Abstract
Infection of erythroid progenitor cells by Friend spleen focus-forming virus (SFFV) leads to acute erythroid hyperplasia and eventually to erythroleukemia in susceptible strains of mice. The viral envelope protein, SFFV gp55, forms a complex with the erythropoietin receptor (EpoR) and a short form of the receptor tyrosine kinase Stk (sf-Stk), activating both and inducing Epo-independent proliferation. Recently, we discovered that coexpression of SFFV gp55 and sf-Stk is sufficient to transform NIH 3T3 and primary fibroblasts. In the current study, we demonstrate that sf-Stk and its downstream effectors are critical to this transformation. Unlike SFFV-derived erythroleukemia cells, which depend on PU.1 expression for maintenance of the transformed phenotype, SFFV gp55-sf-Stk-transformed fibroblasts are negative for PU.1. Underscoring the importance of sf-Stk to fibroblast transformation, knockdown of sf-Stk abolished the ability of these cells to form anchorage-independent colonies. Like SFFV-infected erythroid cells, SFFV gp55-sf-Stk-transformed fibroblasts express high levels of phosphorylated MEK, ERK, phosphatidylinositol 3-kinase (PI3K), Gab1/2, Akt, Jun kinase (JNK), and STAT3, but unlike virus-infected erythroid cells they fail to express phosphorylated STATs 1 and 5, which may require involvement of the EpoR. In addition, the p38 mitogen-activated protein kinase (MAPK) stress response is suppressed in the transformed fibroblasts. Inhibition of either JNK or the PI3K pathway decreases both monolayer proliferation and anchorage-independent growth of the transformed fibroblasts as does the putative kinase inhibitor luteolin, but inhibition of p38 MAPK has no effect. Our results indicate that sf-Stk is a molecular endpoint of transformation that could be targeted directly or with agents against its downstream effectors.
Friend spleen focus-forming virus (SFFV) is a replication-incompetent murine retrovirus that causes a rapid erythroblastosis when injected into mice with its related helper virus Friend MuLV (reviewed in reference 30). Friend SFFV has deletions in its env gene, which give rise to a unique glycoprotein, SFFV gp55. This unique glycoprotein confers pathogenicity on the virus; a vector encoding SFFV gp55 alone is sufficient to induce erythroblastosis in susceptible strains of mice. The Fv-2 gene encodes one of the key susceptibility factors for SFFV-induced erythroid disease (10, 26). Fv-2, which encodes the receptor tyrosine kinase Stk/RON, is unusual in that it has a second internal promoter, which allows for the transcription of two distinct gene products: full-length Stk and a short form, sf-Stk (8, 26). Full-length Stk is a typical receptor tyrosine kinase with an extracellular ligand binding domain, a transmembrane domain, and an intracellular kinase/signaling domain. The short form lacks the extracellular ligand binding domain but retains the transmembrane domain and intracellular kinase/signaling domain. Susceptible strains of mice express both full-length Stk and sf-Stk, but resistant strains express only the full-length form of the kinase (26). The transcript for sf-Stk is rare in normal tissues and is expressed primarily in erythroid progenitors (8), the cells targeted by SFFV. Upon infection of erythroid progenitors with SFFV, the SFFV envelope protein gp55, sf-Stk, and the erythropoietin receptor (EpoR) form a constitutive signaling complex that induces Epo-independent proliferation and hyperplasia (21, 29).
While SFFV-infected primary erythroid cells can proliferate and differentiate independent of Epo, they are not transformed and cannot be cultured indefinitely. In order to obtain an SFFV-derived murine erythroleukemia (MEL) cell line capable of indefinite in vitro growth, infected primary erythroid cells must be serially passaged in vivo to allow for the outgrowth of cells that have undergone a proviral insertion event at the sfpi-1 locus (12, 13, 24, 25). Insertional activation of PU.1 results in changes that block differentiation of the cells even in the presence of Epo (32). Continuous expression of PU.1 is necessary for maintenance of the transformed phenotype of MEL cells (6, 16).
Several signaling pathways and molecules are activated downstream of the EpoR after it binds Epo (reviewed in reference 28), and many of these, such as the JAK/STAT, Ras/Raf/mitogen-activated protein kinase (MAPK), and phosphatidylinositol 3-kinase (PI3K)/Akt pathways, are constitutively activated in EpoR-expressing cells infected with Friend SFFV (14, 15, 19, 23). Initially it was thought that constitutive signals from the EpoR primarily drove SFFV-induced hyperplasia. However, our recent studies (17) demonstrating that coexpression of SFFV gp55 and sf-Stk can transform rodent fibroblasts, which do not express the EpoR, suggested that signals generated from sf-Stk could also play a role in SFFV-induced erythroleukemia. Thus, in the present study, we take advantage of fibroblasts transformed by SFFV gp55/sf-Stk to examine the role of SFFV gp55-activated sf-Stk and its downstream effectors in transformation in the absence of the EpoR. Our studies indicate that sf-Stk expression is required for maintenance of the transformed phenotype of SFFV gp55-expressing fibroblasts, and that PU.1, which is essential for transformation of erythroid cells by SFFV, plays no role in transformation of fibroblasts by SFFV gp55/sf-Stk. We further show that SFFV gp55-activated sf-Stk is capable of activating many, but not all, signal-transducing molecules activated by SFFV gp55 in erythroid cells, and that these transducers can be targeted with small-molecule inhibitors to modulate proliferation and/or transformed growth. Finally, we show that it may be possible to target sf-Stk directly with the flavonoid luteolin. Taken together, these results demonstrate that sf-Stk is a molecular endpoint of transformation that could be targeted directly or with agents against its downstream effectors. Because inappropriate expression of the human homologue of sf-Stk, sf-RON, has been reported in a number of human cancers (2, 3), our studies on SFFV-activated sf-Stk may have relevance for understanding and treating these diseases.
MATERIALS AND METHODS
Cell lines.
NIH 3T3, NIH 3T3/sf-Stk, and NIH 3T3/sf-Stk/SFFV cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum. For serum starvation conditions, cells were grown in DMEM without serum for 24 h. NIH 3T3/sf-Stk and NIH 3T3/sf-Stk/SFFV cells have been described previously (17). The SFFV MEL cell line NP7 (35) was maintained in DMEM supplemented with 10% fetal calf serum. The Epo-dependent erythroid cell line HCD-57 was maintained as previously described (31).
Protein analysis.
