Abstract
The entry of human cytomegalovirus (HCMV) into biologically relevant epithelial and endothelial cells involves endocytosis followed by low-pH-dependent fusion. This entry pathway is facilitated by the HCMV UL128, UL130, and UL131 proteins, which form one or more complexes with the virion envelope glycoprotein gH/gL. gH/gL/UL128-131 complexes appear to be distinct from the gH/gL/gO complex, which likely facilitates entry into fibroblasts. In order to better understand the assembly and protein-protein interactions of gH/gL/UL128-131 complexes, we generated HCMV mutants lacking UL128-131 proteins and nonreplicating adenovirus vectors expressing gH, gL, UL128, UL130, and UL131. Our results demonstrate that UL128, UL130, and UL131 can each independently assemble onto gH/gL scaffolds. However, the binding of individual UL128-131 proteins onto gH/gL can significantly affect the binding of other proteins; for example, UL128 increased the binding of both UL130 and UL131 to gH/gL. Direct interactions between gH/UL130, UL130/UL131, gL/UL128, and UL128/UL130 were also observed. The export of gH/gL complexes from the endoplasmic reticulum (ER) to the Golgi apparatus and cell surface was dramatically increased when all of UL128, UL130, and UL131 were coexpressed with gH/gL (with or without gO expression). Incorporation of gH/gL complexes into the virion envelope requires transport beyond the ER. Thus, we concluded that UL128, UL130, and UL131 must all bind simultaneously onto gH/gL for the production of complexes that can function in entry into epithelial and endothelial cells.
Individuals with undeveloped or compromised immunity are highly sensitive to infection by the ubiquitous herpesvirus human cytomegalovirus (HCMV). Infections acquired during birth can result in cytomegalic (or congenital) inclusion disease, which affects many tissues and organs including the central nervous system, liver, and retina and can lead to multiorgan failure and death (5, 36). AIDS patients are at risk of HCMV pathologies, including retinitis, uveitis, and vitritis (36, 43, 50, 58). Moreover, HCMV infection increases the risk of organ graft loss through transplant vascular sclerosis and restenosis and may increase atherosclerosis in transplant patients and in the general population (1, 15, 36). These pathologies are due in part to the ability of HCMV to enter and replicate in diverse cell types including epithelial cells, endothelial cells, smooth muscle cells, fibroblasts, neurons, and monocytes/macrophages (8, 36, 49).
Laboratory strains of HCMV such as AD169 and Towne have been extensively propagated on fibroblasts and harbor deletions, mutations, and rearrangements in the virus genome that compromise the infection of epithelial and endothelial cells (10, 13, 42). Sinzger et al. showed that loss of virus replication and spread on endothelial cells correlated with genetic alterations occurring during serial passage on fibroblasts (54). Among the more severe genetic modifications are those in the UL128-UL151 or ULb′ region. For example, AD169 has a deletion encompassing all of the UL133-UL150 genes and a frameshift in the UL131 gene (10, 42). Studies by Hahn et al. mapped the ability to infect endothelial cells to the UL128, UL130, and UL131 genes (17). Wang and Shenk repaired the UL131 gene of AD169 and showed that an intact UL128-131 locusis important for infection of both epithelial and endothelial cells (61).
We reported that AD169 and a mutant derived from the clinical strain TR lacking UL128 to UL150 failed to infect epithelial and endothelial cells (51). Treatment with the fusogenic agent polyethylene glycol (PEG) demonstrated that the failure of these viruses was at the stage of entry (51). In the initial studies, PEG did not appear to overcome this entry defect on endothelial cells. However, more-recent studies utilizing an altered experimental design have definitively demonstrated that PEG can promote entry of AD169 and the TR UL128-150 mutant into endothelial cells (51; B. J. Ryckman, M. A. Jarvis, B. L. Rainish, D. D. Drummond, J. A. Nelson, and D. C. Johnson, presented at 31st International Herpesvirus Workshop, Seattle, WA, July 2006). Along with the work of others, these studies indicated that HCMV UL128-131 proteins are necessary for virus entry into both endothelial and epithelial cells. UL128-131 have no apparent role for replication in fibroblasts, and in fact there appears to be a selective advantage for HCMV to lose or inactivate the UL128-131 genes during replication and spread on fibroblasts. Akter et al. reported that HCMV strain Merlin sustained a truncation of the UL128 gene in under three passages on fibroblasts (4), and our UL128-131 mutants replicate to higher titers in fibroblasts than the parental strain, TR (M. Chase, unpublished observations).
The use of alternative entry pathways to infect different cell types is emerging as a common feature of herpesviruses and in some cases involves the use of different receptor binding proteins. Herpes simplex virus (HSV) utilizes three glycoproteins, gB, gD, and gH/gL, to enter some cells by direct fusion with the plasma membrane and other cells by endocytosis and low-pH-dependent fusion (40, 44, 45, 57). The HSV heterodimer gH/gL has been ascribed a direct role in membrane fusion (16, 59) as well as in binding to integrins (47). Epstein-Barr virus (EBV) expresses two forms of gH/gL. Unmodified gH/gL mediates entry into epithelial cells through fusion with the plasma membrane. Alternatively, the gH/gL complex can include gp42, which binds to major histocompatibility complex (MHC) class II proteins and promotes entry into B cells through endocytosis. Importantly, during virion assembly in B cells, gp42 is sequestered by interaction with MHC class II and as a result, progeny virions contain primarily unmodified gH/gL and infect epithelial cells more efficiently (7, 28). Similarly, human herpesvirus 6A produces two types of gH/gL complexes, gH/gL/gO and gH/gL/gQ1/gQ2, each of which may mediate entry into different cell types (3, 41).
The HCMV gH/gL complex was first described based on the recognition that HCMV UL115 is a positional homologue of HSV gL that complexes with gH in a manner that is required for endoplasmic reticulum (ER) export (27, 31, 56). A third glycoprotein, designated gO, was found associated with gH/gL in HCMV-infected cells (25, 37). There is evidence that HCMV gH and gL are essential for virus replication in fibroblasts, and a gO mutant was severely impaired (14, 22). Interestingly, HCMV adaptation to growth in fibroblasts involves not only mutations of UL128-131 but also extensive mutation of gO (up to 20% amino acid variability between strains) (13, 42). As for the corresponding EBV and HSV proteins, there is evidence that HCMV gH/gL binds to integrins (63) and can cause cell-cell fusion of Chinese hamster ovary (CHO) cells (33).
Wang and Shenk made important observations connecting the HCMV UL128-131 genes to gH/gL (62). They described complexes containing gH/gL and the UL128 and UL130 proteins in cells infected with a UL131-repaired AD169 and showed that they are distinct from gH/gL/gO complexes. They also showed that UL128 and UL130 are present in virions and that antibodies against UL128 and UL130 could block HCMV entry into epithelial and endothelial cells but not fibroblasts. Adler et al. extended these studies by showing that UL131 is associated with gH in virions and functions in infection of endothelial cells (2). UL130 is a 35-kDa glycoprotein, while UL128 and UL131 are smaller, 15- to 18-kDa proteins. (2, 48, 62).
Laboratory and clinical isolates of HCMV enter fibroblasts by fusion at the plasma membrane, a process that is not inhibited by lysosomotropic agents (12, 51). By contrast, HCMV TR enters epithelial and endothelial cells by endocytosis and is dependent on endosome acidification (i.e., inhibited by lysosomotropic agents) (51). Other studies have described postentry defects on endothelial cells with fibroblast-passaged HCMV (53). However, at least for mutants of strain TR that lack UL128-131 genes, PEG treatment completely overcomes defects in infection of endothelial cells, suggesting that there are no postentry defects (51; Ryckman et al., presented at 31st International Herpesvirus Workshop, Seattle, WA, July 2006). Altogether, these studies support a model in which HCMV uses gH/gL/UL128-131 complexes to enter epithelial and endothelial cells by endocytosis and low-pH-dependent fusion but enters fibroblasts by direct fusion at the plasma membrane in a process involving gH/gL/gO.
To function in virus entry, gH/gL/gO and gH/gL/UL128-131 complexes must be properly folded and assembled in the ER and must reach modified versions of the Golgi apparatus or trans-Golgi network, where secondary envelopment occurs (23, 52). gH/gL/gO complexes of 250 kDa form in HCMV-infected and -transfected cells, consistent with assembly into trimers and transport to the trans-Golgi network (34). In contrast, little is known about the assembly and intracellular transport of gH/gL/UL128-131 complexes and whether functionally important complexes (i.e., incorporated into virions) include trimers (e.g., gH/gL/UL131), tetramers (e.g., gH/gL/UL128/UL130), or pentamers (gH/gL/UL128/130/131). To study this and to characterize protein-protein interactions, we used HCMV deletion mutants lacking UL128-131 proteins and adenovirus (Ad) vectors that expressed gH, gL, and UL128-131 proteins. Data presented here show that each of UL128, UL130, and UL131 can bind independently to gH/gL but that the binding of one of these proteins can affect the binding of others. Expression of all of UL128, UL130, and UL131 was required for efficient ER export of gH/gL, even in the context of HCMV infection where gO was also present. Therefore, pentameric gH/gL/UL128/130/131 complexes are likely to represent the functionally important structures that are incorporated into the virion envelope.
