Abstract
The endoplasmic reticulum (ER) chaperone BiP/GRP78 regulates ER function and the unfolded protein response (UPR). Human cytomegalovirus infection of human fibroblasts induces the UPR but modifies it to benefit viral replication. BiP/GRP78 protein levels are tightly regulated during infection, rising after 36 h postinfection (hpi), peaking at 60 hpi, and decreasing thereafter. To determine the effects of this regulation on viral replication, BiP/GRP78 was depleted using the SubAB subtilase cytotoxin, which rapidly and specifically cleaves BiP/GRP78. Toxin treatment of infected cells for 12-h periods beginning at 36, 48, 60, and 84 hpi caused complete loss of BiP but had little effect on viral protein synthesis. However, progeny virion formation was significantly inhibited, suggesting that BiP/GRP78 is important for virion formation. Electron microscopic analysis showed that infected cells were resistant to the toxin and showed none of the cytotoxic effects seen in uninfected cells. However, all viral activity in the cytoplasm ceased, with nucleocapsids remaining in the nucleus or concentrated in the cytoplasmic space just outside of the outer nuclear membrane. These data suggest that one effect of the controlled expression of BiP/GRP78 in infected cells is to aid in cytoplasmic virion assembly and egress.
Human cytomegalovirus (HCMV) is the largest of the human herpesviruses, capable of encoding more than 200 proteins, which are expressed in temporal fashion as immediate-early, early, delayed-early, and late genes. The replication cycle of HCMV is slow, and during this protracted cycle the virus must maintain optimal replication conditions in the host cell. However, the increasing strain of the infection will induce cellular stress responses, with consequences that may be deleterious to the progress of the infection; for example, most cellular stress responses inhibit translation (1, 10, 14, 31, 32). Therefore, it is essential that the virus regulate, modulate, or circumvent the effects of cellular stress responses that are activated during infection.
Increasing evidence indicates that HCMV has mechanisms to deal with the deleterious aspects of cellular stress responses while maintaining beneficial ones (4, 6, 12, 13, 15-17, 20, 21, 30). An example of this is the viral control of endoplasmic reticulum (ER) stress and the unfolded protein response (UPR). Due to the number of HCMV proteins that are glycosylated or receive other ER-dependent posttranslational modifications, the load of proteins in the ER can exceed its capacity, resulting in ER stress and the activation of the UPR (13, 29).
The UPR is normally controlled by three transmembrane sensors that initiate the complex UPR signaling cascade when activated by ER stress (reviewed in references 14, 24, 25, and 36). The ER molecular chaperone BiP (immunoglobulin heavy chain-binding protein), also called glucose-regulated protein 78 (GRP78), is believed to bind these sensors and keep them inactive under unstressed conditions. However, upon the accumulation of unfolded proteins in the ER, BiP/GRP78 dissociates from the sensors in order to perform its chaperone function; this allows the sensors to activate. The activation of the first sensor, the PKR-like ER kinase (PERK), leads to the phosphorylation of eIF2α and Nrf2. Phosphorylated eIF2α represses global translation initiation; phosphorylated Nrf2 dissociates from its inhibitor and migrates to the nucleus to activate transcription from promoters containing antioxidant response elements (5). Activation of the second sensor, inosotol-requiring enzyme 1 (IRE-1), stimulates its endonuclease activity, which (i) cleaves nascent mRNAs destined for the ER (11) and (ii) cleaves an intron from the precursor mRNA encoding X-box binding protein 1 (XBP-1) (19, 35). The ensuing splicing produces XBP-1 mRNA that can be productively translated; XBP-1 functions as a transcription factor. BiP/GRP78 dissociation from the third sensor, activating transcription factor 6 (ATF6), exposes a Golgi localization signal, allowing ATF6 to translocate to the Golgi apparatus, where it is cleaved, releasing the active transcription factor, which migrates to the nucleus (27). ATF6 and XBP-1 activate the transcription of genes whose promoters contain ER stress elements. These include the genes for BiP/GRP78 and other ER chaperones, as well as genes encoding degradation factors (34). The degradation factors return to the ER to degrade excess proteins as part of the ER-associated degradation (ERAD) system (33).
Some UPR functions can be deleterious to the HCMV infection, while others can be beneficial. Most notably, the virus would not benefit from translational attenuation caused by activation of PERK or by an increase in the degradation of viral proteins due to activation of the ERAD system. However, an increase in ER chaperone levels might aid in the folding and processing of viral proteins in the ER. Analysis of the UPR during HCMV infection has shown that the virus does regulate the effects of the three ER stress sensors (13). As a result, translational attenuation does not occur, and XBP-1 function is inhibited, as indicated by the fact that ERAD-related genes are not activated. In contrast, the upregulation of chaperones is maintained, as indicated by significantly increased levels of BiP/GRP78 and GRP94 (13).
