Abstract
More than 50 years of genetic analysis has identified a number of host genes that are required for the expansion of infected cells during the progression of Friend-virus-induced erythroleukemia. In this report, we show that Friend virus induces the bone morphogenetic protein 4 (BMP4)-dependent stress erythropoiesis pathway in the spleen, which rapidly amplifies target cells, propagating their infection and resulting in acute splenomegaly. This mechanism mimics the response to acute anemia, in which BMP4 expressed in the spleen drives the expansion of a specialized population of stress erythroid progenitors. Previously we demonstrated that these progenitors, termed stress BFU-E, are targets for Friend virus in the spleen (A. Subramanian, H. E. Teal, P. H. Correll, and R. F. Paulson, J. Virol. 79:14586-14594, 2005). Here, we extend those findings by showing that Friend virus infects two distinct populations of bone marrow cells. One population, when infected, differentiates into mature erythrocytes in an Epo-independent manner, while a second population migrates to the spleen after infection, where it induces BMP4 expression and acts as a reservoir of virus. The activation of the stress erythropoiesis pathway in the spleen by Friend virus results in the rapid expansion of stress BFU-E, providing abundant target cells for viral infection. These observations suggest a novel mechanism by which a virus induces a stress response pathway that amplifies target cells for the virus, leading to acute expansion of infected cells.
Friend erythroleukemia virus (FV) is a complex of two retroviruses, the replication-defective spleen focus-forming virus (SFFV), which is the pathogenic component, and the replication-competent Friend murine leukemia virus. FV induces an acute erythroleukemia that proceeds through a characteristic two-stage progression (4, 35). The initial stage is characterized by the polyclonal expansion of infected cells in the bone marrow and spleen of susceptible mice. In the late stage of disease, a clone of infected cells acquires new mutations, specifically mutation of p53 (34) and proviral insertional activation of Spi1/Pu.1 (33), which leads to the emergence of a leukemic clone and eventually erythroleukemia.
The tropism of the virus for the erythroid lineage and the characteristic two-stage progression has allowed for the identification of a number of host loci involved in the pathogenesis of Friend erythroleukemia (22, 42). Two genes, Fv1 (7) and Fv4 (25), directly affect the ability of the virus to infect target cells by interfering with the retroviral life cycle. Four other genes regulate the expansion of infected cells during the initial stage of Friend disease, the Friend virus susceptibility gene 2 (Fv2) (28), Dominant white spotting (W) (40), Steel (Sl) (5), and flexed-tail (f) (2). Fv2 encodes a naturally occurring truncated form of the macrophage-stimulating 1 receptor (Mst1r), which is referred to as short-form Stk, or Sf-Stk (38). Sf-Stk interacts with the viral envelop glycoprotein from SFFV, gp55, and the erythropoietin receptor, EpoR (16, 36). This complex drives the expansion of infected cells and the Epo-independent differentiation of cells infected with the polycythemia-inducing variant of FV, FVP. Recent work has shown that Sf-Stk can drive the expansion of infected cells in the absence of EpoR in vivo, suggesting that Sf-Stk signaling is responsible for the polyclonal expansion of infected cells during the initial stage of Friend disease (48). Sf-Stk is produced from an alternative promoter located in intron 10 of the gene. Fv2-resistant strains (Fv2r/r) fail to express Sf-Stk due to a mutation in the Sf-Stk promoter and consequently fail to expand infected cells in the spleen (38).
W and Sl encode the Kit receptor tyrosine kinase and its ligand stem cell factor (SCF), respectively (9, 14, 17, 49). Mutation of either locus results in severe macrocytic anemia. The original work demonstrating that W and Sl mice were resistant to FV suggested that the defect in erythropoiesis that leads to the severe anemia was responsible for the resistance (5, 40). However, recent work from our laboratory has demonstrated that pathogenic targets for FV require the Kit/SCF signaling pathway in the spleen but not the bone marrow (45). These studies identified the target cells for FV in the spleen and showed that they were present in the spleen megakaryocyte erythroid progenitor (MEP) population originally described by Akashi et al. (1). W/Wv mutant mice exhibit a 10-fold reduction in spleen MEPs but have normal numbers of bone marrow target cells. These data supported the early work from Mirand and colleagues that showed that a spleen was necessary for the pathogenesis of Friend erythroleukemia; thus, the defect in spleen target cells in W/Wv mice accounts for their resistance (29, 31).
f/f mutant mice exhibit a fetal-neonatal anemia that resolves 2 weeks after birth (19, 20, 23). As adults, the mice exhibit normal steady-state blood parameters and have normal numbers of erythroid progenitors in the bone marrow. However, when challenged with an acute anemia, f/f mice are slow to recover, which suggests that they have a defect in expansive erythropoiesis that occurs at times of acute erythropoietic stress (13, 18). We cloned the f locus and showed that it encoded Madh5 or Smad5, a receptor-activated Smad that acts downstream of the receptors for BMP2, BMP4, and BMP7 (27). Analysis of the f/f mutant phenotype showed that in response to acute anemia, BMP4 is induced in the spleen and drives the expansion of a specialized population of stress erythroid progenitors, which we term stress BFU-E. BMP4 signaling is required only transiently and induces the differentiation of an earlier cell, the BMP4-responsive (BMP4R) cell, into the Epo-responsive stress BFU-E. These progenitors exhibit properties distinct from those of bone marrow steady-state BFU-E in that they require only Epo to rapidly generate large colonies. Analysis of spleen cell populations showed that the BMP4R stress BFU-E are present in the spleen MEP fraction, which are also the targets for FV in the spleen, suggesting that the BMP4-dependent stress erythropoiesis pathway is involved in the pathogenesis of Friend erythroleukemia (45). This link between FV and stress erythropoiesis also is supported by the observation that f/f mice are resistant to FV-induced erythroleukemia (2).
In this report, we show that FV utilizes the BMP4-dependent stress erythropoiesis pathway during the initial stage of Friend erythroleukemia. f/f mutant mice lack target cells in the bone marrow and spleen, which results in resistance to FV. Conditions that lead to the expansion of stress BFU-E, such as the induction of acute anemia in vivo or treatment of spleen cells with BMP4 in vitro, significantly expand the number of target cells in the spleen. Infection with FV leads to BMP4 expression in the spleens of wild-type mice but not f/f mutant mice, suggesting that in addition to the target cell defect, f/f mice have a defect in the spleen microenvironment. Our earlier analysis of W/Wv mice identified MEPs as the target cells in the spleen (45). Here we identify two distinct populations of target cells in the bone marrow, one that expresses Sf-Stk and forms Epo-independent (Epoind) BFU-E following FV infection and a second population that migrates to the spleen following infection and induces BMP4 expression. These latter cells act as infectious center (IC) cells propagating infection of the stress BFU-E, which rapidly expand in response to BMP4. The acute expansion of infected cells in the spleen and the rapid progression to erythroleukemia are a direct result of the ability of FV to activate the BMP4-dependent stress erythropoiesis pathway. These observations suggest a novel mechanism by which a virus induces a physiological response that results in the amplification of target cells, allowing for the acute propagation of the infection.