MATERIALS AND METHODS
Cell lines.
Neonatal normal human dermal fibroblasts were obtained from Cascade Biologics (Portland, OR), and U373-MG microglial cells were obtained from the American Type Culture Collection (Manassas, VA) and grown in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) supplemented with 12% fetal bovine serum (FBS) or 10% bovine growth serum (HyClone). 293 and 293-Cre 4 (46) cells were grown in DMEM plus 10% FBS.
HCMVs.
HCMV TR is a wild-type clinical strain that was derived from the retina and was cloned into a bacterial artificial chromosome (BAC) after limited passage on fibroblasts (42, 55). Derivation of mutant TRΔ4 was described previously (51). TRΔ131 was generated from TR-BAC by use of a two-step recombination technology utilizing the recE and recT genes of the prophage Rac and a negative selection system (64). Briefly, the UL131 gene was first replaced by insertion of the galK gene. A mutant version of the UL131 gene in which the start codon was changed from ATG to ACG (change underlined) was then reinserted in place of the galK gene. Viruses were reconstituted by transfection into permissive human fibroblasts, and the presence of the desired mutation was confirmed by PCR-mediated DNA sequencing of the HCMV genome. HCMV stocks were produced by infecting neonatal normal human dermal fibroblasts by use of 0.1 PFU per cell for 10 to 16 days. To enrich for HCMV particles, infected cells were sonicated, and then large cellular debris was removed by centrifugation at 6,000 × g for 15 min and virus particles were centrifuged through a 20% sorbitol cushion at 50,000 × g for 1 h. Pellets were resuspended in DMEM plus 10% FBS and frozen at −70°C. These stocks contained approximately 50% of the total cell-associated virus and 70% of total cellular calnexin.
Construction of Ad vectors.
Nonreplicating (E1-) Ad vectors expressing HCMV gH, gL, UL128, UL130, or UL131 were generated using a modification of the method of Hardy et al. (18). Briefly, each HCMV open reading frame (from start codon to stop codon) was amplified by PCR from DNA derived from HCMV strain TR and inserted into plasmid pAdTet-7 as described for HCMV US2 (60). UL128 and UL131 proteins are translated from spliced messages (4, 17), and thus reverse transcription-PCR was performed using oligo(dT) in conjunction with primers specific to sequences just upstream of the UL128 and UL131 open reading frames to produce cDNAs corresponding to fully spliced transcripts. To generate an Ad vector expressing soluble gH (sgH), the UL75 (gH)-coding sequence from the start codon to the codon corresponding to amino acid position 718 (Asp) was amplified by PCR and cloned into the pAdTet-7 vector. The downstream PCR primer contained in-frame coding sequence that fused the amino acid sequence RSSTMVRSQPELAPEDPEDSALLEDPV to the C terminus of gH. This sequence is sufficient for recognition by the DL6 monoclonal antibody (MAb) (29). Each pAdTet-7-derived plasmid was verified by DNA sequencing and then cotransfected along with DNA-derived Ad psi-5, which has packaging sequences flanked by loxP sites (18) into 293 cells expressing the Cre-4 recombinase (46). Viruses derived from these cotransfections were passaged at least four times on Cre-4 293 cells to remove psi-5 viruses. U373 cells were infected with Ad vectors (30 to 70 PFU/cell), and HCMV proteins were analyzed by immunoblotting or after radiolabeling cells between 24 and 48 h postinfection. There was little or no cytopathic effect under these conditions.
Antibodies.
MAbs specific for gH, 14-4b, and the major capsid protein 28-4 were kindly provided by Bill Britt (University of Alabama, Birmingham, AL) (6, 11). Anti-UL128 MAb 4B10 was a gift from Tom Shenk (Princeton University, Princeton, NJ) (62). MAb antibody DL6 was provided by Gary Cohen and Roselyn Eisenberg (University of Pennsylvania, Philadelphia, PA). Rabbit polyclonal antisera directed against HCMV gH, gL, UL128, UL130, and UL131 were produced according to standard protocols (19). Briefly, peptides were synthesized by PeptidoGenics (Berkley, CA) as follows: for gH, CSSSGRRDHSLERLTR; for gL, CKQTRVNLPAHSRYGPQAVDAR; for UL128, DQYLESVKKIHKRLDVC; for UL130, SWSTLTANQNPSPPWSKLTYC; and for UL131, SDFRRQNRRGGTNKRTTC. Peptides were coupled to keyhole limpet hemocyanin by use of m-maleimidobenzoyl-N-hydroxysuccinimide ester (MBS; Sigma, St. Louis, MO), mixed with Freund's adjuvant, and used to immunize New Zealand White rabbits. Subsequently, rabbits were boosted with keyhole limpet hemocyanin-conjugated peptides in TiterMax Gold adjuvant (Sigma, St. Louis, MO).
Radiolabeling of cells and immunoprecipitation of proteins.
Cells were washed extensively with medium lacking methionine and cysteine then incubated in this medium for 1 h before the addition of this medium supplemented with [35S]methionine-cysteine (50 to 200 μCi/ml; Amersham). In pulse-chase experiments, radioactivity was chased by incubating cells in medium containing a 10-fold excess of nonradioactive methionine and cysteine. Cell extracts were made with NP-40 lysis buffer (0.5% NP-40 in phosphate-buffered saline supplemented with 1 mg/ml bovine serum albumin and 1 mM phenylmethylsulfonyl fluoride) and clarified by centrifugation at 1,500 × g for 10 min. Culture supernatants and cell extracts were clarified by centrifugation at 60 to 100,000 × g for 30 to 60 min and precleared by incubation with irrelevant rabbit or mouse sera and protein A-agarose beads for 1 to 2 h. Immunoprecipitation involved the addition of ascite fluid or antiserum for 2 h, followed by incubation with protein A-agarose for an additional 2 h. In some cases, the peptide used to produce antipeptide sera (10 μg/ml) was added to cell extracts in conjunction with antisera. For endo-β-N-acetylglucosaminidase H (endo H) treatment, immunoprecipitated proteins bound to protein A-agarose were incubated with 50 U of recombinant endo Hf (New England Biolabs) for 1 h at 37°C in the supplied buffer. Proteins were eluted from protein A-agarose by the addition of 2% sodium dodecyl sulfate (SDS) with or without 2% β-mercaptoethanol and boiling and analyzed by electrophoresis using 4 to 20% gradient gels (Pierce Biotechnology). Gels were fixed in 10% acetic acid and 30% methanol, dried, and then analyzed using a PhosphorImager BAS 2500 (Molecular Dynamics, Sunnyvale, CA).
Immunoblotting and immunoprecipitation-immunoblot analysis.
For direct immunoblotting, preparations of virus particles and microsomes prepared from infected fibroblasts (as described above) were solubilized in NP-40 lysis buffer and separated by SDS-polyacrylamide gel electrophoresis (PAGE). U373 cells were infected with Ad vectors for 24 to 48 h, cell extracts were made using NP-40 lysis buffer, and unlysed cells and nuclei were removed by centrifugation at 1,500 × g for 15 min. Proteins were denatured by boiling in 2% SDS and 2% β-mercaptoethanol and then separated by electrophoresis using 8%, 12%, or 15% SDS-polyacrylamide gels. Proteins were electrophoretically transferred to Immobilon membranes (Millipore) in a buffer containing 25 mM Tris, 192 mM glycine, and 20% methanol. For transfer of UL128 and UL131, a buffer containing 10 mM NaHCO3, 3 mM NaCO3, and 20% methanol was used. Transferred proteins were detected by immunoblotting with rabbit antipeptide antibodies or mouse MAbs by use of standard techniques (26, 66). Immunoprecipitation-immunoblot experiments involved extracts of HCMV/microsome preparations or Ad-infected cells made using NP-40 lysis buffer. Samples were precleared with irrelevant mouse antibody and protein A agarose for 2 h and then immunoprecipitated using anti-gH MAb 14-4b and protein A agarose for 2 h each. Proteins were eluted from protein A agarose by boiling in 2% SDS and 150 mM β-mercaptoethanol and then subjected to electrophoresis and immunoblotting as described above.
RESULTS
Construction and characterization of HCMV mutants lacking UL128-131.
Several questions about the form of gH/gL/UL128-131 complexes have not been resolved, including the following. (i) Do UL128, UL130, and UL131 each independently bind to gH/gL? (ii) Does the binding of any one of the UL128-131 proteins to gH/gL affect the binding of the other UL128-131 proteins? (iii) Which of the possible complexes (i.e., trimers, tetramers, and pentamers) are exported from the ER to the Golgi apparatus and are incorporated into virions? To begin to address some of these questions, we constructed an HCMV UL131-null mutant that expressed UL128 and UL130. HCMV TR is a clinical isolate that was cloned as a BAC after limited propagation in human fibroblasts (42, 55) and infects epithelial and endothelial cells efficiently (51). Previously, a mutant lacking the UL128-UL150 genes (TRΔ4) was constructed and failed to enter epithelial or endothelial cells (51). A second mutant (TRΔ131) was made by first inserting the galK gene in place of UL131 and then replacing the galK gene with a mutated UL131 gene in which the start codon was changed to ACG. Like TRΔ4, TRΔ131 failed to enter epithelial and endothelial cells, and this defect could be completely overcome by PEG treatment (Ryckman et al., presented at 31st International Herpesvirus Workshop, Seattle, WA, July 2006).
