction of the CTL response and, eventually, virus elimination.
MATERIALS AND METHODS
Mice.
IFN-α/β receptor−/−, MyD88−/−, TRIF−/−, TLR2−/−, TLR4−/−, TLR8−/−, and IPS-1−/− mice have been described previously (14, 16, 24, 36, 40). TLR7−/− and TLR9−/− mice were crossed to yield double TLR7- and TLR9-deficient mice (13, 14). Ifna6+/GFP mice were generated as recently described (23).
LCMV infection and virus titration.
LCMV WE and ARM were obtained from T. Otheki (31). For virus propagation, L cells were infected with LCMV and cultured at 37°C for 48 h. Supernatants were diluted in phosphate-buffered saline for infection. Mice were infected intravenously with 3 × 105 PFU LCMV strain WE or ARM after a brief anesthesia with diethyl ether. For analysis of survival rates, mice were infected with 2 × 106 PFU. Virus loads in the spleen and liver were investigated on days 4, 8, and 30 after infection. For virus titration, MC57G cells were inoculated with 10-fold serial dilutions of LCMV-containing supernatants in a 24-well plate, covered with 2% methylcellulose, and incubated at 37°C for 48 h. Cells were then fixed with 4% formalin, permeabilized with 0.5% Triton X, and incubated with 10% fetal bovine serum. After subsequent incubation with murine LCMV immunoglobulin G1 (IgG1; Progen Biotechnik) and anti-mouse IgG-horseradish peroxidase (Amersham Biosciences), plates were stained using an AEC peroxidase substrate kit (Vector Laboratories), and virus plaques were counted for each well.
Induction of specific T-cell response.
Splenocytes were harvested 8 days after infection. To investigate the activation of LCMV-specific T lymphocytes, cells were incubated with T-select H-2Db LCMV tetramer-KAVYNFATC-phycoerythrin (PE) (MBL), CD4-fluorescein isothiocyanate (CD4-FITC), and CD8α-allophycocyanin antibodies (BD Pharmingen). Samples were acquired on a FACSCalibur flow cytometer (BD Biosciences) and analyzed with FlowJo software (TreeStar). IFN-γ induction was analyzed by stimulating splenocytes with an LCMV H-2Db-binding peptide (glycoprotein 33-41 [gp33-41] [KAVYNFATM]; Peptide Institute) and 10 ng/ml murine IL-2 prior to incubation with CD8α antibodies. Cells were then fixed, stained with IFN-γ-FITC antibodies in PermWash solution (BD Pharmingen) according to the manufacturer's recommendation, and assessed by flow cytometry. For determination of cytotoxicity of LCMV-specific T cells, splenocytes were incubated for 5 h with EL-4 target cells that had been loaded with gp33-41 and labeled with 51Cr. The percentage of specific lysis for each sample was calculated as follows: [(sample release − spontaneous release)/(maximal release − spontaneous release)] × 100. To analyze the generation of LCMV-specific memory T lymphocytes, mice were infected with 1 × 105 PFU LCMV WE, and splenocytes were analyzed for activation of specific T memory cells and IFN-γ induction as described below.
Cytokine and type I IFN production.
IL-1β, IL-6, IL-10, IL-12(p40), IL-12(p70), RANTES, and tumor necrosis factor alpha were measured in sera 24 and 48 h after LCMV infection by a multiplex bead-based flow cytometry assay (Bio-Plex cytokine assay; Bio-Rad Laboratories). IFN-α and IFN-β were determined repeatedly between 12 and 96 h by enzyme-linked immunosorbent assay (PBL Biomedical Laboratories).
Expression of green fluorescent protein (GFP) in splenic DCs and macrophages.
Twenty-four and 48 h after LCMV infection, spleens of Ifna6+/GFP mice were injected with 150 U/ml collagenase buffer (Wako Chemicals), 10 μg/ml DNase I (Sigma), and 10% fetal calf serum and incubated for 40 min at 37°C. After the addition of 10 mM EDTA and incubation for another 5 min, spleens were shredded and passed through a nylon mesh. Erythrocytes were lysed, and the single-cell suspension was stained with CD11b-PE, B220-PerCP, and CD11c-allophycocyanin antibodies before fluorescence-activated cell sorter analysis.
Activation of splenic DCs.
Splenocytes were harvested 48 h after infection, incubated with B220-PerCP, CD11c-FITC, and CD40-, CD80-, or CD86-PE antibodies (BD Pharmingen), and analyzed by flow cytometry.
RESULTS
Impaired CTL response to LCMV infection in MyD88−/− mice but not IPS-1−/− mice.