MATERIALS AND METHODS
Mice.
BALB/c and BALB/c-f/f mice were bred and maintained in our colony. For BALB/c-f/f mice, the f mutation was bred for four to five generations onto the BALB/c background. Induction of acute anemia by the injection of phenylhydrazine (PHZ) was done as previously described (27). All research involving the use of mice was performed in strict accordance with protocols approved by the Institutional Animal Care and Use Committee of the Pennsylvania State University.
BFU-E colony assays.
For in vitro BFU-E formation, total bone marrow and spleen cells were harvested from mice. All assays for Epoind BFU-E formation used FVP derived from a single batch of supernatant from FP63 cells. FP63 cells were a kind gift from Alan Bernstein, Mount Sinai Hospital, Toronto, Ontario, Canada. The amount of FP63 supernatant used was determined to produce the maximal number of Epoind BFU-E according to tests using the infection of control bone marrow cells. FP63 supernatant or an equivalent volume of Dulbecco's modified Eagle's medium (DMEM) (mock infection) was incubated with bone marrow (2.5 × 106 cells total) on ice for 1 h or spleen (6.5 × 106 cells total) for 1.5 h. For bone marrow assays, FV-infected cells were plated in Methocult medium (Stem Cell Technologies, Vancouver, Canada) containing interleukin-3 (IL-3) (2.5 ng/ml). Control cells were plated in Methocult medium containing IL-3 (2.5 ng/ml) and Epo (3 U/ml). The cells then were plated in 6-well plates in triplicate at a final concentration of 5 × 105 cells per well. For spleen assays, FV-infected cells were plated in Methocult medium containing IL-3 (2.5 ng/ml) and SCF (100 ng/ml) (Peprotech Inc., Rocky Hill, NJ). Control cells were plated in a solution of IL-3 (2.5 ng/ml), SCF (100 ng/ml), and Epo (3 U/ml). The final concentration of cells was 1.5 × 106 cells per well in triplicate in 6-well plates. The cultures were scored for BFU-E using acid benzidine staining as previously described (16). For assays examining the ability of BMP4 to increase the number of spleen target cells, spleen cells were preincubated with 15 ng/ml BMP4 (R&D Systems, Minneapolis, MN) in DMEM prior to infection. The cells, with or without BMP4, were infected and plated as described above.
IC cell transplantation assays.
Bone marrow cells were isolated from BALB/cJ and BALB/c-f mice. Red blood cells were lysed using 0.16 M NH4Cl. For unfractionated bone marrow cells, the cells were infected on ice with supernatant from FP63 cells as described above for Epoind colony assays. The infected cells were washed three times with phosphate-buffered saline (PBS) to remove surface-bound virus. A total of 4 × 106 infected cells were injected into tail veins of respective recipient mice. Two weeks later, mice were sacrificed and spleens were isolated, weighed, and fixed in Bouin's fixative (Sigma, St. Louis, MO). IC cell transplantation assays with sorted bone marrow cells were done as described above, except that fewer cells were infected and later transplanted. For CD31+ Kit+ Sca1− Lin− cells, 1 × 105 infected cells were transplanted per recipient, and for CD31+ CD41+ Kit+ Sca1− Lin− cells, 5 × 104 infected cells were transplanted.
Analysis of Sf-Stk expression.
Total RNA was isolated from sorted populations of bone marrow and spleen cells. The different sorted populations were lysed in Trizol (Invitrogen, Carlsbad, CA) and reverse transcribed into cDNA. Primer sequences for Sf-Stk were 5′-TCTGGCTGATCCTTCTGTCTG-3′ and 5′-GCAGCAGTGGGACACTTGTCC-3′ (456-bp product) (38). Hypoxanthine phosphoribosyltransferase or β-actin was used as an internal control.
Analysis of BMP4 expression in spleen sections.
Spleens were harvested on the indicated days after FV infection and fixed in 4% paraformaldehyde, and paraffin-embedded tissue sections were cut. Sections were deparaffinized in Histo-clear II (National Diagnostics, Atlanta, GA), rehydrated through an ethanol series to 50% ethanol, blocked in protein-blocking agent (Immunotech, Westbrook, ME) for 1 h to overnight, and rinsed in PBS. Sections were incubated with primary anti-BMP4 antibody (Novocastra Labs, Vector Labs, Burlingame, CA) conjugated to Alexa 667 using the labeling kit from Molecular Probes (Eugene, OR). Some sections also were labeled with mAb34 (12) (provided by K. Hasenkrug, Rocky Mountain Labs, National Institute of Allergy and Infectious Diseases) conjugated to Alexa 488 labeling dye. Labeling antibodies were diluted at a 1:20 concentration. Slides were incubated for 2 to 4 h in a dark humid chamber. Sections were washed in PBS and mounted in Slow Fade (Molecular Probes). Slides were analyzed by confocal microscopy using an Olympus Fluoview 300 confocal laser-scanning microscope.
Fluorescence-activated cell sorter analysis of bone marrow and spleen target populations.
Bone marrow and spleen cells were stained for MEPs as described earlier (1, 27). Bone marrow and spleen cells were sorted for the CD31+ subsets by using biotinylated lineage markers (Molecular Probes) and biotin-conjugated Sca-1 (Pharmingen, San Diego, CA). Sca+ Lin+ cells were removed using streptavidin-conjugated magnetic beads and the Easy-SEP magnet (Stemcell Technologies, Vancouver, Canada). The remaining cells were stained with fluorescein isothiocyanate-conjugated anti-Kit and phycoerythrin-conjugated anti-CD31 (Pharmingen, San Diego, CA). CD41+ subsets were obtained by removing Sca+ Lin+ subsets and staining the remaining cells with fluorescein isothiocyanate-conjugated c-Kit, allophycocyanin-conjugated CD31, and phycoerythrin-conjugated CD41 (Pharmingen). Cells were washed and sorted using a Coulter Elite ESP flow cytometer. For sorting peripheral blood cells, peripheral blood was collected into tubes containing heparin. Peripheral blood mononuclear cells were isolated by layering the blood onto a Ficoll gradient (Histopaque 1077; Sigma) and collecting the cells at the interface following centrifugation. Analysis of CD31+ Kit+ CD41+ Sca1− Lin− cells was done as described above.