In the studies reported here, we examine the expression of BiP/GRP78 and its function in HCMV infection. Besides its role in controlling the UPR, BiP/GRP78 has several other functions. Its chaperone function allows it to bind unfolded proteins in the ER, preventing them from aggregating and keeping them in a foldable state until they are properly folded and exported from the ER. In addition, BiP/GRP78 binds calcium and is involved in maintaining calcium homeostasis in the ER (9, 18). Furthermore, during HCMV infection, BiP/GRP78 binds to the viral proteins US2 and US11; this interaction is necessary for the virus-mediated degradation of major histocompatibility complex classes I and II (8). In the present study, we show that during HCMV infection, BiP/GRP78 expression is tightly regulated, resulting in a significant increase between 24 and 36 h postinfection (hpi), reaching maximal levels between 60 and 72 hpi, and decreasing by 96 hpi. To determine the effects of this increase in BiP/GRP78 levels on viral replication, we depleted BiP/GRP78 by using SubAB subtilase cytotoxin, which rapidly and specifically cleaves BiP/GRP78. Interestingly, an established viral infection was cytoprotective when the toxin was added; infected cells showed little of the cytotoxic effects seen in uninfected cells. In infected cells, a 12-h toxin treatment had little effect on viral protein synthesis but caused virion formation in the cytoplasm to cease, suggesting that BiP/GRP78 plays a significant role in virion formation and cytoplasmic egress.
MATERIALS AND METHODS
Tissue culture and reagents.
Low-passage-number life-extended human foreskin fibroblasts (HFFs) (3) were cultured at 37°C under 5% CO2 in Dulbecco's modified Eagle medium (supplemented with 10% fetal calf serum, 100 U/ml penicillin, 100 g/ml streptomycin, and 2 mM GlutaMAX). Thapsigargin (Calbiochem) was used at 2 μg/ml. SubAB toxin and its nontoxic derivative SubAA272B (22) were purified by nickel-nitrilotriacetic acid chromatography as described previously (23, 28). SubAB toxin and the mutant toxin were used at 100 ng/ml.
Western blotting.
Following a cold phosphate-buffered saline wash, radioimmunoprecipitation assay buffer (1% NP-40, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 0.15 M NaCl, 10 mM sodium phosphate [pH 7.2], 2 mM EDTA, 50 mM NaF, 1 mM phenylmethylsulfonyl fluoride, 1 mM aprotinin, 0.2 mM Na3VO4, 1 μg/ml leupeptin) was added to cells. Cells were then rocked at 4°C for 5 min, scraped into the radioimmunoprecipitation assay buffer, and centrifuged, and the supernatant was collected and frozen. Protein was quantified using a Bio-Rad protein assay. Thirty micrograms of protein was added to 3× loading dye (187.5 mM Tris-HCl [pH 6.8], 6% SDS, 30% glycerol, 0.03% bromophenol blue, 467 mM β-mercaptoethanol) and boiled for 5 min. Proteins were separated by SDS-polyacrylamide gel electrophoresis (10% gels). Following electrophoresis, the gel was transferred to a nitrocellulose filter in transfer buffer (33.3 mM Tris, 256 mM glycine, 20% methanol) under a constant current. Membranes were blocked in 5% milk-Tris-buffered saline with Tween 20 (TBST). Conditions for antibody treatment differed according to vendor instructions. Membranes were washed in TBST and developed with the Lumi-Light Western blotting substrate (Roche). The following primary antibodies were used: antibodies against the C-terminal (sc-1051) and N-terminal (sc-1050) portions of BiP (both from Santa Cruz), anti-pp28 (α-pp28) (C9100-24; U.S. Biological), α-actin (MAB1501; Chemicon), α-exon2/3 (7), and α-p52 (13-131-100; ABI). All horseradish peroxidase-conjugated secondary antibodies were from Pierce.
Virus preparation, titration, growth curves, and infections.
Virus stocks (Towne strain) were prepared as previously described (2) and titered using the 50% tissue culture infective dose (TCID50) method. Growth curves were generated as previously described (12). All experiments were performed using a multiplicity of infection of 3.
shRNA experiments.
Lentivirus vectors expressing BiP/GRP78 short hairpin RNAs (shRNA) (RHS3979 to 9569449; Open Biosystems) or a control shRNA against green fluorescent protein (GFP) were used to infect HFFs in the presence of 8 μg/ml Polybrene. The medium was changed 6 hpi. For coinfection with HCMV, the cells were infected with HCMV 24 h after the lentivirus infection and were prepared for analysis at the indicated times after HCMV infection.
EM.
Following infection and treatment of cells, HFFs were washed with phosphate-buffered saline and fixed with EM fixative (2.5% glutaraldehyde, 2% paraformaldehyde in sodium cacodylate). Cells were prepared for electron microscopy (EM) by the Penn Biomedical Imaging Service. Briefly, cells were pelleted in Eppendorf tubes and postfixed with 1% aqueous OsO4 for 1 h. The cell pellets were dehydrated with ethanol and propylene oxide, embedded in epoxy resin, and polymerized at 65°C for 28 h. Ultrathin (∼80-nm-thick) sections were cut with a diamond knife, mounted on single-slot grids, stained with uranyl acetate and bismuth, and examined with an FEI Tecnai T12 transmission electron microscope.
RESULTS
The levels of BiP/GRP78 are temporally regulated during HCMV infection.