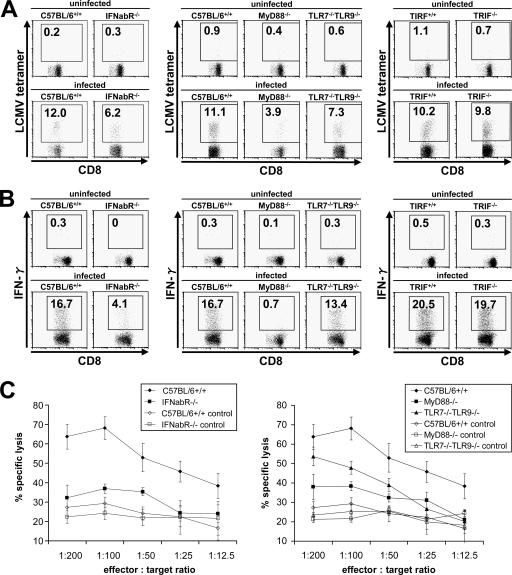
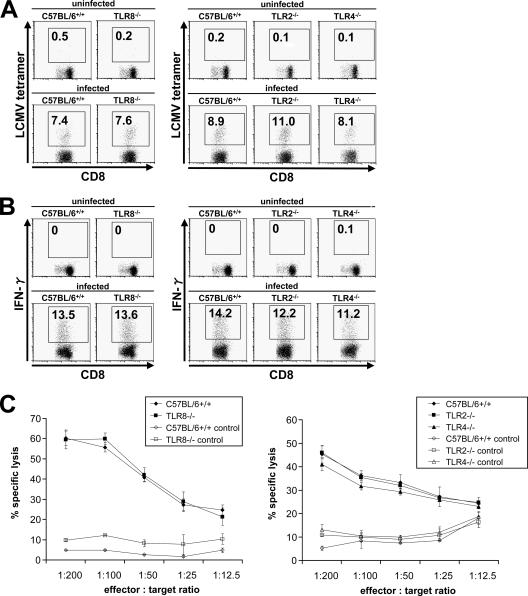
LCMV has been shown to mount a robust CTL response in vivo. Although the involvement of type I IFNs and innate immune cells in activating CTL responses has clearly been demonstrated before, the mechanism of innate recognition of LCMV in vivo is not yet fully understood. Therefore, we first examined the contributions of two major innate viral recognition systems, TLRs and RLHs, to mounting CTL responses against LCMV infection. Eight days after intravenous administration of LCMV, splenocytes of mice were harvested and stained with an H-2Db LCMV-specific tetramer (Fig. 1A) or stimulated with gp33-41 for analysis of IFN-γ production (Fig. 1B), and total and virus-specific CD8+ T lymphocytes per spleen were counted (Table 1). To assess LCMV-specific CTL activity, cells were incubated with gp33-41-loaded, 51Cr-labeled EL-4 target cells and specific lysis was calculated (Fig. 1C). Wild-type mice mounted a vigorous CTL response, demonstrated by massive expansion of CD8+ T cells, significant induction of LCMV-specific CD8+ T cells, strong IFN-γ production, and specific, T-cell-induced lysis of >60% of labeled target cells. IFN-α/β receptor−/− mice and TLR7−/− TLR9−/− mice demonstrated diminished clonal expansion of cytotoxic T cells in response to LCMV infection, whereas DC8+ T cells even decreased in MyD88−/− mice during infection compared to those in uninfected controls, resulting in splenic atrophy. Consistent with previous reports, IFN-α/β receptor−/− mice also showed impaired induction of LCMV-specific T cells as well as decreased IFN-γ production by these cells. Furthermore, cytotoxic T cells derived from IFN-α/β receptor−/− mice failed to effectively lyse target cells that presented an LCMV-specific epitope. MyD88 proved to play a crucial role in this process, as specific T-cell induction, IFN-γ production, and cytotoxicity were severely affected in MyD88−/− mice. In contrast, TRIF−/− mice did not show a defect in the activation of CD8+ T cells, suggesting that TLR3 is not involved in LCMV-induced CTL responses. When mice lacking various TLRs were infected with LCMV, TLR7−/− TLR9−/− mice showed slightly diminished activation of CTL responses. In contrast, TLR2, TLR4, and TLR8 were not involved in the induction of CTL activity (Fig. 2A to C).
FIG. 1.
Induction of LCMV-specific T-cell response via the TLR system. Wild-type, MyD88−/−, IFN-α/β receptor−/−, TLR7−/− TLR9−/−, and TRIF−/− mice were infected intravenously with 3 × 105 PFU LCMV WE, and splenocytes were harvested on day 8 after infection. Cells were stained with LCMV major histocompatibility complex class I tetramer, CD4, and CD8α antibodies (A) or stimulated with gp33-41 prior to CD8α antibody staining (B). Induction of LCMV-specific T lymphocytes and IFN-γ production was analyzed by flow cytometry. (C) Ex vivo CTL activity in splenocytes was determined by a 5-h 51Cr release assay, using gp33-41-loaded EL4 cells as targets. Values are means ± standard deviations (SD) for three mice. Data are representative of three independent experiments.
TABLE 1.
Total and virus-specific numbers of CD8+ T lymphocytes per spleen for uninfected mice and mice that were intravenously infected with 3 × 105 PFU LCMV WEa
| Mouse group and genotype | Total CD8+ cells | Specific CD8+ cells | % of total |
|---|---|---|---|
| Uninfected mice | |||
| C57BL/6+/+ | 6.6 ± 2.6 | 0.03 ± 0.04 | 0.5 |
| IFNabR−/− | 5.4 ± 0.3 | 0.03 ± 0.01 | 0.6 |
| MyD88−/− | 7.1 ± 1.1 | 0.03 ± 0.01 | 0.4 |
| TLR7−/− TLR9−/− | 6.7 ± 2.2 | 0.02 ± 0.02 | 0.3 |
| Infected mice | |||
| C57BL/6+/+ | 19.7 ± 9.2 | 2.19 ± 1.04 | 11.1 |
| IFNabR−/− | 15.7 ± 4.1 | 0.99 ± 0.25 | 6.3 |
| MyD88−/− | 3.6 ± 0.7 | 0.16 ± 0.06 | 4.4 |
| TLR7−/− TLR9−/− | 13.0 ± 2.7 | 0.98 ± 0.23 | 7.5 |
Splenocytes were harvested on day 8 after infection and stained with LCMV major histocompatibility complex class I tetramer and CD8α antibodies. Cell numbers were counted by flow cytometry. Depicted values are multipliers of 106 and indicate means ± standard deviations for nine mice per indicated genotype.