RESULTS
f/f mutant mice exhibit decreased numbers of FV target cells in the bone marrow and spleen.
f/f mice are maintained on the C57BL/6 background, which is Fv2r/r and is resistant to FV. In order to circumvent this problem, we crossed the f mutant allele for four to five generations onto the BALB/c background (BALB/c-f/f), which is Fv2 sensitive (Fv2s/s) and is sensitive to FV infection. In vitro infection of bone marrow and spleen cells from sensitive mice with FVP results in the formation of Epoind BFU-E colonies. These colonies also have been referred to as viral BFU-E or vBFU-E; however, in this paper we will refer to them as Epoind BFU-E (26). Previous work from our laboratory has shown that this assay accurately reflects the in vivo sensitivity of different mouse strains to FV-induced erythroleukemia and is a measure of the number of pathogenic targets for FV (16, 26, 45). We tested whether bone marrow and spleen cells from BALB/c-f/f and BALB/c-f/+ mice could generate Epoind BFU-E after FV infection in vitro. Figure 1A shows that BALB/c-f/f bone marrow and spleen cells generated significantly fewer Epoind BFU-E following FVP infection than the number of BFU-E generated when cells are cultured with Epo. In contrast, bone marrow and spleen cells from BALB/c-f/+ mice exhibited no significant difference between the number of Epoind BFU-E generated following FVP infection and the number of BFU-E generated when Epo was included in the medium. Furthermore, BALB/c-f/+ mice are sensitive to infection in vivo, exhibiting splenomegaly 14 days after infection that is indistinguishable from that of BALB/c control mice (data not shown). These data demonstrate that f/f mice have decreased numbers of target cells in their bone marrow and spleen. This observed decrease in the numbers of target cells could be due to an actual reduction in the number of target cells or an inability of these target cells to respond to FV infection. Previously, we showed that the targets for FV in the spleen are contained in the MEP population (45). We sorted MEPs from BALB/c and BALB/c-f/f mice and observed no difference in the number of spleen MEPs (data not shown). These observations suggest that f/f MEPs must be defective in their ability to respond to FV. Sf-Stk is required for FV to induce Epoind BFU-E formation. Analysis of Sf-Stk expression in the spleens of f/f and control mice demonstrated that f/f spleen MEPs fail to express Sf-Stk. These data suggest that Smad5-dependent signals are required for the proper expression of Sf-Stk in these cells (Fig. 1B). However, despite repeated attempts, treatment of spleen cells with BMP4 in vitro does not induce the expression of Sf-Stk (data not shown), which suggests that the regulation of Sf-Stk by Smad5 is indirect.
FIG. 1.
f/f mice have decreased numbers of target cells in the spleen and bone marrow. (A) BALB/c-f/f and BALB/c-f/+ bone marrow (left) and spleen (right) cells were mock infected or infected with FVP and were plated in the indicated cytokines. BFU-E were scored. (B) RT-PCR analysis of Sf-Stk expression in MEPs that were sorted from BALB/c-f/f and BALB/c spleen. Bars represent the averages ± standard deviations from one representative experiment of four independent experiments.
Acute anemia induces FV target cells in the spleen but not the bone marrow.
PHZ injection induces an acute hemolytic anemia from which control mice take 6 to 7 days to recover. In contrast, f/f mice exhibit a delayed recovery that takes approximately 8 to 9 days. Our analysis of the recovery from acute anemia has demonstrated that a specialized population of stress erythroid progenitors, which we term stress BFU-E, rapidly expands in the spleen during the recovery period. The peak expansion of stress BFU-E is observed at 36 h after PHZ treatment. f/f mutant mice exhibit a delayed expansion of stress BFU-E, which occurs at 4 days after treatment and results in the delayed recovery (27). The number of bone marrow BFU-E does not change significantly during recovery except in the later stages, when their numbers start to decrease (27). Stress BFU-E are contained in the spleen MEP population, which also contains the targets for FV (27, 45). Based on these data, we predict that the treatment of control mice with PHZ causes an expansion of FV target cells at 36 h after treatment; however, in f/f mice the expansion would be delayed until 4 days after treatment. Figure 2A shows that in BALB/c mice, the number of FVP-induced Epoind BFU-E is significantly increased 36 h after PHZ treatment. In contrast, BALB/c-f/f mice show no change in the number of FVP-induced Epoind BFU-E 36 h after treatment with PHZ. However, at 4 days after treatment, BALB/c-f/f mice exhibited a significant increase in Epoind BFU-E following FVP infection, which correlates with the delayed expansion of stress BFU-E we previously observed in f/f mice (27). Control mice exhibit decreased levels of Epoind BFU-E 4 days after PHZ treatment, which also is consistent with our observation that the numbers of stress BFU-E decline 4 days after PHZ treatment of control mice. This expansion of target cells in the spleen of BALB/c-f/f mice following acute anemia induction also translates into sensitivity to FVP-induced disease. BALB/c-f/f mice were treated with PHZ, and 4 days after treatment they were infected with FVP. Two weeks later, these mice developed extensive splenomegaly that was indistinguishable from that observed for control mice infected with FVP. (Average spleen weights were the following: FVP-infected, PHZ-treated BALB/c-f/f mice, 3.15 g; uninfected, PHZ-treated BALB/c-f/f mice, 1.2 g; untreated mice, 0.2 g [n = 2]. For comparison, the average spleen weight for an FVP-infected BALB/c mouse is 2.5 g.) This experiment also shows that FV can replicate in the mutant mice. Thus, defects in viral replication are not the root cause of the resistance in f/f mice. In contrast, the number of bone marrow Epoind BFU-E was unaffected by PHZ treatment (Fig. 2B). These data suggest that the resistance in the f/f mice is caused by a lack of target cells in the spleen. Inducing the expansion of target cells by activating the BMP4-dependent stress erythropoiesis pathway with PHZ renders f/f mice sensitive to FV.
FIG. 2.
PHZ-induced acute anemia expands FV target cells in the spleen but not the bone marrow. (A) BALB/c and BALB/c-f/f mice were treated with PHZ to induce acute anemia. Thirty-six hours and 4 days after treatment, spleen cells were harvested and either mock infected or infected with FVP. The cells then were plated in methylcellulose medium containing Epo, SCF, and IL-3 (mock infected) or SCF and IL-3 (FVP infected), and BFU-E were scored. (B) BALB/c mice were treated with PHZ to induce anemia, and bone marrow cells were harvested 36 h after treatment. The cells were mock infected or infected with FVP and were plated in methylcellulose medium containing Epo, SCF, and IL-3 (mock infected) or SCF and IL-3 (FVP infected), and BFU-E were scored. Significant differences as measured by t tests are indicated. Bars represent the averages ± standard deviations from one representative experiment of four independent experiments.
Treatment of spleen cells with BMP4 in vitro increases the number of target cells.