Cell lysates were prepared by resuspending cells in lysis buffer (1% Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate in phosphate-buffered saline) supplemented with 1.5× protease inhibitor cocktail set I (Calbiochem, La Jolla, CA) and 5 mM sodium orthovanadate, followed by vortexing and incubation on ice for 20 min. Insoluble materials were removed by centrifugation. Protein concentrations of the clarified lysates were estimated using the Bio-Rad assay (Bio-Rad Laboratories, Hercules, CA). Samples of 30 to 75 μg of protein were used, depending upon the experiment. Proteins were separated by electrophoresis on 8, 10, or 10 to 20% Tris glycine gels (Invitrogen, Carlsbad, CA) and then transferred to Immobilon-P membranes (Millipore, Billerica, MA) by the semidry method. Membranes to be assayed for phosphoproteins were blocked with 5% bovine serum albumin (Sigma-Aldrich Co., St. Louis, MO) in Tris-buffered saline with 0.1% Tween 20 (TBST). Other membranes were blocked with 5% dried milk (Carnation, Solon, OH) in TBST. Antibodies specific for the phosphorylated forms of MEK1, p44/42 MAPK, stress-activated protein kinase (SAPK)/JNK, Gab 1, Gab 2, Akt, STAT1, STAT3, STAT5, and p38 MAPK were obtained from Cell Signaling (Beverly, MA). Antibodies specific for total MEK1, p44/42 MAPK, SAPK/JNK, Gab 1, Gab 2, Akt, STAT1, STAT3, and p38 MAPK were also obtained from Cell Signaling. Antibodies specific for STAT5 A and B and PU.1 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Antisera specific for phosphatidylinositol 3-kinase (PI3K) and an antiphosphotyrosine antibody, 4G10, were obtained from Upstate Biotechnology (Lake Placid, NY). An antibody specific for β-tubulin was obtained from Sigma (St. Louis, MO). The polyclonal antiserum against sf-Stk and the monoclonal antibody against SFFV gp55 (7C10) have both been described previously (21, 34). Blots were stripped with 0.5 M NaOH for 15 min at room temperature and then washed twice with TBST before reblocking and reprobing. Immunoblotting experiments were performed two or more times and representative blots are shown in the figures.
shRNA plasmids.
A short hairpin RNA (shRNA) vector against sf-Stk was generated by inserting annealed paired oligonucleotides based on a sequence from the sf-Stk cDNA (3′-ACACTCTCTGACATCAACGATACA-5′) into the pGSU6-GFP vector using the GeneSilencer kit (Gene Therapy Systems, Inc., San Diego, CA). A luciferase shRNA vector, which served as a vector control, was included with the kit. Cells were transfected with the shRNA constructs using Lipofectamine 2000 (Invitrogen) following the manufacturer's instructions. Because the pGSU6-GFP vector includes a neomycin resistance cassette, stable cell lines were selected by both growth in G418-containing medium and by fluorescence-activated cell sorter analysis for green fluorescent protein (GFP) expression.
Proliferation and anchorage independence assays.
For monolayer proliferation assays, 3 × 103 NIH 3T3 or NIH 3T3/sf-Stk/SFFV cells per well were seeded in quadruplicate wells in 96-well plates with or without various inhibitors. Forty-eight hours after plating, the colorimetric reagent WST-1 (Roche Diagnostics, Mannheim, Germany) was added and proliferation was assessed by measuring absorbance at 450 nm. Mean data were normalized to untreated cells. For anchorage independence assays, 5 × 103 NIH 3T3 or NIH 3T3/sf-Stk/SFFV cells were suspended in 5 ml of a 0.35% SeaPlaque agarose suspension with or without inhibitor. The cells were fed with additional agar suspension weekly, but no additional drug was added. Two weeks after plating, anchorage-independent colonies were counted.
Inhibitors.
SP600125, MEK inhibitor 1, LY249002, SB203580, and luteolin were obtained from Calbiochem (Darmstadt, Germany). Rapamycin was purchased from Biomol (Plymouth Meeting, PA). All were dissolved in dimethyl sulfoxide (DMSO) (Sigma, St. Louis, MO). Vehicle controls included DMSO only.
RESULTS
PU.1 is not required for gp55-sf-Stk transformation of fibroblasts.
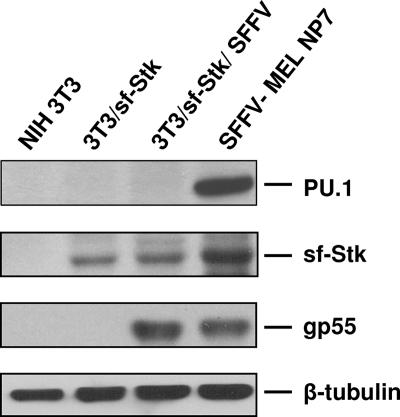
MEL cells derived from mice infected with Friend SFFV inappropriately express the myeloid transcription factor PU.1 (Fig. 1) due to a proviral insertion event (12, 13, 24, 25). Since expression of PU.1 is critical to the transformed phenotype of SFFV-derived erythroleukemia cells and because there are data suggesting that expression of PU.1 may occur independently of proviral insertion in SFFV disease (1), we tested NIH 3T3 cells engineered to express sf-Stk for expression of PU.1 before and after infection with SFFV. Figure 1 shows that although the SFFV-derived MEL line NP7 expresses a high level of PU.1, none could be detected in transformed fibroblasts coexpressing SFFV gp55 and sf-Stk, although both lines express high levels of SFFV gp55 and sf-Stk. Thus, transformation of sf-Stk-expressing rodent fibroblasts by SFFV, unlike SFFV transformation of erythroid cells, may be due primarily to its activation of sf-Stk.
FIG. 1.
PU.1 expression is not induced in NIH 3T3 cells by sf-Stk or SFFV-activated sf-Stk. Western analysis was performed on lysates from NIH 3T3, 3T3/sf-Stk, 3T3/sf-Stk/SFFV, and SFFV-MEL NP7 cells with antibodies against PU.1, sf-Stk, SFFV gp55, and β-tubulin.
Anchorage-independent transformation requires sf-Stk expression.