FIG. 2.
Involvement of other TLRs in T-cell response. Wild-type, TLR2−/−, TLR4−/−, and TLR8−/− mice were infected intravenously with 3 × 105 PFU LCMV WE, and splenocytes were harvested on day 8 after infection. Induction of LCMV-specific T lymphocytes (A), IFN-γ production (B), and CTL activity (C) were analyzed. Values are means ± SD for three mice.
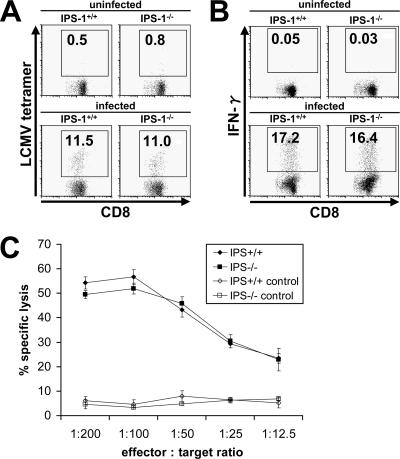
Next, we investigated the role of RLHs in LCMV-induced CD8+ T-cell activation. The analysis of IPS-1−/− splenocytes revealed a normal T-cell response after LCMV infection. Specific T-cell activation, IFN-γ production, and specific lysis were comparable to those of wild-type cells (Fig. 3), suggesting that RLHs are not involved in adaptive immunity after LCMV infection. In general, the results obtained herein showed no difference between the WE and ARM strains of LCMV (data for LCMV ARM are not shown).
FIG. 3.
T-cell response via the RLH system. Wild-type and IPS-1−/− mice were infected intravenously with 3 × 105 PFU LCMV WE, and splenocytes were harvested on day 8 after infection. Induction of LCMV-specific T lymphocytes (A), IFN-γ production (B), and CTL activity (C) were analyzed as described in the legend to Fig. 1. Values are means ± SD for three mice. Data are representative of three independent experiments.
Role of TLRs and RLHs in the production of type I IFN and proinflammatory cytokines in response to LCMV infection.
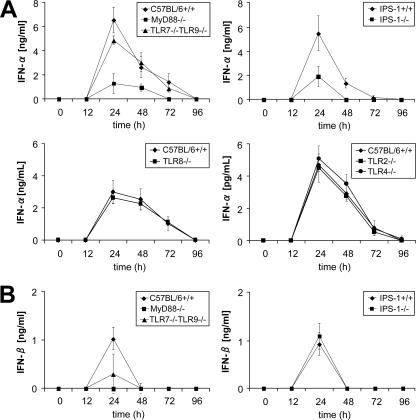
We further investigated how the TLR system contributes to the activation of CTL responses. Given the importance of type I IFN receptors in the development of LCMV-induced T-cell activity, we hypothesized that MyD88 is involved in the production of type I IFNs, although previous reports have shown that IFN-α production is not impaired in MyD88−/− mice with LCMV infection. We therefore measured serum levels of IFN-α and cytokines between 12 h and 96 h after intravenous LCMV challenge. In wild-type mice, IFN-α and IFN-β peaked 24 h after infection, and IFN-α levels gradually decreased after 48 h. IFN-α production was severely impaired in MyD88−/− mice (Fig. 4A), and IFN-β production was abolished (Fig. 4B), suggesting that TLRs play a critical role in type I IFN production in response to LCMV. In contrast, IFN-α production was not impaired in the absence of TRIF (data not shown), showing that TLR3 is not involved in LCMV-induced type I IFN production. Mice deficient in both TLR7 and TLR9 showed a partially impaired type I IFN response. TLR2, TLR4, and TLR8 did not play any role in the IFN response (data not shown). Thus, it is possible that a combination of TLRs might be important for the recognition of whole LCMV via MyD88 in vivo. We then investigated the involvement of RLHs by using IPS-1−/− mice. Although LCMV-induced IFN-α production was modestly impaired (Fig. 4A), IFN-β production was not impaired in IPS-1−/− mice (Fig. 4B), suggesting that the contribution of RLHs to LCMV-induced type I IFN production is smaller than that of TLRs. In general, no strain-specific differences were observed between LCMV WE and ARM infections (data for LCMV ARM are not shown).
FIG. 4.
Type I IFN response to LCMV infection. The indicated mice were infected intravenously with 3 × 105 PFU LCMV WE, and IFN-α (A) and IFN-β (B) levels in sera were measured by enzyme-linked immunosorbent assay between 12 and 96 h after infection. Data show means ± SD for three mice and are representative of three independent experiments.
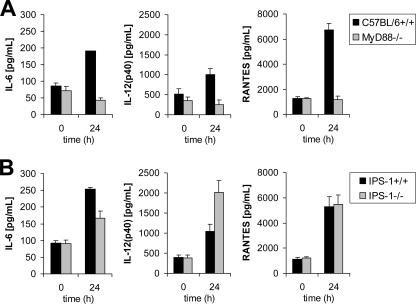
Furthermore, we examined the levels of proinflammatory cytokines in sera. The production of IL-6, IL-12(p40), and RANTES was abolished in MyD88−/− mice (Fig. 5A and B). Conversely, IPS-1 deficiency did not significantly alter the production of these cytokines, suggesting that the TLR system, but not RLHs, plays an important role in the production of cytokines.
FIG. 5.
Cytokine production. IL-6, IL-12(p40), and RANTES levels in sera of MyD88−/− (A) and IPS-1−/− (B) mice after intravenous infection with 3 × 105 PFU LCMV WE were assessed after 24 h by a multiplex bead-based assay. Data show means ± SD for three mice.
