Abstract
Early in infection, herpes simplex virus type 1 (HSV-1) immediate-early (IE) proteins ICP0 and ICP4 localize to the nucleus, where they stimulate viral transcription. Later in infection, ICP0 and to a lesser extent ICP4 accumulate in the cytoplasm, but their biological role there is unknown. Previously, it was shown that the cytoplasmic localization of ICP0/4 requires the multifunctional IE protein ICP27, which is itself an activator of viral gene expression. Here, we identify a viral ICP27 mutant, d3-4, which is unable to efficiently localize ICP0 and ICP4 to the cytoplasm but which otherwise resembles wild-type HSV-1 in its growth and viral gene expression phenotypes. These results genetically separate the function of ICP27 that affects ICP0/4 localization from its other functions, which affect viral growth and gene expression. As both ICP0 and ICP4 are known to be minor virion components, we used d3-4 to test the hypothesis that the cytoplasmic localization of these proteins is required for their incorporation into viral particles. Consistent with this conjecture, d3-4 virions were found to lack ICP0 in their tegument and to have greatly reduced levels of ICP4. Thus, the cytoplasmic localization of ICP0 and ICP4 appears to be a prerequisite for the assembly of these important transcriptional regulatory proteins into viral particles. Furthermore, our results show that ICP27 plays a previously unrecognized role in determining the composition of HSV-1 virions.
Viral proteins that are present in the cell at the very earliest stages of infection can have critical regulatory functions, e.g., to counteract host immunity or to transactivate viral gene expression. For herpes simplex virus type 1 (HSV-1), there are two categories of viral regulatory polypeptides which are present very early in infection: those which enter the cell as components of the virion tegument and those which are expressed immediately upon infection as immediate-early (IE) proteins. Interestingly, two HSV-1 proteins, ICP0 and ICP4, fall into both categories, as they are abundantly expressed IE proteins as well as minor components of the tegument layer of virions (12, 64, 65).
The virions of HSV-1 and other herpesviruses are among the most complex viral particles known, consisting of more than 30 viral proteins as well as some cellular components (36). HSV-1 virions are composed of four morphologically distinct structures: core, capsid, tegument, and envelope. The inner nucleoprotein core containing the 152-kbp viral double-stranded DNA genome is enclosed in a capsid which is surrounded by a proteinaceous layer known as the tegument, which in turn is enclosed in a host cell-derived lipid envelope containing numerous viral glycoproteins. The tegument (reviewed in reference 34) consists of more than 15 proteins, several of which are known to have regulatory roles in the newly infected cell. It interacts with the capsid on one side and the cytoplasmic tails of envelope glycoproteins on the other to secure the integrity of the virus particle. Although the process of tegument acquisition is not well understood, it involves a highly ordered network of protein-protein interactions (35, 58) and occurs sequentially at multiple sites in the cell, beginning in the nucleus (4, 39) and culminating on cytoplasmic membranes when fully tegumented capsids acquire their final envelope.
As mentioned above, the IE proteins ICP0 and ICP4 are minor tegument components (100 to 200 copies per virion) (12, 64, 65). Although the biological role of virion-localized ICP0 and ICP4 is unclear, the functions of ICP0 and ICP4 as IE proteins have been well described: both play important roles in activating HSV-1 gene expression. ICP4 is the major transcriptional activator of HSV-1 early (E) and late (L) genes and is essential for viral growth under all known conditions (8). ICP0 (reviewed in reference 14) is also an important activator of viral genes and is required for growth during low-multiplicity infections. ICP0's gene activation function correlates with its E3 ubiquitin ligase activity that disrupts host cell ND10 domains and targets certain host proteins for proteasomal degradation.
ICP0 and ICP4 interact functionally, and in the case of ICP4 physically, with another IE protein, ICP27 (30, 42, 51). ICP27 is a nucleocytoplasmic shuttling protein that performs a number of functions during infection, most notably the induction of several E and L viral genes (reviewed in references 50 and 53). As described below, ICP27 also determines the intracellular localization of ICP0 and ICP4. These two proteins are characteristically nuclear in wild-type (WT) HSV-1 infected cells early in infection (25) but become progressively more cytoplasmic as infection continues (12, 21, 27, 57). In the case of ICP0, the extent of relocalization can be dramatic, with many infected cells showing predominant cytoplasmic localization late in infection. The relocalization of ICP0 and ICP4 to the cytoplasm during infection has been shown to be dependent on ICP27 (27, 69, 70). Moreover, ICP27 can promote the cytoplasmic localization of ICP4 and ICP0 even when these proteins are coexpressed in the absence of infection (68, 70). Thus, the mechanism by which ICP27 promotes ICP0/4 cytoplasmic localization does not require the environment of the infected cell or other viral factors.
Since many viral proteins are incorporated into the tegument in the cytoplasm (34), we speculated that the ICP27-dependent localization of ICP0 and ICP4 to the cytoplasm could be required for their incorporation into virions. However, this hypothesis is difficult to address, as most ICP27 mutants do not produce significant levels of infectious progeny due to their severe defects in viral gene expression (29, 46, 47). In an attempt to explore the role of ICP27 in virion composition, we surveyed a set of viable or semiviable ICP27 mutants which have short in-frame deletions in the amino-terminal portion of the ICP27 gene (26). Among these, we identified a mutant, d3-4, which is unable to localize ICP0 and ICP4 to the cytoplasm but which otherwise replicates similarly to the WT virus. The phenotype of this mutant thus genetically separates ICP27's ability to affect ICP0/4 localization from its ability to stimulate viral gene expression. It also allowed us to address the role of ICP27 in the virion incorporation of ICP0 and ICP4.
MATERIALS AND METHODS
Cells, viruses, and infections.
African green monkey kidney cells (Vero) were obtained from the American Type Culture Collection. They were grown in Dulbecco modified Eagle medium containing 5% heat-inactivated fetal calf serum (FCS), 50 U/ml penicillin, and 50 μg/ml streptomycin. All tissue culture reagents were purchased from Life Technologies/Invitrogen (Carlsbad, CA), except FCS, which was purchased from Atlas Biologicals (Fort Collins, CO).
The WT virus used in these studies was HSV-1 strain KOS1.1. The ICP27 mutants used were d1-2 (48), d2-3 and d6-7 (3, 26), d3-4 and d4-5 (31), and d5-6 (26, 33). All infections were carried out as previously described (26) at a multiplicity of infection (MOI) of 10 or 0.1, as indicated.
Immunoblotting analysis.
Protein extracts were prepared from infected or transfected cells as described previously (43). The purification of virions is described below. Protein samples were separated by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes (Trans-Blot; Bio-Rad, Hercules, CA). Immunoblotting was performed as previously described (43). The primary antibodies used included anti-ICP27 monoclonal antibody (MAb) H1119, diluted 1:5,400; anti-ICP0 MAb H1112, diluted 1:1,000; anti-ICP4 MAb H114, diluted 1:5,000; anti-gD MAb H1103, diluted 1:3,000; and anti-gC MAb H1104, diluted 1:300. These were purchased from Rumbaugh-Goodwin Institute for Cancer Research (Plantation, FL.). A MAb specific for capsid protein VP5 was purchased from Abcam (Cambridge, MA.) and used at a dilution of 1:1,000. Rabbit antipeptide serum against VP16 (60) was used at a dilution of 1:10,000. A MAb specific for cellular endosomal antigen EEA1 was purchased from BD Biosciences (San Jose, CA) and used at a dilution of 1:500. Rabbit antiserum specific for the HSV-1 VP22 protein (13) was provided by Gillian Elliott (Imperial College, London, United Kingdom) and was used at a dilution of 1:50,000. Immunoreactive proteins were detected by enhanced chemiluminescence (ECL detection kit; Amersham, Piscataway, NJ).
Immunofluorescence.
