Abstract
Adenoviral infections in the immunocompromised host are associated with significant morbidity and mortality. Although the adoptive transfer of adenovirus-specific T cells may prevent and treat such infections, the T-cell immune response to the multiplicity of adenovirus serotypes and subspecies that infect humans has not been well characterized, impeding the development of such approaches. We have, therefore, analyzed the specificities of T-cell responses to the viral capsid hexon antigen, since this structure is highly conserved in human pathogens. We screened 25 human cytotoxic T-cell lines with adenovirus specificity to extensively characterize their responses to adenoviral hexon and to identify a panel of novel CD4+ and CD8+ T-cell epitopes. Using a peptide library spanning the entire sequence of the hexon protein, we confirmed the responsiveness of these cytotoxic T-cell lines to seven peptides described previously and also identified 33 new CD4- or CD8-restricted hexon epitopes. Importantly, the majority of these epitopes were shared among different adenovirus subspecies, suggesting that T cells with such specificities could recognize and be protective against multiple serotypes, simplifying the task of effective adoptive transfer or vaccine-based immunotherapy for treating infection by this virus.
Adenovirus (Adv) infections are associated with significant morbidity and high mortality rates in the immunocompromised human host (12, 14, 19). The current preemptive or prophylactic pharmacotherapy is ineffective, so there is interest in developing immunity-based approaches. Treatment of Epstein-Barr virus (EBV) and cytomegalovirus (CMV) diseases in immunocompromised patients has been accomplished with adoptively transferred virus-specific T cells (24, 34), but Adv is a greater challenge due to the multiplicity of different adenoviral serotypes and subspecies that can cause disease in humans. Many of the expressed antigens that are potential T-cell targets are highly polymorphic, and preparing discrete cytotoxic T lymphocyte (CTL) lines that could recognize every species for each patient is impractical. Fortunately, however, regions of the capsid protein hexon are well conserved among serotypes and species, and there are increasingly strong preclinical and clinical data to show that CTLs directed to hexon are indeed protective (3, 4, 8, 13, 15, 16, 20, 33).
Although hexon is both a conserved and an immunodominant T-cell target antigen, it has been difficult to date to take full advantage of these characteristics. Only eight CD8+ epitopes from hexon have been identified, presented in the context of HLA-A1, HLA-A2 (three epitopes), HLA-A24, HLA-B7 (two epitopes), and HLA-B13/49 (16, 31); CD4+ T-cell reactivity is even less well characterized, with one HLA-DP (30) and four HLA-DR-restricted epitopes identified (8, 30). If we could identify a broader panel of hexon epitopes, the task of immunotherapy would be simplified. We would be able to derive reagents such as multimers and peptides that would allow characterizing and tracking adoptively transferred Adv-specific T cells. We would also be able to design multipeptide- or multiepitope-based vaccines that would generate both CD4 and CD8 responses with the potential to protect patients irrespective of their HLA backgrounds and with a reduced risk of viral escape resulting from epitope mutation.
We therefore screened 26 Adv-specific CTL lines from healthy donors with diverse HLA phenotypes, using a library of 20-mer peptides covering the entire hexon protein and overlapping by 15 amino acids (aa), and thereby identified both CD4+ and CD8+ T-cell epitopes. We discovered 5 new hexon-derived HLA class I epitopes as well as 28 new class II-restricted epitopes.
MATERIALS AND METHODS
CTL lines.
CTL lines are defined as polyclonal antigen-specific T-cell lines containing both CD4+ and CD8+ cells. The lines studied were prepared from stem cell donors who gave informed consent upon enrollment in our clinical trials of virus-specific T cells for the treatment of CMV infections and Adv-associated diseases (13). All protocols were approved by the Baylor College of Medicine institutional review boards and the National Marrow Donor Program. For the purposes of this analysis, we have characterized 26 of these CTL lines.
Adv-specific cell lines.
Details of cell line preparation have been described previously (13). In brief, we transduced 5 × 107 donor peripheral blood mononuclear cells (PBMC) with an Adv vector (Ad5f35pp65 or Ad5f35 null) at a multiplicity of infection of 1,000 or 200 virus particles, as described previously (13). Starting on day 9 posttransduction, the cells were restimulated weekly (for 2 to 4 weeks) with an irradiated EBV-lymphoblastoid cell line (LCL) transduced with the same vector used to initiate the culture, at a responder/stimulator ratio of 4:1. After a total of 3 or 4 stimulations, the CTLs were cryopreserved, and the antigen specificity of each CTL line was analyzed with a standard 4-h chromium-51 release assay and an enzyme-linked immunospot (ELISPOT) assay. All cell lines demonstrated specific cytolytic activity (data not shown). ELISPOT analysis of gamma interferon (IFN-γ) production was used to determine the frequency of peptide-responsive Adv-specific T cells (11, 16, 29). Plates were sent for evaluation to Zellnet Consulting. Spot-forming cells (SFC) and input-cell numbers were plotted.
Hexon peptides.
We purchased a peptide library covering the complete sequence of hexon (Adv serotype 5), which consisted of 188 20-mer peptides overlapping by 15 amino acids (Alta Bioscience, University of Birmingham, Edgbaston, Birmingham, United Kingdom). Lyophilized peptides were reconstituted with 5 mg/ml dimethyl sulfoxide and pooled in a total of 11 pools: pools 1 to 10 each contained 17 contiguous peptides, while pool 11 contained the remaining 18 peptides. These 11 hexon pools were used for the initial screening. To determine the 20 mer(s) containing the stimulating peptide, CTL lines were screened against individual peptides contained within the positive pools. To identify the minimal epitope sequences, additional shorter peptides were obtained from Genemed Synthesis, Inc. (South San Francisco, CA) and reconstituted with 10 mg/ml dimethyl sulfoxide. Aliquots of peptides were stored at −80°C.
Multimer staining.