Identification of IFN-α-producing cells during LCMV infection.
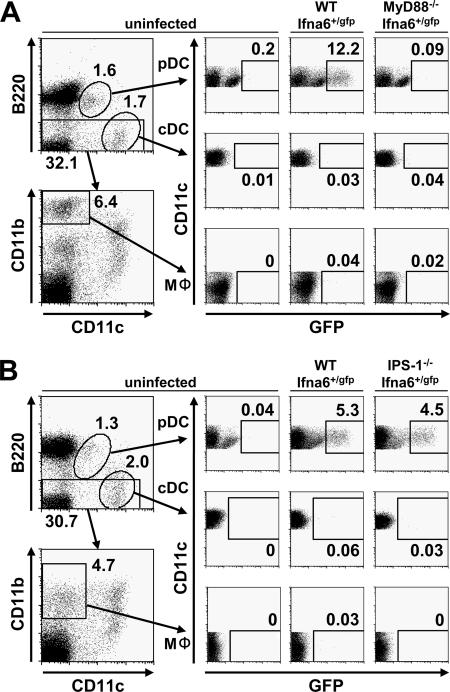
Previous reports pointed out that cells other than pDCs are responsible for IFN-α production during LCMV infection. Therefore, we further investigated IFN-α-producing cells in LCMV infection by using Ifna6+/GFP reporter mice. We found that 24 and 48 h after intravenous LCMV infection, GFP+ cells were most frequently observed in the spleen (Fig. 6A and B) and, to a lesser extent, in inguinal lymph nodes, bone marrow, and the liver (data not shown). LCMV infection intensively increased the number of GFP+ B220+ CD11cdull pDCs, indicating that they were the major IFN-α producers in response to LCMV infection. LCMV also modestly increased the numbers of GFP+ B220− CD11c+ cDCs and GFP+ CD11c− CD11b+ macrophages in the spleen (Fig. 6A and B), although this phenomenon was not observed in other organs (data not shown). Expression of GFP+ pDCs was abolished in the absence of MyD88, whereas no significant difference was observed between wild-type and IPS-1−/− mice, providing evidence that pDCs utilize the TLR system to produce IFN-α. The number of GFP+ cDCs and macrophages was slightly but constantly reduced in Ifna6+/GFP reporter mice lacking IPS-1 but was normal in MyD88−/− mice, suggesting that IFN-α production by these cell types in response to LCMV requires the RLH system and is independent of TLRs (Fig. 6A and B). Taken together, these data indicate that pDCs are the main type I IFN producers during LCMV infection through virus recognition by the TLR-MyD88 system.
FIG. 6.
Expression of GFP in splenic DCs and macrophages. Ifna6+/GFP mice lacking MyD88 (A) or IPS-1 (B) as well as wild-type mice were infected intravenously with 3 × 105 PFU LCMV WE. Splenocytes were harvested 24 and 48 h after infection, and the expression of GFP in pDCs, cDCs, and macrophages was analyzed by fluorescence-activated cell sorter analysis. Data are representative of two experiments.
Activation of DCs in response to LCMV infection.
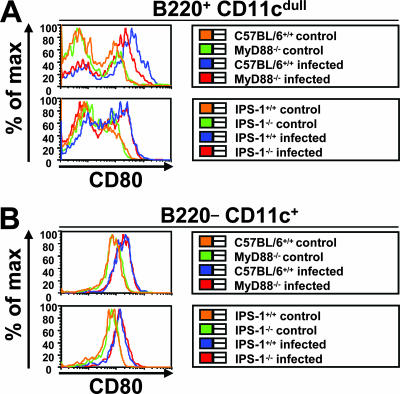
DCs have been shown to be important for the activation of adaptive immunity. One attribute of DC activation is the surface upregulation of costimulatory molecules (29). To analyze whether different DC subsets were activated in response to LCMV infections, we assessed the expression of surface CD80, CD86, and CD40 on splenic pDCs and cDCs 24 and 48 h after infection. In wild-type mice, DC expression of CD80 (Fig. 7A and B), CD86, and CD40 (data not shown) was enhanced after infection. While no differences in the induction of these surface molecules were found between wild-type and MyD88- or IPS-1-deficient cDCs, upregulation was impaired in pDCs in MyD88- but not IPS-1-deficient mice, presenting further evidence that MyD88 plays an important role in the pDC-mediated innate response to LCMV infections.
FIG. 7.
Activation of splenic DCs. Mice were infected intravenously with 3 × 105 PFU LCMV WE. Splenocytes of MyD88- and IPS-1-deficient mice were investigated for the activation of CD80 on pDCs (A) and cDCs (B) by flow cytometry 48 h after infection. Data shown are representative of three mice.
Enhanced susceptibility of MyD88−/− mice to LCMV infection.