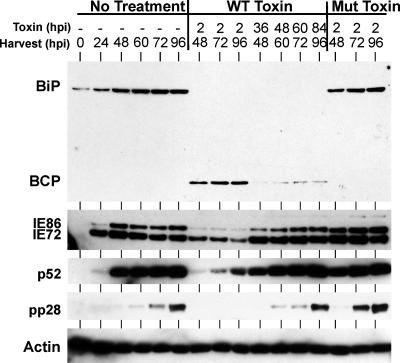
Figure 1 shows a viral infection time course in HFFs in which the levels of BiP/GRP78 protein and viral proteins were quantitated at each time point by Western blot analysis. Two antibodies were used to detect BiP/GRP78: one directed to the C terminus and the other directed to the N terminus. Both antibodies show that BiP/GRP78 protein levels are significantly increased by 36 hpi, maximal levels are seen at 60 to 72 hpi, and levels decrease by 96 hpi. With the C-terminal antibody, the levels attained at 60 and 72 hpi approach those seen in uninfected cells in which the UPR has been robustly activated by a 24-h treatment with the drug thapsigargin. The exposure using the C-terminal antibody is short, and BiP/GRP78 is not seen in mock-infected cells or at earlier times in infection. The longer exposure of the Western blot using the N-terminal antibody shows endogenous BiP/GRP78 at earlier times and in mock-infected samples. It should be noted that the precise timing of the rise and fall can be influenced by the multiplicity of infection (data not shown). Figure 1 also shows the expression of representative immediate-early (IE72 and IE86), early (p52), and late (pp28) proteins for temporal comparison with the expression of BiP/GRP78. The peak of expression at 60 and 72 hpi correlates with the late phase of infection, when the BiP/GRP78 chaperone function may aid in the synthesis and processing of viral glycoproteins. As shown below, the peak also corresponds to a period of significant virion formation.
FIG. 1.
HCMV temporally regulates BiP/GRP78 protein levels. Proteins were harvested from HCMV-infected HFFs at the indicated times postinfection or from uninfected cells treated for 24 h with thapsigargin (Thaps). Thirty micrograms of extracted protein from each sample was analyzed by Western blot analysis as described in Materials and Methods. BiP-C and BiP-N, antibodies directed to the C terminus and N terminus of BiP, respectively.
Depletion of BiP/GRP78 in an established infection does not alter steady-state viral protein levels.
The data reported above show that HCMV carefully regulates BiP/GRP78 protein levels during infection. The peak of BiP/GRP78 protein corresponds to the period of intense virion protein production and virion assembly. Thus, BiP/GRP78, an ER chaperone, may function in virion protein folding and assembly. To examine the importance of BiP/GRP78 for the infection, we depleted it in infected HFFs by using the subtilase cytotoxin SubAB (wild-type [WT] toxin), which has been shown to specifically cleave and inactivate BiP/GRP78 within 1 to 2 h in cultured cells (22). SubAB is the prototype of a new family of AB5 toxins produced by certain strains of Shiga-toxigenic E. coli (23). Its 35-kDa A subunit is responsible for BiP/GRP78 cleavage, while its pentameric B subunit directs binding to surface receptors and trafficking of the holotoxin within the cell. A mutant toxin with a single amino acid change (Ser272-Ala) in the active site of the A subunit, SubAA272B (Mut toxin), cannot cleave BiP/GRP78 (22) and is not cytotoxic (23).
The rapid cleavage of BiP/GRP78 provides a means to obtain a snapshot of BiP/GRP78 function at different times during HCMV infection. HFFs were infected with HCMV to generate a normal growth time course (with no treatment) and were harvested at 24, 48, 60, 72, or 96 hpi; in addition, infected cells were treated with either WT toxin or Mut toxin at 2 hpi and were harvested at 48, 72, or 96 hpi. It was anticipated that depletion of BiP/GRP78 at 2 hpi might prevent the viral infection from becoming established and that lengthy toxin treatment might be deleterious to the cells. To circumvent these problems, a different treatment strategy was used: SubAB was added to infected cells for 12-h periods just prior to harvesting at 48, 60, 72, or 96 hpi. This approach allowed the infection to become established prior to the depletion of BiP/GRP78.
Figure 2 shows the efficacy of the toxin. The no-treatment lanes show the kinetics of expression of BiP/GRP78, the major immediate-early proteins (MIEPs), p52, and pp28 during the normal 96-h HCMV infection time course. In all samples treated with the WT toxin, BiP/GRP78 was completely depleted. This was the case whether the toxin was added at 2 hpi or for 12-h periods (36 to 48, 48 to 60, 60 to 72, and 84 to 96 hpi) before harvest. The BiP/GRP78 C-terminal cleavage product, detected by the C-terminal antibody, can be observed in the treated samples. Not surprisingly, MIEP and p52 protein expression was significantly lowered in cells treated with the WT toxin at 2 hpi, and pp28 gene expression was undetectable. Samples similarly treated with the Mut toxin showed no effect on BiP/GRP78, MIEP, p52, or pp28 levels. Interestingly, in each of the samples treated for 12 h before harvest, the steady-state levels of the MIEPs, p52, and pp28 were not significantly different from those for the no-treatment controls, despite the complete depletion of BiP/GRP78. This suggests that in an established infection, the loss of BiP/GRP78 has little effect on viral protein synthesis.