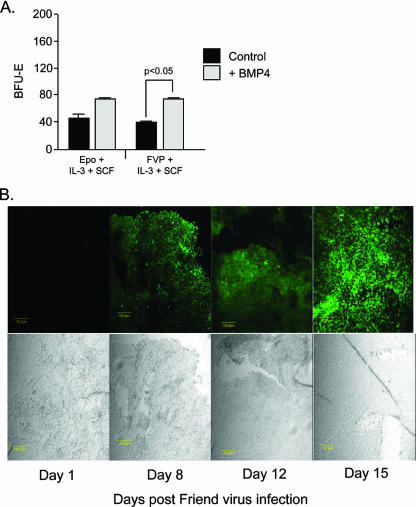
Acute anemia induces the expression of BMP4 in the spleen, where it acts on an immature cell, the BMP4R cell, causing it to become an Epo-responsive stress BFU-E. Treatment of spleen cells in vitro with BMP4 results in a significant expansion of stress BFU-E (27). Based on our observation that acute anemia increases the number of target cells in the spleen, we next tested whether culturing spleen cells with BMP4 could increase the number of FV target cells in vitro. Spleen cells from BALB/c mice were cultured in medium containing BMP4 (15 ng/ml) for 24 h and then tested for the ability of FVP to induce Epoind BFU-E. As shown in Fig. 3A, culturing cells in BMP4 significantly increased the number of Epoind BFU-E following infection with FVP in BALB/c mice. Thus, acute anemia expands the number of FV target cells in vivo and treatment with BMP4 in vitro increases the number of FV targets, which further implicates the BMP4-dependent stress erythropoiesis pathway in the pathogenesis of Friend erythroleukemia.
FIG. 3.
BMP4 treatment increases the number of target cells in the spleen, and its expression is induced in the spleen by FVP infection. (A) BALB/c spleen cells were mock infected or infected with FVP and were plated in methylcellulose medium containing Epo, SCF, and IL-3 (mock infected) or SCF and IL-3 (FVP infected), with or without added BMP4 (15 ng/ml). BFU-E were scored. Significant differences as measured by t tests are indicated. Bars indicate averages ± standard deviations from one representative experiment of three independent experiments. (B) Spleen sections of BALB/c mice isolated on the indicated days postinfection with FVP. The sections are stained with anti-BMP4 antibodies. The lower images are bright-field pictures.
FV infection induces the expression of BMP4 in the spleen.
The expansion of stress BFU-E in the spleen during the recovery from acute anemia is driven by the expression of BMP4. If FV utilizes this pathway, we predict that FV infection leads to a similar induction of BMP4 in the spleen. We tested whether BMP4 is expressed in the spleen after infection with FVP by staining spleen sections with anti-BMP4 antibodies at different times postinfection. No expression of BMP4 is seen at 1 day postinfection, but by days 8 and 12 after infection, BMP4 is expressed in small patches in the red pulp of spleen. By day 15, BMP4 expression is observed throughout the red pulp of the spleen (Fig. 3B). The kinetics of BMP4 expression correlates with our previous data showing that the expansion of FV-infected Epoind BFU-E in the spleen does not occur until after day 6 (45). These data demonstrate that FV infection can induce the expression of BMP4 in the spleen, which suggests that the activation of the BMP4-dependent stress erythropoiesis pathway plays a role in the pathogenesis of FV.
FVP-infected bone marrow cells cannot induce BMP4 expression or splenomegaly when transplanted into f/f mice.
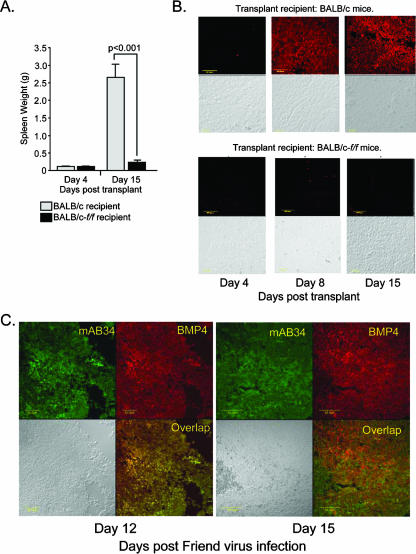
Early work showed that bone marrow cells infected in vitro with FV could be transplanted into susceptible mice, leading to the propagation of infection in the spleen. Surprisingly, these early experiments showed that the transplanted cells did not expand in the spleen but rather acted as a reservoir of virus that infected recipient spleen cells, resulting in splenomegaly (41, 44). These cells were referred to as IC cells. In the above sections (and corresponding figures), we showed that signals induced by acute anemia and, in particular, BMP4 increased the number of target cells in the spleen and that FV infection of control mice induced the expression of BMP4 in the spleen. These observations suggest that FV induces the BMP4-dependent stress pathway to amplify target cells in the spleen. Based on these observations, we predict that control bone marrow IC cells infected in vitro with FV could not propagate an infection when transplanted into f/f mice, because the recipient spleen cells are unable to respond to BMP4. We tested this hypothesis by transplanting FVP-infected control bone marrow cells into f/f and control mice and assaying whether IC cells could propagate an infection that would lead to splenomegaly. BALB/c bone marrow cells were infected in vitro with FVP and were transplanted in BALB/c control or BALB/c-f/f mice. The spleen weights were measured at days 4 and 15 after transplantation. FVP-infected bone marrow cells very efficiently induced splenomegaly by day 15 after being transplanted into control recipients, but no splenomegaly was observed after infected bone marrow cells were transplanted into f/f recipients (Fig. 4A). The homing of bone marrow cells to f/f spleens is similar to the homing of bone marrow cells to control spleens, so a defect in homing to the spleen cannot explain these observations (O. Harandi and R. F. Paulson, unpublished data).
FIG. 4.
FVP-infected bone marrow cells transplanted into f/f recipients cannot propagate the infection in the spleen. BALB/c control bone marrow cells were infected in vitro with FVP and were transplanted into BALB/c-f/f or BALB/c control recipients. (A) On the indicated days, spleens were removed and weighed to determine splenomegaly. Significant differences, as measured by t tests, are indicated. (B) Spleen sections were stained with anti-BMP4 antibodies to determine BMP4 expression. (C) Sections from spleens isolated from BALB/c mice infected with FVP on day 12 (top) or day 15 (bottom) after infection were stained with anti-BMP4 and mAB34, which recognizes FV-infected cells. BMP4 staining is shown in red, mAB34 staining in green, and the overlap is shown in yellow. A bright-field image is included in the lower left panel for each day.
We next examined whether this defect in the ability of IC cells to transplant infection was due to a defect in the progenitor cells or due to a defect in the expression of BMP4. During the recovery from acute anemia, the expression of BMP4 is very tightly regulated in the spleen. However, previous work from our laboratory has shown that f/f mice exhibit a delayed expression of BMP4, which suggests that Smad5-dependent signals regulate BMP4 expression in the spleen stroma (27). We tested the expression of BMP4 in the spleens of control and f/f mice transplanted with control bone marrow cells infected in vitro with FVP. Figure 4B shows that the spleens of control mice transplanted with control FVP-infected cells express BMP4 at high levels at days 8 and 15 posttransplantation, but f/f mice transplanted with FVP-infected bone marrow cells expressed little to no BMP4 at any of the time points. Based on these observations, we propose that an impaired BMP4 stress erythropoiesis pathway in the spleen results in the resistance of f/f mice to FV infection. This defect is caused by a combination of the inability of f/f progenitor cells to respond to BMP4 and the inability of IC cells to induce BMP4 expression. Together, these defects block the rapid expansion of target cells, leading to resistance.