Vero cells, grown on coverslips, were either infected with HSV-1 or transfected and then were processed for indirect immunofluorescence at the times indicated. Cells were fixed in 3.7% formaldehyde, followed by acetone permeabilization (44). Coverslips were incubated for 1 h at 37°C with anti-ICP0 MAb H1112, diluted 1:1,000; anti-ICP4 MAb H1114, diluted 1:800; or anti-ICP27 MAb H1119, diluted 1:1,600. Secondary staining was done with Cy3-conjugated goat anti-mouse immunoglobulin G (heavy plus light chains; Jackson Immunoresearch Laboratories, West Grove, PA), diluted 1:400. Nuclei were stained with Hoechst dye 33258 (0.5 μg/ml) during the secondary antibody incubation. The cells were examined using a fluorescence microscope (Olympus BX60) linked to a video camera. Images were captured as TIFF files using CG7 frame grabber (Scion). Images from each experiment were adjusted for brightness and contrast in parallel using Adobe Photoshop Elements software.
Analysis of transfected cells.
Analysis of the effect of ICP27 on ICP0 and ICP4 in transfected cells was done by a method similar to that described by Zhu et al. (68, 70). Briefly, at 1 day prior to transfection, Vero cells were plated on coverslips in 12-well trays. Transfection of slightly subconfluent monolayers was carried out using Lipofectamine 2000 (Life Technologies/Invitrogen, Carlsbad, CA) as per the manufacturer's instructions. The total amount of DNA per well was 1.6 μg, consisting of 0.5 μg of ICP0- or ICP4-encoding plasmid and, where applicable, 1.1 μg of ICP27-encoding plasmid. In transfections where ICP27 plasmid was not used, 1.1 μg of salmon sperm DNA was used instead. The ICP0-encoding plasmid was pSHZ (38), whereas the ICP4-encoding plasmid was pK1-2 (9). The plasmids used for WT and d3-4 ICP27 expression were pM27 (47) and pMd3-4 (31), respectively. All of the HSV-1 IE genes on the plasmids listed above utilize the native IE gene promoters to drive gene expression. At 24 h posttransfection, cells were fixed, permeabilized, and processed for immunofluorescence. Localization patterns in the transfected cells were assessed in a blinded fashion, and at least 400 positive cells were scored for each combination of plasmids.
Purification and analysis of extracellular virions.
The protocol of Szilagyi and Cunningham (56) was adapted to purify extracellular virions. Briefly, monolayers of Vero cells cultured in 150-cm2 flasks were infected at a MOI of 0.1. Ten flasks were used for WT HSV-1 virion preparations; however, because d3-4 produces slightly lower levels of infectious progeny than does the WT, 30 flasks were used for the d3-4 preparations. After incubation at 37°C for 2 days, when extensive cytopathic effect was observed, the media were combined, cell debris was removed by low-speed centrifugation (1000 × g for 30 min at 4°C), and virus particles were pelleted by centrifugation (23,000 × g for 2 h at 4°C). The pellet was then gently resuspended in 1 ml of modified 199 medium (without phenol red and FCS) and layered onto 35-ml preformed gradients of 5 to 15% Ficoll 400 (Sigma) suspended in modified 199 medium. After centrifugation using a swinging-bucket rotor (26,000 × g for 2 h at 4°C), one predominant light-scattering band was observed and collected by side puncture using a needle and syringe. The particles were pelleted by centrifugation (80,000 × g for 2 h at 4°C) and resuspended overnight at 4°C in 200 μl radioimmunoprecipitation assay (RIPA) buffer (10 mM Tris [pH 7.4], 150 mM NaCl, 5 mM EDTA, 1% deoxycholate, 0.1% SDS) or gently resuspended in phosphate-buffered saline (PBS) (pH 7.4) and stored at −80°C. In one experiment (see Fig. 5B and C), virions were isolated by two successive rounds of Ficoll gradient purification. The protein content of virion lysate samples was determined using a DC protein assay kit (Bio-Rad, Hercules, CA) as per the manufacturer's instructions. Protein extracts, prepared as described above, were obtained from the same infected-cell monolayers as the extracellular virions.
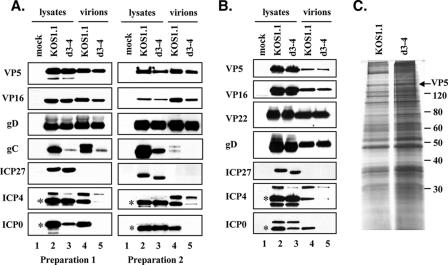
FIG. 5.
Analysis of purified WT HSV-1 and d3-4 virions. (A and B) Immunoblotting analysis. Extracellular virions were prepared as described in Materials and Methods, using either one (A) or two (B) rounds of 5 to 15% Ficoll gradient purification. For comparison, total protein extracts from the same infected cells are also shown. Virions and cell lysates were analyzed by immunoblotting using antibodies specific for the viral proteins listed. In panel A, the results from two separate virion preparations are shown. For ICP0 and ICP4, asterisks mark the positions of the major 110-kDa and 175-kDa species, respectively. (C) Protein profiles. Equivalent amounts of the twice-gradient-purified virions used for panel B were separated by SDS-PAGE and visualized by silver staining. The positions of molecular weight standards are shown, as is the band corresponding to major capsid protein VP5 (arrow).
To test for the localization of HSV-1 proteins in virions, purified HSV-1 virions were treated with 0.1 mg of trypsin per ml in either the presence or absence of 1% Triton X-100 for 20 min at 37°C. The proteolysis reaction was terminated by the addition of 1 mg of soybean-trypsin inhibitor per ml and 25 μg of phenylmethylsulfonyl fluoride per ml.
RESULTS
HSV-1 ICP27 mutant d3-4 fails to localize ICP0 and ICP4 to the cytoplasm.
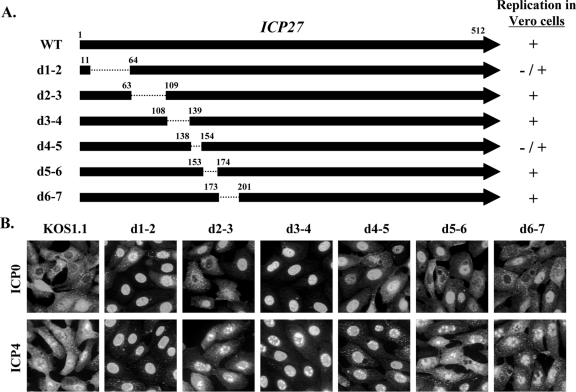
Previous work has shown that ICP27 promotes the cytoplasmic localization of ICP0 and ICP4 and that the C-terminal part of ICP27 is required for this activity (68, 70). A recent study from our laboratory indicated that the N-terminal half of ICP27 also affects this activity, as a viral ICP27 mutant with a methionine-to-threonine substitution at residue 50 shows reduced cytoplasmic localization of ICP4 and ICP0 (27). To look more extensively at the contribution of the N-terminal portion of ICP27, we examined ICP0/4 localization in cells infected with viruses having short in-frame deletions in this region of the gene (Fig. 1A). These mutants fall into two classes with respect to their replication in Vero cells: d1-2 and d4-5 show moderate growth defects (1- to 2-log reductions in viral growth in a single-cycle replication assay), whereas d2-3, d3-4, d5-6, and d6-7 grow comparably to the WT virus (26). Infected Vero cells were fixed at 6 h postinfection (hpi) and processed for immunofluorescence to look at ICP0 (Fig. 1B, top panels) and ICP4 (bottom panels). In WT-infected cells at this time point, ICP0 was localized predominantly to the cytoplasm of many cells, although other cells showed significant nuclear staining. Among the ICP27 mutants, d2-3, d5-6, and d6-7 displayed ICP0 localization that was similar to that with the WT virus. In contrast, cells infected with d1-2 and in particular d3-4 showed predominantly nuclear ICP0. Mutant d4-5 exhibited an intermediate phenotype. Similar results were seen when ICP4 was examined. That is, in WT-infected cells, as well as in cells infected with d2-3, d5-6, and d6-7, ICP4 was localized to both the nucleus and cytoplasm of most cells at 6 hpi. However, in d1-2-, d3-4-, and d4-5-infected cells, ICP4 was localized primarily to the nucleus. These results indicate that two N-terminal regions of ICP27, corresponding to residues 12 to 63 and 109 to 153, are important for ICP27's ability to promote the cytoplasmic localization of ICP0 and ICP4. These results do not readily correlate with ICP27's ability to efficiently exit the nucleus as part of its nucleocytoplasmic shuttling activity. That is, among the three mutants which are defective for localizing ICP0/4 to the cytoplasm, d1-2 is deficient for nuclear export whereas d3-4 and d4-5 are export competent (5, 32).