FIG. 2.
Depletion of BiP by SubAB toxin does not alter the steady-state levels of viral proteins. Proteins were harvested from HCMV-infected HFFs that were either left untreated (no treatment) or treated with WT or Mut toxin. The times (hours postinfection) of toxin addition and protein harvest are given. Proteins were analyzed by Western blot analysis as described in Materials and Methods. BCP, the BiP/GRP78 C-terminal cleavage product.
Depletion of BiP/GRP78 inhibits the production of infectious virions.
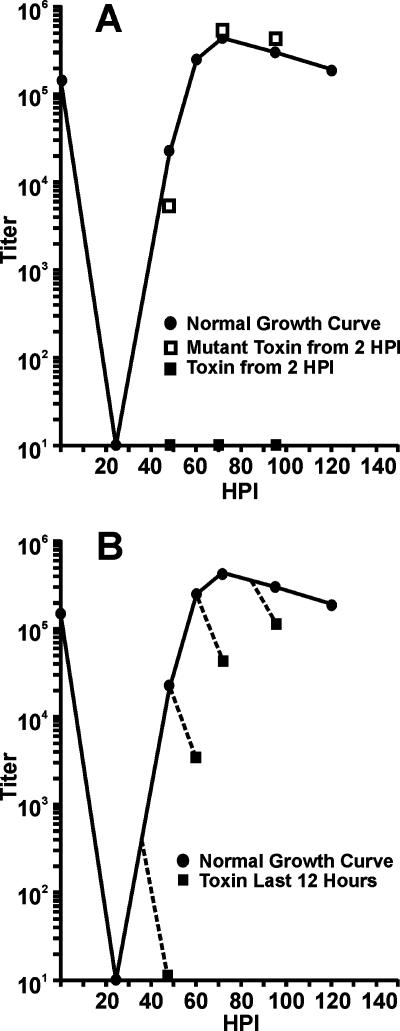
Viral growth curves were generated from infected cells treated with WT or Mut toxin in the time course patterns described above. Figure 3A shows the normal growth curve for untreated cells. The addition of the Mut toxin at 2 hpi and harvest at 48, 72, or 96 hpi had no effect on viral growth compared to that of untreated samples. However, similar treatment with the WT toxin allowed no infectious virions to be produced. Figure 3B shows that the addition of the WT toxin for 12-h periods prior to harvest severely inhibited virion production. This contrasts with the observation that steady-state levels of representative viral proteins were not affected during the 12-h treatment periods (Fig. 2).
FIG. 3.
Depletion of BiP/GRP78 inhibits production of infectious HCMV virions. (A) HFFs were infected with HCMV, and infectious virions were harvested at the indicated times postinfection (normal growth curve). Virus was also harvested at 48, 72, and 86 hpi from infected HFFs that had been treated with either SubAB (toxin) or SubAA272B (mutant toxin) at 2 hpi. Viral titers were determined using the TCID50 method. (B) Growth curves were performed as described for panel A, except that SubAB was added 12 h before the harvesting of virus at 48, 60, 72, or 96 hpi.
HCMV infection protects cells from the deleterious effects of BiP/GRP78 depletion; however, cytoplasmic virion egress and envelopment are inhibited.
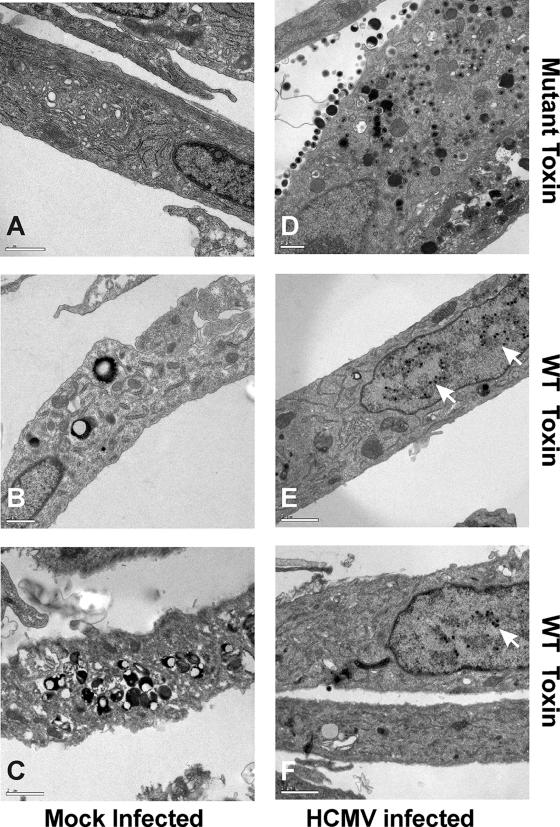
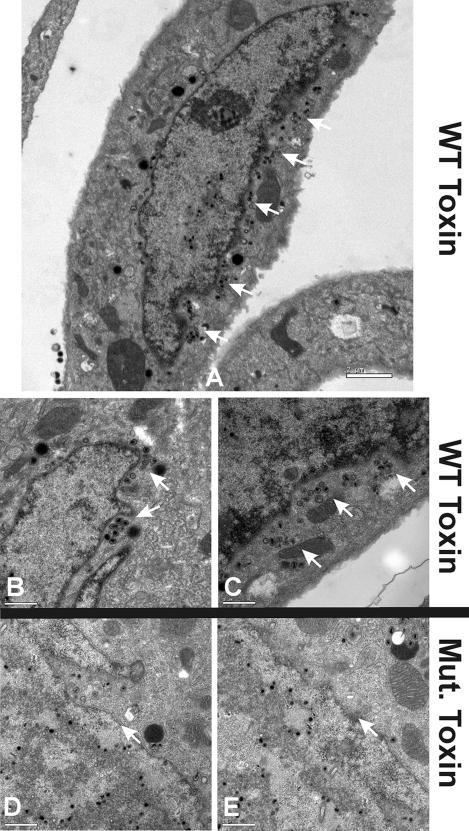
The data reported above indicate that the depletion of BiP/GRP78 in an established infection does not disturb viral protein synthesis but does inhibit virion formation. To further examine this, we performed EM on infected and mock-infected cells treated with WT or Mut toxin. Figure 4A shows a representative mock-infected cell treated with Mut toxin for 12 h. The cell looks normal and is indistinguishable from an untreated, uninfected cell (data not shown). Figure 4B and C show two examples of uninfected cells that have been treated for 12 h with the WT toxin; the cytotoxic effect of the toxin is quite evident, especially the marked formation of secondary lysosomes and autophagic vesicles. Figure 4D shows a cell infected with HCMV for 96 h and treated with Mut toxin from 84 to 96 hpi. There is extensive viral activity in the cytoplasm, identical to that seen in an untreated infected cell (data not shown). Figure 4E and F show infected