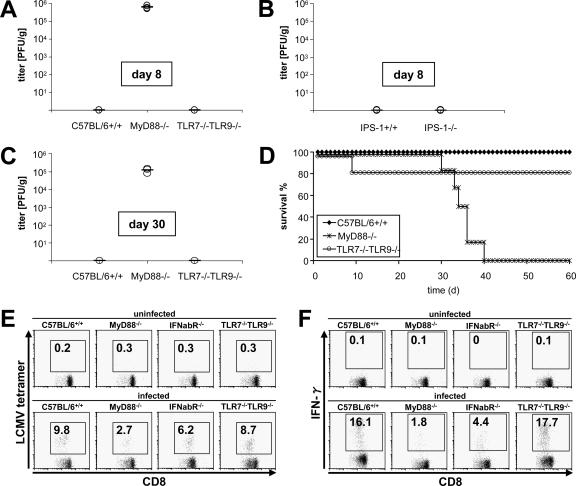
When wild-type mice were challenged intravenously with LCMV, the virus was cleared by the activation of CTLs, leading to full recovery. Because MyD88−/− mice failed to mount a sufficient T-cell response, we further investigated the long-term outcome of LCMV infections. As expected, IPS-1−/− mice effectively cleared the virus from the spleen (Fig. 8B) and other organs (data not shown) within 1 week after infection, in a manner similar to that of wild-type mice. In contrast, the absence of MyD88 resulted in a failure of virus elimination, with viral titers persisting for several weeks, independent of TLR7 and TLR9 (Fig. 8A and C). This observation was in accordance with a long-lasting deficiency in T-cell function (Fig. 8E and F). The severe defects in virus clearance and in the generation of LCMV-specific T memory cells were underlined by the finding that MyD88−/− mice died between 30 and 40 days after challenge with higher virus titers (Fig. 8D). These results demonstrate that MyD88 is essential for the survival of mice with LCMV infection by enabling the activation of acquired immune responses.
FIG. 8.
Long-term sequelae after LCMV infection. Wild-type, MyD88−/−, and TLR7−/− TLR9−/− mice or IPS-1−/− mice were infected intravenously with 3 × 105 PFU LCMV WE, and virus titers were measured in spleens on day 8 (A and B) and day 30 (C) by a methylcellulose titration assay. Results for three mice are displayed, with means. Data are representative of three independent experiments. (D) Survival rates were assessed after infecting six mice per indicated genotype with 2 × 106 PFU LCMV WE in two independent experiments. (E and F) Generation of LCMV-specific memory CD8+ T lymphocytes in MyD88−/−, TLR7−/− TLR9−/−, and IFN-α/β receptor−/− mice following infection with 1 × 105 PFU LCMV WE was analyzed as described above. Indicated values are means ± SD for three mice and are representative of two independent experiments.
DISCUSSION
LCMV has been studied intensively to understand the mechanisms of CTL activation following recognition of specific antigens. However, the role of innate immunity in the activation of CTL responses as well as in the elimination of the virus has yet to be clarified. When analyzing mice deficient in MyD88 or IPS-1, we clearly showed that the TLR system, but not the RLH system, is critical for the development of adaptive immunity against LCMV infection. Since the expression of IFN-α/β receptor on antigen-specific CD8+ T cells is important for their expansion, activation, and memory formation after viral infection (22), the contribution of MyD88 to this process seems to be via the initial control of type I IFN production. We demonstrate that both IFN-α and IFN-β are mainly controlled by MyD88-dependent pathways. IFN-α and IFN-β share the IFN-α/β receptor for signaling, and mice deficient in IFN-α/β receptor showed impaired CTL activity during LCMV infection. Therefore, it is presumable that both IFN-α and IFN-β contribute to the generation of a CTL response to LCMV. Notably, it appears that MyD88−/− mice have a more severe defect in CTL responses than do IFN-α/β receptor−/− mice, a phenomenon also observed for CD8+ T memory cells. Furthermore, IPS-1−/− mice did not show a defect in CTL responses, although they showed partially impaired serum IFN-α levels after LCMV infection. Thus, it is possible that the development of LCMV-specific T cells is not solely regulated by type I IFNs. In fact, at least two different pathways for LCMV-induced T-cell IFN-γ production have been described (7). Under normal conditions, the IFN-γ response is dependent on type I IFN, without a contribution of IL-12. However, mice deficient in IFN-α/β receptor elicit elevated levels of IL-12, and this may overcome the defect caused by IFN-α/β deficiency on CD8+ T cells. MyD88 regulates the production of various cytokines, such as IL-1, IL-6, IL-12, IL-18, and RANTES, in addition to type I IFNs, and these cytokines likely contribute to the MyD88-dependent development of LCMV-specific T cells in vivo.
Previous reports have shown that the production of type I IFNs in response to LCMV infection is not impaired in pDC-depleted mice (8) and that splenic cDCs from infected mice produce IFN-α (10). On the other hand, it was also suggested that splenic pDCs can produce IFN-α in response to LCMV infection (29). In this study, we identified pDCs as the predominant IFN-α producers by infecting Ifna6+/GFP mice with LCMV. We also observed a modest expression of GFP+ cDCs and macrophages in LCMV-infected reporter mice; however, this expression was clearly subordinate to that of pDCs. Other cell types, such as T and B cells, did not express GFP in early LCMV infection (data not shown), and no type I IFNs could be detected in sera later than 72 h postinfection, indicating that lymphocytes are not IFN-α producers in response to LCMV. Furthermore, we found that IFN-α production in pDCs was exclusively dependent on MyD88 but not on IPS-1. Consistently, IFN-α and IFN-β levels in sera were severely impaired in MyD88−/− mice. These results are in contradiction to a previous report showing that the production of type I IFNs in response to LCMV was MyD88 independent (43). Although we do not have an explanation for this discrepancy, we believe that the involvement of MyD88 as well as pDCs in the IFN response is well grounded based on the data from measurements of serum IFN and our novel reporter mice. On the other hand, cDCs and macrophages from Ifna6+/GFP mice lacking IPS-1 showed reduced GFP expression in response to LCMV compared to those from wild-type mice, although the frequency of GFP+ cells was much lower than that for pDCs. However, given that the total number of cDCs and macrophages in the body by far exceeds the number of pDCs, it can be presumed that the impaired IFN-α levels in sera in IPS-1−/− mice are a result of the failure to produce IFN in these cell types.