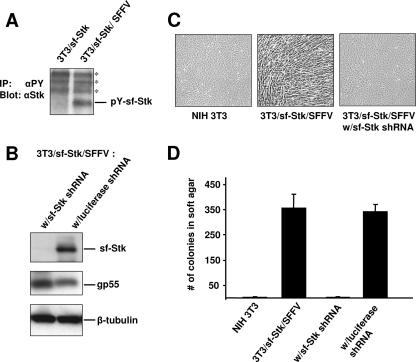
If SFFV is transforming sf-Stk-expressing fibroblasts by activating sf-Stk, the kinase should be phosphorylated only in virus-infected cells. As shown in Fig. 2A, sf-Stk becomes tyrosine phosphorylated in NIH 3T3/sf-Stk cells only after infection with SFFV, consistent with our earlier findings that the kinase domain of sf-Stk is required for the transformation of fibroblasts (17). NIH 3T3 cells that coexpress sf-Stk and SFFV (NIH 3T3/sf-Stk/SFFV) become transformed and are capable of forming anchorage-independent colonies in soft agar and subcutaneous tumors in nude mice (17). In order to demonstrate that sf-Stk expression is necessary for maintaining the transformed phenotype, an shRNA construct was used to knock down sf-Stk in these cells. Stable lines were established from NIH 3T3/sf-Stk/SFFV cells that were transfected with an sf-Stk shRNA construct or with a luciferase shRNA construct, which served as a vector control. Western analysis of the shRNA stable lines indicated that the cells transfected with the sf-Stk shRNA construct exhibited knockdown of sf-Stk protein to below the level of detection (Fig. 2B), while the luciferase shRNA line continued to express sf-Stk robustly. In contrast, expression of SFFV gp55 and β-tubulin was comparable in both shRNA lines, indicating a specific knockdown of sf-Stk rather than a general decrease in protein expression. The sf-Stk shRNA stable line reverted to a normal phenotype as assessed by its appearance in monolayer culture (Fig. 2C). In order to test the relevance of sf-Stk expression to anchorage-independent growth, a hallmark of transformation, the sf-Stk shRNA line and the luciferase shRNA line were plated in soft agar in parallel with NIH 3T3/sf-Stk/SFFV and low-passage parental NIH 3T3 cells, as positive and negative controls, respectively. As previously shown (17), NIH 3T3/sf-Stk/SFFV cells formed numerous anchorage-independent colonies within 2 weeks, but low-passage parental NIH 3T3 cells formed very few to none (Fig. 2D). However, the sf-Stk shRNA line showed a dramatic reduction in the number of anchorage-independent colonies, down to levels comparable to that of low-passage parental NIH 3T3 cells (Fig. 2D). The luciferase shRNA line had colony counts comparable to the 3T3/sf-Stk/SFFV cells, indicating that the decrease in colony formation in the sf-Stk shRNA line was not the result of nonspecific vector toxicity. The loss of anchorage-independent growth with sf-Stk knockdown coupled with the absence of PU.1 expression indicates that activation of sf-Stk by SFFV gp55 in itself is capable of transforming fibroblasts.
FIG. 2.
Sf-Stk is phosphorylated only in SFFV-infected fibroblasts, and its knockdown abolishes anchorage-independent growth. (A) Lysates from 3T3/sf-Stk and 3T3/sf-Stk/SFFV cells were immunoprecipitated with a monoclonal antibody against phosphotyrosine (4G10), and then the immunoprecipitates were immunoblotted with sf-Stk antiserum. Asterisks represent nonspecific bands. (B) Western analysis of 3T3/sf-Stk/SFFV cell lines stably transfected with sf-Stk or luciferase shRNA with antibodies against sf-Stk, SFFV gp55, and β-tubulin. (C) Photographs of NIH 3T3, 3T3/sf-Stk/SFFV, and 3T3/sf-Stk/SFFV w/sf-Stk shRNA cells in monolayer culture. (D) Cells of the lines NIH 3T3, 3T3/sf-Stk/SFFV, 3T3/sf-Stk/SFFV with sf-Stk shRNA, and 3T3/sf-Stk/SFFV with luciferase shRNA were plated in soft agar in triplicate. Two weeks later, total colonies were counted. Average counts of colonies from three dishes/cell type were graphed. Error bars show standard error of the mean.
MAPKs and PI3K signaling pathway molecules are constitutively phosphorylated in NIH 3T3 cells coexpressing sf-Stk and SFFV gp55.
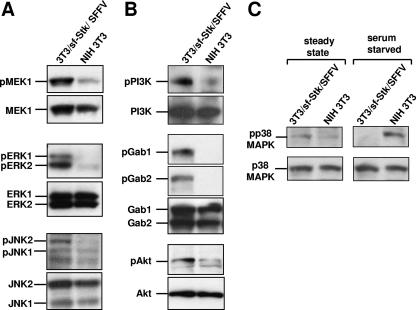
Many signaling molecules are constitutively activated in hematopoietic cells infected with Friend SFFV, including STATs 1, 3, and 5; p44/42 MAPK (ERK 1/2); PI3K; and Jun kinase (JNK) (14, 15, 19, 23). In erythroid cells expressing SFFV gp55, both the EpoR and sf-Stk are constitutively active, but which signals are generated by each of these receptors is unknown. The SFFV-sf-Stk-transformed fibroblast system enables us to determine the origins of the signals by separating SFFV gp55-activated sf-Stk from SFFV gp55-activated EpoR. In order to determine which signaling cascades are initiated by SFFV gp55-activated sf-Stk, total cell lysates of SFFV gp55-sf-Stk-transformed fibroblasts, parental NIH 3T3 cells, and NIH 3T3/sf-Stk cells were assayed with antibodies specific for the phosphorylated forms of signaling molecules. As shown in Fig. 3, many signaling molecules known to be activated in SFFV-infected erythroid cells are also activated in SFFV gp55/sf-Stk-transformed fibroblasts. Levels of phosphorylated MEK1 and ERK 1/2 were significantly higher in NIH 3T3/sf-Stk/SFFV cells than in parental NIH 3T3 cells (Fig. 3A) or in NIH 3T3 cells expressing only sf-Stk (data not shown). JNK activity, which has been demonstrated to be critical to the survival of SFFV MEL cells (20), was also shown to be increased in SFFV gp55/sf-Stk-transformed fibroblasts, as demonstrated by higher levels of phosphorylated JNK1/2 than in parental NIH 3T3 cells (Fig. 3A) or NIH 3T3/sf-Stk cells (data not shown). As shown in Fig. 3B, levels of phospho-p85α PI3K, phospho-Akt, phospho-Gab1, and phospho-Gab2 were also higher in NIH 3T3/sf-Stk/SFFV cells than in parental NIH 3T3 cells (Fig. 3B) or NIH 3T3/sf-Stk cells (data not shown). Thus, the MAPKs and various components of the PI3K-Akt pathway, all of which support growth and/or survival and may contribute to transformation, are activated downstream of SFFVgp55-activated sf-Stk.
FIG. 3.