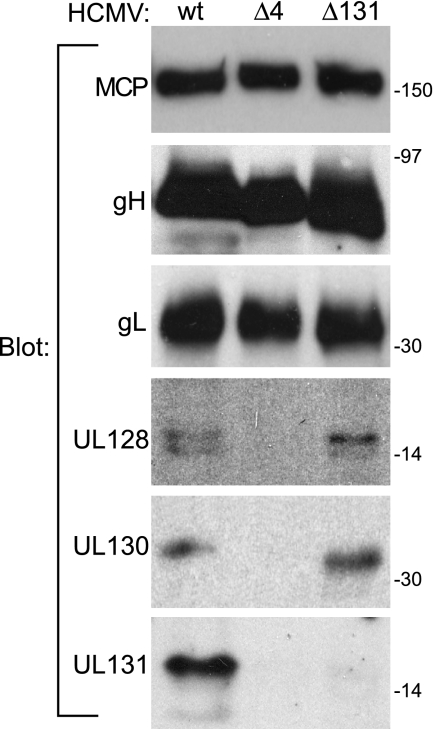
A new panel of rabbit antipeptide antibodies specific for gH, gL, UL128, UL130, and UL131 was prepared and used to characterize the expression of these proteins by the HCMV mutants. HCMV particles were enriched for and concentrated by centrifugation through a 20% sorbitol cushion. These preparations contained approximately 50% of the total infectious HCMV as well as 70% of the ER-resident protein calnexin (data not shown). Proteins were resolved by SDS-PAGE and detected by immunoblotting (Fig. 1). As expected, extracts from TRΔ4-infected cells contained gH and gL but no UL128, UL130, or UL131, and TRΔ131 expressed UL128 and UL130 but not UL131. A previously described UL131 mutant failed to express UL130, probably because the insertion mutagenesis used in that case destabilized a common transcript encoding UL130 and UL131 (17). Apparently, the mutation of the start codon used for construction of TRΔ131 avoided this issue.
FIG. 1.
Expression of gH, gL, UL128, UL130, and UL131 proteins by wild-type and mutant HCMV. Human fibroblasts were infected with HCMV mutant TRΔ4 (lacking UL128, UL130, and UL131) or TRΔ131 (lacking UL131 only) or wild-type (wt) TR at 0.1 PFU/cell; cells were harvested after 10 to 16 days and sonicated; larger cellular debris was removed by centrifugation at 6,000 × g; and then virus particles and microsomes were pelleted through a 20% sorbitol cushion. These preparations were enriched for viral proteins but also contained approximately 70% of the cellular calnexin, an ER protein. Proteins were analyzed by immunoblotting with anti-major capsid protein (MCP) MAb 28-4 or with rabbit antipeptide antibodies directed against gH, gL, UL128, UL130, or UL131.
Characterization of gH/gL/UL128-UL131 complexes in HCMV-infected cells.
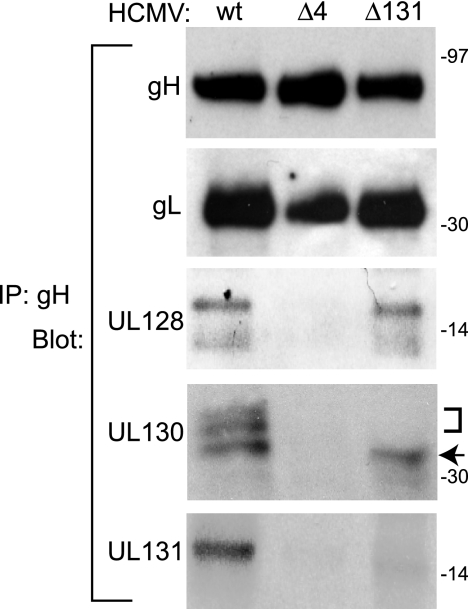
We analyzed gH/gL complexes that were immunoprecipitated from TRΔ131- and TRΔ4-infected cells by immunoblotting to determine whether gL, UL128, and UL130 were present when UL131 or UL128-131 was absent (Fig. 2). As expected, gL was consistently observed with or without the UL128-131 proteins. Wild-type TR gH/gL complexes also contained two electrophoretically separable forms of UL128, three forms of UL130, and a single form of UL131. TRΔ4 gH/gL complexes did not contain UL128, UL130, or UL131, whereas TRΔ131 expressed gH/gL complexes containing UL128 and UL130 but not UL131. Interestingly, the slower-migrating forms of UL130 observed for TR gH/gL complexes were not observed for TRΔ131 gH/gL complexes.
FIG. 2.
Characterization of gH/gL/UL128/UL130/UL131 complexes in cells infected with wild-type and mutant HCMV. Human fibroblasts were infected with mutant TRΔ4 or TRΔ131, or wild-type (wt) TR. Virion/microsome preparations (as described for Fig. 1) were solubilized in 0.5% NP-40 lysis buffer, and gH/gL complexes were immunoprecipitated (IP) using anti-gH MAb 14-4b followed by immunoblotting with rabbit antipeptide antibodies specific for gH, gL, UL130, or UL131 or with anti-UL128 MAb 4B10. The bracket and arrow indicate slower- and faster-migrating forms of UL130, respectively.
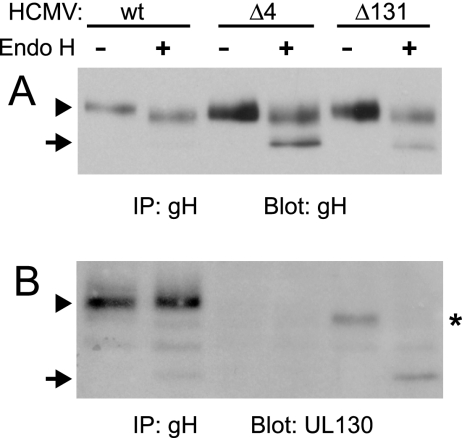
Both gH and UL130 are modified with N-linked oligosaccharides (48), and thus it appeared that faster-migrating forms of these glycoproteins represented unprocessed forms containing immature, high-mannose N-linked oligosaccharides. Transport of herpesvirus glycoproteins to the Golgi apparatus is associated with the processing of N-linked oligosaccharides to complex oligosaccharides that are resistant to endo H (30). To address defects in the intracellular transport of gH/gL complexes and of UL130, gH/gL complexes were immunoprecipitated from extracts of HCMV-infected cells and then treated with endo H. About half of the gH produced in TRΔ4- and TRΔ131-infected cells exhibited dramatically increased electrophoretic mobility compared with untreated gH (Fig. 3A). This fast-migrating form of gH was similar to the radiolabeled gH produced in a short pulse (see Fig. 6), suggesting that this gH contained only endo H-sensitive N-linked oligosaccharides. By contrast, gH produced in wild-type HCMV-infected cells migrated with a very low electrophoretic mobility compared to what was seen for the faster-migrating species observed for TRΔ4- and TRΔ131-infected cells (Fig. 3A). It appears that gH produced in wild-type HCMV-infected cells acquires substantial endo H resistance (compared with the highly endo H-sensitive gH in TRΔ4- and TRΔ131-infected cells), consistent with all or most of the glycoprotein reaching the Golgi apparatus. The small shift in gH produced in wild-type HCMV-infected cells is likely due to a small fraction of N-linked oligosaccharides that are not processed to complex oligosaccharides, as was observed with HSV gB (65). However, the salient point is that half of the gH produced in TRΔ4- and TRΔ131-infected cells is highly endo H sensitive, with no evidence of any endo H resistance, and this suggests that half of the gH is ER retained. The other half of gH that is processed in TRΔ4- and TRΔ131-infected cells likely reflects expression of gO, which can complex with gH/gL and promote transport to the Golgi apparatus (24).
FIG. 3.
endo H treatment of gH and UL130 in extracts of mutant and wild-type HCMV-infected cells. Human fibroblasts were infected with mutant TRΔ4 or TRΔ131, or wild-type (wt) TR. gH complexes were immunoprecipitated (IP) from extracts of virion/microsome preparations (as described for Fig. 1) using anti-gH MAb 14-4b. Precipitated proteins were treated with endo H (+) or incubated in buffer alone (−) and then analyzed by immunoblotting using antipeptide antibodies directed against gH (A) or UL130 (B). Arrowheads indicate endo H-resistant forms of gH and UL130, arrows indicate endo H-sensitive forms, and the asterisk marks a faster-migrating form UL130 precipitated with gH complexes from cells infected with TRΔ131 not treated with endo H.