FIG. 1.
Effect of N-terminal ICP27 deletions on the nucleocytoplasmic distribution of ICP0 and ICP4 during viral infection. (A) Diagram of the ICP27 polypeptides produced by the viral deletion mutants used in this study. The top arrow represents the 512-residue ICP27 polypeptide, and the arrows below represent deleted proteins encoded by the various viral mutants. The residues bordering the deleted regions (dotted lines) are indicated. The ability of the mutants to replicate in Vero cells (26) is shown at right. (B) ICP0 and ICP4 localization. Vero cells were infected at an MOI of 10 with WT HSV-1 or the mutant viruses as shown. Cells were fixed at 6 hpi and processed for ICP0 and ICP4 immunofluorescence.
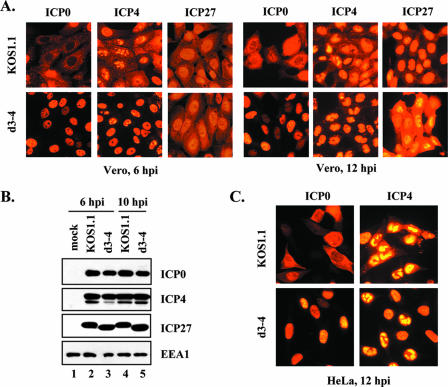
The apparent inability of d3-4 to affect ICP0 and ICP4 localization is noteworthy, as this mutant is competent for viral gene expression and growth in Vero cells (26) (although in some analyses it demonstrates a slight [∼3-fold] replication defect compared to the WT virus [31; L. Sedlackova and S. Rice, unpublished data]). We considered the possibility that d3-4-infected cells might simply have a delay in localizing ICP0 and ICP4 to the cytoplasm. To address this, we infected Vero cells with WT or d3-4 virus and examined ICP0/4 localization at both an early and late time point (6 and 12 hpi, respectively). In WT-infected cells, both ICP0 and ICP4 showed appreciable cytoplasmic localization at both time points (Fig. 2A), whereas in d3-4 infections, ICP0 and ICP4 remained predominantly nuclear, even at 12 hpi. Thus, ICP0/4 appear to be restricted to the nucleus in d3-4-infected cells throughout the course of infection.
FIG. 2.
HSV-1 ICP27 mutant d3-4 fails to localize ICP0 and ICP4 to the cytoplasm. (A) Protein localization. Vero cells were infected with WT HSV-1 or d3-4 at an MOI of 10. Cells were fixed at 6 or 12 hpi and processed for ICP0, ICP4, and ICP27 immunofluorescence. (B) Protein accumulation. Vero cells were mock infected or infected with WT HSV-1 or d3-4 at an MOI of 10, and total proteins were harvested at the indicated times. ICP0, ICP4, and ICP27 levels were determined by immunoblotting. As a loading control, the levels of cellular protein EEA1 were also analyzed. (C) Localization of ICP0 and ICP4 in infected HeLa cells. The experiment was conducted as for panel A, except HeLa cells were infected and fixed at 12 hpi.
It was of interest to see how the localization of the ICP27 protein itself correlated with its effects on the localization of ICP0/4. Therefore, in the above-described experiment, the localization of ICP27 was also determined (Fig. 2A). At 6 hpi, the localization of ICP27 was similar in WT- and d3-4-infected cells, showing strong nuclear localization and a smaller amount of cytoplasmic localization. However, at 12 hpi, there was a significant difference in that WT ICP27 was predominantly nuclear, whereas much more of the d3-4 ICP27 was located in the cytoplasm. This result is consistent with previous studies which show that d3-4 ICP27 has a defect in nuclear localization (26, 31). Thus, at least at late times, there appears to be an inverse correlation between the localization of ICP27 and ICP0/4, in that strong nuclear localization of ICP27 correlates with an increased level of cytoplasmic localization for ICP0 and ICP4.
We next considered whether the differences in ICP0/4 localization could be secondary effects related to protein abundance. To investigate this, immunoblotting analysis was carried out on proteins harvested from infected Vero cells at 6 and 10 hpi. Similar amounts of ICP0 and ICP4 were seen in both WT- and d3-4-infected cells (Fig. 2B). Additionally, ICP27 levels in the two infections were comparable. As a protein loading control, we examined levels of the cellular protein EEA1, the abundance of which does not change during HSV-1 infection (15). Similar EEA1 levels were seen in all lanes, demonstrating that comparable amounts of protein were loaded. Thus, the nearly exclusive nuclear localization of ICP0 and ICP4 in d3-4-infected cells cannot be attributed to differences in protein abundance.
As cell type could potentially affect the nucleocytoplasmic localization of ICP0/4, we also examined ICP0 and ICP4 localization in two other cell lines. As can be seen in Fig. 2C, a significant amount of the ICP0 and ICP4 in WT-infected HeLa cells at 12 hpi was cytoplasmic, but both proteins remained predominantly nuclear in d3-4-infected HeLa cells. Similar results were seen in human U2OS osteosarcoma cells (data not shown). Thus, three distinct primate cell lines gave similar results with respect to ICP27's effects on ICP0/4 localization.
In summary, the above analyses indicate that the HSV-1 ICP27 mutant d3-4 is defective in its ability to promote the cytoplasmic localization of ICP0 and ICP4. Since d3-4 is competent for viral gene expression and growth, these results provide genetic evidence that the function of ICP27 which affects ICP0/4 nucleocytoplasmic distribution is distinct from its other functions involved in viral gene induction.
d3-4 ICP27 fails to modulate ICP0/4 localization in transfected cells.
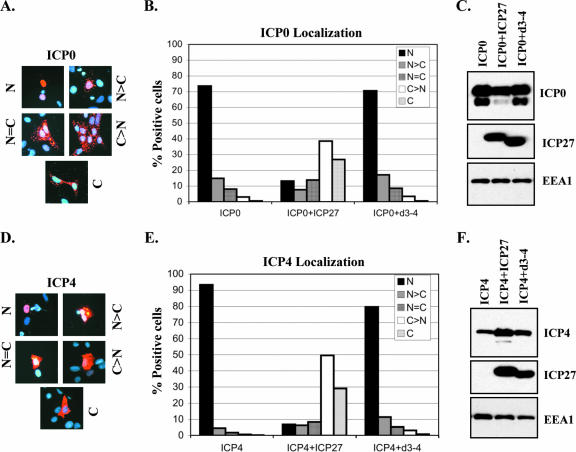
Transfection experiments have shown that ICP27 can promote the cytoplasmic localization of ICP0 and ICP4 even in uninfected cells (26, 68, 70). Based on the viral infection experiments shown above, we predicted that d3-4-encoded ICP27 would be unable to modulate ICP0/4 localization in transfected cells. To test this, plasmid cotransfection experiments were carried out, using plasmids having cloned IE genes under the control of their native promoters. In the first set of experiments, an ICP0 plasmid was transfected into Vero cells with or without a plasmid expressing either WT- or d3-4-encoded ICP27. The cells were fixed 24 h later and stained for ICP0. The ICP0 immunofluorescence patterns were assessed in a blinded fashion and divided into five categories: predominantly nuclear, predominantly cytoplasmic, or mixed. Representative staining patterns are shown in Fig. 3A. As previously found, localization of ICP0 in the absence of ICP27 was predominantly nuclear, whereas coexpression of WT ICP27 dramatically shifted its distribution to the cytoplasm (Fig. 3B). However, this effect was not observed when ICP0 was coexpressed with d3-4 ICP27. The inability of d3-4 ICP27 to affect ICP0 localization was not due to lack of its expression, as it could be readily detected by immunoblotting in a control transfection (Fig. 3C). Similar results were observed when ICP4 was examined (Fig. 3D to F); i.e., when expressed alone, ICP4 was predominantly nuclear, whereas WT but not d3-4 ICP27 coexpression caused a dramatic shift of ICP4 to the cytoplasm. Thus, this experiment confirms that d3-4-encoded ICP27 lacks the regulatory activity which promotes the cytoplasmic accumulation of ICP0 and ICP4.