To detect peptide-specific T cells in the CTL lines and the PBMC, we used the soluble unlabeled pentamers HLA-B*3501-MPNRPNYIAF, HLA-A*2401-TYFSLNNKF, HLA-A1-TDLGQNLLY, and HLA-B*0702-KPYSGTAYNAL (all prepared by Proimmune, Inc.) or the phycoerythrin (PE)-conjugated tetramers HLA-B*3501-IPYLDGTFY, HLA-B53-IPYLDGTFY, HLA-A*0201-GLRYRSMLL, and HLA B53-LPGSYTYEW (constructed by MHC Tetramer Core Laboratory, Houston, TX). Peptides were synthesized by the Baylor College of Medicine Protein Core Facility or by Genemed Synthesis, Inc. (San Antonio, TX). We stained CTLs (5 × 105 cells) with multimers as described previously (6, 13). Briefly, for pentamer staining, we incubated CTLs with unlabeled pentamer, followed by Pro5 Flurotag (PE-conjugated) (Proimmune, Inc.) according to the manufacturer's instructions. We used tetramers at a 1:100 final dilution. After the cells were incubated for 30 min at 4°C in the dark, we washed the cells twice and then fixed and analyzed them. From each sample, we acquired 100,000 live events.
Determining HLA restriction of identified epitopes.
To confirm the HLA restriction of the newly identified T-cell epitopes, CD4+ and CD8+ T cells were isolated by using a cell sorter (MoFlo cell sorter; DAKO) or by magnetic cell-sorting-positive (autoMACS; Miltenyi Biotec, Germany) selection columns, according to the manufacturers' instructions, and were used as the responders in an IFN-γ ELISPOT assay. The isolated T-cell fractions were stimulated using the stimulating 20-mer peptides, which were identified as immunogenic in the initial CTL screening experiments. IFN-γ release was detected in either the CD4+ or the CD8+ T-cell fraction, identifying the stimulating peptides as either HLA class I restricted or class II restricted.
Identifying the minimal epitope peptides and the restricting allele of novel CD8+ T-cell epitopes.
Minimal CD8+ T-cell epitopes were identified by IFN-γ ELISPOT using isolated CD8+ T cells as responders. These CD8+ T cells were plated at 1 × 103 to 1 × 104 cells/well and then stimulated with 1 μg/ml individual 9-mer peptides, which overlapped each other by 8 aa and spanned the original 20-mer peptide(s) recognized by the CTL line. Only one 9-mer peptide was capable of stimulating CD8+ T cells to produce IFN-γ, thereby identifying the minimal epitope peptide. HLA restriction was identified by using autologous LCLs and a panel of partially matched allogeneic EBV-transformed LCLs as antigen-presenting cells (APCs) in an IFN-γ ELISPOT assay. The LCLs were loaded with 1 μg/ml minimal epitope peptide for 1 h, excess peptide was washed off, and then these peptide-loaded LCLs were used as stimulator cells in the ELISPOT assay at 1 × 105 cells/well, with LCL alone as a negative control. Isolated CD8+ T cells were plated at 1 × 103 to 1 × 104 cells/well as responders. Only the autologous peptide-loaded LCL and the allogeneic LCLs that shared the restricting HLA type were capable of stimulating T cells, thereby allowing the identification of the HLA-restricting molecule.
RESULTS
Magnitude and specificity of Adv reactivity within patient CTL lines.
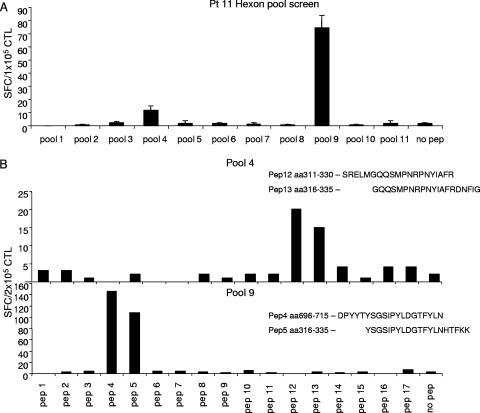
To map the Adv epitope specificity of the CTL lines, we used an overlapping peptide library representing the entire sequence of the Adv serotype 5 hexon protein, which we and others have previously shown is responsible for the majority of T-cell reactivity to the Ad5f35 vector (7, 16). For the initial screening, CTL lines were stimulated by overnight incubation with the 188 overlapping hexon peptides, divided into 11 pools, containing 17 or 18 peptides per pool. Hexon-specific reactivity was detected in 25/26 CTL lines. An example of the screening process is shown in Fig. 1A, in which a CTL line generated from donor 11 reacted against pools 4 and 9. Subsequent screening with each of the 20-mer peptides contained in these pools is shown in Fig. 1B, where the CTL reactivity in pool 4 could be mapped to overlapping peptides 12 (aa 311 to 330, SRELMGQQSMPNRPNYIAFR) and 13 (aa 316 to 335, GQQSMPNRPNYIAFRDNFIG), while the CTL reactivity directed against pool 9 could be mapped to overlapping peptides 4 (aa 696 to 715, DPYYTYSGSIPYLDGTFYLN) and 5 (aa 701 to 720, YSGSIPYLDGTFYLNHTFKK).
FIG. 1.
Specificity of an Adv-specific CTL line for patient 11. Adv-specific CTL lines were screened by an ELISPOT assay against 11 pools of hexon peptides (20-mer overlapping by 15 aa) to identify which peptide pools were being recognized by each CTL line. (A) The CTL line generated for patient 11 showed specific IFN-γ release against peptide pools 4 and 9. Results are expressed as numbers of spot-forming cells (SFC)/1 × 105 CTL. (B) To identify the stimulating 20-mer peptides, the CTL line for patient 11 was rescreened against the individual peptides contained in pools 4 and 9. The CTL reactivity against pool 4 mapped to the overlapping peptides 12 (SRELMGQQSMPNRPNYIAFR) and 13 (GQQSMPNRPNYIAFRDNFIG), while the reactivity in pool 9 mapped to peptides 4 and 5. Results are expressed as numbers of SFC/2 × 105 CTL.