MAPKs and the PI3K-Akt pathway, but not p38 MAPK, are activated in SFFVgp55/sf-Stk-transformed fibroblasts. Whole-cell lysates were prepared from 3T3/sf-Stk/SFFV and parental NIH 3T3 cells which were serum starved for 24 h prior to lysis in order to eliminate background phosphorylation in response to serum factors and highlight constitutive phosphorylation. (A) The ERK 1/2 pathway was examined by immunoblotting using antibodies specific for phosphoserines 217/221 of MEK1 and for phosphothreonine 202/phosphotyrosine 204 of ERK 1/2. Levels of phospho-JNK were assessed using an antibody specific for phosphothreonine 183/phosphotyrosine 185 JNK/SAPK. (B) p85α PI3K was immunoprecipitated, and the immunoprecipitate was assessed for phosphotyrosine with a monoclonal antibody (4G10). Levels of phosphorylated Gab1 and Gab2 were examined by immunoblotting using antibodies specific for phosphotyrosine 627 of Gab1 and phosphotyrosine 452 of Gab2. Levels of phospho-Akt were examined by immunoblotting with an antibody specific for phosphoserine 473 of Akt. (C) In addition to serum-starved lysates, whole-cell lysates were prepared from 3T3/sf-Stk/SFFV and parental NIH 3T3 cells at steady state. Levels of phosphorylated p38 MAPK protein were assessed by immunoblotting using antibodies specific for phosphothreonine 180/phosphotyrosine 182 of p38 MAPK. For all panels, blots were stripped and reprobed with antibodies for the total forms.
p38 MAPK is not phosphorylated in response to serum starvation stress in NIH 3T3 cells coexpressing sf-Stk and SFFV gp55.
As shown in Fig. 3C, both NIH 3T3 cells and those coexpressing sf-Stk and SFFV gp55 express low phosphorylated levels of the stress kinase p38 MAPK under steady-state conditions. While NIH 3T3 cells respond to stress induced by serum starvation by upregulating phosphorylation of p38 MAPK, NIH 3T3 cells coexpressing sf-Stk and SFFV gp55 do not (Fig. 3C). Like parental NIH 3T3 cells, NIH 3T3/sf-Stk cells maintain the p38 MAPK phosphorylation response to serum starvation (data not shown). Interestingly, a similar phenomenon is observed in Epo-dependent HCD-57 cells. These cells normally show enhanced phosphorylation of p38 MAPK when Epo is withdrawn, but after the cells are infected with SFFV, p38 MAPK phosphorylation in response to Epo withdrawal is lost (T. Yugawa and S. Ruscetti, unpublished data).
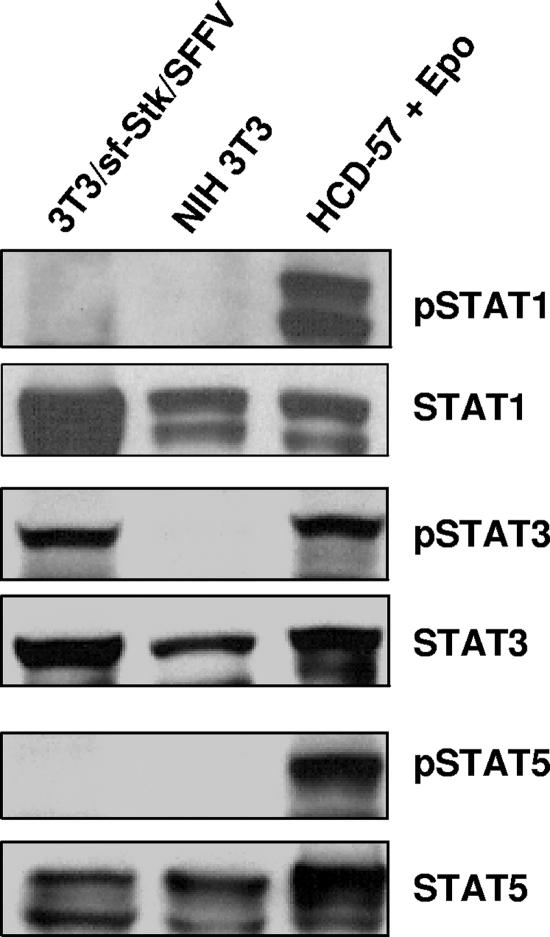
STAT1 and STAT5 are not phosphorylated in NIH 3T3 cells coexpressing sf-Stk and SFFV gp55.
It is well established that JAK-STAT signaling occurs downstream of the EpoR when it is activated by Epo (28). Previous studies have shown that infection of erythroid cells with SFFV results in the constitutive activation of STATs 1, 3, and 5 (22, 23). In order to test whether the JAK-STAT pathway could be activated by SFFV gp55-activated sf-Stk in the absence of the EpoR, we assessed the levels of phosphorylated STAT1, -3, and -5 proteins in NIH3T3/sf-Stk/SFFV cells by Western analysis. As shown in Fig. 4, neither STAT1 nor STAT5 was phosphorylated in NIH 3T3/sf-Stk/SFFV or NIH 3T3 cells. The anti-phospho-STAT antibodies used were capable of detecting phospho-STATs since positive control lysates from HCD-57 cells stimulated with Epo gave robust phospho-STAT1 and -5 signals (Fig. 4). Furthermore, NIH 3T3/sf-Stk/SFFV cells, NIH 3T3 cells, and HCD-57 cells express comparable amounts of total STAT1 and -5 proteins. In contrast, STAT3 was strongly phosphorylated in NIH 3T3/sf-Stk/SFFV cells (Fig. 4). Phosphorylated STAT3 could not be detected in NIH 3T3 cells (Fig. 4) or NIH 3T3 cells expressing only sf-Stk (data not shown), although both cell lines expressed abundant total STAT3. These results indicate that while phosphorylation of STAT3 is induced downstream of SFFV gp55-activated sf-Stk, phosphorylation of STATs 1 and 5 is not.
FIG. 4.
STAT3 but not STAT1 or STAT5 is phosphorylated in SFFVgp55/sf-Stk-transformed fibroblasts. Levels of phosphorylated STAT proteins in 3T3/sf-Stk/SFFV and parental NIH 3T3 cells were assessed by immunoblotting using antibodies specific for phosphotyrosine 701 of STAT1, phosphotyrosine 705 of STAT3, and phosphotyrosine 694 of STAT5. A lysate of the Epo-dependent cell line HCD-57 that had been stimulated with Epo was used as a positive control for the phospho-specific antibodies. All blots were stripped and reprobed with antibodies for the total forms.
Inhibition of JNK, MEK, PI3K, or mTOR, but not inhibition of p38 MAPK, decreases anchorage-independent growth of NIH 3T3 cells coexpressing SFFV gp55 and sf-Stk.
Having identified the ERK 1/2 pathway, the PI3K-Akt pathway, and JNK1/2 as being activated in the SFFVgp55-sf-Stk-transformed fibroblasts, we sought to evaluate the importance of these pathways to transformed growth using commercially available small-molecule inhibitors. All the inhibitors were first tested in a monolayer proliferation assay in order to assess toxicity over a wide range of drug concentrations and to compare the effect of the drugs on the parental NIH 3T3 cells versus the transformed cells. Drug concentrations that induced a ≤50% decrease in monolayer proliferation were then tested in the soft agar assay to assess the impact on anchorage-independent growth.