cells treated with the WT toxin from 84 to 96 hpi. Note that the infected cells do not show the cytotoxic effects of the toxin seen in uninfected cells; the cytoplasms are relatively normal in appearance. In addition, there is very little viral activity in the cytoplasm of infected cells treated with the WT toxin; however, there are significant numbers of nucleocapsids in the nuclei. These data suggest that (i) HCMV infection is cytoprotective, allowing cells to resist the cytotoxic effects of toxin treatment, and (ii) the cytoplasmic events involved in virion formation cease when BiP/GRP78 is depleted (this would agree with the inhibition of infectious virion formation seen in the growth curve when the WT toxin was added [Fig. 3B]).
FIG. 4.
HCMV infection protects HFFs from the cytotoxic effects of BiP/GRP78 depletion by SubAB; however, viral activity in the cytoplasm is inhibited. Mock-infected (A to C) and HCMV-infected (D to F) HFFs were treated with either SubAA272B (mutant toxin) (A and D) or SubAB (WT toxin) (B, C, E, and F) at 84 hpi and were harvested at 96 hpi for analysis by EM. Arrows point to nucleocapsids in the nucleus.
The effects of depleting BiP/GRP78 by shRNA are similar to those of depletion by the WT toxin.
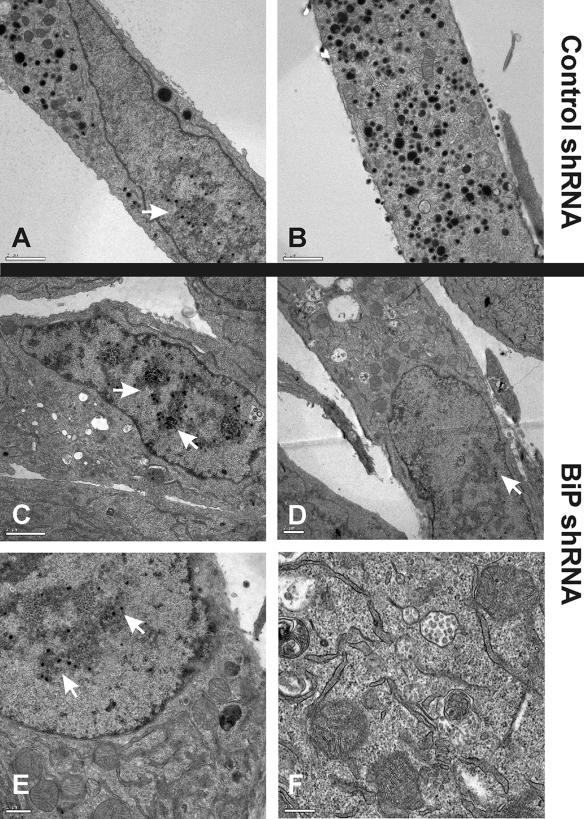
Although the SubAB toxin appears to be rather specific for BiP/GRP78 cleavage (22), it is possible that other, yet to be determined activities of the toxin could contribute to the viral phenotype in infected cells. Therefore, we used shRNA expressed from lentivirus vectors to deplete BiP/GRP78 in infected cells. HFFs were infected with the lentivirus vector expressing BiP/GRP78 shRNA or a control shRNA (GFP) for 24 h; then the cells were infected with HCMV and prepared for EM analysis 96 h post-HCMV infection. We found that the depletion of BiP/GRP78 was slow, presumably due to its long half-life. BiP/GRP78 reached its minimum levels (10 to 20% WT) between 48 and 72 h post-HCMV infection (data not shown). Hence, the viral infection could be established before minimal BiP/GRP78 depletions occurred, making the experiment somewhat similar to the toxin depletion experiment. With the shRNA, the BiP/GRP78 levels were lowered by 80 to 90% (data not shown); thus, some BiP/GRP78 remained, whereas a short treatment with WT toxin (Fig. 2) eliminated all detectable BiP/GRP78. Nevertheless, Fig. 5 shows that infected cells treated with BiP/GRP78 shRNA show a phenotype at 96 hpi similar to that of those treated with WT toxin. The EM analysis shows normal viral activity in cells treated with the control shRNA (Fig. 5A and B); however, in cells treated with the BiP/GRP78 shRNA, viral activity in the cytoplasm was significantly reduced, while nucleocapsids were detected in the nuclei (Fig. 5C, D, E, and F). The similarity of the viral phenotypes seen in BiP/GRP78 shRNA- and WT toxin-treated cells confirms that the effect of the WT toxin is the result of BiP/GRP78 cleavage and inactivation.