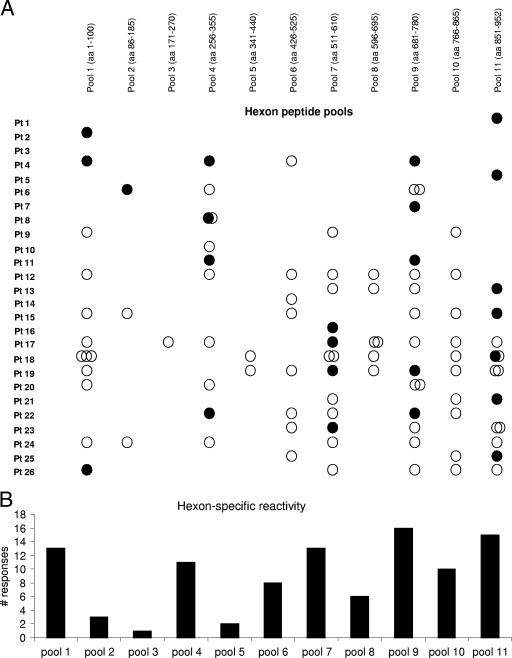
Results from the Adv hexon peptide pool screening carried out on all 26 CTL lines are shown in Fig. 2A. Seven of the 26 CTL lines had reactivity against only one epitope, whereas the remaining 18 CTL lines recognized between 2 and 10 different epitopes. The pools most frequently recognized were 9 and 11, but all pools were recognized by at least one CTL line (Fig. 2B). In total, 98 responses were detected in the 26 CTL lines (Fig. 2A), and the reactivity was mediated by both CD4+ and CD8+ T cells. Overall, 24/98 (24%) responses were CD8 restricted (Fig. 2A, filled circles), and 74/98 (76%) were CD4 restricted (Fig. 2A, open circles). Eleven of the 24 CD8 responses were targeted toward previously described epitopes, including the peptides HLA-A24-TYFSLNNKF (aa 37 to 45), contained in pool 1; the peptides HLA-B7-KPYSGTAYNAL (aa 114 to 124), contained in pool 2; and the peptides HLA-A1-TDLGQNLLY (aa 886 to 894) and HLA-A2-YVLFEVFDV (aa 917 to 925), both contained in pool 11.
FIG. 2.
Hexon reactivity detected in 26 CTL lines. (A) Adv hexon-specific CD4+ (○) and CD8+ (•) T-cell reactivity detected in 26 CTL lines. Pt, patient. (B) The frequency of responses detected in each CTL line directed against each of the 11 hexon peptide pools is shown.
CD4+ T-cell target epitopes are mostly hexon derived.
The majority of T-cell responses detected in the CTL lines were directed against CD4+ T-cell epitopes; however, it is difficult to determine the minimal sequences and the restriction patterns of CD4+ T-cell epitopes, since they are often promiscuously presented in the context of multiple HLA class II alleles. Hence, we mapped these CD4 responses to the stimulatory 20-mer peptides, but the epitopes were not minimized. A list of the immunogenic CD4 peptides is shown in Table 1. We identified a total of 31 CD4+ T-cell epitopes, only three of which have been described previously (20, 30). A number of these hexon-specific CD4+ T-cell epitopes were recognized by multiple CTL lines, including aa 556 to 580 (VPFHIQVPQKFFAIKNLLLLPGSYT), which was recognized by six different lines, whereas reactivity against 15 of these peptides was detected in only one CTL line, suggesting that there is a hierarchy of immunodominance among the identified CD4+ T-cell epitopes.
TABLE 1.
Immunogenic CD4 peptides
| aa positions | Sequence | No. of responders |
|---|---|---|
| 11-20 | SYMHISGQDA | 1 |
| 16-40 | SGQDASEYLSPGLVQFARATETYFS | 1 |
| 31-55 | FARATETYFSLNNKFRNPTVAPTHD | 1 |
| 56-80 | VTTDRSQRLTLRFIPVDREDTAYSY | 5 |
| 71-95 | VDREDTAYSYKARFTLAVGDNRVLD | 2 |
| 111-135 | PTFKPYSGTAYNALAPKGAPNPCEW | 2 |
| 201-225 | QPEPQIGESQWYETEINHAAGRVLK | 1 |
| 316-335 | GQQSMPNRPNYIAFRDNFIG | 6 |
| 391-415 | VDSYDPDVRIIENHGTEDELPNYCF | 1 |
| 396-420 | PDVRIIENHGTEDELPNYCFPLGGV | 1 |
| 456-475 | NNFAMEINLNANLWRNFLYS | 2 |
| 466-490 | ANLWRNFLYSNIALYLPDKLKYSPS | 3 |
| 501-510 | YDYMNKRVVA | 1 |
| 521-545 | GARWSLDYMDNVNPFNHHRNAGLRY | 1 |
| 556-580 | VPFHIQVPQKFFAIKNLLLLPGSYT | 6 |
| 566-590 | FFAIKNLLLLPGSYTYEWNFRKDVN | 1 |
| 591-615 | MVLQSSLGNDLRVDGASIKFDSICL | 1 |
| 646-670 | YLSAANMLYPIPANATNVPISIPSR | 3 |
| 661-680 | TNVPISIPSRNWAAFRGWAF | 2 |
| 671-680 | NWAAFRGWAF | 2 |
| 691-715 | LGSGYDPYYTYSGSIPYLDGTFYLN | 6 |
| 706-715 | PYLDGTFYLN | 1 |
| 711-730 | TFYLNHTFKKVAITFDSSVS | 1 |
| 736-760 | RLLTPNEFEIKRSVDGEGYNVAQCN | 1 |
| 756-775 | VAQCNMTKDWFLVQMLANYN | 2 |
| 786-810 | SYKDRMYSFFRNFQPMSRQVVDDTK | 2 |
| 791-815 | MYSFFRNFQPMSRQVVDDTKYKDYQ | 6 |
| 811-835 | YKDYQQVGILHQHNNSGFVGYLAPT | 2 |
| 856-880 | VDSITQKKFLCDRTLWRIPFSSNFM | 1 |
| 861-885 | QKKFLCDRTLWRIPFSSNFMSMGAL | 1 |
| 906-930 | EVDPMDEPTLLYVLFEVFDVVRVHRPHR | 6 |
Identification of novel CD8+ T-cell epitopes.