Since type I IFN production in response to LCMV infection was mainly dependent on MyD88, we investigated the contribution of each TLR to the recognition of LCMV infection. Although the involvement of TLR2 was reported previously (43), we did not detect a defect in TLR2−/−, TLR4−/−, or TLR8−/− mice. TLR1 and TLR6 both form heterodimers with TLR2 to recognize bacterial components, and they are likely not involved in LCMV recognition, since TLR2−/− mice responded appropriately to LCMV infection. Also, TLR5 is unlikely to contribute to MyD88-dependent LCMV recognition, as it does not activate IFN-inducible genes and is only marginally expressed in splenic cells (37). TLR7−/− TLR9−/− mice showed only partial impairment in type I IFN levels and CTL activity compared to MyD88−/− mice, and TRIF was not involved in innate and adaptive immune responses to LCMV. Taken together, the data show that other unknown MyD88-dependent but TLR7- and TLR9-independent receptors may contribute to the complex orchestration of LCMV signal recognition in vivo. Another possibility is that pDCs are indirectly activated by LCMV-infected cells and that MyD88-dependent signaling is required in other cell types. Indeed, pDCs induced from bone marrow failed to produce large amounts of IFN-α in response to LCMV infection in vitro, whereas infection with Newcastle disease virus, Sendai virus, and influenza virus was reported to induce large amounts of IFN-α in bone marrow-derived pDCs (19). Thus, it is possible that IFN-α production in response to LCMV is mediated through a mechanism different from that in response to other RNA viruses. Further studies are required to clarify the mechanism of IFN-α production in pDCs via the MyD88-dependent pathway.
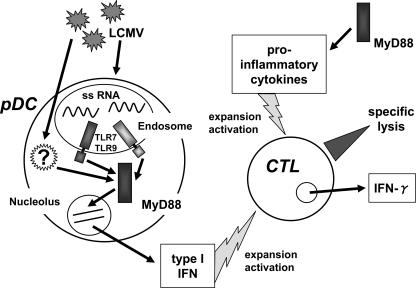
DCs are reported to be critical for the development of antigen-specific T-cell responses against viral infection (3). In response to LCMV infection, cDCs upregulated costimulatory molecules, namely, CD40, CD80, and CD86, even in the absence of MyD88 or IPS-1. In contrast, costimulatory molecule expression in pDCs was impaired in MyD88-deficient mice. The critical role of MyD88-dependent signaling in CD8 T-cell activation and its role in IFN production in pDCs suggested that pDCs are essential for inducing the development of LCMV-specific CTL responses. However, it is not clear if pDCs play a direct role in the presentation of LCMV antigen to CD8 T cells. A possible explanation is that pDCs act to facilitate CTL activation by producing type I IFN rather than directly presenting antigens. A model depicting the modulation of the CTL response to LCMV by pDCs via MyD88 is shown in Fig. 9.
FIG. 9.
Innate immune modulation of CTL response to LCMV through MyD88 signaling. MyD88 mediates recognition of LCMV by TLR7/TLR9 and possibly other, unknown receptors via pDCs. Type I IFNs produced by infected pDCs result in activation and clonal expansion of virus-specific CTLs. In an alternative pathway, CTLs are activated by proinflammatory cytokines in an MyD88-dependent manner. Activated CTLs mount effector functions that finally contribute to virus elimination.
MyD88-deficient mice were highly susceptible to infection with LCMV and showed long-lasting virus persistence. It seems that the defect in the acute-phase innate response cannot explain the cause of death observed for MyD88−/− mice, considering that all MyD88-deficient mice died after 30 to 40 days of infection with higher virus titers. These results are similar to the effects of LCMV infection in perforin-deficient (Perf−/−) mice. Perf−/− mice have been shown to enhance T-cell expansion and activation in response to persistent LCMV infection, leading to immunomediated tissue damage and increased mortality (5, 26). Thus, it has been suggested that the pathology and fatality of LCMV infection are most likely not direct results of the infection but, rather, are due to virus-induced immunopathology. Therefore, although LCMV-mediated CTL responses, including the formation of CD8α+ T memory cells, were impaired in MyD88−/− mice, the persistent T-cell response may eventually have caused enough pathology to result in the death of the mice. However, further studies are required to clarify the precise cause of death observed for MyD88−/− mice.
In summary, our results demonstrate the importance of MyD88-dependent signaling in the production of type I IFNs in pDCs. Consistently, MyD88 is critical for the activation of CTL responses. Furthermore, MyD88 controls various proinflammatory cytokines that are likely to contribute to the activation of CTL responses independently of type I IFN. However, the cell-specific contributions of TLRs and cytoplasmic RLHs to the activation of acquired immunity appear to be different in response to various viruses. Future studies will clarify how these two innate viral recognition pathways are involved in T-cell activation depending on different viral pathogens.
Acknowledgments
We thank Y. Fujiwara, M. Shiokawa, and A. Shibano for skillful technical assistance, N. Kitagaki for an excellent methodological tutorial, and P. Lee for critically reading the manuscript. M. Hashimoto deserves special appreciation for distinguished organizational support and secretarial assistance. T. Otheki kindly provided L cells and MC57G cells. EL4 cells were gratefully obtained from H. Tsutsui.
This work was supported in part by grants from the Yokochi Fund of the Kanehara Ichiro Foundation, from the Ministry of Education, Culture, Sports, Science and Technology in Japan, from the 21st Century Center of Excellence Program of Japan, and from the NIH (AI070167).
We have no competing financial interests.
Footnotes
Published ahead of print on 17 October 2007.