FIG. 3.
The d3-4 ICP27 polypeptide is unable to promote cytoplasmic localization of ICP0 and ICP4 in transfected cells. Vero cells were transfected with ICP0-expressing (A and B) or ICP4-expressing (D and E) plasmids, with or without plasmids encoding either WT ICP27 or d3-4 ICP27. Cells were fixed 1 day later and processed for ICP0 or ICP4 immunofluorescence. Localization patterns of ICP0 and ICP4 were scored as predominantly nuclear (N), predominantly cytoplasmic (C), or mixed (N = C, N>C, or C>N). Representative staining patterns for ICP0 and ICP4 are shown in panels A and D, respectively. The data in panels B and E represent the mean values from two independent experiments. (C and F) Immunoblot analysis of ICP0 and ICP4 in transfected cells. Vero cells were transfected as for panels A, B, D, and E, and accumulation of ICP0, ICP4, and ICP27 at 1 day posttransfection was analyzed by immunoblotting. As a loading control, the levels of cellular protein EEA1 were also determined.
ICP27 is required for the efficient virion incorporation of ICP0 and ICP4.
As ICP0 and ICP4 are incorporated at low levels into the tegument layer of HSV-1 virions, we hypothesized that the cytoplasmic localization of these proteins is necessary for their virion incorporation. This is consistent with current models of alphaherpesviral particle assembly, in which the majority of viral tegument proteins are incorporated in the cytoplasm (36). If this hypothesis is correct, it would be predicted that d3-4 virions should be deficient in ICP0 and ICP4. Before examining this, however, we wished to confirm that we could detect ICP0 and ICP4 in the tegument layer of WT virions. Therefore, Vero cells were infected at a MOI of 0.1, and cell-free supernatants were collected when extensive cytopathic effect was observed. Virions were pelleted from this material and purified by centrifugation through a 5 to 15% Ficoll 400 gradient. One light-scattering virus band was observed, consistent with the published analysis of extracellular virions prepared from Vero cells by this procedure (63). The purified virions were then analyzed by immunoblotting, along with lysates from infected cells which had been prepared in parallel. As expected, the major capsid protein VP5 and envelope glycoprotein gD could be readily detected in the purified virions, in RIPA or PBS (Fig. 4, lanes 3 and 4, respectively). Furthermore, ICP0 and ICP4 were also detected, suggesting that they are contained in virions. To verify that virion-associated ICP0 and ICP4 are located within virus particles, virions were treated with trypsin in either the absence or presence of detergent as described in Materials and Methods. The proteolysis reaction was terminated by the addition of trypsin inhibitors, and equal amounts of proteins were analyzed by immunoblotting. The results show that envelope protein gD was sensitive to trypsin in both the absence (Fig. 4, lane 5) and presence (lane 6) of detergent, as expected given its location on the exterior of the virion. In contrast, the major capsid protein VP5 was resistant to trypsin even in the presence of detergent (lane 6), consistent with recent work showing that VP5 and several other capsid proteins are resistant to trypsin when they are assembled into capsids (40). Importantly, ICP0 and ICP4 were completely sensitive to trypsin treatment, but only when detergent was present (compare lanes 5 and 6). These results verify that ICP0 and ICP4 are present in the tegument layer of HSV-1 virion preparations, consistent with the work of Courtney and others (10, 12, 41, 64, 65).
FIG. 4.
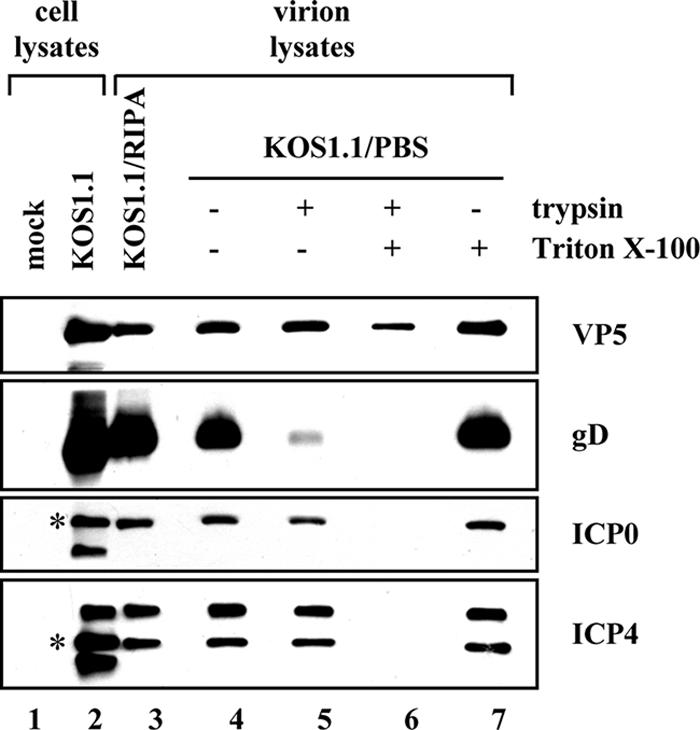
ICP0 and ICP4 are localized to the tegument layer of HSV-1 virions. Vero cells were infected with WT HSV-1, and extracellular virions were purified on a Ficoll gradient as described in Materials and Methods. Virions were either solubilized in RIPA buffer or resuspended in PBS. Those resuspended in PBS were then treated with trypsin (0.1 mg/ml) in either the presence or absence of 1% Triton X-100 for 20 min at 37°C. Equivalent amounts of cell lysates as well as virion samples were analyzed by immunoblotting for the viral proteins indicated. For ICP0 and ICP4, asterisks mark the positions of the major 110-kDa and 175-kDa species, respectively.
Next, we purified WT and d3-4 virions in parallel. After collection of the virion bands, they were pelleted and their compositions were compared by immunoblotting. Figure 5A shows the results from two independent virion preparations. In both cases, analysis of VP5 showed that the WT and d3-4 virion samples were approximately equally loaded on the gel. ICP0 and ICP4 were clearly present in WT virions (lanes 4), but no ICP0 was detected in d3-4 virions, and the level of ICP4 was significantly reduced (lanes 5). The apparent failure of ICP0 and ICP4 to be efficiently incorporated into d3-4 virions was not due to their lack of expression, as both proteins were readily detected in the infected-cell lysates (lanes 2 and 3). We also noted that there were other forms of ICP0 and ICP4 observed in addition to the major characterized species, which have apparent molecular sizes of 110 and 175 kDa, respectively. While the faster-migrating species probably represent proteolytic fragments of ICP0 and ICP4, the origin of the more slowly migrating species is currently unclear. In addition to VP5, ICP0, and ICP4, the purified virions were also examined for the presence of several other viral proteins. As can be seen in Fig. 5A, both WT and d3-4 mutant virions showed comparable levels of envelope protein gD and tegument protein VP16. Analysis of glycoprotein C (gC) revealed an unexpected difference, however, in that d3-4 virions contained significantly less of this envelope constituent than did WT virions (see below). ICP27 was not detected in either virion preparation, consistent with the previous findings demonstrating that it is not a virion component (65).