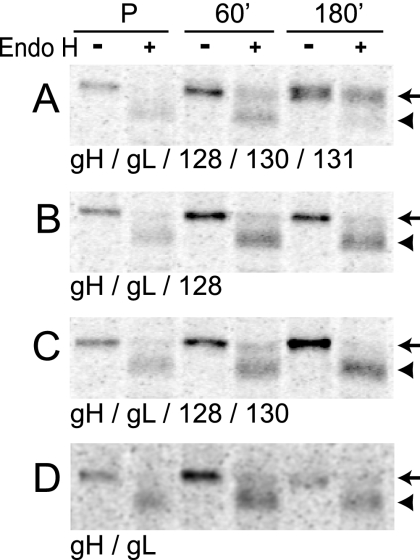
FIG. 6.
Coexpression of all of UL128, UL130, and UL131 increases ER-to-Golgi apparatus export of gH/gL complexes. U373 cells were infected with Ad vectors expressing different combinations of gH/gL and UL128-131 proteins. Cells were pulse-labeled with [35S]methionine-cysteine for 15 min (P), and radioactivity was chased for 60 or 180 min. gH was immunoprecipitated with MAb 14-4b, treated with endo H, and separated by SDS-PAGE under reducing conditions. Arrows and arrowheads indicate endo H-resistant and -sensitive forms of gH, respectively.
The fraction of UL130 associated with gH/gL from TRΔ131-infected cells was entirely endo H sensitive (Fig. 3B). gL, UL128, and UL131 were not analyzed because they are not predicted to contain extensive glycosylation. Together, these results indicate that when either UL131 or all of UL128, UL130, and UL131 are absent, a substantial population of gH/gL complexes is retained in the ER. The subset of gH/gL that is transported to the Golgi compartment in the absence of UL128-131 likely represents gH/gL complexes containing gO (24).
Construction and characterization of Ad vectors expressing gH/gL and UL128-131.
To further characterize the effects of UL128-131 on the transport and assembly of gH/gL complexes and to investigate protein-protein interactions, Ad vectors expressing these proteins were constructed. Ad vectors allow the expression of different protein combinations for characterization of direct interactions (e.g., UL130 binding to UL131 without gH/gL). This is difficult to study in the context of HCMV infection because mutants lacking gH, gL, or gO have not been constructed or replicate poorly and because the assembly of gH/gL/UL128-131 may be complicated by the presence of gO. Additionally, it can be difficult to metabolically label HCMV proteins, especially small proteins like UL131, which contain few methionines and cysteines.
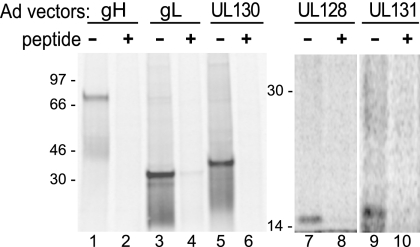
U373 glioblastoma cells were chosen for these analyses because we have extensive experience working with Ad vectors and HCMV in these cells (20, 21). A second reason for choosing this cell line is based on observations that MHC class II proteins in B cells interfere with assembly of the EBV gH/gL/gp42 complex (7, 28). Similarly, since HCMV UL128-131 functions in the entry of epithelial and endothelial cells, it is possible that the assembly of HCMV gH/gL/UL128-131 complexes might be affected by molecules expressed in these cells. This is less likely in U373 cells, which HCMV infects in a UL128-131-independent manner (B. Ryckman, unpublished observations). Expression of HCMV proteins by Ad vectors was analyzed by labeling U373 cells with [35S]methionine-cysteine and by immunoprecipitation of gH, gL, UL128, UL130, and UL131. Proteins of the expected sizes (2, 62) were detected and efficiently competed when antipeptide sera were premixed with the synthetic peptides (Fig. 4).
FIG. 4.
Expression of gH, gL, UL128, UL130, and UL131 by nonreplicating Ad vectors. U373 cells were infected with Ad vectors and labeled with [35S]methionine-cysteine. Cell extracts were immunoprecipitated with rabbit antipeptide antibodies specific for gH, gL, UL128, UL130, or UL131 and analyzed by SDS-PAGE under reducing conditions. Immunoprecipitation was performed in the presence (+) or absence (−) of the synthetic peptide used to generate each antibody.
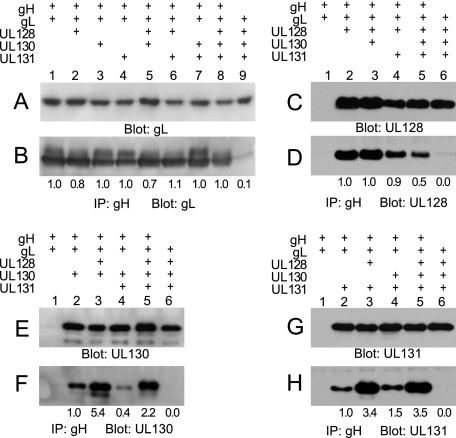
Binding of UL128-131 proteins to gH/gL and effects on intracellular transport.
U373 cells were infected with Ad vectors and extracts were either analyzed directly by immunoblotting (Fig. 5A, C, E, and G) or by immunoprecipitation with anti-gH antibodies followed by immunoblotting for the presence of gL, UL128, UL130, or UL131 (Fig. 5B, D, F, and H). Expression of each protein varied by less than 1.5-fold when other proteins were expressed (Fig. 5A, C, E, and G), and negligible amounts of gL, UL128, UL130, or UL131 were precipitated by anti-gH antibodies in the absence of gH expression (Fig. 5B, D, F, and H). Consistent with the hypothesis that the gH/gL dimer is the foundation for these complexes, expression of any combination of the UL128, UL130, and UL131 proteins did not alter the quantities of gL bound to gH (Fig. 5B, lanes 1 to 8). Each of UL128, UL130, and UL131 could independently bind to gH/gL (Fig. 5D, F, and H, lanes 2). However, the amount of each of the UL128-131 proteins bound to gH/gL was often markedly influenced by the presence of the others as follows. (i) The binding of UL128 to gH/gL was reduced by half when both UL130 and UL131 were also present (Fig. 5D, lanes 2 and 5). (ii) The presence of UL128 increased binding of UL130 to gH/gL by fivefold (Fig. 5F, lanes 2 and 3) and increased binding of UL131 to gH/gL by threefold (Fig. 5H, lanes 2 and 3). (iii) The presence of UL131, in the absence of UL128, reduced the binding of UL130 to gH/gL (Fig. 5F, lanes 2 and 4). Additionally, the electrophoretic mobility of the UL130 protein that was bound to gH/gL was low when both UL128 and UL131 were also present compared to expression conditions in which either UL128 or UL131 was absent (Fig. 5F, lanes 2 to 5). The latter observation was consistent with the analysis of HCMV-infected cells, in which gH/gL complexes containing UL130 produced by TRΔ131 were inefficiently transported through the secretory pathway (Fig. 3B).
FIG. 5.
gH/gL/UL128/UL130/UL131 complexes formed by Ad vector expression. U373 cells were infected with Ad vectors expressing different combinations of gH, gL, UL128, UL130, and UL131. Extracts made using 0.5% NP-40 lysis buffer were analyzed directly by immunoblotting (A, C, E, and G) or by immunoprecipitation (IP) with anti-gH MAb 14-4b followed by immunoblotting with anti-gL, -UL130, or -UL131 rabbit antipeptide antibodies or anti-UL128 MAb 4B10 (B, D, F, and H). The numbers below the panels represent the relative signal intensities of gL, UL128, UL130, and UL131 detected in gH/gL complexes normalized to the total amounts of these proteins in expressed in cells (measured by direct immunoblotting in panels A, C, E, and G).
To further analyze the influence of UL128-131 proteins on the intracellular transport and posttranslational processing of gH/gL complexes, pulse-chase experiments were performed. Cells were labeled with [35S]methionine-cysteine for 15 min, the label was chased for 60 or 180 min, and then gH/gL complexes were immunoprecipitated and treated with endo H. Following the 180-min chase period, approximately 80% of the gH was endo H resistant when all of gL, UL128, UL130, and UL131 were present in the cells (Fig. 6A). By contrast, when UL131 alone, UL130 and UL131, or all of UL128-131 was omitted, less than 25% of the gH became endo H resistant (Fig. 6B, C, and D). These results suggest that all of UL128, UL130, and UL131 are required in order for efficient transport beyond the ER to the Golgi apparatus when gH/gL is expressed without gO. However, the combinations tested do not exclude the possibility that UL131 is sufficient for gH/gL ER export.
Secretion of sgH/gL complexes.