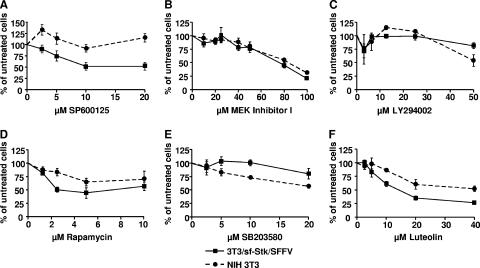
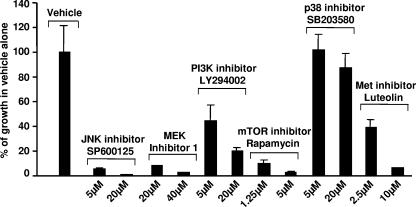
As shown in Fig. 5A, the JNK inhibitor SP600125 significantly reduced the proliferation of NIH 3T3/sf-Stk/SFFV cells at 5, 10, and 20 μM, while the proliferation of parental NIH 3T3 cells was not affected. When tested in the soft agar assay (Fig. 6), 5 μM SP600125 reduced the number of anchorage-independent colonies to about 5% of that observed with the vehicle control, and 20 μM reduced the number of colonies to levels comparable to those of low-passage NIH 3T3 cells. These data suggest that JNK activity contributes to both the proliferation and the anchorage-independent transformation of the NIH 3T3/sf-Stk/SFFV cells, consistent with an earlier study demonstrating that SP600125 is very effective at inhibiting the proliferation of SFFV-derived erythroleukemia cells (20).
FIG. 5.
Small-molecule inhibitors against downstream effectors of sf-Stk inhibit monolayer proliferation of SFFVgp55/sf-Stk-transformed fibroblasts. NIH 3T3 and 3T3/sf-Stk/SFFV cells were seeded in quadruplicate wells in monolayer proliferation assays with various concentrations of (A) the JNK inhibitor SP600125, (B) MEK inhibitor I, (C) the PI3K inhibitor LY294002, (D) the mTOR inhibitor rapamycin, (E) the p38 MAPK inhibitor SB203580, or (F) the putative Met inhibitor luteolin. Forty-eight hours after plating, the colorimetric reagent WST-1 was added and the absorbance at 450 nm was determined. Average absorbance readings were normalized to the untreated control. Error bars show standard error of the mean.
FIG. 6.
Small-molecule inhibitors against downstream effectors of sf-Stk inhibit anchorage-independent growth of SFFVgp55/sf-Stk-transformed fibroblasts. 3T3/sf-Stk/SFFV cells were plated in soft agar in triplicate with various concentrations of the JNK inhibitor SP600125, MEK inhibitor I, the PI3K inhibitor LY294002, the mTOR inhibitor rapamycin, the p38 MAPK inhibitor SB203580, or the putative Met inhibitor luteolin. Two weeks later, total colonies were counted. Average counts of colonies from three dishes/drug concentration were normalized to the DMSO vehicle control. Error bars show the standard error of the mean.
Inhibition of MEK, unlike inhibition of JNK, had no differential effect on the growth of NIH 3T3/sf-Stk/SFFV cells compared to parental NIH 3T3 cells in the monolayer proliferation assay (Fig. 5B). Both the transformed and parental NIH 3T3 lines showed a mild response at 40 μM or less to Calbiochem's MEK inhibitor I and strong inhibition at 80 μM or greater. When tested in the soft agar assay (Fig. 6), 20 and 40 μM concentrations of MEK inhibitor I were very effective at decreasing anchorage-independent growth of SFFVgp55/sf-Stk-transformed fibroblasts, decreasing the number of colonies to about 8% and 3% of those of the vehicle control, respectively. Similar results were obtained with another commercial MEK inhibitor, PD98059 (data not shown). These data suggest that MEK contributes primarily to anchorage-independent transformation.
To test the importance of the PI3K/Akt/mTOR pathway to monolayer proliferation and anchorage-independent transformation, we used the PI3K inhibitor LY294002 and the mTOR inhibitor rapamycin. LY294002, like MEK inhibitor I, did not have a differential effect on the growth of NIH 3T3/sf-Stk/SFFV cells compared to parental NIH 3T3 cells in the proliferation assay (Fig. 5C). Five and 20 μM concentrations of LY294002 reduced the numbers of SFFVgp55/sf-Stk-transformed fibroblasts forming anchorage-independent colonies in a dose-dependent manner to about 45% and 20% of those of the vehicle control, respectively (Fig. 6). Rapamycin effectively inhibited the monolayer proliferation of NIH 3T3/sf-Stk/SFFV cells at 2 μM, while the proliferation of NIH 3T3 cells was inhibited to a much lesser extent (Fig. 5D). Rapamycin concentrations of 1.25 and 5 μM reduced the number of anchorage-independent colonies formed by the SFFVgp55/sf-Stk-transformed fibroblasts to about 10% and 3% of those of the vehicle control, respectively (Fig. 6). These data suggest that the PI3K/Akt/mTOR pathway contributes to both the proliferation and the anchorage-independent transformation of the NIH 3T3/sf-Stk/SFFV cells.
We have demonstrated that while many other signaling molecules are constitutively phosphorylated in NIH 3T3/sf-Stk/SFFV cells, phosphorylation of p38 MAPK is suppressed. Therefore, we hypothesized that inhibition of p38 MAPK would have little or no effect on the monolayer proliferation or anchorage-independent transformation of NIH 3T3/sf-Stk/SFFV cells. Consistent with this hypothesis, the p38 MAPK inhibitor SB203580 had a mild effect on the monolayer proliferation of NIH 3T3/sf-Stk/SFFV cells (Fig. 5E), much less than that observed with NIH 3T3 cells. This is in contrast to all of the other compounds tested, which were always more or equivalently inhibitory to the SFFVgp55/sf-Stk-transformed cells compared to the parental NIH 3T3 cells. In addition, SB203580 did not greatly reduce the number of anchorage-independent colonies formed by 3T3/sf-Stk/SFFV cells (Fig. 6), and several unusually large colonies were observed at low concentrations of SB203580. Thus, as predicted, suppression of p38 MAPK did not inhibit either the monolayer proliferation or the anchorage-independent proliferation of NIH 3T3/sf-Stk/SFFV cells but rather slightly enhanced the anchorage-independent growth as manifested by unusually large colonies.