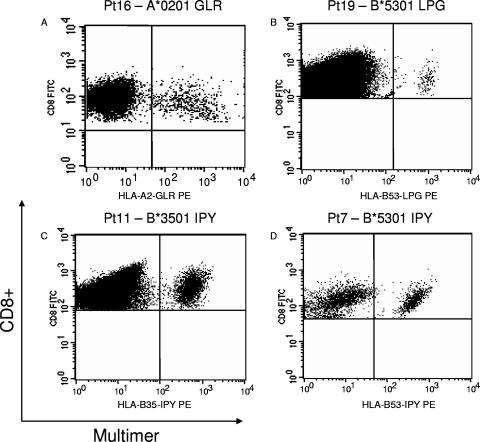
We next sought to identify the minimal CD8+ T-cell epitopes that were detected using the overlapping 20-mer library in order to allow the subsequent generation of multimers. CD8 reactivity against pool 7 was detected in four CTL lines from donors 16, 17, 19, and 23. Using the same screening process shown in Fig. 1, we found that the CTL from patients (pts.) 16, 17, and 23 responded to the overlapping 20-mer peptides (aa 536 to 555, NHHRNAGLRYRSMLLGNGRY; and aa 541 to 560, AGLRYRSMLLGNGRYVPFHI). Therefore, we synthesized the 15-mer peptide spanning the overlapping region (aa 541 to 555, AGLRYRSMLLGNGRY) as well as a panel of 9-mer peptides overlapping by 8 aa, starting from aa 541 to 549 (AGLRYRSML) to aa 548 to 556 (MLLGNGRYV), and we found that the minimal peptide recognized by all of the CTL lines was the 9-mer peptide from aa 542 to 550 (GLRYRSMLL), presented in the context of HLA-A2 (data not shown). In contrast, patient 19 responded to the overlapping peptides from aa 566 to 585 (FFAIKNLLLLPGSYTYEWNF) and aa 571 to 590 (NLLLLPGSYTYEWNFRKDVN), and the mimimal epitope peptide mapped to aa 575 to 583 (LPGSYTYEW), presented in the context of HLA-B53. Staining of the CTL lines from patients 16 and 19 with the GLR and the LPG tetramers is shown in Fig. 3A and B, respectively.
FIG. 3.
Tetramer analysis of Adv-specific CTL lines. (A) The CTL line for patient (pt) 16 was incubated with the HLA-A2 GLR tetramer. (B) Staining of pt 19 CTL line with the HLA-B53 LPG tetramer. (C) Staining of pt 11 CTL line with the HLA-B35 IPY tetramer. (D) Staining of pt 7 CTL line with the HLA-B53 IPY tetramer.
Using a similar approach, we identified the CD8 epitope in pool 9, which was recognized by CTL lines from pts. 4, 7, 11, 19, and 22. The overlapping 20-mer peptides (aa 696 to 715, DPYYTYSGSIPYLDGTFYLN; and aa 701 to 720, YSGSIPYLDGTFYLNHTFKK) from pool 9 were recognized by all five lines. To identify the minimal epitope peptide, we synthesized the 15-mer peptide spanning the overlapping region (aa 701 to 715, YSGSIPYLDGTFYLN), as well as a panel of 9-mer peptides starting from aa 700 to 709 (TYSGSIPYLT) to aa 708 to 721 (LDGTFYLNH), and we found that the minimal peptide recognized by all the CTL lines was the 9-mer peptide from aa 705 to 713 (IPYLDGTFY). We found that no single HLA-A or HLA-B allele was shared by all; however, three donors carried the HLA-B35 allele, while the other two donors carried HLA-B53. Since HLA-B35 and HLA-B53 are closely related alleles and both have a preference for a proline residue at position 2 and a tyrosine at the C-terminal position, we examined whether the IPY peptide was being recognized in the context of both HLA-B35 and HLA-B53. We initially generated an HLA-B35 tetramer and used it to stain CTL lines from pts. 4, 11, and 23, all of whom carried the HLA-B35 allele, and we were able to identify a tetramer-positive population in all three lines (Fig. 3C). When we folded the same peptide in the context of the HLA-B53 allele and used it to stain the CTL lines from pts. 7 and 20, who carried the HLA-B53 allele, we were also able to identify a tetramer-positive population (Fig. 3D). Therefore, the IPY peptide (aa 705 to 713) is immunogenic when it is presented in the context of either HLA-B35 or HLA-B53. This phenomenon has been previously reported for the human papillomavirus (HPV) peptide (aa 407 to 417) derived from the EBV-associated EBNA1 protein.
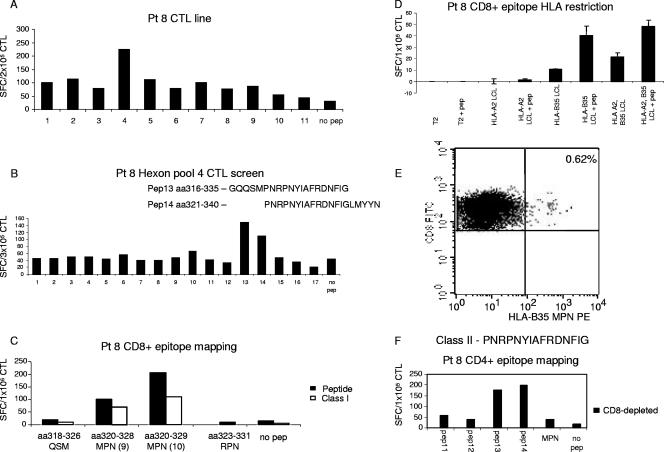
Finally, we mapped the CD8+ T-cell reactivity against pool 4 in the CTL lines from donors 4, 8, 11, and 22 (Fig. 4A). The individual 20-mer screening showed that all four lines specifically recognized peptides 13 (aa 316 to 335, GQQSMPNRPNYIAFRDNFIG) and 14 (aa 321 to 340, PNRPNYIAFRDNFIGLMYYN). The screening results from patient 8 are shown in Fig. 4B. Interestingly, we found that these two peptides, spanning aa 316 to 340, stimulated both CD4+ and CD8+ T cells from donor 8, whereas only the CD8+ T cells from pts. 4, 11, and 22 reacted to these peptides (data not shown). Thus, the CTL from donor 8 recognized an overlapping CD4 and CD8 T-cell epitope. To identify the minimal CD8+ T-cell epitope recognized by the CTL lines from all four donors, we generated a panel of short 9-mer or 10-mer peptides spanning this region and used them to stimulate isolated CD8+ T cells in the presence or absence of a major histocompatibility complex class I (MHC-I) blocking antibody. The peptides we synthesized were aa 318 to 326 (QSMPNRPNY), aa 320 to 328 (MPNRPNYIA [9 mer]), aa 320 to 329 (MPNRPNYIAF [10 mer]), and aa 323 to 331 (RPNYIAFRD). Only the 9-mer and 10-mer MPN peptides induced CD8+ T cells to produce IFN-γ in an ELISPOT assay, an effect abrogated by the addition of MHC-I blocking antibody. The 10-mer MPN peptide (aa 320 to 329) was the minimal epitope, since this epitope stimulated the maximum number of reactive T cells to produce IFN-γ. Results from donor 8 are shown in Fig. 4C. To identify the HLA restriction of this peptide epitope, we used partially matched LCL lines pulsed with the 10-mer MPN peptide as APCs in an ELISPOT assay (Fig. 4D). T cells secreted IFN-γ upon stimulation with peptide-pulsed APCs matched for the HLA-B35 allele but not HLA-A2. HLA-B35 restriction was confirmed by the identification of a T-cell population within the CTL line from donor 8, which stained positive with the HLA-B35-MPNRPNYIAF pentamer (Fig. 4E). All of the CTL lines from donors 4, 11, and 22 that also demonstrated CD8+ T-cell reactivity against pool 4 carried the HLA-B35 allele and showed reactivity against the minimal MPN peptide by ELISPOT and pentamer staining (data not shown). Thus, we were successful in identifying the minimal epitope peptides for all five novel CD8+ T-cell responses detected in the current study, and the resultant multimers were able to specifically bind to antigen-specific T cells. These reagents will provide important tools for CTL characterization and immunomonitoring studies.