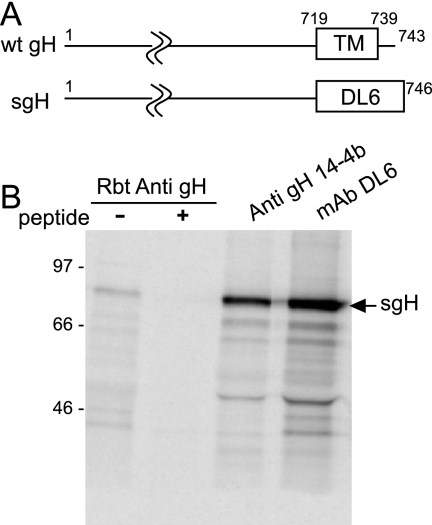
To characterize the transport of gH/gL/UL128-131 complexes by another approach, we analyzed the secretion of a soluble form of gH/gL. Of the gH, gL, UL128, UL130, and UL131 proteins, only gH is predicted to be anchored in membranes. Therefore, we replaced the transmembrane and cytoplasm domains of gH with an epitope tag (for anti-HSV gD MAb, DL6) and expressed the protein (designated sgH) by use of an Ad vector (Fig. 7A). sgH was radiolabeled in U373 cells and immunoprecipitated with (i) a gH-specific antipeptide antibody; (ii) anti-gH MAb 14-4b, which is specific for a conformational epitope, not recognized in Western blots (6); and (iii) MAb DL6, specific for the epitope tag (Fig. 7B).
FIG. 7.
Construction and expression of sgH. (A) Schematic representation of the HCMV gH protein. Indicated are transmembrane (TM) and cytoplasmic (CT) coding (amino acids 719 to 743) sequences. In sgH, both the TM and CT domains were replaced with a 27-amino-acid sequence derived from HSV type 1 gD that is recognized by MAb DL6. wt, wild type. (B) sgH was expressed in U373 cells and radiolabeled with [35S]methionine-cysteine, and cell extract was analyzed by immunoprecipitation with rabbit (Rbt) anti-gH peptide sera with and without the gH peptide anti-gH MAb 14-4b or MAb DL6.
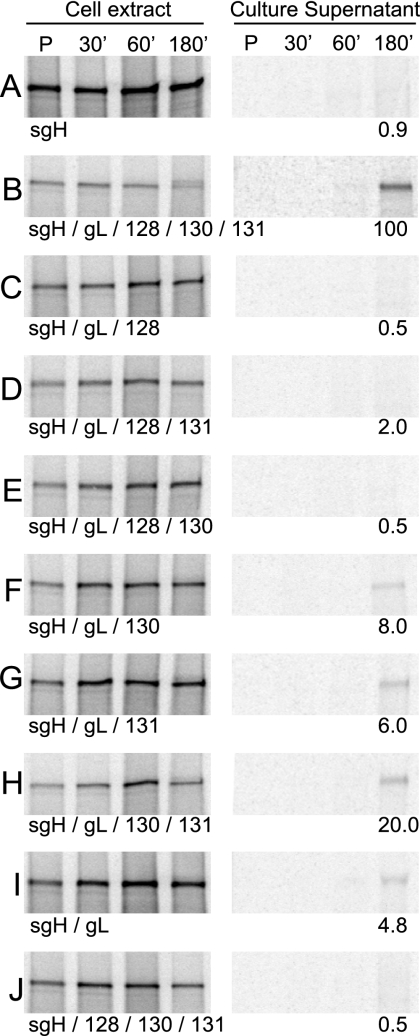
U373 cells infected with various combinations of sgH, gL, and UL128-131 were radiolabeled in a pulse-chase format, and sgH complexes were immunoprecipitated from either the culture medium or cell extracts (Fig. 8). Consistent with previous data (31, 56), sgH alone was not secreted, and the presence of gL promoted the secretion of some sgH (Fig. 8A and I). However, when gL, UL128, UL130, and UL131 were all coexpressed, sgH was very efficiently secreted compared to what was seen for sgH/gL alone (Fig. 8B and I). When any one of UL128, UL130, or UL131 was omitted, secretion of sgH was dramatically reduced compared with what was seen for sgH/gL/UL128/130/131 (Fig. 8C through H). Of particular interest were conditions in which UL128 was present but UL130, UL131, or both were absent (Fig. 8C, D, and E). In these conditions, the secretion of sgH was similar to what was seen for sgH alone. Conversely, when only UL128 was lacking, sgH was secreted with approximately 20% efficiency (Fig. 8H). Furthermore, the presence of UL128, UL130, and UL131 did not alleviate the necessity of gL for the secretion of sgH (Fig. 8J), again highlighting the gH/gL heterodimer as the foundation for these complexes. These data support the hypothesis that all three of UL128, UL130, and UL131 are required for the efficient intracellular transport of gH/gL complexes beyond the ER when gO is not present.
FIG. 8.
Secretion of sgH coexpressed with gL, UL128, UL130, and UL131 proteins. U373 cells were infected with Ad vectors expressing sgH together with different combinations of gL, UL128, UL130, and UL131. Cells were pulse-labeled (P) with [35S]methionine-cysteine for 15 min, and radioactivity was chased for 30, 60, or 180 min. sgH was immunoprecipitated from cell extracts or culture supernatants by using MAb DL6 and analyzed by SDS-PAGE under reducing conditions. The amount of sgH detected in culture supernatants under each condition was determined and normalized to the value obtained when all five proteins were expressed (panel B).
Interactions between pairs of proteins.
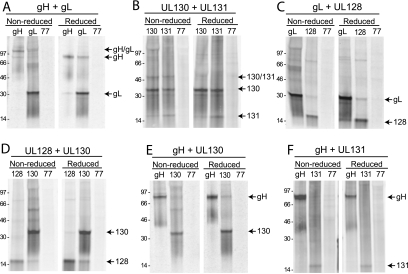
To address the organization of the gH/gL/UL128-131 complex, i.e., which proteins bind to which other proteins, pairs of proteins were expressed using Ad vectors. Cells were metabolically labeled and analyzed by coimmunoprecipitation followed by SDS-PAGE under reducing or nonreducing conditions. HCMV gH and gL form a disulfide-linked heterodimer (31, 56), and this complex served as the benchmark for these analyses. Both anti-gH and anti-gL antibodies immunoprecipitated a protein species that migrated at 120 kDa under nonreducing conditions (Fig. 9A). Under reducing conditions, the 120-kDa protein disappeared, and only 90-kDa gH and 30-kDa gL were observed, confirming the formation of a disulfide-linked gH/gL heterodimer in this expression system.
FIG. 9.
Coimmunoprecipitation analysis of interactions between pairs of gH, gL, UL128, UL130, and UL131 proteins. Pairs of HCMV proteins (indicated in the text above the panels) were expressed from Ad vectors in U373 cells. The cells were labeled with [35S]methionine-cysteine, cell extracts were made using 0.5% NP-40 lysis buffer, and then HCMV proteins were immunoprecipitated with appropriate antibodies (as indicated at the tops of the panels) or with an irrelevant antibody (anti-UL77) followed by SDS-PAGE under reducing or nonreducing conditions.
Similar analyses were performed for the other nine pairs of gH, gL, and UL128-131 proteins. When UL131 and UL130 were coexpressed, both UL130 and UL131 antibodies precipitated bands of 35 and 18 kDa, corresponding to UL130 and UL131 under reducing conditions. Under nonreducing conditions, an additional band of approximately 50 kDa was observed with both anti-UL130 and anti-UL131 antibodies, consistent with the predicted size of a UL131-UL130 dimer. Since the 50-kDa band was detected only under nonreducing conditions, these data support a disulfide interaction between UL130 and UL131. (Fig. 9B). For gL and UL128, gL was precipitated by anti-UL128 antibodies and UL128 was precipitated by anti-gL antibodies, and neither protein was precipitated by an irrelevant antibody (Fig. 9C). Since the same bands were observed under both reducing and nonreducing gel conditions, these results suggest a noncovalent interaction between gL and UL128. A noncovalent interaction was also observed between gH and UL130, where anti-UL130 antibodies precipitated gH but an irrelevant antibody did not (Fig. 9E, especially reduced lanes). In this case, however, there was no obvious UL130 precipitated by anti-gH antibodies. These results are similar to those reported for gH/gL complexes, where a very low amount of the smaller gL protein was observed compared with what was seen for the larger gH protein (31). gH is also larger and contains more methionines and cysteines than UL130. Thus, we would expect that the amount of radioactive label in the UL130 that coprecipitated with gH would be small compared to that in the gH band. Similarly, anti-UL128 antibodies precipitated a small amount of UL130 (Fig. 9D, especially reduced lanes). There was more clearly visible UL128 precipitated by anti-UL130 antibodies (Fig. 9D). Again, the quantity of radioactivity in UL128 is lower given its smaller size. Other interactions between different proteins were not detected, either because detergents disrupted protein-protein interactions or because the proteins do not contact one another. For example, there was no obvious UL131 precipitated with anti-gH or vice versa (Fig. 9F). Interestingly, although we could not detect a direct interaction between UL131 and either gH or gL independently, UL131 was immunoprecipitated by anti-gH antibodies when both gH and gL were expressed (Fig. 5H, lane 2). Therefore, it appears that UL131 binds to a surface created by the dimerization of gH/gL. These data are summarized schematically in Fig. 10.
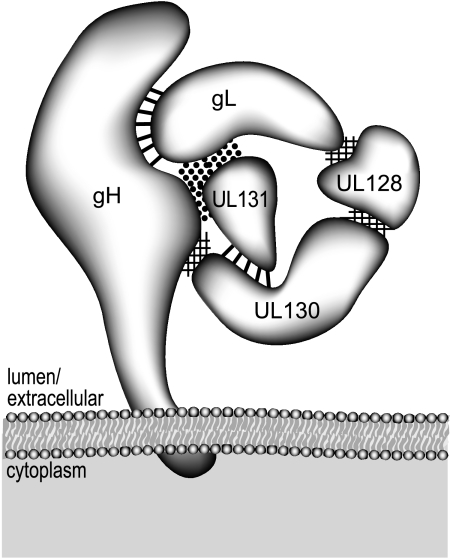
FIG. 10.