FIG. 5.
Depletion of BiP/GRP78 by shRNA has the same effect as depletion by SubAB toxin. HFFs were infected with HCMV 24 h after infection with lentivirus vectors expressing shRNA against GFP (control shRNA) (A and B) or BiP/GRP78 (C to F). The cells were harvested at 96 h post-HCMV infection for analysis by EM. White arrows indicate the presence of nucleocapsids in the nucleus.
In BiP/GRP78-depleted cells, nucleocapsids accumulate outside of the outer nuclear envelope and fail to move into the cytoplasm.
The effects of the toxin and shRNA on the viral infection suggest that BiP/GRP78 may be necessary for either the egress of nucleocapsids from the nucleus or the progression to tegumentation and envelopment in the cytoplasm. Further EM examination of samples infected for 96 h and treated with toxin from 84 to 96 h revealed cells that were sectioned such that we could detect nucleocapsid accumulation just outside of the outer nuclear envelope. These nucleocapsids appeared to be halted at this point, unable to move further in the process of tegumentation, envelopment, and cytoplasmic egress. Figure 6A shows an example of this arrest. The cytoplasm of the cell shows little viral activity; however, nucleocapsids are heavily concentrated just outside of the outer nuclear envelope on one side of the nucleus. Figure 6B and C show additional examples. Such a concentration of nucleocapsids was not detected in infected cells that were treated with Mut toxin (examples are shown in Fig. 6D and E).
FIG. 6.
Nucleocapsids accumulate outside of the nuclear envelope in BiP/GRP78-depleted cells. HFFs were infected with HCMV, treated with either WT toxin (A, B, and C) or Mut toxin (D and E) at 84 hpi, and harvested at 96 hpi for analysis by EM. White arrows in panels A to C show accumulation of virion particles outside of the nuclear membrane. White arrows in panels D and E indicate the nuclear membrane.
DISCUSSION
In the studies presented here, we show that the expression of the ER chaperone BiP/GRP78 is closely regulated during HCMV infection. This results in a temporally precise increase and decrease in BiP/GRP78 levels: levels begin to increase at 36 hpi, peak by 60 to 72 hpi, and decrease thereafter. The peak of BiP/GRP78 protein levels in infected cells corresponds to a period of intense virion structural protein and glycoprotein synthesis/processing and virion assembly. This would be a period in which the BiP/GRP78 chaperone function would be needed and during which the UPR could be induced. Thus, additional BiP/GRP78 may provide a needed chaperone function while maintaining a pool of BiP/GRP78 sufficient to exert some control on PERK, ATF6, and XBP-1, thus limiting UPR activation. In this regard we have previously shown that HCMV infection manipulates UPR signaling such that translational inhibition is circumvented (13). Additionally, this HCMV-induced maintenance of translation is effective even under conditions of robust activation of the UPR by the drug thapsigargin, which causes Ca2+ to be released from the ER to the cytoplasm (12). Since BiP/GRP78 is also a Ca2+ binding protein, its increased levels in infected cells may maintain ER function and translation by buffering the ER from Ca2+ loss due to thapsigargin treatment or, more relevantly, due to the ER Ca2+ release mediated by the HCMV UL37x1 protein during HCMV infection (26).
Growing evidence shows that HCMV infection modulates not only the UPR but also many other cellular stress signaling pathways (4, 6, 15-17, 20, 21, 30). These data suggest that a major goal of the virus is to make the host cell ignore signs of severe stress. By circumventing the effects of cellular stress signaling, the virus maintains needed cellular metabolic and synthetic functions and avoids apoptosis. This is clearly demonstrated in the present study, where we find that the complete depletion of BiP/GRP78 during HCMV infection has very little adverse effect on HCMV-infected cells, though it is very deleterious to uninfected cells. Just as the viral infection can circumvent the inhibition of viral protein synthesis by thapsigargin (12), it can also maintain protein synthesis after the depletion of BiP/GRP78 by the SubAB toxin. However, upon addition of the toxin, the continued formation of infectious virions is inhibited. We have previously reported that this also happens upon addition of thapsigargin (12).