Unfortunately there are no commercially available inhibitors that are selective for Stk or its human equivalent, RON. However, there are strong data that the flavonoid luteolin can inhibit Stk's closest receptor tyrosine kinase relative, Met (9), making it a good candidate for a potential Stk/RON inhibitor. When tested in the proliferation assay, luteolin greatly inhibited the proliferation of NIH 3T3/sf-Stk/SFFV cells at concentrations of 20 μM or greater but had much less of an inhibitory effect on the parental NIH 3T3 cells (Fig. 5F). Luteolin concentrations of 5 and 10 μM reduced the number of anchorage-independent colonies formed to about 24% and 5% of those of the vehicle control, respectively (Fig. 6). Thus, luteolin decreases both the proliferation and the anchorage-independent transformation of NIH 3T3/sf-Stk cells, as would be expected if luteolin inhibited sf-Stk itself.
DISCUSSION
Work from our laboratory has demonstrated that the SFFV envelope protein, SFFV gp55, can mediate the transformation of fibroblasts provided that they express sf-Stk (17). The transformation of fibroblasts by SFFV gp55 and sf-Stk is cooperative; neither component can transform on its own. Overexpression of sf-Stk alone is insufficient to induce transformation of fibroblasts (17), and when sf-Stk is knocked down in the NIH 3T3/sf-Stk/SFFV cells, as shown in the present study, the viral proteins continue to be expressed, but the cells lose their transformed phenotype (i.e., they can no longer form anchorage-independent colonies). SFFV gp55 has no inherent kinase/signaling activity and, therefore, must recruit a partner molecule that can signal, either sf-Stk or EpoR or both, in order to induce constitutive signals in cells to drive hyperplasia or transformation.
Inappropriate expression of the transcription factor PU.1 is required for the transformed phenotype of SFFV-derived erythroleukemia cell lines (6, 12, 13, 32). PU.1 is expressed in SFFV-transformed erythroid cells because of a proviral insertion event (12, 24, 25). Because the NIH 3T3/sf-Stk/SFFV cells were generated by infecting NIH 3T3/sf-Stk cells with SFFV, it was possible that a proviral insertion event could have activated PU.1 in these cells as well, but PU.1 expression was not detected in these cells, suggesting that such an insertion event had not likely occurred. Rather, our data showing the loss of anchorage independence with sf-Stk knockdown in NIH 3T3/sf-Stk/SFFV cells and the absence of PU.1 indicate that activation of sf-Stk in itself may be capable of inducing the transformation of fibroblasts.
We found that sf-Stk is phosphorylated in NIH 3T3 cells only when they coexpress SFFV gp55. Because phosphorylation is required for activation of kinase activity, these results are consistent with our earlier findings that the kinase domain of sf-Stk is required for transformation, and that NIH 3T3/sf-Stk cells are not capable of transformed growth (17). It is also consistent with the fact that we observed high levels of constitutively phosphorylated signaling molecules in NIH 3T3/sf-Stk/SFFV cells, but not in NIH 3T3/sf-Stk cells. NIH 3T3/sf-Stk/SFFV cells have higher levels of phosphorylated MEK1, ERK 1/2, Gab 1/2, Akt, PI3K, and JNK 1/2. These effectors all contribute to growth/survival and are frequently upregulated in cancers. These effectors also have the potential to be activated downstream of the EpoR (28). Overlapping signals from SFFVgp55-activated sf-Stk and SFFV gp55-activated EpoR probably act together to push SFFV-infected erythroid cells over a threshold into full-blown hyperplasia. Signals from SFFV gp55-activated EpoR appear to be insufficient to reach this threshold since sf-Stk−/− mice do not develop splenomegaly in response to SFFV despite virus in the spleen (10, 26). However, in NIH 3T3 cells, signals generated from SFFV-gp55-activated sf-Stk are sufficient to induce transformation.
A notable difference in the constitutive signaling profile of SFFV-infected erythroid cells and NIH 3T3/sf-Stk/SFFV cells is the absence of phosphorylated STATs 1 and 5 in the latter cells. This suggests that interaction of SFFV gp55 with the EpoR, which NIH 3T3 cells lack, may be required for the activation of these STATs. To test this, we introduced an EpoR expression vector (5) into NIH 3T3, NIH 3T3/sf-Stk, and NIH 3T3/sf-Stk/SFFV cells. Although all three lines expressed EpoR robustly, as assessed by Western analysis (data not shown), none showed any STAT1 or STAT5 phosphorylation before or after stimulation with Epo (data not shown). Although the EpoR construct that we used was biologically active when expressed in hematopoietic cells, it may require a critical component lacking in fibroblasts to signal. We previously demonstrated that NIH 3T3 cells express JAK2 (17), the major regulator of STAT protein tyrosine phosphorylation in erythroid cells (33). However, there may be other components of EpoR signaling that are missing in rodent fibroblasts. Although we were unable to show definitively that the failure of SFFV gp55-activated sf-Stk to activate STATs 1 and 5 in NIH 3T3 cells was due to the absence of the EpoR in these cells, it remains a likely possibility that activation of these STATs in SFFV-infected erythroid cells is due to the involvement of the EpoR in the disease complex. In contrast to STATs 1 and 5, STAT3 is activated in NIH 3T3/sf-Stk/SFFV cells, suggesting that activation of this STAT may not require the EpoR but may be activated directly by sf-Stk, as occurs with sf-Stk's close relative Met (4) or may be activated downstream of sf-Stk by binding to Gab2 (16).
Our data indicate that the activation of STATs 1 and 5 is not required for transformation of rodent fibroblasts by SFFV-activated sf-Stk, consistent with our prior finding that SFFVgp55-activated sf-Stk can transform JAK2−/− murine embryonic fibroblasts (17). This result is also consistent with recent studies from our laboratory (18) indicating that STAT1 activation is not required for maintenance of the transformed phenotype of SFFV-derived erythroleukemia cells. In fact, our data suggest that SFFV-infected erythroid cells become transformed only when erythroid differentiation signals activated by STAT1 during SFFV-induced erythroid hyperplasia are blocked due to high levels of the hematopoietic phosphatase SHP-1 induced by inappropriate expression of the PU.1 protein (18).
The whole-cell lysates used for the Western blots assessing phosphoproteins were prepared from both steady-state and serum-starved cells. In most cases, the overall results were the same for both conditions, but the differences in phosphorylation levels in the transformed and parental lines were more prominent in the serum-starved samples, due to the elimination of background phosphorylation in response to serum factors. The one instance in which this was not the case was that of phosphorylated p38 MAPK. While both the transformed and parental NIH 3T3 cells had low levels of phosphorylated p38 MAPK at steady state, only the parental NIH 3T3 cells heavily phosphorylated p38 MAPK in response to serum starvation. Indeed, this stress response was essentially absent in the 3T3/sf-Stk/SFFV cells. Suppression of p38 MAPK has been observed in fibroblasts and epithelial cells transformed by other molecular mechanisms (7, 11, 27). Thus, in addition to the activation of progrowth/survival pathways, SFFVgp55-sf-Stk-transformed cells suppress the proapoptotic p38 MAPK stress response.