Model for the protein-protein interactions that make up the gH/gL/UL128/UL130/UL131 complex. The HCMV gH protein is predicted to be inserted in the virion envelope and interacts with gL through disulfide linkage(s) (indicated by straight dark lines). Interactions between UL130 and UL131 also involve a disulfide bond(s). UL128 interacts with both UL130 and gL through noncovalent interactions (indicated by cross-hatching), and a surface created by both gH and gL is required for UL131 binding (indicated by dark dots).
DISCUSSION
Previous reports have indicated that the UL128, UL130, and UL131 proteins bind in some manner to gH/gL to form complexes that mediate entry into epithelial and endothelial cells and are distinct from the gH/gL/gO complex, which may mediate entry into fibroblasts (2, 17, 48, 51, 61, 62). There may be analogies with the dual receptor-binding gH/gL complexes of EBV and human herpesvirus 6 (7, 41). An important aspect of this model that had not been addressed was whether gH/gL and UL128-131 assemble to form one complex consisting of all five proteins or whether gH/gL assemble with subsets of UL128, UL130, and UL131 to form several different complexes. We reasoned that any complex of gH/gL and UL128-131 that is important for entry into epithelial and endothelial cells must be assembled in the ER and transported to sites of virus envelopment, which are likely derived from the Golgi apparatus (23, 52).
The transport of membrane protein complexes from the ER to the Golgi apparatus and then on to cell surfaces or other compartments is intimately tied to or dependent upon proper protein folding, posttranslational modifications, and assembly into multiprotein complexes. These processes involve the activity of ER chaperones such as BiP, calnexin, and protein disulfide isomerase, which recognize immaturity in the form of exposed hydrophobic surfaces or specific oligosaccharide structures and help to promote folding and assembly (39). When any single component of a multiprotein complex is missing or misfolded, the entire complex is frequently retained in the ER or is degraded by ER-associated degradation.
Based on this understanding, we analyzed the intracellular transport of gH/gL complexes in cells infected with HCMV mutants lacking UL128-150 or just UL131 and found that approximately half of the gH was highly endo H sensitive compared to what was seen for cells infected with wild-type TR, where the majority of gH was endo H resistant. The high endo H sensitivity of gH produced in TRΔ4- and TRΔ131-infected cells reflected ER-retained gH/gL and could be discerned by comparing the electrophoretic mobility of this species to that of the gH produced in pulse samples as shown in Fig. 6. Together, these results strongly support our conclusion that approximately half of the gH/gL complexes in HCMV-infected cells require the incorporation of the UL128-131 proteins for efficient export from the ER. The loss of UL131 was as deleterious as the loss of all of UL128-131 in this respect, suggesting either that all of the UL128-131 proteins are required or that UL131 by itself is sufficient to facilitate the transport of gH/gL. The population of gH/gL that acquired endo H resistance in TRΔ4- and TRΔ131-infected cells likely represents the formation of gH/gL/gO complexes that also occurs in these HCMV-infected cells, and gH/gL/gO can clearly reach the Golgi apparatus (24). It might be somewhat surprising that such a large fraction of gH remained endo H sensitive even when gO was expressed. It is possible that the quantities of gO and UL128-131 expressed in HCMV-infected cells are limiting. However, comparing these results to those involving Ad vectors (where very little gH attained endo H resistance without UL128-131), gO can clearly function to promote the export of some gH/gL out of the ER. In these experiments, UL130 behaved differently from gH, remaining entirely endo H sensitive in HCMV-infected cells lacking UL131. This is consistent with a larger complex containing gH/gL and UL128-131 proteins which, when produced without UL131, is retained in the ER. These results also fit with previous observations that UL130 is retained in the ER when expressed in the absence of other HCMV proteins (48).
To examine the effects of UL128, UL130, and UL131 on the intracellular transport of gH/gL complexes without the complicating effects of gO, we used Ad vectors to express these proteins. When gH/gL was expressed in the absence of all the UL128-131 proteins, or without UL131 or UL130 and UL131, most of the gH remained endo H sensitive, whereas in the presence of all three of UL128-131, the majority of gH was endo H resistant. This left open the possibility that UL131 was by itself sufficient for gH/gL ER export. However, the lack of any one of UL128, UL130, or UL131 dramatically reduced the secretion of soluble gH/gL. Together, these experiments strongly support the hypothesis that gH/gL complexes containing all of UL128, UL130, and UL131 must be assembled in the ER before there is export to the Golgi apparatus and sites of virus assembly. Complexes lacking any one of the UL128-131 proteins are inefficiently exported from the ER and are therefore unlikely to be incorporated into the virion. Consistent with this, Wang and Shenk showed that AD169, which is effectively a UL131-null mutant, fails to incorporate UL128 and UL130 into virions (62).
Prior to the discovery of UL128-131 in clinical strains, the transport of gH to cell surfaces was observed with the coexpression of gL alone (31, 56), although immunofluorescence studies indicated that the majority of HCMV gH remained in cytoplasmic vesicles (31). More recently, gH/gL was expressed without gO (or UL128-131)-mediated cell-cell fusion, again indicating some transport to cell surfaces (33). Our results indicate that the transport of gH/gL out of the ER is inefficient in the absence of UL128-131. However, it is possible that gH/gL is more efficiently exported from the ER in transfected CHO and BHK cells or that there are compensatory mutations in AD169 gH or gL that promote more-efficient intracellular transport.
UL128-131, gO, and gL clearly promote ER export and may affect gH/gL folding but should not be considered as chaperones. Unlike chaperones, these proteins remain bound onto gH, are incorporated into the virion envelope, and are required for function. Supporting this view, Cairns et al. recently described HSV gH mutants that were transported to Golgi compartments and incorporated into virions in the absence of gL but still failed to function in entry, consistent with HSV gH/gL functioning as one protein (9). Thus, gH/gL complexes might be better described as hetero-oligomers.
The expression of gH/gL and UL128-131 proteins by use of Ad vectors allowed an analysis of protein-protein interactions. In these experiments, the interaction between gH and gL was not affected by the addition of any combination of UL128, UL130, or UL131, suggesting that the UL128-131 proteins are assembled onto a gH/gL core. This is consistent with the observation that gO is added subsequent to the formation of gH/gL dimers and with reports about other herpesvirus gH/gL complexes (24, 27, 32, 38). Additionally, UL128, UL130, and UL131 could each individually bind to gH/gL, indicating that there are independent binding sites for each of the UL128-131 proteins on gH, gL, or gH/gL. This hypothesis was supported by analyses of interactions between of pairs of gH, gL, UL128, UL130, and UL131. We observed direct, noncovalent interactions between UL128 and gL and between UL130 and gH (Fig. 10). By contrast, UL131 did not bind detectably to either gH or gL; thus, the binding site for UL131 apparently involves a surface created when gH and gL complex to each other. Moreover, UL130 and UL131 formed a disulfide-linked dimer that might strengthen UL131 binding to gH/gL. Interestingly, the binding of certain of the UL128-131 proteins to gH/gL affected the binding of others. For example, UL128 binding onto gH/gL dramatically enhanced the binding of UL130 and UL131. One interpretation of these results is that UL128 stabilizes interactions between a disulfide-linked UL130/131 heterodimer and gH/gL (Fig. 10). However, the binding of UL128 to gH/gL complexes was reduced by 50% when UL130/UL131 was present. This might indicate that there are multiple UL128 binding sites on gH/gL, some of which are occluded by UL130/131. Importantly, gO might compete with UL128-131 for gH/gL binding at any of these sites, affecting the assembly and transport of gH/gL in HCMV-infected cells.
The stoichiometry of the gH/gL/UL128-131 complex is not yet defined. Kirschner et al. were able to show that the EBV gH/gL/gp42 complex is formed in a 1:1:1 ratio (35). However, the small size of the UL128 and UL131 proteins has made attempts at analyzing the stoichiometry of gH/gL/UL128-131 complexes by gel filtration or in cross-linking experiments difficult. For example, we chemically cross-linked soluble gH/gL/UL128-131 complexes and observed bands consistent with a pentamer (≈190 kDa) (results not shown). However, given the sizes of UL128 and UL131 (15 to 17 kDa), it was impossible to determine whether there were one or two copies of these proteins present in putative pentameric bands.
In summary, our results support the hypothesis that the complex that facilitates entry into epithelial and endothelial cells is one comprised of gH/gL and all of UL128, UL130, and UL131. One interesting issue that remains to be investigated is the influence of the gH/gL/gO and gH/gL/UL128/UL130/UL131 complexes on one another. One could imagine a situation in which the timing and magnitude of expression of gO versus UL128-131 might vary in different cell types and thereby skew the ratio of the two complexes that are incorporated into the virion envelope. Related to this is the apparent strong selective disadvantage for maintaining expression of UL128-131 during the propagation of HCMV in fibroblasts. Together with the identification of receptors, understanding the assembly of the different gH/gL complexes will help clarify the tropisms of HCMV for different cells.