FIG. 4.
Overlapping CD4+ and CD8+ T-cell epitopes. (A) The CTL line generated for patient (pt) 8 showed specific IFN-γ release against peptide pool 4. Results are expressed as numbers of SFC/2 × 105 CTL. (B) To identify the stimulating 20-mer peptides, the CTL line for pt 8 was rescreened against the individual peptides contained in pool 4. The CTL reactivity against pool 4 mapped to the overlapping peptides 13 (GQQSMPNRPNYIAFRDNFIG) and 14 (PNRPNYIAFRDNFIGLMYYN). Results are expressed as numbers of SFC/3 × 105 CTL. (C) To identify the minimal CD8+ T-cell epitope, the CTL was screened against a panel of shorter peptides, and the 10-mer MPN peptide (aa 320 to 329) was identified as the minimal epitope that stimulated the maximum number of reactive T cells to produce IFN-γ. This effect was blocked by the addition of an MHC-I blocking antibody. (D) To identify the HLA restriction of the CD8-restricted MPN peptide, partially matched LCL lines were pulsed with this peptide and used as APCs in an ELISPOT assay. Only lines that were HLA-B35-matched LCL lines were capable of inducing IFN-γ secretion. (E) The CTL line for pt 8 was stained with the HLA-B35 MPN tetramer, and 0.62% of the CD8+ T cells were tetramer positive. (F) CD8-depleted CTL from pt 8 responded to PNRPNYIAFRDNFIG (aa 321 to 335) but showed no reactivity against the minimal CD8+ MPN peptide. Results are expressed as numbers of SFC/1 × 105 CTL.
Identification of overlapping CD4+ and CD8+ T-cell epitopes.
Five CD8+ T-cell epitopes (HLA-A24 TYF, HLA-B35 MPN, HLA-B35 LPG, HLA-B35/53 IPY, and HLA-A2 YVL) were found to overlap directly with CD4+ T-cell epitopes (aa 31 to 55, FAR; aa321 to 335, PNP; aa 566 to 590, FFA; aa 691 to 715, LGS; and aa 906 to 930, EVD). An example is shown in Fig. 4F. The CTL line from donor 8 was found to respond to an overlapping CD4/CD8 epitope. The CD8+ T-cell epitope MPN was presented to T cells in the context of HLA-B35 (Fig. 4E); however, the overlapping CD4+ T-cell epitope that stimulated CTL reactivity mapped to PNRPNYIAFRDNFIG (aa 321 to 335), the region of overlap between peptides 13 (aa 316 to 335) and 14 (aa 321 to 340) (data not shown). There was no recognition of the minimal CD8+ T-cell epitope MPN peptide (aa 320 to 329) by isolated CD4+ T cells (Fig. 4F). Thus, five of the immunogenic peptides are capable of stimulating both CD4+ and CD8+ T-cell reactivity and are ideal candidates for vaccination strategies aiming to stimulate a polyclonal antigen-specific T-cell response in vivo.
Conserved regions of the hexon protein stimulate both CD4+ and CD8+ T cells.
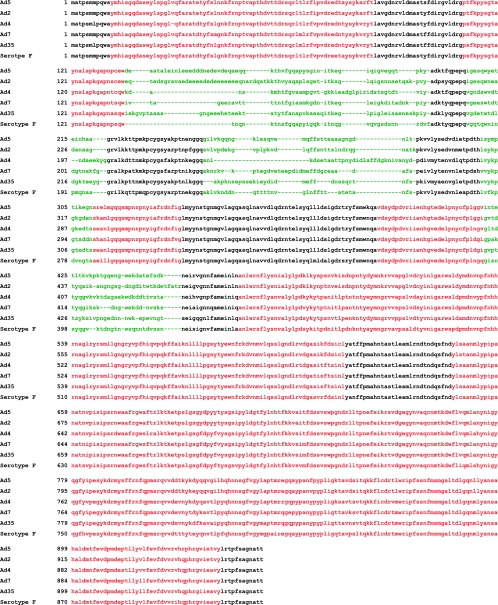
The mapping of hexon-specific T-cell reactivity in these 26 CTL lines identified immunogenic regions of the hexon protein. To determine the conservation of the epitopes identified among Adv serotypes, we compared the sequences of hexons from Adv serotype 5 (species C), serotype 2 (species C), serotype 4 (species E), serotype 7 (species B), serotype 35 (species B), and species F. By highlighting the conserved regions and the hypervariable regions among the different serotypes and species and the locations of both the CD4+ T-cell epitopes and the CD8+ T-cell epitopes, we determined that the cellular immune response targeting hexon is directed against regions of the molecule which are highly conserved through different Adv serotypes and species (Fig. 5).
FIG. 5.
Conservation of the novel Adv epitopes. Epitope alignment of sequences from the indicated Adv serotypes. Hypervariable regions are shown in green, and the conserved regions are shown in black, while the identified epitopes are highlighted in red.