Targeting the downstream effectors of SFFV gp55-activated sf-Stk with small-molecule inhibitors proved to be very effective at decreasing the monolayer and anchorage-independent growth of NIH 3T3/sf-Stk/SFFV cells. Of all the inhibitors tested, the JNK inhibitor SP600125 and the putative kinase inhibitor luteolin were the most effective both in the monolayer proliferation assay and the anchorage independence assay. In addition, both showed a selectivity for NIH 3T3/sf-Stk/SFFV cells over the parental NIH 3T3 cells. The effectiveness of SP600125 in both assays indicates that JNK contributes to both proliferation and anchorage independence. Since the cells in the latter assay are more effectively inhibited by lower concentrations of SP600125, JNK may contribute more to transformation than to proliferation, but this could also reflect the longer exposure of the cells to the drug in the soft agar assay (14 days versus 48 h), which could amplify its effect. Because luteolin suppresses the phosphorylation of the Stk-related kinase Met (9) and was effective in decreasing both the monolayer and anchorage-independent growth of NIH 3T3/sf-Stk/SFFV cells, it may be blocking transformation by inhibiting the activity of sf-Stk, which is necessary for the transformation of fibroblasts by SFFV.
In contrast, inhibition of MEK with two different compounds had little effect on monolayer proliferation, but at the same concentrations had a profound effect on anchorage-independent growth. This indicates that the Ras/Raf/MAPK pathway primarily drives anchorage independence in NIH 3T3/sf-Stk/SFFV cells. The p38 MAPK inhibitor SB203580 had little effect on the number of anchorage-independent colonies observed, but several of these colonies were unusually large, indicating that inhibition of basal p38 MAPK enhances growth of NIH 3T3/sf-Stk/SFFV cells. In the monolayer proliferation assay, this effect is more pronounced, with NIH 3T3/sf-Stk/SFFV cells showing significantly less inhibition of proliferation in the presence of SB203580 than do parental NIH 3T3 cells. These results fit with the Western blot data showing suppression of p38 MAPK phosphorylation in NIH 3T3/sf-Stk/SFFV cells and with the finding that SB203580 enhances the transformation of fibroblasts and epithelial cells by other retroviruses (11, 27).
In summary, we examined the molecular mechanisms behind the transformation of fibroblasts by SFFVgp55-activated sf-Stk. We found that several progrowth/survival pathways are stimulated by SFFV gp55-activated sf-Stk and that a proapoptotic pathway, p38 MAPK, is suppressed. Transformation can be reversed by knockdown of sf-Stk and transformed growth can be decreased by the use of small-molecule inhibitors against downstream effectors or with luteolin, which may inhibit sf-Stk itself. Our results show that sf-Stk is critical to the transformation of fibroblasts that express SFFVgp55 and, by analogy, suggest that sf-Stk signals are critical to reaching a threshold to push erythroid cells into hyperplasia in SFFV disease. Inappropriate expression of the human homolog of sf-Stk, sf-RON, in human tumors (2, 3) indicates that this key component of SFFV disease is a molecular endpoint of interest in human cancer as well. Furthermore, the transformed fibroblast system could be useful for screening anti-sf-Stk/RON therapeutic compounds.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Footnotes
Published ahead of print on 24 October 2007.
REFERENCES
- 1.Afrikanova, I., E. Yeh, D. Bartos, S. S. Watowich, and G. D. Longmore. 2002. Oncogene cooperativity in Friend erythroleukemia: erythropoietin receptor activation by the env gene of SFFV leads to transcriptional upregulation of PU.1, independent of SFFV proviral insertion. Oncogene 211272-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angeloni, D., A. Danilkovitch-Miagkova, T. Ivanova, E. Braga, E. Zabarovsky, and M. I. Lerman. 2007. Hypermethylation of Ron proximal promoter associates with lack of full-length Ron and transcription of oncogenic short-Ron from an internal promoter. Oncogene 264499-4512. [DOI] [PubMed] [Google Scholar]
- 3.Bardella, C., B. Costa, P. Maggiora, S. Patane', M. Olivero, G. N. Ranzani, M. De Bortoli, P. M. Comoglio, and M. F. Di Renzo. 2004. Truncated RON tyrosine kinase drives tumor cell progression and abrogates cell-cell adhesion through E-cadherin transcriptional repression. Cancer Res. 645154-5161. [DOI] [PubMed] [Google Scholar]
- 4.Boccaccio, C., M. Ando, L. Tamagnone, A. Bardelli, P. Michieli, C. Battistini, and P. M. Comoglio. 1998. Induction of epithelial tubules by growth factor HGF depends on the STAT pathway. Nature 391285-288. [DOI] [PubMed] [Google Scholar]
- 5.D'Andrea, A., H. F. Lodish, and G. G. Wong. 1989. Expression cloning of the murine erythropoietin receptor. Cell 57277-285. [DOI] [PubMed] [Google Scholar]
- 6.Delgado, M., M. Dolores, M. Hallier, P. Meneceur, A. Tavitian, and F. Moreau-Gachelin. 1994. Inhibition of Friend cells proliferation by spi-1 antisense oligodeoxynucleotides. Oncogene 91723-1727. [PubMed] [Google Scholar]
- 7.Dermott, J. M., J. H. Ha, C. H. Lee, and N. Dhanasekaran. 2004. Differential regulation of Jun N-terminal kinase and p38MAP kinase by Gα12. Oncogene 23226-232. [DOI] [PubMed] [Google Scholar]
- 8.Iwama, A., K. Okano, T. Sudo, Y. Matsuda, and T. Suda. 1994. Molecular cloning of a novel receptor tyrosine kinase gene, STK, derived from enriched hematopoietic stem cells. Blood 833160-3169. [PubMed] [Google Scholar]
- 9.Lee, W., L. Wu, W. Chen, C. Wang, and T. Tseng. 2006. Inhibitory effect of luteolin on hepatocyte growth factor/scatter factor-induced HepG2 cell invasion involving both MAPK/ERKs and PI3K-Akt pathways. Chem. Biol. Interact. 160123-133. [DOI] [PubMed] [Google Scholar]
- 10.Lilly, F. 1970. Fv-2: identification and location of a second gene governing the spleen focus response to Friend leukemia virus in mice. J. Natl. Cancer Inst. 45163-169. [PubMed] [Google Scholar]
- 11.Maeda, N., W. Fu, A. Ortin, M. de las Heras, and H. Fan. 2005. Roles of the ras-MEK-mitogen-activated protein kinase and phosphatidylinositol 3-kinase-Akt-mTOR pathways in jaagsiekte sheep retrovirus-induced transformation of rodent fibroblast and epithelial cell lines. J. Virol. 794440-4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreau-Gachelin, F., D. Ray, M. Mattei, P. Tambourin, and A. Tavitian. 1989. The putative oncogene Spi-1: murine chromosomal localization and transcriptional activation in murine acute erythroleukemias. Oncogene 41449-1456. [PubMed] [Google Scholar]