Acknowledgments
This work was supported by National Institutes of Health grants AI73996 and EY11245 (to D.C.J.) and AI21640 (to J.A.N. and M.A.J.). B.J.R. was supported by a Ruth L. Kirschstein National Research Service Award from the NEI (EY015965).
We are very grateful to Gary Cohen, Roselyn Eisenberg, Tom Shenk, and William Britt for supplying important monoclonal antibodies and to Don Court (National Cancer Institute, Frederick, MD) for the galK mutagenesis system. We also thank Tiffani Howard for computer graphics and Chris Langford for help with online manuscript submission. Finally, we appreciate the support and advice of all the members of the Johnson and Nelson laboratories.
Footnotes
Published ahead of print on 17 October 2007.
REFERENCES
- 1.Adam, E., J. L. Melnick, and M. E. DeBakey. 1997. Cytomegalovirus infection and atherosclerosis. Cent. Eur. J. Public Health 599-106. [PubMed] [Google Scholar]
- 2.Adler, B., L. Scrivano, Z. Ruzcics, B. Rupp, C. Sinzger, and U. Koszinowski. 2006. Role of human cytomegalovirus UL131A in cell type-specific virus entry and release. J. Gen. Virol. 872451-2460. [DOI] [PubMed] [Google Scholar]
- 3.Akkapaiboon, P., Y. Mori, T. Sadaoka, S. Yonemoto, and K. Yamanishi. 2004. Intracellular processing of human herpesvirus 6 glycoproteins Q1 and Q2 into tetrameric complexes expressed on the viral envelope. J. Virol. 787969-7983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akter, P., C. Cunningham, B. P. McSharry, A. Dolan, C. Addison, D. J. Dargan, A. F. Hassan-Walker, V. C. Emery, P. D. Griffiths, G. W. Wilkinson, and A. J. Davison. 2003. Two novel spliced genes in human cytomegalovirus. J. Gen. Virol. 841117-1122. [DOI] [PubMed] [Google Scholar]
- 5.Bissinger, A. L., C. Sinzger, E. Kaiserling, and G. Jahn. 2002. Human cytomegalovirus as a direct pathogen: correlation of multiorgan involvement and cell distribution with clinical and pathological findings in a case of congenital inclusion disease. J. Med. Virol. 67200-206. [DOI] [PubMed] [Google Scholar]
- 6.Bogner, E., M. Reschke, B. Reis, E. Reis, W. Britt, and K. Radsak. 1992. Recognition of compartmentalized intracellular analogs of glycoprotein H of human cytomegalovirus. Arch. Virol. 12667-80. [DOI] [PubMed] [Google Scholar]
- 7.Borza, C. M., and L. M. Hutt-Fletcher. 2002. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nat. Med. 8594-599. [DOI] [PubMed] [Google Scholar]
- 8.Britt, W. J., and C. A. Alford. 1996. Cytomegaloviruses, p. 2493-2523. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Press, Philadelphia, PA.
- 9.Cairns, T. M., L. S. Friedman, H. Lou, J. C. Whitbeck, M. S. Shaner, G. H. Cohen, and R. J. Eisenberg. 2007. N-terminal mutants of herpes simplex virus type 2 gH are transported without gL but require gL for function. J. Virol. 815102-5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cha, T., E. Tom, G. Kemble, G. Duke, E. Mocarski, and R. Spaete. 1996. Human cytomegalovirus clinical isolates carry at least 19 genes not found in laboratory strains. J. Virol. 7078-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chee, M., S. A. Rudolph, B. Plachter, B. Barrell, and G. Jahn. 1989. Identification of the major capsid protein gene of human cytomegalovirus. J. Virol. 631345-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Compton, T., R. R. Nepomuceno, and D. M. Nowlin. 1992. Human cytomegalovirus penetrates host cells by pH-independent fusion at the cell surface. Virology 191387-395. [DOI] [PubMed] [Google Scholar]
- 13.Dolan, A., C. Cunningham, R. D. Hector, A. F. Hassan-Walker, L. Lee, C. Addison, D. J. Dargan, D. J. McGeoch, D. Gatherer, V. C. Emery, P. D. Griffiths, C. Sinzger, B. P. McSharry, G. W. G. Wilkinson, and A. J. Davison. 2004. Genetic content of wild-type human cytomegalovirus. J. Gen. Virol. 851301-1312. [DOI] [PubMed] [Google Scholar]
- 14.Dunn, W., C. Chou, L. Hong, R. Hai, D. Patterson, V. Stolc, H. Zhu, and F. Liu. 2003. Functional profiling of a human cytomegalovirus genome. Proc. Natl. Acad. Sci. USA 10014223-14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epstein, S. E., Y. F. Zhou, and J. Zhu. 1999. Potential role of cytomegalovirus in the pathogenesis of restenosis and atherosclerosis. Am. Heart J. 138S476-S478. [DOI] [PubMed] [Google Scholar]
- 16.Gianni, T., P. L. Martelli, R. Casadio, and G. Campadelli-Fiume. 2005. The ectodomain of herpes simplex virus glycoprotein H contains a membrane alpha-helix with attributes of an internal fusion peptide, positionally conserved in the Herpesviridae family. J. Virol. 792931-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hahn, G., M. G. Revello, M. Patrone, E. Percivalle, G. Campanini, A. Sarasini, M. Wagner, A. Gallina, G. Milanesi, U. Koszinowski, F. Baldanti, and G. Gerna. 2004. Human cytomegalovirus UL131-128 genes are indispensable for virus growth in endothelial cells and virus transfer to leukocytes. J. Virol. 7810023-10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardy, S., M. Kitamura, T. Harris-Stansil, Y. Dai, and M. L. Phipps. 1997. Construction of adenovirus vectors through Cre-lox recombination. J. Virol. 711842-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harlow, E., and D. Lane. 1999. Using antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 20.Hegde, N. R., M. S. Chevalier, T. W. Wisner, M. C. Denton, K. Shire, L. Frappier, and D. C. Johnson. 2006. The role of BiP in endoplasmic reticulum-associated degradation of major histocompatibility complex class I heavy chain induced by cytomegalovirus proteins. J. Biol. Chem. 28120910-20919. [DOI] [PubMed] [Google Scholar]
- 21.Hegde, N. R., and D. C. Johnson. 2003. Human cytomegalovirus US2 causes similar effects on both major histocompatibility complex class I and II proteins in epithelial and glial cells. J. Virol. 779287-9294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hobom, U., W. Brune, M. Messerle, G. Hahn, and U. H. Koszinowski. 2000. Fast screening procedures for random transposon libraries of cloned herpesvirus genomes: mutational analysis of human cytomegalovirus envelope glycoprotein genes. J. Virol. 747720-7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Homman-Loudiyi, M., K. Hultenby, W. Britt, and C. Soderberg-Naucler. 2003. Envelopment of human cytomegalovirus occurs by budding into Golgi-derived vacuole compartments positive for gB, Rab 3, trans-Golgi network 46, and mannosidase II. J. Virol. 773191-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huber, M. T., and T. Compton. 1999. Intracellular formation and processing of the heterotrimeric gH-gL-gO (gCIII) glycoprotein envelope complex of human cytomegalovirus. J. Virol. 733886-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huber, M. T., and T. Compton. 1998. The human cytomegalovirus UL74 gene encodes the third component of the glycoprotein H-glycoprotein L-containing envelope complex. J. Virol. 728191-8197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huber, M. T., T. W. Wisner, N. R. Hegde, K. A. Goldsmith, D. A. Rauch, R. J. Roller, C. Krummenacher, R. J. Eisenberg, G. H. Cohen, and D. C. Johnson. 2001. Herpes simplex virus with highly reduced gD levels can efficiently enter and spread between human keratinocytes. J. Virol. 7510309-10318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hutchinson, L., H. Browne, V. Wargent, N. Davis-Poynter, S. Primorac, K. Goldsmith, A. C. Minson, and D. C. Johnson. 1992. A novel herpes simplex virus glycoprotein, gL, forms a complex with glycoprotein H (gH) and affects normal folding and surface expression of gH. J. Virol. 662240-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hutt-Fletcher, L. M. 2007. Epstein-Barr virus entry. J. Virol. 817825-7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isola, V. J., R. J. Eisenberg, G. R. Siebert, C. J. Heilman, W. C. Wilcox, and G. H. Cohen. 1989. Fine mapping of antigenic site II of herpes simplex virus glycoprotein D. J. Virol. 632325-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson, D. C., and P. G. Spear. 1983. O-linked oligosaccharides are acquired by herpes simplex virus glycoproteins in the Golgi apparatus. Cell 32987-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaye, J. F., U. A. Gompels, and A. C. Minson. 1992. Glycoprotein H of human cytomegalovirus (HCMV) forms a stable complex with the HCMV UL115 gene product. J. Gen. Virol. 732693-2698. [DOI] [PubMed] [Google Scholar]