REFERENCES
- 1.Aichele, P., H. Unsoeld, M. Koschella, O. Schweier, U. Kalinke, and S. Vucikuja. 2006. Cutting edge: CD8 T cells specific for lymphocytic choriomeningitis virus require type I IFN receptor for clonal expansion. J. Immunol. 1764525-4529. [DOI] [PubMed] [Google Scholar]
- 2.Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124783-801. [DOI] [PubMed] [Google Scholar]
- 3.Belz, G. T., K. Shortman, M. J. Bevan, and W. R. Heath. 2005. CD8α dendritic cells selectively present MHC class I-restricted noncytological viral and intracellular bacterial antigens in vivo. J. Immunol. 175196-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beutler, B. 2004. Interferences, questions and possibilities in Toll-like receptor signaling. Nature 230257-263. [DOI] [PubMed] [Google Scholar]
- 5.Binder, D., M. F. van den Broek, D. Kaegi, H. Bluethmann, J. Fehr, H. Hnegartner, and R. M. Zinkernagel. 1989. Aplastic anemia rescued by exhaustion of cytokine-secreting CD8+ T cells in persistent infection with lymphocytic choriomeningitis virus. J. Exp. Med. 1871902-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colonna, M., A. Krug, and M. Cella. 2002. Interferon-producing cells: on the front line in immune responses against pathogens. Curr. Opin. Immunol. 14373-379. [DOI] [PubMed] [Google Scholar]
- 7.Cousens, L. P., R. Peterson, S. Hsu, A. Dorner, J. D. Altman, R. Ahmed, and C. A. Biron. 1999. Two roads diverged: interferon α/β- and interleukin 12-mediated pathways in promoting T cell interferon γ responses during viral infection. J. Exp. Med. 1891315-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalod, M., T. P. Salazar-Mather, L. Malmgard, C. Lewis, C. Asselin-Paturel, F. Brière, G. Trinchieri, and C. A. Brion. 2002. Interferon α/β and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. J. Exp. Med. 195517-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalod, M., T. Hamilton, R. Salomon, T. P. Salazar-Mather, S. C. Henry, J. D. Hamilton, and C. A. Biron. 2003. Dendritic cell responses to early cytomegalovirus infection: subset functional specialization and differential regulation by interferon α/β. J. Exp. Med. 197885-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diebold, S. S., M. Montoya, H. Unger, L. Alexopoulou, P. Roy, L. E. Haswell, A. Al-Shamkhani, R. Flavell, P. Borrow, and C. Reis e Sousa. 2003. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature 424324-328. [DOI] [PubMed] [Google Scholar]
- 11.Gitlin, L., W. Barchet, S. Gilfillan, M. Cella, B. Beutler, R. A. Flavell, M. S. Diamond, and M. Colonna. 2006. Essential role of MDA5 in type I IFN responses to polyriboinosinic acid and encephalomyocarditis picornavirus. Proc. Natl. Acad. Sci. USA 1038459-8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guermonprez, P., J. Valladeau, L. Zitvogel, C. Thery, and S. Amigorena. 2002. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 20621-676. [DOI] [PubMed] [Google Scholar]
- 13.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408740-745. [DOI] [PubMed] [Google Scholar]
- 14.Hemmi, H., T. Kaisho, O. Takeuchi, S. Sato, H. Sanjo, K. Hoshino, T. Horiuchi, H. Tomizawa, K. Takeda, and S. Akira. 2002. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 3196-200. [DOI] [PubMed] [Google Scholar]
- 15.Honda, K., H. Yanai, A. Takaoka, and T. Taniguchi. 2005. Regulation of type I IFN induction: a current view. Int. Immunol. 171367-1378. [DOI] [PubMed] [Google Scholar]
- 16.Hoshino, K., T. Kaisho, T. Iwabe, O. Takeuchi, and S. Akira. 2002. Differential involvement of IFN-beta in Toll-like receptor-stimulated dendritic cell activation. Int. Immunol. 141225-1231. [DOI] [PubMed] [Google Scholar]
- 17.Iwasaki, A., and R. Medzhitov. 2004. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5987-995. [DOI] [PubMed] [Google Scholar]
- 18.Kato, H., O. Takeuchi, S. Sato, M. Yoneyama, M. Yamamoto, K. Matsui, S. Uematsu, A. Jung, T. Kawai, O. Yamaguchi, K. Ohtsu, T. Tsujimura, C. S. Koh, C. Reis e Sousa, Y. Matsuura, T. Fujita, and S. Akira. 2006. Differential role of MDA5 and RIG-I in the recognition of RNA viruses. Nature 141101-105. [DOI] [PubMed] [Google Scholar]
- 19.Kato, H., S. Sato, M. Yoneyama, M. Yamamoto, S. Uematsu, K. Matsui, T. Tsujimura, K. Takeda, T. Fujita, O. Takeuchi, and S. Akira. 2005. Cell-type specific involvement of RIG-I in antiviral response. Immunity 2319-28. [DOI] [PubMed] [Google Scholar]
- 20.Kawai, T., and S. Akira. 2006. Innate immune recognition of viral infection. Nat. Immunol. 7131-137. [DOI] [PubMed] [Google Scholar]
- 21.Kawai, T., K. Takahashi, S. Sato, C. Coban, H. Kumar, H. Kato, K. J. Ishii, O. Takeuchi, and S. Akira. 2005. IPS-1, an adaptor triggering RIG-I- and MDA5-mediated type I interferon induction. Nat. Immunol. 61074-1076. [DOI] [PubMed] [Google Scholar]