The induction of BMP4 expression in the spleen by FV infection is puzzling. Our previous analysis showed that hypoxia was capable of inducing BMP4 expression in spleen stromal cells. However, because of the increased red cell production caused by FV, it is unlikely that hypoxia induces BMP4 expression following FV infection. Furthermore, the observation that FVP-infected control bone marrow cells were unable to induce the expression of BMP4 when transplanted into f/f mice suggested that the mutant mice also have a defect in the cells expressing BMP4. We examined spleen sections to determine which cells were expressing BMP4 in the infected spleens. At days 12 and 15 after infection, we stained spleen sections with anti-BMP4 antibodies and mAB34, a monoclonal antibody that recognizes FV-infected cells (12, 45). At day 12, there is clear overlap between infected cells and BMP4-expressing cells; however, by day 15 BMP4-expressing cells are distinct from FV-infected cells (Fig. 4C). These observations suggest that FV infection could itself induce BMP4 expression. However, when we examined the expression of BMP4 in bone marrow cells infected in vitro with FV, we could not detect any expression (data not shown). Taken together, these data suggest a model in which FVP-infected bone marrow cells migrate into the spleen, where they encounter a signal in the spleen microenvironment that induces BMP4 expression by the infected cells. The subsequent expression of BMP4 by the surrounding stromal cells is Smad5 dependent and is defective in f/f mice.
Bone marrow CD31+ Kit+ Sca1− Lin− cells form Epoind BFU-E following FVP infection in vitro.
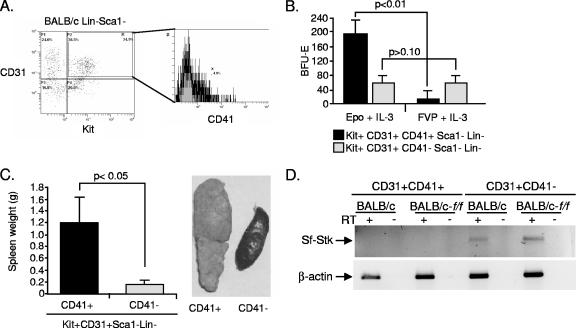
Previous work from our laboratory showed that the spleen MEP population contained the targets for FV, while the bone marrow MEP population did not contain targets for FV, as they failed to form Epoind BFU-E following infection in vitro (45). Recent work from Bauman et al. identified a new population of progenitor cells that exhibit erythroid potential (3). These cells are characterized by their expression of the endothelial cell marker CD31 or PECAM. This work showed that CD31+ Kit+ Sca1− Lin− cells sorted from bone marrow were able to form BFU-E and provide short-term erythroid radioprotection when transplanted into irradiated recipients. They also showed that these cells were distinct from the MEP population in the bone marrow. We tested whether these progenitors were targets for FV infection by sorting CD31+ Kit+ Sca1− Lin− cells from the bone marrow of BALB/c mice. Approximately 38% of Lin−Sca1− cells were CD31+ Kit+ in the bone marrow. These cells were infected with FVP and scored for Epoind BFU-E in methylcellulose assays. Figure 5A shows that this population contains FV target cells; in fact, the CD31− fraction failed to form Epoind BFU-E (data not shown), suggesting that this population contains most, if not all, of the target cells for FV in the bone marrow. We also sorted CD31+ Kit+ Sca1− Lin− cells from spleen and found that these cells do not form Epoind BFU-E following FVP infection (Fig. 5B). These data show that the target cells for FV in the bone marrow are distinct from the target cells in the spleen.
FIG. 5.
CD31+ Kit+ Sca1− Lin− cells are FV target cells in the bone marrow. (A) The scatter plot on the left depicts flow cytometry analysis of BALB/c Lin− Sca1− cells analyzed for the expression of CD31 and Kit. The box indicates the CD31+ Kit+ Sca1− Lin− population. The graph on the right depicts CD31+ Kit+ Sca1− Lin− cells that were sorted from BALB/c bone marrow either mock infected or infected with FVP and plated in methylcellulose medium containing Epo, SCF, and IL-3 (mock infected) or SCF and IL-3 (FVP infected), and BFU-E were scored. (B) Spleen CD31+ Kit+ Sca1− Lin− cells were mock infected or infected with FVP and were plated in methylcellulose medium containing Epo, SCF, and IL-3 (mock infected) or SCF and IL-3 (FVP infected), and BFU-E were scored. (C) The scatter plot on the left shows the flow cytometry analysis of BALB/c-f/f Lin− Sca1− cells analyzed for expression of CD31 and Kit. The box indicates the CD31+ Kit+ Sca1− Lin− population. The graph on the right depicts CD31+ Kit+ Sca1− Lin− cells that were sorted from BALB/c-f/f bone marrow and either mock infected or infected with FVP and plated in methylcellulose medium containing Epo, SCF, and IL-3 (mock infected) or SCF and IL-3 (FVP infected), and were BFU-E scored. (D) RT-PCR analysis of Sf-Stk expression in CD31+ Kit+ Sca1− Lin− cells isolated from BALB/c-f/f and BALB/c control mice. Significant differences, as measured by t tests, are indicated. The bars represent averages ± standard deviations from one representative experiment of three independent experiments.
f/f bone marrow contains few functional FV target cells. We tested whether the mutation in Smad5 resulted in a decrease in the number of CD31+ Kit+ Sca1− Lin− cells in the bone marrow or an inability of these cells to respond to the virus. Flow cytometry analysis of f/f bone marrow showed that these mice had slightly fewer CD31+ Kit+ Sca1− Lin− cells, and approximately 33% of the Sca1− Lin− cells were CD31+ Kit+. However, when this population was sorted and infected in vitro, f/f CD31+ Kit+ Sca1− Lin− cells generated significantly fewer Epoind BFU-E (Fig. 5C). When we analyzed the expression of Sf-Stk in CD31+ Kit+ Sca1− Lin− cells from f/f and control mice, we observed that the expression in the mutant cells was indistinguishable from that of the control (Fig. 5D). We next tested whether CD31+ Kit+ Sca1− Lin− cells from BALB/c-f/f mice could be infected by FV. Analysis of f/f bone marrow cells infected in vitro by FV showed that CD31+ Kit+ Sca1− Lin− cells were infected as measured by flow cytometry with mAB34 (12, 45; also data not shown). These data show that Smad5-dependent signaling is not required in the bone marrow for the development of these target cells or for Sf-Stk expression but is required for FVP-induced BFU-E formation. At the present time, we do not understand why f/f CD31+ Kit+ Sca1− Lin− cells that express Sf-Stk fail to form Epoind BFU-E following FVP infection. Further work will be needed to explain the role of Smad5-dependent signals in these cells.