DISCUSSION
Clinical trials of immunotherapy to prevent or treat the severe adenoviral infections that occur in the immunocompromised host (4) are hampered by a lack of identification of the target epitopes that are shared by a broad range of pathogenic adenoviruses. We have stimulated Adv-reactive CTL lines with an overlapping hexon peptide library, using IFN-γ secretion as the readout with which to evaluate CD4 and CD8 T-cell responses against the viral hexon protein and to map their specificities. These responses were detectable in 25/26 CTL lines, and the stimulating peptides identified 5 new HLA class I-restricted (CD8) and 28 new class II-restricted epitopes (Table 1). Moreover, five of these epitopes stimulated both CD4 and CD8 cells and may be particularly suited as vaccine antigens.
There were significantly fewer Adv epitope-specific responses detected in the trivirus-specific CTL lines generated using the Ad5f35pp65 (lines 1 to 13) vector than in the bivirus-specific CTL lines generated using the Ad5f35null (lines 13 to 26) vector. Thus, all 13 bivirus-specific CTL lines contained Adv reactivity, and 11 had reactivity against at least three distinct epitopes, while 1 trivirus-specific line did not exhibit a detectable Adv-specific component, and 5 reacted with only one Adv epitope. It is not clear if this was due to competition between the high- and low-frequency responder T cells or between pp65 and hexon peptides for HLA molecules on the stimulator cells. This situation is further complicated at the second and the subsequent stimulations when EBV epitopes are also competing with the Adv and CMV epitopes for HLA molecules. It has previously been demonstrated that high-affinity T cells can outgrow low-affinity T cells, particularly when a common APC is stimulating the responses (9, 10). Nevertheless, in our clinical trial, the infusion of the CTL line for patient 2, which reacted with only one CD8+ epitope, was associated with a rapid Adv clearance and resolution of Adv disease, suggesting that CTL lines with a wide breadth of reactivity are not crucial for in vivo efficacy (13).
Adenovirus is a lytic DNA virus that expresses a number of potential target antigens. However, in order to activate an effective and protective serotype-cross-reactive immune response, the target antigen(s) must be conserved among the different serotypes. Mouse models indicated that the well-conserved immediate-early proteins E1A (22, 25, 26) and E2A (17, 23) were strong targets for specific T cells. Unfortunately, this observation did not hold true for humans, in whom virion proteins, particularly hexon, are stronger targets for antigen-specific T cells (3, 5, 15, 16, 28). While penton and fiber elements show significant heterogeneity among types and species, the viral capsid hexon contains regions which are highly conserved among the different serotypes (16, 27). Data from our group and those of other groups had suggested that the cellular immune response directed against this protein is highly cross-reactive and specifically targeted against these conserved regions (8, 30, 31). The current more extensive analysis confirms that the immunogenic epitope peptides lie in the conserved regions of the hexon protein rather than in the hypervariable regions, which are frequently the targets of serotype-specific antibody responses (Fig. 5). Thus, hexon is a potent target for antigen-specific T cells and, as such, serves as an ideal target, both for vaccine protocols aimed at recruiting antigen-specific T cells in vivo and for adoptive immunotherapy using transfer of Adv-specific T cells.
The phenomenon of overlapping CD4+ and CD8+ T-cell epitopes has previously been described for the tumor setting where MART-1-specific CD8+ T cells recognize the HLA-A2-restricted peptide EAA (aa 26 to 35), which has direct sequence overlap with an HLA-DR11 epitope (aa 25 to 36), and this peptide has been widely used in the melanoma setting in vaccination protocols (1, 2). The results of our current study suggest that for active Adv vaccination strategies, the best candidate peptides are the five immunodominant peptides that contain an overlapping CD4 and CD8 T-cell epitope. Alternatively, since we have demonstrated that the conserved regions of hexon are immunogenic, an overlapping 30-mer peptide library spanning these regions could also be synthesized for use in a vaccination protocol (32, 33). For the generation of Adv-specific T cells for adoptive immunotherapy, the relevant CD8+ T-cell peptides (based on HLA restriction) can be combined with the immunodominant CD4+ T-cell peptides to activate specific T cells. Alternatively, for the vaccine strategy, a peptide library spanning the conserved regions of the hexon protein can be used to reactivate T cells in an HLA-unrestricted manner. Ultimately, the epitope peptides will also allow precise and extensive characterization of CTL lines prior to adoptive transfer, and for both vaccine and adoptive immunotherapy studies, these peptides will allow the immune response to be monitored using the specific immunogenic peptides as stimulators in immunoassays.
There is increasing evidence that an optimal antiadenoviral response requires the integration of both CD4+ and CD8+ T-cell responses (1, 2, 13, 18). Hence, irrespective of whether adoptive transfer or vaccination is the primary therapeutic strategy, it is likely that both CD4+ and CD8+ responses will be desired. In 13 of the 26 CTL lines screened in the current study, we found a mixture of CD4+ and CD8+ Adv-reactive T cells, though the breadth of the reactivity differed. Of the 19 lines containing an Adv-specific CD4+ T-cell component, only 4/19 recognized one single CD4+ T-cell epitope, whereas 14 of these lines had reactivity against ≥3 distinct CD4+ T-cell epitopes. In contrast, of the 19 CTL lines with a specific CD8+ T-cell component, 15 recognized a single CD8+ epitope. In total, nine CD8+ T-cell epitopes were recognized by the CTL lines screened in the current study, four of which had been previously published and five of which were novel epitopes. So, although we identified epitopes that stimulated either CD4+ or CD8+ cells, the immune response directed against the hexon protein seems to be dominated by CD4+ T cells directed against multiple MHC-II epitopes (8, 21, 30, 33), whereas CD8+ reactivity was less frequent and recognized fewer epitopes, presented by a more limited range of class I molecules. Nonetheless, five of the immunogenic peptides were able to stimulate both CD4+ and CD8+ T cells.
In summary, we have identified a panel of CD4+ and CD8+ T-cell epitopes which can be used in vaccination studies to prime antigen-specific T cells as well as to provide a source of antigen to challenge adoptively transferred T cells in vivo. These epitopes also provide us with valuable tools for immune monitoring prior to and following immunotherapeutic interventions.
Acknowledgments
We thank V. Torrano and Y. Lin for research coordination, T. Gotsolva and F. Jimenez for technical assistance, and T. Lopez and staff of the Good Manufacturing Practice facilities for assisting with CTL preparation and quality assurance.