- 22.Kolumam, G. A., T. Sunil, L. J. Thompson, J. Sprent, and K. Murali-Krishna. 2005. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J. Exp. Med. 202637-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumagai, Y., O. Takeuchi, H. Kato, H. Kumar, K. Matsui, E. Morii, K. Aozasa, T. Kawai, and S. Akira. 2007. Alveolar macrophages are the primary interferon-α producer in pulmonary infection with RNA viruses. Immunity 27240-252. [DOI] [PubMed] [Google Scholar]
- 24.Kumar, H., T. Kawai, H. Kato, S. Sato, K. Takahashi, C. Coban, M. Yamamoto, S. Uematsu, K. J. Ishii, O. Takeuchi, and S. Akira. 2006. Essential role of IPS-1 in innate immune responses against RNA viruses. J. Exp. Med. 2031795-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Bon, A., and D. F. Tough. 2002. Links between innate and adaptive immunity via type-I interferon. Curr. Opin. Immunol. 14432-436. [DOI] [PubMed] [Google Scholar]
- 26.Matloubian, M., M. Suresh, A. Glass, M. Galvan, K. Chow, J. K. Whitmire, C. M. Walsh, W. R. Clark, and R. Ahmed. 1999. A role for perforin in downregulating T cell responses during chronic viral infections. J. Virol. 732527-2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meylan, E., J. Curran, K. Hofmann, D. Moradpour, M. Binder, R. Bartenschlager, and J. Tschopp. 2005. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 4371167-1172. [DOI] [PubMed] [Google Scholar]
- 28.Meylan, E., J. Tschopp, and M. Karin. 2006. Intracellular pattern recognition receptors in the host response. Nature 44239-44. [DOI] [PubMed] [Google Scholar]
- 29.Montoya, M., M. J. Edwards, D. M. Reid, and P. Borrow. 2005. Rapid activation of spleen dendritic cell subsets following lymphocytic choriomeningitis virus infection of mice: analysis of the involvement of type I IFN. J. Immunol. 1741851-1861. [DOI] [PubMed] [Google Scholar]
- 30.Oldstone, M. B. A. 2002. Biology and pathogenesis of lymphocytic choriomeningitis virus infection. Curr. Top. Microbiol. Immunol. 26383-117. [DOI] [PubMed] [Google Scholar]
- 31.Otheki, T., M. Parsons, A. Zakarian, R. G. Jones, L. T. Nguyen, J. R. Woodgett, and P. S. Ohashi. 2000. Negative regulation of T cell proliferation and interleukin 2 production by the serine threonine kinase GSK-3. J. Exp. Med. 19299-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ou, R., S. Zhou, L. Huang, and D. Moskophidis. 2001. Critical role for alpha/beta and gamma interferons in persistence of lymphocytic choriomeningitis virus by clonal exhaustion of cytotoxic T cells. J. Virol. 758407-8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seth, R. B., L. Sun, C. K. Ea, and Z. J. Chen. 2005. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF3. Cell 122669-682. [DOI] [PubMed] [Google Scholar]
- 34.Stetson, D. B., and R. Medzhitov. 2006. Type I interferons in host defense. Immunity 25373-381. [DOI] [PubMed] [Google Scholar]
- 35.Sun, Q., L. Sun, H. H. Liu, X. Chen, R. B. Seth, J. Forman, and Z. J. Chen. 2006. The specific and essential role of MAVS in antiviral innate immune responses. Immunity 24633-642. [DOI] [PubMed] [Google Scholar]
- 36.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11443-451. [DOI] [PubMed] [Google Scholar]
- 37.Uematsu, S., M. H. Jang, N. Chevrier, Z. Guo, Y. Kumagai, M. Yamamoto, H. Kato, N. Sougawa, H. Matsui, H. Kuwata, H. Hemmi, C. Coban, T. Kawai, K. Ishii, O. Takeuchi, M. Miyasaka, K. Takeda, and S. Akira. 2006. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat. Immunol. 7868-874. [DOI] [PubMed] [Google Scholar]
- 38.Uematsu, S., S. Sato, M. Yamamoto, T. Hirotani, H. Kato, F. Takeshita, M. Matsuda, C. Coban, K. Ishii, T. Kawai, O. Takeuchi, and S. Akira. 2005. Interleukin-1 receptor-associated kinase-1 plays an essential role for Toll-like receptor (TLR)7- and TLR9-mediated interferon-α induction. J. Exp. Med. 201915-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu, L. G., Y. Y. Wang, K. J. Han, L. Y. Li, Z. Zhai, and H. B. Shu. 2005. VISA is an adaptor protein required for virus-triggered IFN-β signaling. Mol. Cell 19727-740. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto, M., S. Sato, H. Hemmi, K. Hoshino, T. Kaisho, H. Sanjo, O. Takeuchi, M. Sugiyama, M. Okabe, K. Takeda, and S. Akira. 2003. Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science 301640-643. [DOI] [PubMed] [Google Scholar]
- 41.Yoneyama, M., M. Kikuchi, K. Matsumoto, T. Imaizumi, M. Miyagishi, K. Taira, E. Foy, Y. M. Loo, M. Gale, S. Akira, S. Yonehara, A. Kato, and T. Fujita. 2005. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J. Immunol. 1752851-2858. [DOI] [PubMed] [Google Scholar]
- 42.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5730-737. [DOI] [PubMed] [Google Scholar]
- 43.Zhou, S., E. A. Kurt-Jones, L. Mandell, A. Cerny, M. Chan, D. T. Golenbock, and R. W. Finberg. 2005. MyD88 is critical for the development of innate and adaptive immunity during acute lymphocytic choriomeningitis virus infections. Eur. J. Immunol. 35822-830. [DOI] [PubMed] [Google Scholar]