The results described above suggest that d3-4 virions lack ICP0 and have reduced but detectable levels of ICP4. However, it is possible that the small amount of ICP4 found in the d3-4 virion fraction could be due to contaminating nonvirion protein. To address this, we carried out an additional experiment in which we isolated WT and d3-4 virions using two sequential rounds of Ficoll gradient centrifugation. These more highly purified virions were then analyzed by immunoblotting as before (Fig. 5B). Again, both WT and d3-4 virions showed comparable levels of virion structural proteins VP5, VP16, and gD and lacked the nonstructural protein ICP27. Additionally, we examined the levels of the tegument protein VP22, which has been implicated in the virion incorporation of ICP0 and possibly ICP4 (12). It was present at similar levels in the two virion preparations. Importantly, the results for ICP0 and ICP4 were essentially the same as those observed for the virions isolated after a single round of Ficoll gradient purification; i.e., d3-4 virions lacked detectable ICP0 and contained reduced but detectable levels of ICP4.
As a more general method of comparing the protein compositions of the d3-4 and WT virions, we also analyzed the twice-gradient-purified virions by SDS-PAGE and silver staining (Fig. 5C). Although the d3-4 virions appeared to be somewhat overloaded relative to the WT sample, the two preparations appeared to exhibit roughly comparable protein patterns. This indicates that the overall protein contents of WT and d3-4 virions are similar. Of note is the fact that ICP0 and ICP4 are minor components of the virion and are not readily identified by this technique (12, 64).
Taken together, the experiments described above demonstrate that d3-4 virions differ from WT virions in that they lack ICP0 and have significantly reduced levels of ICP4. In addition, there is a reduction in the level of gC. Since d3-4 is defective at localizing ICP0/4 to the cytoplasm, these results indicate that cytoplasmic localization of ICP0 and ICP4 is required for their efficient incorporation into virus particles.
d3-4 expresses reduced levels of gC.
The failure of d3-4 virions to efficiently incorporate gC appeared to correlate with poor gC expression in d3-4-infected cells (Fig. 5). Although it has been previously shown that expression of the gC gene is highly dependent on ICP27 during infection (46, 52), these results were unexpected since d3-4 is replication competent. Furthermore, d3-4-infected Vero cells exhibited apparently normal expression of viral proteins in a metabolic labeling assay, although gC was not specifically examined (26). To ensure that reduced expression of gC in d3-4-infected cells is attributable to the deletion in the ICP27 gene and is not the result of an unrelated secondary mutation, we examined gC expression in cells infected with d3-4b, a genetically independent isolate of d3-4 (31). To replicate the conditions used for the virion preparations, the infections were carried out at an MOI of 0.1. Total infected-cell proteins were isolated at 24 and 48 hpi. The results of an immunoblotting analysis (Fig. 6) indicated that there was indeed a significant reduction in gC expression in both d3-4- and d3-4b-infected cells at both 24 and 48 hpi compared to WT-infected cells. In contrast, neither gD nor VP22 levels were reduced in the d3-4 infections. Regarding gD, the WT-encoded gD isolated at 48 hpi appeared to migrate a bit faster than did d3-4-encoded gD. However, this appears to be a gel anomaly, as WT and d3-4 gD comigrated in several other analyses (e.g., see the cell lysate data in Fig. 5). These results indicate that the d3-4 deletion in the ICP27 gene leads to a specific defect in expression of the gC gene. Moreover, the reduced expression of gC in d3-4-infected cells can readily explain its decreased incorporation into virions. This is in contrast to the results with ICP0 and ICP4, which are not packaged efficiently in d3-4 virions despite their normal levels of expression.
FIG. 6.
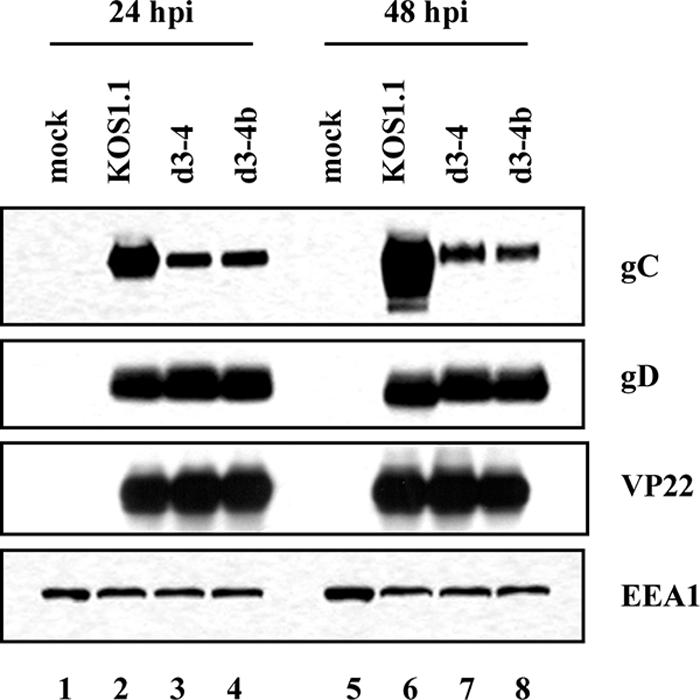
Analysis of gC expression in infected cells. Vero cells were infected with WT, d3-4, or d3-4b virus at an MOI of 0.1. Total proteins from equivalent numbers of cells were isolated at 24 and 48 hpi and analyzed for gC, gD, VP22, and EEA1 levels by immunoblotting.
DISCUSSION
Cytoplasmic localization of ICP0 and ICP4 is required for their incorporation into virions.
Although the nuclear regulatory functions of the HSV-1 transcriptional regulatory proteins ICP0 and ICP4 are well documented, it has not been clear what functions, if any, these two IE proteins might have in the cytoplasm at late times after infection. It has been suggested that the cytoplasmic localization of ICP0 might allow it to regulate translation via its documented physical interaction with translation elongation factor EF-1δ (21). The results of this study provide another explanation for why HSV-1 has evolved a mechanism to localize ICP0 and ICP4 to the cytoplasm late in infection: this allows these important transcriptional regulatory proteins to be incorporated into infectious virions. Our findings in this regard are consistent with the known assembly pathway of alphaherpesviral virions, in which many if not most tegument proteins are incorporated into virus particles either in the cytoplasm or on cytoplasmic membranes (34). It is also consistent with recent work suggesting that the virion packaging of ICP0 requires its localization to specific punctate sites in the cytoplasm (12).
In addition to mature virions, also termed heavy (H) particles, HSV-1 has the potential to produce virion-like particles, termed light (L) particles (49, 56, 63). L particles contain many HSV-1 envelope and tegument proteins, including ICP0 and ICP4, but lack capsids and genome-containing cores. They appear to arise via a capsid-independent pathway of tegumentation and envelopment (35). It has been shown previously that the host cell influences the degree of L particle formation but that in Vero cells, WT HSV-1 produces mostly H particles which yield a single light-scattering band when extracellular virions are purified on 5 to 15% Ficoll gradients (63). Consistent with this, we observed single virion bands for both the WT and d3-4 on our Ficoll gradients. It is worth noting that in some but not all of our experiments the single band of d3-4 virions appeared to be somewhat more dispersed than the WT band. Although the basis of this physical heterogeneity is unknown, our immunoblotting results show comparable levels of major capsid protein VP5 in the WT and d3-4 virion preparations (Fig. 5). This suggests that most of the particles in the d3-4 preparations contain capsids and are therefore H particles. At present, however, we cannot rule out the possibility that d3-4 produces some abnormal particles. We also have documented a slight virion release defect for d3-4, in that the ratio of secreted to cell-associated progeny in Vero cells is ∼7-fold lower for d3-4 than it is for WT HSV-1 (L. Sedlackova and S. Rice, unpublished data).