CD31+ Kit+ CD41+ Sca1− Lin− cells act as IC cells.
FV target cells can be subdivided into two populations: target cells capable of forming Epoind BFU-E following FVP infection and target cells that are capable of acting as IC cells, propagating the infection in the spleen. Early work that separated cells in the bone marrow and spleen by velocity sedimentation analysis showed that these two target cell populations were distinct (43). We fractionated bone marrow and spleen cells into MEPs (Kit+ CD34− FcgRlo Sca1− IL-7Rα− Lin−) or CD31+ Kit+ Sca1− Lin− cells and tested whether these fractions, when infected in vitro with FVP, could act as IC cells by inducing splenomegaly when transplanted into susceptible control mice. Despite the fact that earlier work showed that IC cells were distinct from Epoind BFU-E-forming cells, we observed that bone marrow CD31+ Kit+ Sca1− Lin− cells functioned very efficiently as IC cells (Fig. 6A), while bone marrow or spleen MEPs failed to induce splenomegaly. Furthermore, bone marrow CD31+ Kit+ Sca1− Lin− cells induced BMP4 expression 14 days posttransplantation, while spleen MEPs, the targets of FVP in the spleen, failed to induce BMP4 expression (Fig. 6B).
FIG. 6.

CD31+ Kit+ Sca1− Lin− cells act as IC cells. (A) Bone marrow cells from BALB/c control mice were sorted into CD31+ Kit+ Sca1− Lin− (CD31+) or MEP (Kit+ CD34− FcγRlo IL-7Rα− Sca1− Lin−) populations, and spleen MEPs were isolated. The sorted cells were infected in vitro with FVP and transplanted into BALB/c control mice. Fourteen days later, the spleens were removed and weighed to test for splenomegaly. Significant differences are indicated by t tests. The data represent the averages ± standard deviations from three independent experiments. (B) Spleen sections from mice transplanted with FVP-infected bone marrow CD31+ and spleen MEP cells were stained with anti-BMP4 antibodies. Bright-field images are presented in the lower panels.
Given that earlier work showed that IC cells were distinct from Epoind colony-forming cells, we further subdivided the CD31+ Kit+ Sca1− Lin− cells. We chose CD41 as an additional marker. CD41, which encodes platelet α integrin IIb, originally was shown to play a role in platelet function. Recent work, however, demonstrated that CD41 also is expressed on progenitor cells in the yolk sac and fetal liver in a pattern similar to what was observed for CD31 (15, 30, 32). Approximately 5% of the CD31+ Kit+ Sca1− Lin− cells were CD41+ (Fig. 7A). Sorted CD31+ Kit+ CD41+ Sca1− Lin− (CD41+) and CD31+ Kit+ CD41− Sca1− Lin− (CD41−) cells were infected in vitro with FVP and plated in methylcellulose medium to test for Epoind BFU-E formation or were transplanted into susceptible control mice to assay for IC cell activity. When cells were plated for Epoind BFU-E formation, the CD41− cells readily formed Epoind BFU-E, while the CD41+ fraction did not (Fig. 7B). However, the opposite was true in the IC cell assay, in which the CD41+ population induced splenomegaly while the CD41− population had no effect (Fig. 7C). The spleen also contains IC cells (44), so we tested whether the CD31+ Kit+ CD41+ Sca1− Lin− cells from the spleen also could propagate an infection when infected in vitro and transplanted into susceptible recipients. The number of CD41+ cells in the spleen was significantly less than what was observed in the bone marrow. Despite the decreased numbers, spleen CD41+ cells infected in vitro with FVP were able to propagate the infection and induce splenomegaly when transplanted into BALB/c mice (data not shown). Sf-Stk is required for FV to induce Epoind BFU-E formation. We tested Sf-Stk expression by reverse transcription-PCR (RT-PCR) in bone marrow CD31+ Kit+ Sca1− Lin− cells fractionated for CD41 expression. Figure 7D shows that the CD41− cells express Sf-Stk and form Epoind BFU-E, while the CD41+ cells fail to express Sf-Stk and their role is limited to that of an IC cell. The lack of Sf-Stk expression by the CD41+ IC cells is consistent with results of earlier work that showed that IC cells from Fv2r/r congenic mice could propagate infection when transplanted into Fv2s/s recipients (10).
FIG. 7.
CD41 expression marks the IC cells present in the bone marrow CD31+ Kit+ Sca1− Lin− population. (A) Flow cytometry analysis of CD41 expression in the bone marrow CD31+ Kit+ Sca1− Lin− population of cells. (B) Bone marrow CD31+ Kit+ Sca1− Lin− cells were sorted into CD41+ and CD41− populations. Each population was tested for the ability to form Epoind BFU-E following infection in vitro with FVP. Cells were mock infected or infected with FVP and were plated in methylcellulose medium containing the indicated cytokines. BFU-E were scored. (C) Bone marrow CD31+ Kit+ Sca1− Lin− cells were sorted into CD41+ and CD41− populations. Each population was tested for the ability to propagate infection in the spleen after infection with FVP in vitro and were transplanted into BALB/c control mice. Spleens were removed and weighed 14 days posttransplantation (left). Pictures of representative spleens are shown (right). (D) RT-PCR analysis of Sf-Stk expression in CD31+ Kit+ Sca1− Lin− cells sorted into CD41+ or CD41− populations. The expression was analyzed in each population from both BALB/c and BALB/c-f/f mice. Significant differences, as measured by t tests, are indicated. The bars represent the averages ± standard deviations from one representative experiment of two independent experiments.
BALB/c-f/f mice have a defect in target cells in the bone marrow, and control cells infected in vitro are unable to propagate an infection in an f/f spleen. We next investigated whether f/f mice also have a defect in IC cells. CD31+ Kit+ CD41+ Sca1− Lin− cells were sorted from bone marrow, and approximately 7.6% of CD31+ Kit+ Sca1− Lin− cells were CD41+. However, given the fact that BALB/c-f/f mice have slightly fewer CD31+ Kit+ Sca1− Lin− cells than control mice, the CD41+ populations in the two strains, as measured by the percentage of Sca1− Lin− cells, is approximately equal (1.9% in BALB/c mice and 2.4% in BALB/c-f/f mice) (Fig. 8A). We further tested whether the f/f mutation could affect the ability of f/f bone marrow cells infected in vitro with FVP to propagate infection in the spleen when transplanted into control recipient mice. Fifteen days after transplantation, FVP-infected f/f bone marrow could induce splenomegaly in recipient mice. However, the extent of splenomegaly was slightly but significantly less. In addition, the kinetics of splenomegaly was significantly slower in the mice transplanted with f/f-infected bone marrow cells than in mice with control bone marrow cells (Fig. 8B). The expansion of infected cells in the f/f transplants is delayed and the majority of the expansion occurs in the final 3 days, while in the control transplants the expansion begins at day 8 and continues through day 15. These observations are consistent with the possibility that f/f mutant IC cells have a defect in their ability to propagate infection in the spleen. Two alternative explanations could account for these data. First, f/f mutant IC cells could have a defect in homing to the spleen. We have examined this possibility in other experiments and have observed no difference between the homing of f/f mutant bone marrow cells to the spleen and that of control cells (O. Harandi and R. F. Paulson, unpublished). Second, as discussed earlier, f/f mice can replicate FV in the spleen; however, decreased viral titers in the mutant IC cells also could lead to a delay in the expansion of infected cells in the spleen. This possibility cannot be ruled out by our experiments.