This work was supported in part by a Production Assistance for Cellular Therapies grant (contract NO1HB37163-C001) from the National Heart, Lung, and Blood Institute (to C.M.R. and A.P.G.), a Specialized Centers for Cell-Based Therapy grant, NIH-NHLBI 1 U54 HL1081007, grants from the National Gene Vector Laboratories (NIH-NCRR U42 RR16578) and the General Clinical Research Center at Baylor College of Medicine (RR00188), and a Doris Duke Distinguished Clinical Scientist award (to H.E.H.), an Amy Strelzer Manasevit Scholar Award (to C.M.B.), and an award from the Dana Foundation (to C.M.R. and A.M.L.).
Footnotes
Published ahead of print on 17 October 2007.
REFERENCES
- 1.Bioley, G., C. Jandus, S. Tuyaerts, D. Rimoldi, W. W. Kwok, D. E. Speiser, J. M. Tiercy, K. Thielemans, J. C. Cerottini, and P. Romero. 2006. Melan-A/MART-1-specific CD4 T cells in melanoma patients: identification of new epitopes and ex vivo visualization of specific T cells by MHC class II tetramers. J. Immunol. 1776769-6779. [DOI] [PubMed] [Google Scholar]
- 2.Boon, T., P. G. Coulie, B. J. Van den Eynde, and P. van der Bruggen. 2006. Human T cell responses against melanoma. Annu. Rev. Immunol. 24175-208. [DOI] [PubMed] [Google Scholar]
- 3.Feuchtinger, T., P. Lang, K. Hamprecht, M. Schumm, J. Greil, G. Jahn, D. Niethammer, and H. Einsele. 2004. Isolation and expansion of human adenovirus-specific CD4+ and CD8+ T cells according to IFN-gamma secretion for adjuvant immunotherapy. Exp. Hematol. 32282-289. [DOI] [PubMed] [Google Scholar]
- 4.Feuchtinger, T., S. Matthes-Martin, C. Richard, T. Lion, M. Fuhrer, K. Hamprecht, R. Handgretinger, C. Peters, F. R. Schuster, R. Beck, M. Schumm, R. Lotfi, G. Jahn, and P. Lang. 2006. Safe adoptive transfer of virus-specific T-cell immunity for the treatment of systemic adenovirus infection after allogeneic stem cell transplantation. Br. J. Haematol. 13464-76. [DOI] [PubMed] [Google Scholar]
- 5.Flomenberg, P., V. Piaskowski, R. L. Truitt, and J. T. Casper. 1995. Characterization of human proliferative T cell responses to adenovirus. J. Infect. Dis. 1711090-1096. [DOI] [PubMed] [Google Scholar]
- 6.Foster, A. E., A. M. Leen, T. Lee, T. Okamura, A. Lu, J. Vera, R. Atkinson, C. M. Bollard, G. Dotti, and C. M. Rooney. 2007. Autologous designer antigen-presenting cells by gene modification of T lymphocyte blasts with IL-7 and IL-12. J. Immunother. (1997). 30506-516. [DOI] [PubMed] [Google Scholar]
- 7.Hamel, Y., N. Blake, S. Gabrielsson, T. Haigh, K. Jooss, C. Martinache, S. Caillat-Zucman, A. B. Rickinson, S. Hacein-Bey, A. Fischer, and M. Cavazzana-Calvo. 2002. Adenovirally transduced dendritic cells induce bispecific cytotoxic T lymphocyte responses against adenovirus and cytomegalovirus pp65 or against adenovirus and Epstein-Barr virus EBNA3C protein: a novel approach for immunotherapy. Hum. Gene Ther. 13855-866. [DOI] [PubMed] [Google Scholar]
- 8.Heemskerk, B., T. van Vreeswijk, L. A. Veltrop-Duits, C. C. Sombroek, K. Franken, R. M. Verhoosel, P. S. Hiemstra, D. van Leeuwen, M. E. Ressing, R. E. Toes, M. J. van Tol, and M. W. Schilham. 2006. Adenovirus-specific CD4+ T cell clones recognizing endogenous antigen inhibit viral replication in vitro through cognate interaction. J. Immunol. 1778851-8859. [DOI] [PubMed] [Google Scholar]
- 9.Kedl, R. M., W. A. Rees, D. A. Hildeman, B. Schaefer, T. Mitchell, J. Kappler, and P. Marrack. 2000. T cells compete for access to antigen-bearing antigen-presenting cells. J. Exp. Med. 1921105-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kedl, R. M., B. C. Schaefer, J. W. Kappler, and P. Marrack. 2002. T cells down-modulate peptide-MHC complexes on APCs in vivo. Nat. Immunol. 327-32. [DOI] [PubMed] [Google Scholar]
- 11.Kern, F., N. Faulhaber, C. Frommel, E. Khatamzas, S. Prosch, C. Schonemann, I. Kretzschmar, R. Volkmer-Engert, H. D. Volk, and P. Reinke. 2000. Analysis of CD8 T cell reactivity to cytomegalovirus using protein-spanning pools of overlapping pentadecapeptides. Eur. J. Immunol. 301676-1682. [DOI] [PubMed] [Google Scholar]
- 12.Kojaoghlanian, T., P. Flomenberg, and M. S. Horwitz. 2003. The impact of adenovirus infection on the immunocompromised host. Rev. Med. Virol. 13155-171. [DOI] [PubMed] [Google Scholar]
- 13.Leen, A. M., G. D. Myers, U. Sili, M. H. Huls, H. Weiss, K. S. Leung, G. Carrum, R. A. Krance, C. C. Chang, J. J. Molldrem, A. P. Gee, M. K. Brenner, H. E. Heslop, C. M. Rooney, and C. M. Bollard. 2006. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat. Med. 121160-1166. [DOI] [PubMed] [Google Scholar]
- 14.Leen, A. M., and C. M. Rooney. 2005. Adenovirus as an emerging pathogen in immunocompromised patients. Br. J. Haematol. 128135-144. [DOI] [PubMed] [Google Scholar]
- 15.Leen, A. M., U. Sili, B. Savoldo, A. M. Jewell, P. A. Piedra, M. K. Brenner, and C. M. Rooney. 2004. Fiber-modified adenoviruses generate subgroup cross-reactive, adenovirus-specific cytotoxic T lymphocytes for therapeutic applications. Blood 1031011-1019. [DOI] [PubMed] [Google Scholar]