- 13.Moreau-Gachelin, F., A. Tavitian, and P. Tambourin. 1988. Spi-1 is a putative oncogene in virally induced murine erythroleukemias. Nature 331277-280. [DOI] [PubMed] [Google Scholar]
- 14.Muszynski, K. W., T. Ohashi, C. Hanson, and S. K. Ruscetti. 1998. Both the polycythemia- and anemia-inducing strains of Friend spleen focus-forming virus induce constitutive activation of the Raf-1/mitogen-activated protein kinase signal transduction pathway. J. Virol. 72919-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muszynski, K. W., D. Thompson, C. Hanson, R. Lyons, A. Spadaccini, and S. K. Ruscetti. 2000. Growth factor-independent proliferation of erythroid cells infected with Friend spleen focus-forming virus is protein kinase C dependent but does not require Ras-GTP. J. Virol. 748444-8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ni, S., C. Zhao, G.-S. Feng, R. F. Paulson, and P. H. Correll. 2007. A novel Stat3 binding motif in Gab2 mediates transformation of primary hematopoietic cells by the Stk/Ron receptor tyrosine kinase in response to Friend virus infection. Mol. Cell. Biol. 273708-3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishigaki, K., C. Hanson, T. Jelacic, D. Thompson, and S. Ruscetti. 2005. Friend spleen focus-forming virus transforms rodent fibroblasts in cooperation with a short form of the receptor tyrosine kinase Stk. Proc. Natl. Acad. Sci. USA 10215488-15493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishigaki, K., C. Hanson, T. Ohashi, A. Spadaccini, and S. Ruscetti. 2006. Erythroblast transformation by the Friend spleen focus-forming virus is associated with a block in erythropoietin-induced STAT1 phosphorylation and DNA binding and correlates with high expression of the hematopoietic phosphatase SHP-1. J. Virol. 805678-5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishigaki, K., C. Hanson, T. Ohashi, D. Thompson, K. Muszynski, and S. Ruscetti. 2000. Erythroid cells rendered erythropoietin independent by infection with Friend spleen focus-forming virus show constitutive activation of phosphatidylinositol 3-kinase and Akt kinase: involvement of insulin receptor substrate-related adapter proteins. J. Virol. 743037-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishigaki, K., C. Hanson, D. Thompson, T. Yugawa, and S. Ruscetti. 2005. Activation of the Jun N-terminal kinase pathway by Friend spleen focus-forming virus and its role in the growth and survival of Friend virus-induced erythroleukemia cells. J. Virol. 7912752-12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishigaki, K., D. Thompson, C. Hanson, T. Yugawa, and S. Ruscetti. 2001. The envelope glycoprotein of Friend spleen focus-forming virus covalently interacts with and constitutively activates a truncated form of the receptor tyrosine kinase Stk. J. Virol. 757893-7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohashi, T., M. Masuda, and S. K. Ruscetti. 1997. Constitutive activation of Stat-related DNA-binding proteins in erythroid cells by the Friend spleen focus-forming virus. Leukemia 11251-254. [PubMed] [Google Scholar]
- 23.Ohashi, T., M. Masuda, and S. K. Ruscetti. 1995. Induction of sequence-specific DNA-binding factors by erythropoietin and the spleen focus-forming virus. Blood 851454-1462. [PubMed] [Google Scholar]
- 24.Paul, R., S. Schuetze, S. L. Kozak, and D. Kabat. 1989. A common site for immortalizing proviral integrations in Friend erythroleukemia: molecular cloning and characterization. J. Virol. 634958-4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paul, R., S. Schuetze, S. L. Kozak, C. A. Kozak, and D. Kabat. 1991. The Sfpi-1 proviral integration site of Friend erythroleukemia encodes the ets-related transcription factor PU.1. J. Virol. 65464-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Persons, D. A., R. F. Paulson, M. R. Loyd, M. T. Herley, S. M. Bodner, A. Bernstein, P. H. Correll, and P. A. Ney. 1999. Fv2 encodes a truncated form of the Stk receptor tyrosine kinase. Nat. Genet. 23159-165. [DOI] [PubMed] [Google Scholar]
- 27.Pruitt, K., W. M. Pruitt, G. K. Bilter, J. K. Westwick, and C. J. Der. 2002. Raf-independent deregulation of p38 and JNK mitogen-activated protein kinases are critical for Ras transformation. J. Biol. Chem. 27731808-31817. [DOI] [PubMed] [Google Scholar]
- 28.Richmond, T. D., M. Chohan, and D. L. Barber. 2005. Turning cells red: signal transduction mediated by erthropoietin. Trends Cell Biol. 15146-155. [DOI] [PubMed] [Google Scholar]
- 29.Rulli, K., T. Yugawa, C. Hanson, D. Thompson, S. Ruscetti, and K. Nishigaki. 2004. Ex vivo and in vivo biological effects of a truncated form of the receptor tyrosine kinase Stk when activated by interaction with the Friend spleen focus-forming virus envelope glycoprotein or by point mutation. J. Virol. 784573-4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruscetti, S. K. 1999. Deregulation of erythropoiesis by the Friend spleen focus-forming virus. Int. J. Biochem. Cell Biol. 311089-1109. [DOI] [PubMed] [Google Scholar]
- 31.Ruscetti, S. K., N. J. Janesch, A. Chakraborti, S. T. Sawyer, and W. D. Hankins. 1990. Friend spleen focus-forming virus induces factor independence in an erythropoietin-dependent erythroleukemia cell line. J. Virol. 641057-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuetze, S., R. Paul, B. C. Gliniak, and D. Kabat. 1992. Role of the PU.1 transcription factor in controlling differentiation of Friend erythroleukemia cells. Mol. Cell. Biol. 122967-2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Witthuhn, B. A., F. W. Quelle, O. Silvennoinen, T. Yi, B. Tang, O. Miura, and J. N. Ihle. 1993. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell 74227-236. [DOI] [PubMed] [Google Scholar]
- 34.Wolff, L., R. Koller, and S. Ruscetti. 1982. Monoclonal antibody to spleen focus-forming virus-encoded gp52 provides a probe for the amino-terminal region of retroviral envelope proteins that confers dual tropism and xenotropism. J. Virol. 43472-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolff, L., P. Tambourin, and S. Ruscetti. 1986. Induction of the autonomous stage of transformation in erythroid cells infected with SFFV: helper virus is not required. Virology 152272-276. [DOI] [PubMed] [Google Scholar]