Role of ICP27 in virion incorporation of ICP0 and ICP4.
A second and somewhat surprising conclusion of our study is that the IE protein ICP27, itself not a virion protein, is a required cofactor for the efficient virion incorporation of ICP0 and ICP4. Analysis of the ICP27 deletion mutant d3-4 demonstrates that this assembly function of ICP27 is genetically separate from its other activities involved in viral gene induction. Given that ICP27 can promote the cytoplasmic accumulation of ICP0/4 even in the absence of infection, it is likely that ICP27's role in virion assembly is indirect. In other words, there is little evidence to suggest that ICP27 has a direct role in the virion assembly process. Rather, ICP27 may simply promote the cytoplasmic accumulation of ICP0 and ICP4, and once in the cytoplasm of infected cells, ICP0/4 may diffuse to sites of tegumentation or interact with viral components that traffic to such sites. In this regard, recent data suggest that tegument protein VP22 has a critical role in the assembly of ICP0 and possibly ICP4 into virions, possibly by recruiting these proteins into the punctate cytoplasmic domains which are associated with virion assembly (12).
The mechanism by which ICP27 promotes the cytoplasmic accumulation of ICP0/4 is unknown. Since ICP27 can affect ICP0/4 localization even in transfected cells, the mechanism clearly does not require the environment of the infected cell or other viral factors. One possible model is based on ICP27's nucleocytoplasmic shuttling activity and the fact that it can physically interact with ICP4 (42), which itself can interact with ICP0 (66). Thus, it is possible that ICP27 directly mediates the transport of ICP4 and ICP0 to the cytoplasm. It is worth pointing out, however, that d3-4 ICP27 is fully competent for nuclear export in an in vitro assay (5). Thus, if the above model is correct, it is not clear why d3-4 ICP27 would be unable to direct the movement of ICP0/4 to the cytoplasm, unless its mutation abrogates its interaction with ICP0/4. These issues are complicated by the fact that d3-4 ICP27 is partially defective for nuclear import, due to the deletion of its major nuclear localization signal (26, 31). We examined the localization of d3-4 ICP27 and found that, as expected, at late times after infection it shows an enhanced degree of cytoplasmic localization compared to WT ICP27. Further studies will be required to see if and how ICP27's shuttling activity affects the cellular distribution of ICP0 and ICP4.
Another possible model by which ICP27 could affect the nucleocytoplasmic localization of ICP0/4 was originally put forward by Zhu et al. (68, 70), who suggested that ICP27 might mediate its effects indirectly by modulating the phosphorylation of ICP0 and ICP4. There are many data to support such a model. First, it has been shown that the nucleocytoplasmic transport of proteins is often regulated by phosphorylation (19, 20). Second, ICP0 and ICP4, as well as ICP27, are all phosphoproteins with complex modification patterns, and in the case of ICP0 and ICP4, the patterns differ between the cytoplasm and nucleus (1, 2, 6, 61, 62, 67). Third, ICP27 alters the electrophoretic mobility of ICP4 in infected and transfected cells, likely via an effect on its phosphorylation (30, 45, 54). Fourth, ICP27 physically associates with and modulates the activity of a cellular protein kinase, CK2 (formerly called casein kinase II) (59), that plays an important role in numerous cellular processes, including signal transduction, transcriptional control, apoptosis, and cell cycle regulation (28). Moreover, both ICP0 and ICP4 have potential CK2 phosphorylation sites (7, 62). We have found that inhibition of CK2 activity by chemical agents delays but ultimately does not prevent the cytoplasmic localization of ICP0 and ICP4 in HSV-1-infected Vero cells (data not shown). This suggests a possible involvement of CK2 that merits further investigation. Finally, during HSV-1 infection, ICP27 has been implicated in activating a number of cellular signal transduction pathways (16, 17) which could affect the phosphorylation of ICP0 and ICP4 via cellular protein kinases.
It is interesting to compare our study on the nucleocytoplasmic distribution of ICP0/4 with studies on the ICP4 homolog of varicella-zoster virus (VZV), IE62. This polypeptide is an abundant tegument protein (23), and like both ICP0 and ICP4, it localizes to the nucleus early in lytic infection but to the cytoplasm at later times (24). It has been shown that protein kinase encoded by the VZV ORF66 gene, which is homologous to the HSV-1 US3 protein kinase, induces the cytoplasmic localization of IE62 by directly phosphorylating it near its major nuclear localization signal (11, 22). Thus, it appears that both HSV-1 and VZV utilize a common strategy to enable the virion incorporation of important IE transcriptional regulatory proteins, i.e., they express a viral cofactor (ICP27 and ORF66, respectively) which promotes the cytoplasmic localization of these proteins late in infection.
Role of ICP27 in virion incorporation of gC.
We were surprised to find that the d3-4 mutant virions contained less gC than WT virions. The reduced levels of virion gC can be readily explained by the fact that gC expression is significantly reduced in d3-4-infected cells. This is contrast to the situation for ICP0 and ICP4, which are not packaged into virions despite normal expression. Why is gC expression so poor in the d3-4 infection when most other viral proteins appear to be expressed normally? Recent studies from our laboratory have begun to provide an explanation. We have found that ICP27 suppresses the splicing of a cryptic 225-nucleotide intron in gC mRNA (K. Perkins et al., unpublished data). When excised, the spliced message encodes a gC variant that lacks the normal carboxyl-terminal coding region of gC, including the transmembrane domain. We have found that d3-4 and several other N-terminal mutants express enhanced levels of spliced gC mRNA and hence predominantly produce the secreted form of gC, which does not efficiently associate with the virion envelope (L. Sedlackova and S. Rice, unpublished data). Thus, it appears that ICP27 can affect the composition of both the tegument and envelope, but by apparently distinct mechanisms.
Potential biological functions of virion-localized ICP0 and ICP4.
It is unknown whether the relatively small amounts of virion-associated ICP0 and ICP4 (100 to 150 copies per virion produced in Vero or Hep-2 cells) (64, 65) could have regulatory functions in the newly infected cell. Clearly, our data with the d3-4 mutant indicate that replication in Vero cells is not greatly affected by virion ICP0/4. However, the same may not be true during infection of other cell types or in natural human infections. Interestingly, recent data suggest that the virion-localized ICP4 plays a role in genome circularization early in infection (55). Furthermore, as both ICP0 and ICP4 are powerful transcriptional activators, one could envision that virion-associated ICP0/4 could help stimulate viral gene expression at the very earliest stages of infection. ICP0 has been shown to be an important factor in allowing HSV-1 to resist the antiviral effects of type I interferons, one of which is to prevent the efficient expression of HSV-1 IE genes (18, 37). Thus, it is conceivable that in an infected host, virion-associated ICP0 could help the virus overcome a preexisting interferon blockade. We expect that the d3-4 mutant will be a useful tool in testing some of these ideas.
Finally, it is interesting to note that the host cell influences the degree to which ICP0 and ICP4 are packaged into virions (63). Based on our results, this difference may correlate with the extent to which ICP0 and ICP4 localize to the cytoplasm, which in turn is dependent on ICP27. If this function of ICP27 is mediated by its interaction with host cell factors, then the levels of ICP0 and ICP4 in progeny virions could vary in an infected host according to the identity or physiology of the particular cells which are infected. In this way, ICP27 may help to modulate the biological phenotype of infectious viral progeny.
Acknowledgments
We are grateful to Joy Lengyel and Oksana Goldman for excellent technical assistance, and we thank Gill Elliott for generously providing the VP22 antisera.
This research was supported by a grant from the NIH (R01-AI42737).
Footnotes
Published ahead of print on 24 October 2007.