- 32.Khattar, S. K., S. van Drunen Littel-van den Harke, S. K. Attah-Poku, L. A. Babiuk, and S. K. Tikoo. 1996. Identification and characterization of a bovine herpesvirus-1 (BHV-1) glycoprotein gL which is required for proper antigenicity, processing, and transport of BHV-1 glycoprotein gH. Virology 21966-76. [DOI] [PubMed] [Google Scholar]
- 33.Kinzler, E. R., and T. Compton. 2005. Characterization of human cytomegalovirus glycoprotein-induced cell-cell fusion. J. Virol. 797827-7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kinzler, E. R., R. N. Theiler, and T. Compton. 2002. Expression and reconstitution of the gH/gL/gO complex of human cytomegalovirus. J. Clin. Virol. 25(Suppl. 2)S87-S95. [DOI] [PubMed] [Google Scholar]
- 35.Kirschner, A. N., J. Omerovic, B. Popov, R. Longnecker, and T. S. Jardetzky. 2006. Soluble Epstein-Barr virus glycoproteins gH, gL, and gp42 form a 1:1:1 stable complex that acts like soluble gp42 in B-cell fusion but not in epithelial cell fusion. J. Virol. 809444-9454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Landolfo, S., M. Gariglio, G. Gribaudo, and D. Lembo. 2003. The human cytomegalovirus. Pharmacol. Ther. 98269-297. [DOI] [PubMed] [Google Scholar]
- 37.Li, L., J. A. Nelson, and W. J. Britt. 1997. Glycoprotein H-related complexes of human cytomegalovirus: identification of a third protein in the gCIII complex. J. Virol. 713090-3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li, Q., C. Buranathai, C. Grose, and L. M. Hutt-Fletcher. 1997. Chaperone functions common to nonhomologous Epstein-Barr virus gL and varicella-zoster virus gL proteins. J. Virol. 711667-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma, Y., and L. M. Hendershot. 2004. ER chaperone functions during normal and stress conditions. J. Chem. Neuroanat. 2851-65. [DOI] [PubMed] [Google Scholar]
- 40.Milne, R. S., A. V. Nicola, J. C. Whitbeck, R. J. Eisenberg, and G. H. Cohen. 2005. Glycoprotein D receptor-dependent, low-pH-independent endocytic entry of herpes simplex virus type 1. J. Virol. 796655-6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mori, Y., P. Akkapaiboon, S. Yonemoto, M. Koike, M. Takemoto, T. Sadaoka, Y. Sasamoto, S. Konishi, Y. Uchiyama, and K. Yamanishi. 2004. Discovery of a second form of tripartite complex containing gH-gL of human herpesvirus 6 and observations on CD46. J. Virol. 784609-4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murphy, E., D. Yu, J. Grimwood, J. Schmutz, M. Dickson, M. A. Jarvis, G. Hahn, J. A. Nelson, R. M. Myers, and T. E. Shenk. 2003. Coding potential of laboratory and clinical strains of human cytomegalovirus. Proc. Natl. Acad. Sci. USA 10014976-14981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mutimer, H. P., Y. Akatsuka, T. Manley, E. L. Chuang, M. Boeckh, R. Harrington, T. Jones, and S. R. Riddell. 2002. Association between immune recovery uveitis and a diverse intraocular cytomegalovirus-specific cytotoxic T cell response. J. Infect. Dis. 186701-705. [DOI] [PubMed] [Google Scholar]
- 44.Nicola, A. V., J. Hou, E. O. Major, and S. E. Straus. 2005. Herpes simplex virus type 1 enters human epidermal keratinocytes, but not neurons, via a pH-dependent endocytic pathway. J. Virol. 797609-7616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nicola, A. V., A. M. McEvoy, and S. E. Straus. 2003. Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells. J. Virol. 775324-5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parks, R. J., L. Chen, M. Anton, U. Sankar, M. A. Rudnicki, and F. L. Graham. 1996. A helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc. Natl. Acad. Sci. USA 9313565-13570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parry, C., S. Bell, T. Minson, and H. Browne. 2005. Herpes simplex virus type 1 glycoprotein H binds to alphavbeta3 integrins. J. Gen. Virol. 867-10. [DOI] [PubMed] [Google Scholar]
- 48.Patrone, M., M. Secchi, L. Fiorina, M. Ierardi, G. Milanesi, and A. Gallina. 2005. Human cytomegalovirus UL130 protein promotes endothelial cell infection through a producer cell modification of the virion. J. Virol. 798361-8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Plachter, B., C. Sinzger, and G. Jahn. 1996. Cell types involved in replication and distribution of human cytomegalovirus. Adv. Virus Res. 46195-261. [DOI] [PubMed] [Google Scholar]
- 50.Robinson, M. R., G. Reed, K. G. Csaky, M. A. Polis, and S. M. Whitcup. 2000. Immune-recovery uveitis in patients with cytomegalovirus retinitis taking highly active antiretroviral therapy. Am. J. Ophthalmol. 13049-56. [DOI] [PubMed] [Google Scholar]
- 51.Ryckman, B. J., M. A. Jarvis, D. D. Drummond, J. A. Nelson, and D. C. Johnson. 2006. Human cytomegalovirus entry into epithelial and endothelial cells depends on genes UL128 to UL150 and occurs by endocytosis and low-pH fusion. J. Virol. 80710-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanchez, V., K. D. Greis, E. Sztul, and W. J. Britt. 2000. Accumulation of virion tegument and envelope proteins in a stable cytoplasmic compartment during human cytomegalovirus replication: characterization of a potential site of virus assembly. J. Virol. 74975-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sinzger, C., M. Kahl, K. Laib, K. Klingel, P. Rieger, B. Plachter, and G. Jahn. 2000. Tropism of human cytomegalovirus for endothelial cells is determined by a post-entry step dependent on efficient translocation to the nucleus. J. Gen. Virol. 813021-3035. [DOI] [PubMed] [Google Scholar]
- 54.Sinzger, C., K. Schmidt, J. Knapp, M. Kahl, R. Beck, J. Waldman, H. Hebart, H. Einsele, and G. Jahn. 1999. Modification of human cytomegalovirus tropism through propagation in vitro is associated with changes in the viral genome. J. Gen. Virol. 802867-2877. [DOI] [PubMed] [Google Scholar]
- 55.Smith, I. L., I. Taskintuna, F. M. Rahhal, H. C. Powell, E. Ai, A. J. Mueller, S. A. Spector, and W. R. Freeman. 1998. Clinical failure of CMV retinitis with intravitreal cidofovir is associated with antiviral resistance. Arch. Ophthalmol. 116178-185. [DOI] [PubMed] [Google Scholar]
- 56.Spaete, R. R., K. Perot, P. I. Scott, J. A. Nelson, M. F. Stinski, and C. Pachl. 1993. Coexpression of truncated human cytomegalovirus gH with the UL115 gene product or the truncated human fibroblast growth factor receptor results in transport of gH to the cell surface. Virology 193853-861. [DOI] [PubMed] [Google Scholar]
- 57.Spear, P. G., and R. Longnecker. 2003. Herpesvirus entry: an update. J. Virol. 7710179-10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Springer, K. L., and A. Weinberg. 2004. Cytomegalovirus infection in the era of HAART: fewer reactivations and more immunity. J. Antimicrob. Chemother. 54582-586. [DOI] [PubMed] [Google Scholar]
- 59.Subramanian, R. P., and R. J. Geraghty. 2007. Herpes simplex virus type 1 mediates fusion through a hemifusion intermediate by sequential activity of glycoproteins D, H, L, and B. Proc. Natl. Acad. Sci. USA 1042903-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tomazin, R., J. Boname, N. R. Hegde, D. M. Lewinsohn, Y. Altschuler, T. R. Jones, P. Cresswell, J. A. Nelson, S. R. Riddell, and D. C. Johnson. 1999. Cytomegalovirus US2 destroys two components of the MHC class II pathway, preventing recognition by CD4+ T cells. Nat. Med. 51039-1043. [DOI] [PubMed] [Google Scholar]
- 61.Wang, D., and T. Shenk. 2005. Human cytomegalovirus UL131 open reading frame is required for epithelial cell tropism. J. Virol. 7910330-10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang, D., and T. Shenk. 2005. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc. Natl. Acad. Sci. USA 10218153-18158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang, X., D. Y. Huang, S. M. Huong, and E. S. Huang. 2005. Integrin alphavbeta3 is a coreceptor for human cytomegalovirus. Nat. Med. 11515-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Warming, S., N. Costantino, D. L. Court, N. A. Jenkins, and N. G. Copeland. 2005. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 33e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wenske, E. A., M. W. Bratton, and R. J. Courtney. 1982. Endo-beta-N-acetylglucosaminidase H sensitivity of precursors to herpes simplex virus type 1 glycoproteins gB and gC. J. Virol. 44241-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wisner, T., C. Brunetti, K. Dingwell, and D. C. Johnson. 2000. The extracellular domain of herpes simplex virus gE is sufficient for accumulation at cell junctions but not for cell-to-cell spread. J. Virol. 742278-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]