FIG. 8.
BALB/c-f/f mice exhibit a defect in IC cells in the bone marrow. (A) Flow cytometry analysis of CD41+ cells in the CD31+ Kit+ Sca1− Lin− cells in the bone marrow of BALB/c-f/f mice. (B) BALB/c-f/f and BALB/c control bone marrow cells were infected in vitro with FVP and transplanted into BALB/c control mice. On the indicated days, spleens were isolated and weighed. Significant differences, as measured by t tests, are indicated. The bars represent averages ± standard deviations from one representative experiment of two independent experiments.
CD31+ Kit+ CD41+ Sca1− Lin− cells increase in the peripheral blood during FV infection.
The pathogenesis of FV infection requires the expansion of infected target cells in the spleen (29, 31, 45), and our data demonstrate that the activation of the BMP4-dependent stress erythropoiesis pathway is required for this expansion. The mechanism by which the infection spreads to the spleen is not clear. IC cells from the bone marrow, when infected in vitro and transplanted into susceptible mice, propagate the infection in the spleen. However, during the natural course of infection, do infected IC cells migrate to the spleen to initiate infection? We tested whether the number of CD31+ Kit+ CD41+ cells in the peripheral blood increased after FVP infection in vivo. Figure 9 shows that there is a sixfold increase in the number of CD31+ Kit+ CD41+ cells in the peripheral blood of BALB/c mice infected with FVP compared to the level of these cells in uninfected controls. This observation supports a model in which IC cells infected in the bone marrow migrate to the spleen, induce BMP4 expression, and infect stress BFU-E. However, we also have observed IC cells in the spleen, which could be directly infected by FV and contribute to the expansion of target cells. Our earlier data showing that the expansion of infected Epoind BFU-E in the spleen does not occur until after day 6 postinfection suggests that immediate direct infection of spleen IC cells does not make a significant contribution to the initial expansion of target cells (45).
FIG. 9.
CD31+ Kit+ CD41+ Sca1− Lin− cells are mobilized into the peripheral blood during infection with FVP in vivo. Peripheral blood mononuclear cells from uninfected (top) and infected (bottom) BALB/c control mice were analyzed by flow cytometry for CD31+ Kit+ CD41+ Sca1− Lin− cells.
DISCUSSION
FV is capable of inducing an acute splenomegaly caused by the rapid expansion of infected cells, which progresses to erythroleukemia. Our data show that FV activates the BMP4-dependent stress erythropoiesis pathway and takes advantage of the ability of this pathway to rapidly expand a specialized population of stress BFU-E, which are the targets for FV in the spleen. Based on these observations, we propose a new model for the pathogenesis of FV in which the bone marrow contains two populations of target cells. One population, CD31+ Kit+ CD41−Sca1− Lin− cells, expresses Sf-Stk and forms Epoind BFU-E upon infection with FVP. The second population, CD31+ Kit+ CD41+ Sca1− Lin− cells, does not express Sf-Stk. These cells act as IC cells during infection that migrate to the spleen following infection. Once in the spleen, IC cells respond to signals in the spleen microenvironment and begin expressing BMP4. We speculate that the expression of BMP4 in the spleen stroma is initiated and maintained by a BMP4/Smad5-dependent mechanism. BMP4 causes the expansion of stress BFU-E, which are the targets for FV in the spleen (27, 45). The BMP4 stress erythropoiesis pathway in the spleen has enormous proliferative potential, as demonstrated by our observation that stress BFU-E expand 48-fold 36 h after anemia induction. The ability of FV to activate this pathway and utilize stress BFU-E as targets results in the acute splenomegaly, polycythemia, and rapid progression to erythroleukemia associated with FVP infection. This new model proposes that FV manipulates a physiological stress response pathway to amplify target cells, resulting in an acute expansion of infected cells.
The close relationship between FV and stress erythropoiesis is further strengthened by reexamining previous work in light of this new model. The four host genes that are required for the expansion of FV-infected cells all appear to play a role in the BMP4-dependent stress erythropoiesis pathway. Our earlier work on the resistance of W and Sl mice showed that the defect in these mice was caused by a lack of target cells in the spleen (45). Similarly, we have demonstrated that W mutant mice have a severe defect in the expansion of stress BFU-E in response to acute anemia, and in vitro, SCF plays a key role in the expansion of stress BFU-E in culture (37). Sf-Stk is absolutely required for the expansion of infected cells (38), and preliminary data suggest that Sf-Stk is up-regulated in the spleen during the recovery from acute anemia, in which it appears to play a role in the differentiation of stress BFU-E (O. Harandi, L. Shi, and R. F. Paulson, unpublished data). Based on these observations, our identification of two target cell populations in the bone marrow suggests that these cells also are involved in the BMP4-dependent stress erythropoiesis pathway. Although both of these populations exhibit erythroid potential in vitro, their exact roles in stress or steady-state erythropoiesis will await further investigation.
Our model proposes that IC cells must migrate to the spleen to induce BMP4 expression and act as reservoirs of infectious virus. Our observation that the infection of bone marrow cells with FVP does not induce BMP4 expression suggests that the induction of BMP4 expression in the spleen is indirect. We propose that it is the interaction between infected IC cells and the spleen microenvironment that induces the expression of BMP4. This situation is very similar to what we have observed for the maintenance of the BMP4-dependent stress erythropoiesis pathway. Our data show that transplanted bone marrow cells can home to the spleen and replenish the BMP4R stress BFU-E. Donor bone marrow cells express BMP4 when they migrate to the spleen, which is very similar to what we observed 12 days after FVP infection of BALB/c control mice. However, by 15 days after infection, we observed that FVP-infected cells no longer express BMP4. Taken together, these data support a model in which initial BMP4 expression by infected cells is induced by interactions with the spleen microenvironment, and the subsequent expression of BMP4 by the spleen stroma requires BMP4/Smad5-dependent signals. The ability of BMP4 to induce its own expression in neighboring cells and expand the area of BMP4 expression has been observed with Dpp, the Drosophila ortholog of BMP4, and this autoregulation is responsible for the spread and maintenance of Dpp expression (11, 21, 24).