REFERENCES
- 1.Ackermann, M., D. K. Braun, L. Pereira, and B. Roizman. 1984. Characterization of herpes simplex virus 1 alpha proteins 0, 4, and 27 with monoclonal antibodies. J. Virol. 52108-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Advani, S. J., R. Hagglund, R. R. Weichselbaum, and B. Roizman. 2001. Posttranslational processing of infected cell proteins 0 and 4 of herpes simplex virus 1 is sequential and reflects the subcellular compartment in which the proteins localize. J. Virol. 757904-7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aubert, M., S. A. Rice, and J. A. Blaho. 2001. Accumulation of herpes simplex virus type 1 early and leaky-late proteins correlates with apoptosis prevention in infected human HEp-2 cells. J. Virol. 751013-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bucks, M. A., J. O'Regan, K., M. A. Murphy, J. W. Wills, and R. J. Courtney. 2007. Herpes simplex virus type 1 tegument proteins VP1/2 and UL37 are associated with intranuclear capsids. Virology 361316-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, I. H., L. Li, L. Silva, and R. M. Sandri-Goldin. 2005. ICP27 recruits Aly/REF but not TAP/NXF1 to herpes simplex virus type 1 transcription sites although TAP/NXF1 is required for ICP27 export. J. Virol. 793949-3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davido, D. J., D. A. Leib, and P. A. Schaffer. 2002. The cyclin-dependent kinase inhibitor roscovitine inhibits the transactivating activity and alters the posttranslational modification of herpes simplex virus type 1 ICP0. J. Virol. 761077-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davido, D. J., W. F. von Zagorski, W. S. Lane, and P. A. Schaffer. 2005. Phosphorylation site mutations affect herpes simplex virus type 1 ICP0 function. J. Virol. 791232-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeLuca, N. A., A. M. McCarthy, and P. A. Schaffer. 1985. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J. Virol. 56558-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeLuca, N. A., and P. A. Schaffer. 1987. Activities of herpes simplex virus type 1 (HSV-1) ICP4 genes specifying nonsense peptides. Nucleic Acids Res. 154491-4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duffy, C., J. H. Lavail, A. N. Tauscher, E. G. Wills, J. A. Blaho, and J. D. Baines. 2006. Characterization of a UL49-null mutant: VP22 of herpes simplex virus type 1 facilitates viral spread in cultured cells and the mouse cornea. J. Virol. 808664-8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisfeld, A. J., S. E. Turse, S. A. Jackson, E. C. Lerner, and P. R. Kinchington. 2006. Phosphorylation of the varicella-zoster virus (VZV) major transcriptional regulatory protein IE62 by the VZV open reading frame 66 protein kinase. J. Virol. 801710-1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elliott, G., W. Hafezi, A. Whiteley, and E. Bernard. 2005. Deletion of the herpes simplex virus VP22-encoding gene (UL49) alters the expression, localization, and virion incorporation of ICP0. J.Virol. 799735-9745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elliott, G., and P. O'Hare. 1997. Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell 88223-233. [DOI] [PubMed] [Google Scholar]
- 14.Everett, R. D. 2006. The roles of ICP0 diuring HSV-1 infection., p. 39-63. In R. M. Sandri-Goldin (ed.), Alpha herpesviruses: molecular and cellular biology. Caister Academic Press, Norwich, United Kingdom.
- 15.Fraser, K. A., and S. A. Rice. 2005. Herpes simplex virus type 1 infection leads to loss of serine-2 phosphorylation on the carboxyl-terminal domain of RNA polymerase II. J. Virol. 7911323-11334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hargett, D., T. McLean, and S. L. Bachenheimer. 2005. Herpes simplex virus ICP27 activation of stress kinases JNK and p38. J. Virol. 798348-8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hargett, D., S. Rice, and S. L. Bachenheimer. 2006. Herpes simplex virus type 1 ICP27-dependent activation of NF-κB. J. Virol. 8010565-10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harle, P., B. Sainz, Jr., D. J. Carr, and W. P. Halford. 2002. The immediate-early protein, ICP0, is essential for the resistance of herpes simplex virus to interferon-alpha/beta. Virology. 293295-304. [DOI] [PubMed] [Google Scholar]
- 19.Harreman, M. T., T. M. Kline, H. G. Milford, M. B. Harben, A. E. Hodel, and A. H. Corbett. 2004. Regulation of nuclear import by phosphorylation adjacent to nuclear localization signals. J. Biol. Chem. 27920613-20621. [DOI] [PubMed] [Google Scholar]
- 20.Hood, J. K., and P. A. Silver. 1999. In or out? Regulating nuclear transport. Curr. Opin. Cell Biol. 11241-247. [DOI] [PubMed] [Google Scholar]
- 21.Kawaguchi, Y., R. Bruni, and B. Roizman. 1997. Interaction of herpes simplex virus 1 alpha regulatory protein ICP0 with elongation factor 1δ: ICP0 affects translational machinery. J. Virol. 711019-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinchington, P. R., K. Fite, A. Seman, and S. E. Turse. 2001. Virion association of IE62, the varicella-zoster virus (VZV) major transcriptional regulatory protein, requires expression of the VZV open reading frame 66 protein kinase. J. Virol. 759106-9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinchington, P. R., J. K. Hougland, A. M. Arvin, W. T. Ruyechan, and J. Hay. 1992. The varicella-zoster virus immediate-early protein IE62 is a major component of virus particles. J. Virol. 66359-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinchington, P. R., and S. E. Turse. 1998. Regulated nuclear localization of the varicella-zoster virus major regulatory protein, IE62. J. Infect. Dis. 178(Suppl. 1)S16-S21. [DOI] [PubMed] [Google Scholar]
- 25.Knipe, D. M., D. Senechek, S. A. Rice, and J. L. Smith. 1987. Stages in the nuclear association of the herpes simplex virus transcriptional activator protein ICP4. J. Virol. 61276-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lengyel, J., C. Guy, V. Leong, S. Borge, and S. A. Rice. 2002. Mapping of functional regions in the amino-terminal portion of the herpes simplex virus ICP27 regulatory protein: importance of the leucine-rich nuclear export signal and RGG box RNA-binding domain. J. Virol. 7611866-11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lengyel, J., A. K. Strain, K. D. Perkins, and S. A. Rice. 2006. ICP27-dependent resistance of herpes simplex virus type 1 to leptomycin B is associated with enhanced nuclear localization of ICP4 and ICP0. Virology 352368-379. [DOI] [PubMed] [Google Scholar]
- 28.Litchfield, D. W. 2003. Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem. J. 3691-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCarthy, A. M., L. McMahan, and P. A. Schaffer. 1989. Herpes simplex virus type 1 ICP27 deletion mutants exhibit altered patterns of transcription and are DNA deficient. J. Virol. 6318-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McMahan, L., and P. A. Schaffer. 1990. The repressing and enhancing functions of the herpes simplex virus regulatory protein ICP27 map to C-terminal regions and are required to modulate viral gene expression very early in infection. J. Virol. 643471-3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mears, W. E., V. Lam, and S. A. Rice. 1995. Identification of nuclear and nucleolar localization signals in the herpes simplex virus regulatory protein ICP27. J. Virol. 69935-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mears, W. E., and S. A. Rice. 1998. The herpes simplex virus immediate-early protein ICP27 shuttles between nucleus and cytoplasm. Virology 242128-137. [DOI] [PubMed] [Google Scholar]
- 33.Mears, W. E., and S. A. Rice. 1996. The RGG box motif of the herpes simplex virus ICP27 protein mediates an RNA-binding activity and determines in vivo methylation. J. Virol. 707445-7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mettenleiter, T. C. 2002. Herpesvirus assembly and egress. J. Virol. 761537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mettenleiter, T. C. 2006. Intriguing interplay between viral proteins during herpesvirus assembly or: the herpesvirus assembly puzzle. Vet. Microbiol. 113163-169. [DOI] [PubMed] [Google Scholar]