- 16.Leen, A. M., U. Sili, E. F. Vanin, A. M. Jewell, W. Xie, D. Vignali, P. A. Piedra, M. K. Brenner, and C. M. Rooney. 2004. Conserved CTL epitopes on the adenovirus hexon protein expand subgroup cross-reactive and subgroup-specific CD8+ T cells. Blood 1042432-2440. [DOI] [PubMed] [Google Scholar]
- 17.Mulbacher, A., A. J. D. Bellett, and R. T. Hla. 1989. The murine cellular immune response to adenovirus type 5. Immunol. Cell Biol. 6731-39. [DOI] [PubMed] [Google Scholar]
- 18.Myers, G. D., C. M. Bollard, M. F. Wu, H. Weiss, C. M. Rooney, H. E. Heslop, and A. M. Leen. 2007. Reconstitution of adenovirus-specific cell-mediated immunity in pediatric patients after hematopoietic stem cell transplantation. Bone Marrow Transplant. 39677-686. [DOI] [PubMed] [Google Scholar]
- 19.Myers, G. D., R. A. Krance, H. Weiss, I. Kuehnle, G. Demmler, H. E. Heslop, and C. M. Bollard. 2005. Adenovirus infection rates in pediatric recipients of alternate donor allogeneic bone marrow transplants receiving either antithymocyte globulin (ATG) or alemtuzumab (Campath). Bone Marrow Transplant. 361001-1008. [DOI] [PubMed] [Google Scholar]
- 20.Olive, M., L. Eisenlohr, N. Flomenberg, S. Hsu, and P. Flomenberg. 2002. The adenovirus capsid protein hexon contains a highly conserved human CD4+ T-cell epitope. Hum. Gene Ther. 131167-1178. [DOI] [PubMed] [Google Scholar]
- 21.Olive, M., L. C. Eisenlohr, and P. Flomenberg. 2001. Quantitative analysis of adenovirus-specific CD4+ T-cell responses from healthy adults. Viral Immunol. 14403-413. [DOI] [PubMed] [Google Scholar]
- 22.Pereira, D. S., K. L. Rosenthal, and F. L. Graham. 1995. Identification of adenovirus E1A regions which affect MHC class I expression and susceptibility to cytotoxic T lymphocytes. Virology 211268-277. [DOI] [PubMed] [Google Scholar]
- 23.Rawle, F. C., B. B. Knowles, R. P. Ricciardi, V. Brahmacheri, P. Duerksen-Hughes, W. S. Wold, and L. R. Gooding. 1991. Specificity of the mouse cytotoxic T lymphocyte response to adenovirus 5. E1A is immunodominant in H-2b, but not in H-2d or H2k mice. J. Immunol. 1463977-3984. [PubMed] [Google Scholar]
- 24.Rooney, C. M., C. A. Smith, C. Ng, S. K. Loftin, C. Li, R. A. Krance, M. K. Brenner, and H. E. Heslop. 1995. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr virus-related lymphoproliferation. Lancet 3459-13. [DOI] [PubMed] [Google Scholar]
- 25.Routes, J. M., D. Bellgrau, W. J. McGrory, D. S. Bautista, F. L. Graham, and J. L. Cook. 1991. Anti-adenovirus type 5 cytotoxic T lymphocytes: immunodominant epitopes are encoded by the E1A gene. J. Virol. 651450-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Routes, J. M., B. A. Metz, and J. L. Cook. 1993. Endogenous expression of E1A in human cells enhances the effect of adenovirus E3 on class I major histocompatibility complex antigen expression. J. Virol. 673176-3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rux, J. J., P. Kuser, and R. M. Burnett. 2003. Structural and phylogenetic analysis of adenovirus hexons by use of high-resolution X-ray crystallographic, molecular modeling, and sequence-based methods. J. Virol. 779553-9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith, C. A., L. S. Woodruff, G. R. Kitchingman, and C. M. Rooney. 1996. Adenovirus-pulsed dendritic cells stimulate human virus-specific T-cell responses in vitro. J. Virol. 706733-6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Straathof, K. C., A. M. Leen, E. L. Buza, G. Taylor, M. H. Huls, H. E. Heslop, C. M. Rooney, and C. M. Bollard. 2005. Characterization of latent membrane protein 2 specificity in CTL lines from patients with EBV-positive nasopharyngeal carcinoma and lymphoma. J. Immunol. 1754137-4147. [DOI] [PubMed] [Google Scholar]
- 30.Tang, J., M. Olive, K. Champagne, N. Flomenberg, L. Eisenlohr, S. Hsu, and P. Flomenberg. 2004. Adenovirus hexon T-cell epitope is recognized by most adults and is restricted by HLA DP4, the most common class II allele. Gene Ther. 111408-1415. [DOI] [PubMed] [Google Scholar]
- 31.Tang, J., M. Olive, R. Pulmanausahakul, M. Schnell, N. Flomenberg, L. Eisenlohr, and P. Flomenberg. 2006. Human CD8+ cytotoxic T cell responses to adenovirus capsid proteins. Virology 350312-322. [DOI] [PubMed] [Google Scholar]
- 32.Vambutas, A., J. DeVoti, M. Nouri, J. W. Drijfhout, G. B. Lipford, V. R. Bonagura, S. H. van der Burg, and C. J. Melief. 2005. Therapeutic vaccination with papillomavirus E6 and E7 long peptides results in the control of both established virus-induced lesions and latently infected sites in a pre-clinical cottontail rabbit papillomavirus model. Vaccine 235271-5280. [DOI] [PubMed] [Google Scholar]
- 33.Veltrop-Duits, L. A., B. Heemskerk, C. C. Sombroek, T. van Vreeswijk, S. Gubbels, R. E. Toes, C. J. Melief, K. L. Franken, M. Havenga, M. J. van Tol, and M. W. Schilham. 2006. Human CD4+ T cells stimulated by conserved adenovirus 5 hexon peptides recognize cells infected with different species of human adenovirus. Eur. J. Immunol. 362410-2423. [DOI] [PubMed] [Google Scholar]
- 34.Walter, E. A., P. D. Greenberg, M. J. Gilbert, R. J. Finch, K. S. Watanabe, E. D. Thomas, and S. R. Riddell. 1995. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N. Engl. J. Med. 3331038-1044. [DOI] [PubMed] [Google Scholar]