EM analysis of infected cells depleted of BiP/GRP78 shows that viral activity in the cytoplasm ceases after only 12 h of toxin treatment during an established infection. A similar result is seen at 96 hpi in cells depleted of BiP/GRP78 by using shRNA. This is significant because it indicates that the toxin's effects on viral replication are, in fact, due to the depletion of BiP/GRP78 and not to an uncharacterized effect of the toxin. It is also important to point out the differences between these two depletion approaches. The toxin depletes very rapidly and completely due to its cleavage efficiency. With the shRNA, depletion of BiP/GRP78 is slow and not as complete; the lowest levels of BiP/GRP78 occur 48 to 72 h after lentivirus infection. Thus, HCMV, which is added 24 h after the lentivirus vector, has sufficient levels of BiP/GRP78 to initiate the infection before the levels drop to the point where the effects on cytoplasmic egress can be seen. The fact that the same effect can be seen after only a few hours of toxin treatment indicates how quickly the loss of BiP/GRP78 can be manifested.
Besides the lack of viral activity in the cytoplasm of BiP/GRP78-depleted cells, we also noted that nucleocapsids accumulated just outside of the outer nuclear membrane. This would suggest that in the absence of BiP/GRP78, the nucleocapsids can depart from the nucleus, although we cannot rule out the possibility that this process may be debilitated. However, the accumulation outside of the outer nuclear membrane suggests that without BiP/GRP78, movement away from the outer nuclear membrane is inhibited; correspondingly, the cytoplasmic processes of tegumentation, envelopment, and egress do not occur. In sum, these data suggest that HCMV precisely controls the expression of BiP/GRP78, which aids in a number of functions needed by the virus, including cytoplasmic viral egress.
Acknowledgments
We thank William Britt (University of Alabama) for helpful discussions and advice and Q. C. Yu of the Penn Biomedical Imaging Facility for EM expertise. We also thank all the members of the Alwine laboratory for critical evaluation of the data and the manuscript. Cheers to all.
This work was supported by the National Institutes of Health through Public Health Service grant CA28379, awarded to J.C.A. by the National Cancer Institute.
Footnotes
Published ahead of print on 17 October 2007.
REFERENCES
- 1.Arsham, A. M., J. J. Howell, and M. C. Simon. 2003. A novel hypoxia-inducible factor-independent hypoxic response regulating mammalian target of rapamycin and its targets. J. Biol. Chem. 27829655-29660. [DOI] [PubMed] [Google Scholar]
- 2.Barrasa, M. I., N. Y. Harel, and J. C. Alwine. 2005. The phosphorylation status of the serine-rich region of the human cytomegalovirus 86-kDa major immediate-early protein IE2/IEP86 affects temporal viral gene expression. J. Virol. 791428-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bresnahan, W. A., G. E. Hultman, and T. Shenk. 2000. Replication of wild-type and mutant human cytomegalovirus in life-extended human diploid fibroblasts. J. Virol. 7410816-10818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Child, S. J., M. Hakki, K. L. De Niro, and A. P. Geballe. 2004. Evasion of cellular antiviral responses by human cytomegalovirus TRS1 and IRS1. J. Virol. 78197-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cullinan, S. B., D. Zhang, M. Hannink, E. Arvisais, R. J. Kaufman, and J. A. Diehl. 2003. Identification of Nrf2 as a novel PERK substrate and critical effector of PERK-dependent cell survival. Mol. Cell. Biol. 237198-7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hakki, M., E. E. Marshall, K. L. De Niro, and A. P. Geballe. 2006. Binding and nuclear relocalization of protein kinase R by human cytomegalovirus TRS1. J. Virol. 8011817-11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harel, N. Y., and J. C. Alwine. 1998. Phosphorylation of the human cytomegalovirus 86-kilodalton immediate-early protein IE2. J. Virol. 725481-5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hegde, N. R., M. S. Chevalier, T. W. Wisner, M. C. Denton, K. Shire, L. Frappier, and D. C. Johnson. 2006. The role of BiP in endoplasmic reticulum-associated degradation of major histocompatibility complex class I heavy chain induced by cytomegalovirus proteins. J. Biol. Chem. 28120910-20919. [DOI] [PubMed] [Google Scholar]
- 9.Hendershot, L. M. 2004. The ER function BiP is a master regulator of ER function. Mt. Sinai J. Med. 71289-297. [PubMed] [Google Scholar]
- 10.Holcik, M., and N. Sonenberg. 2005. Translational control in stress and apoptosis. Nat. Rev. Mol. Cell Biol. 6318-327. [DOI] [PubMed] [Google Scholar]
- 11.Hollien, J., and J. S. Weissman. 2006. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science 313104-107. [DOI] [PubMed] [Google Scholar]