- 36.Mettenleiter, T. C., B. G. Klupp, and H. Granzow. 2006. Herpesvirus assembly: a tale of two membranes. Curr. Opin. Microbiol. 9423-429. [DOI] [PubMed] [Google Scholar]
- 37.Mossman, K. L., H. A. Saffran, and J. R. Smiley. 2000. Herpes simplex virus ICP0 mutants are hypersensitive to interferon. J. Virol. 742052-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nabel, G. J., S. A. Rice, D. M. Knipe, and D. Baltimore. 1988. Alternative mechanisms for activation of human immunodeficiency virus enhancer in T cells. Science 2391299-1302. [DOI] [PubMed] [Google Scholar]
- 39.Naldinho-Souto, R., H. Browne, and T. Minson. 2006. Herpes simplex virus tegument protein VP16 is a component of primary enveloped virions. J. Virol. 802582-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Newcomb, W. W., F. P. Booy, and J. C. Brown. 2007. Uncoating the herpes simplex virus genome. J. Mol. Biol. 370633-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orlando, J. S., J. W. Balliet, A. S. Kushnir, T. L. Astor, M. Kosz-Vnenchak, S. A. Rice, D. M. Knipe, and P. A. Schaffer. 2006. ICP22 is required for wild-type composition and infectivity of herpes simplex virus type 1 virions. J. Virol. 809381-9390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Panagiotidis, C. A., E. K. Lium, and S. J. Silverstein. 1997. Physical and functional interactions between herpes simplex virus immediate-early proteins ICP4 and ICP27. J. Virol. 711547-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perkins, K. D., J. Gregonis, S. Borge, and S. A. Rice. 2003. Transactivation of a viral target gene by herpes simplex virus ICP27 is posttranscriptional and does not require the endogenous promoter or polyadenylation site. J. Virol. 779872-9884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quinlan, M. P., L. B. Chen, and D. M. Knipe. 1984. The intranuclear location of a herpes simplex virus DNA-binding protein is determined by the status of viral DNA replication. Cell 36857-868. [DOI] [PubMed] [Google Scholar]
- 45.Rice, S. A., and D. M. Knipe. 1988. Gene-specific transactivation by herpes simplex virus type 1 alpha protein ICP27. J. Virol. 623814-3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rice, S. A., and D. M. Knipe. 1990. Genetic evidence for two distinct transactivation functions of the herpes simplex virus alpha protein ICP27. J. Virol. 641704-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rice, S. A., and V. Lam. 1994. Amino acid substitution mutations in the herpes simplex virus ICP27 protein define an essential gene regulation function. J. Virol. 68823-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rice, S. A., V. Lam, and D. M. Knipe. 1993. The acidic amino-terminal region of herpes simplex virus type 1 alpha protein ICP27 is required for an essential lytic function. J. Virol. 671778-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rixon, F. J., C. Addison, and J. McLauchlan. 1992. Assembly of enveloped tegument structures (L particles) can occur independently of virion maturation in herpes simplex virus type 1-infected cells. J. Gen. Virol. 73277-284. [DOI] [PubMed] [Google Scholar]
- 50.Sandri-Goldin, R. M. 2006. The functions and activities of herpes simplex virus protein ICP27, a multifunctional regulator of gene expression p. 65-83. In R. M. Sandri-Goldin (ed.), Alpha herpesviruses: molecular and cellular biology. Caister Academic Press, Norfolk, United Kingdom.
- 51.Sekulovich, R. E., K. Leary, and R. M. Sandri-Goldin. 1988. The herpes simplex virus type 1 alpha protein ICP27 can act as a trans-repressor or a trans-activator in combination with ICP4 and ICP0. J. Virol. 624510-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith, I. L., M. A. Hardwicke, and R. M. Sandri-Goldin. 1992. Evidence that the herpes simplex virus immediate early protein ICP27 acts post-transcriptionally during infection to regulate gene expression. Virology 18674-86. [DOI] [PubMed] [Google Scholar]
- 53.Smith, R. W., P. Malik, and J. B. Clements. 2005. The herpes simplex virus ICP27 protein: a multifunctional post-transcriptional regulator of gene expression. Biochem. Soc. Trans. 33499-501. [DOI] [PubMed] [Google Scholar]
- 54.Su, L., and D. M. Knipe. 1989. Herpes simplex virus alpha protein ICP27 can inhibit or augment viral gene transactivation. Virology 170496-504. [DOI] [PubMed] [Google Scholar]
- 55.Su, Y. H., X. Zhang, X. Wang, N. W. Fraser, and T. M. Block. 2006. Evidence that the immediate-early gene product ICP4 is necessary for the genome of the herpes simplex virus type 1 ICP4 deletion mutant strain d120 to circularize in infected cells. J. Virol. 8011589-11597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Szilagyi, J. F., and C. Cunningham. 1991. Identification and characterization of a novel non-infectious herpes simplex virus-related particle. J. Gen. Virol. 72661-668. [DOI] [PubMed] [Google Scholar]
- 57.Van Sant, C., P. Lopez, S. J. Advani, and B. Roizman. 2001. Role of cyclin D3 in the biology of herpes simplex virus 1 ICPO. J. Virol. 751888-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vittone, V., E. Diefenbach, D. Triffett, M. W. Douglas, A. L. Cunningham, and R. J. Diefenbach. 2005. Determination of interactions between tegument proteins of herpes simplex virus type 1. J. Virol. 799566-9571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wadd, S., H. Bryant, O. Filhol, J. E. Scott, T. Y. Hsieh, R. D. Everett, and J. B. Clements. 1999. The multifunctional herpes simplex virus IE63 protein interacts with heterogeneous ribonucleoprotein K and with casein kinase 2. J. Biol. Chem. 27428991-28998. [DOI] [PubMed] [Google Scholar]
- 60.Weinheimer, S. P., B. A. Boyd, S. K. Durham, J. L. Resnick, and D. R. O'Boyle II. 1992. Deletion of the VP16 open reading frame of herpes simplex virus type 1. J. Virol. 66258-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilcox, K. W., A. Kohn, E. Sklyanskaya, and B. Roizman. 1980. Herpes simplex virus phosphoproteins. I. Phosphate cycles on and off some viral polypeptides and can alter their affinity for DNA. J. Virol. 33167-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xia, K., N. A. DeLuca, and D. M. Knipe. 1996. Analysis of phosphorylation sites of herpes simplex virus type 1 ICP4. J. Virol. 701061-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang, T. Y., and R. J. Courtney. 1995. Influence of the host cell on the association of ICP4 and ICP0 with herpes simplex virus type 1. Virology 211209-217. [DOI] [PubMed] [Google Scholar]
- 64.Yao, F., and R. J. Courtney. 1989. A major transcriptional regulatory protein (ICP4) of herpes simplex virus type 1 is associated with purified virions. J. Virol. 633338-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yao, F., and R. J. Courtney. 1992. Association of ICP0 but not ICP27 with purified virions of herpes simplex virus type 1. J. Virol. 662709-2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yao, F., and P. A. Schaffer. 1994. Physical interaction between the herpes simplex virus type 1 immediate-early regulatory proteins ICP0 and ICP4. J. Virol. 688158-8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhi, Y., and R. M. Sandri-Goldin. 1999. Analysis of the phosphorylation sites of herpes simplex virus type 1 regulatory protein ICP27. J. Virol. 733246-3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu, Z., W. Cai, and P. A. Schaffer. 1994. Cooperativity among herpes simplex virus type 1 immediate-early regulatory proteins: ICP4 and ICP27 affect the intracellular localization of ICP0. J. Virol. 683027-3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu, Z., N. A. DeLuca, and P. A. Schaffer. 1996. Overexpression of the herpes simplex virus type 1 immediate-early regulatory protein, ICP27, is responsible for the aberrant localization of ICP0 and mutant forms of ICP4 in ICP4 mutant virus-infected cells. J. Virol. 705346-5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu, Z., and P. A. Schaffer. 1995. Intracellular localization of the herpes simplex virus type 1 major transcriptional regulatory protein, ICP4, is affected by ICP27. J. Virol. 6949-59. [DOI] [PMC free article] [PubMed] [Google Scholar]