- 12.Isler, J. A., T. G. Maguire, and J. C. Alwine. 2005. Production of infectious human cytomegalovirus virions is inhibited by drugs that disrupt calcium homeostasis in the endoplasmic reticulum. J. Virol. 7915338-15397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isler, J. A., A. H. Skalet, and J. C. Alwine. 2005. Human cytomegalovirus infection activates and regulates the unfolded protein response. J. Virol. 796890-6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufman, R. J., D. Scheuner, M. Schroder, X. Shen, K. Lee, C. Y. Liu, and S. M. Arnold. 2002. The unfolded protein response in nutrient sensing and differentiation. Nat. Rev. Mol. Cell Biol. 3411-421. [DOI] [PubMed] [Google Scholar]
- 15.Kudchodkar, S., Y. Yu, T. Maguire, and J. C. Alwine. 2004. Human cytomegalovirus infection induces rapamycin-insensitive phosphorylation of downstream effectors of mTOR kinase. J. Virol. 7811030-11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kudchodkar, S. B., G. Q. Del Prete, T. G. Maguire, and J. C. Alwine. 2007. AMPK-mediated inhibition of mTOR kinase is circumvented during immediate-early times of human cytomegalovirus infection. J. Virol. 813649-3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kudchodkar, S. B., Y. Yu, T. G. Maguire, and J. C. Alwine. 2006. Human cytomegalovirus infection alters the substrate specificities and rapamycin sensitivities of raptor- and rictor-containing complexes. Proc. Natl. Acad. Sci. USA 10314182-14187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee, A. S. 2007. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 673496-3499. [DOI] [PubMed] [Google Scholar]
- 19.Lee, K., W. Tirasophon, X. Shen, M. Michalak, R. Prywes, T. Okada, H. Yoshida, K. Mori, and R. J. Kaufman. 2002. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev. 16452-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohr, I. 2006. Phosphorylation and dephosphorylation events that regulate viral mRNA translation. Virus Res. 11989-99. [DOI] [PubMed] [Google Scholar]
- 21.Moorman, N. J., I. Cristea, S. Terhune, M. R. Rout, B. Chait, and T. Shenk. Human cytomegalovirus pUL38 antagonizes the tuberous sclerosis complex. Cell Host Microbe, in press. [DOI] [PMC free article] [PubMed]
- 22.Paton, A. W., T. Beddoe, C. M. Thorpe, J. C. Whisstock, M. C. Wilce, J. Rossjohn, U. M. Talbot, and J. C. Paton. 2006. AB5 subtilase cytotoxin inactivates the endoplasmic reticulum chaperone BiP. Nature 443548-552. [DOI] [PubMed] [Google Scholar]
- 23.Paton, A. W., P. Srimanote, U. M. Talbot, H. Wang, and J. C. Paton. 2004. A new family of potent AB5 cytotoxins produced by Shiga toxigenic Escherichia coli. J. Exp. Med. 20035-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rutkowski, D. R., and R. J. Kaufman. 2004. A trip to the ER: coping with stress. Trends Cell Biol. 1420-28. [DOI] [PubMed] [Google Scholar]
- 25.Schröder, M., and R. J. Kaufman. 2006. Divergent roles of IRE1α and PERK in the unfolded protein response. Curr. Mol. Med. 65-36. [DOI] [PubMed] [Google Scholar]
- 26.Sharon-Friling, R., J. Goodhouse, A. M. Colberg-Poley, and T. Shenk. 2006. Human cytomegalovirus pUL37x1 induces the release of endoplasmic reticulum calcium stores. Proc. Natl. Acad. Sci. USA 10319117-19122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen, J., X. Chen, L. M. Hendershot, and R. Prywes. 2002. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev. Cell 399-111. [DOI] [PubMed] [Google Scholar]
- 28.Talbot, U. M., J. C. Paton, and A. W. Paton. 2005. Protective immunization of mice with an active-site mutant of subtilase cytotoxin of Shiga toxin-producing Escherichia coli. Infect. Immun. 734432-4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tirosh, B., N. N. Iwakoshi, B. N. Lilley, A. Lee, L. H. Glimcher, and H. L. Ploegh. 2005. Human cytomegalovirus protein US11 provokes an unfolded protein response that may facilitate the degradation of class1 major histocompatibility complex products. J. Virol. 792768-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walsh, D., C. Perez, J. Notary, and I. Mohr. 2005. Regulation of the translation initiation factor eIF4F by multiple mechanisms in human cytomegalovirus-infected cells. J. Virol. 798057-8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wek, R. C., H. Y. Jiang, and T. G. Anthony. 2006. Coping with stress: eIF2 kinases and translational control. Biochem. Soc. Trans. 347-11. [DOI] [PubMed] [Google Scholar]
- 32.Wouters, B. G., T. van den Beucken, M. G. Magagnin, M. Koritzinsky, D. Fels, and C. Koumenis. 2005. Control of the hypoxic response through regulation of mRNA translation. Semin. Cell Dev. Biol. 16487-501. [DOI] [PubMed] [Google Scholar]
- 33.Yoshida, H. 2007. ER stress and diseases. FEBS J. 274630-658. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida, H., K. Haze, H. Yanagi, T. Yura, and K. Mori. 1998. Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. J. Biol. Chem. 27333741-33749. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida, H., T. Matsui, A. Yamamoto, T. Okada, and K. Mori. 2001. Xbp1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107881-891. [DOI] [PubMed] [Google Scholar]
- 36.Zhang, K., and R. J. Kaufman. 2006. The unfolded protein response: a stress signaling pathway critical for health and disease. Neurology 66S102-S109. [DOI] [PubMed] [Google Scholar]