An essential component of our model proposes that bone marrow IC cells infected with virus migrate to the spleen, where they induce BMP4 expression and act as reservoirs of virus for the infection of the expanding stress BFU-E. Earlier work in the field investigated the ability of helper-free SFFV to cause disease in mice (6, 8, 39, 46, 47). In some instances, infection with helper-free SFFV was able to induce disease (splenomegaly, polycythemia, and erythroleukemia) (39, 46, 47). On the surface, these results would appear to be inconsistent with our model. If direct infection of spleen targets could result in disease, then IC cells are not necessary to acts as reservoirs of virus or to induce the BMP4-dependent expansion of target cells in the spleen. However, on closer inspection of the data, helper-free SFFV causes disease in mice only when they have been treated with PHZ prior to infection. PHZ treatment activates the BMP4-dependent stress erythropoiesis pathway leading to the expansion of stress BFU-E, which can be directly infected by helper-free virus.
In summary, we have demonstrated that FV utilizes the BMP4-dependent stress erythropoiesis pathway during the early stages of Friend erythroleukemia. We have identified two distinct target cells for FV in the bone marrow, one that forms Epoind BFU-E and a second that functions as an IC cell propagating infection in the spleen. These data led us to propose a new model for FV pathogenesis in which infection of cells in the bone marrow leads to either Epo-independent erythropoiesis or migration of infected IC cells to the spleen. Once in the spleen, these cells induce BMP4 expression, which leads to the expansion of stress BFU-E, the target cells for FV. This model suggests a novel mechanism by which FV activates the stress erythropoiesis pathway, resulting in the rapid amplification of target cells, leading to acute splenomegaly and polycythemia.
Acknowledgments
We dedicate this paper to Alan Bernstein on the occasion of his retirement as Director of the Canadian Institutes of Health Research, in honor of his service to Canadian science and his contributions to FV research. We thank Andy Henderson for comments on the manuscript.
This work was funded by Public Health Service grant HL070720 from the National Heart Lung and Blood Institute (R.F.P.). This project is funded, in part, under a grant with the Pennsylvania Department of Health using Tobacco Settlement Funds.
The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations, or conclusions.
Footnotes
Published ahead of print on 17 October 2007.
REFERENCES
- 1.Akashi, K., D. Traver, T. Miyamoto, and I. Weissman. 2000. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 404193-197. [DOI] [PubMed] [Google Scholar]
- 2.Axelrad, A. 1969. Genetic and cellular basis of susceptibility or resistance to Friend leukemia virus infection in mice. Proc. Can. Cancer Conf. 8313-343. [PubMed] [Google Scholar]
- 3.Baumann, C. I., A. S. Bailey, W. Li, M. J. Ferkowicz, M. C. Yoder, and W. H. Fleming. 2004. PECAM-1 is expressed on hematopoietic stem cells throughout ontogeny and identifies a population of erythroid progenitors. Blood 1041010-1016. [DOI] [PubMed] [Google Scholar]
- 4.Ben-David, Y., and A. Bernstein. 1991. Friend virus induced erythroleukemia and the multistage nature of cancer. Cell 66831-834. [DOI] [PubMed] [Google Scholar]
- 5.Bennett, M., R. Steeves, G. Cudkowicz, E. Mirand, and L. Russell. 1968. Mutant Sl alleles of mice affect the susceptibility to Friend spleen focus forming virus. Science 162564-565. [DOI] [PubMed] [Google Scholar]
- 6.Berger, S. A., N. Sanderson, A. Bernstein, and W. D. Hankins. 1985. Induction of the early stages of Friend erythroleukemia with helper-free Friend spleen focus-forming virus. Proc. Natl. Acad. Sci. USA 826913-6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Best, S., P. Le Tissier, G. Towers, and J. P. Stoye. 1996. Positional cloning of the mouse retrovirus restriction gene Fv1. Nature 382826-829. [DOI] [PubMed] [Google Scholar]
- 8.Bestwick, R. K., W. D. Hankins, and D. Kabat. 1985. Roles of helper and defective retroviral genomes in murine erythroleukemia: studies of spleen focus-forming virus in the absence of helper. J. Virol. 56660-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chabot, B., D. A. Stephenson, V. M. Chapman, P. Besmer, and A. Bernstein. 1988. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature 33588-89. [DOI] [PubMed] [Google Scholar]
- 10.Chantratita, W., and C. Yoosook. 1982. Use of in vivo infected spleen cells to study Fv-2r-mediated resistance of mice to Friend spleen focus-forming virus. Leukemia Res. 6595-600. [DOI] [PubMed] [Google Scholar]
- 11.Chanut, F., and U. Heberlein. 1997. Role of decapentaplegic in initiation and progression of the morphogenetic furrow in the developing Drosophila retina. Development 124559-567. [DOI] [PubMed] [Google Scholar]
- 12.Chesebro, B., K. Wehrly, M. Cloyd, W. Britt, J. Portis, J. Collins, and J. Nishio. 1981. Characterization of mouse monoclonal antibodies specific for Friend murine leukemia virus-induced erythroleukemia cells: Friend-specific and FMR-specific antigens. Virology 112131-144. [DOI] [PubMed] [Google Scholar]
- 13.Coleman, D., E. Russell, and E. Levin. 1969. Enzymatic studies of the hemopoietic defect in flexed mice. Genetics 61631-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Copeland, N. G., D. J. Gilbert, B. C. Cho, P. J. Donovan, N. A. Jenkins, D. Cosman, D. Anderson, S. D. Lyman, and D. E. Williams. 1990. Mast cell growth factor maps near the steel locus on mouse chromosome 10 and is deleted in a number of steel alleles. Cell 63175-183. [DOI] [PubMed] [Google Scholar]
- 15.Ferkowicz, M. J., M. Starr, X. Xie, W. Li, S. A. Johnson, W. C. Shelley, P. R. Morrison, and M. C. Yoder. 2003. CD41 expression defines the onset of primitive and definitive hematopoiesis in the murine embryo. Development 1304393-4403. [DOI] [PubMed] [Google Scholar]
- 16.Finkelstein, L., P. Ney, Q. Liu, R. Paulson, and P. Correll. 2002. Sf-Stk kinase activity and the Grb2 binding site are required for Epo-independent growth of primary erythroblasts infected with Friend virus. Oncogene 213562-3570. [DOI] [PubMed] [Google Scholar]
- 17.Geissler, E. N., M. A. Ryan, and D. E. Housman. 1988. The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell 55185-192. [DOI] [PubMed] [Google Scholar]
- 18.Gregory, C., E. McCulloch, and J. Till. 1975. The cellular basis for the defect in haematopoiesis in flexed-tail mice. III. Restriction of the defect to erythropoietic progenitors capable of transient colony formation in vivo. Br. J. Haematol. 30401-410. [DOI] [PubMed] [Google Scholar]
- 19.Gruneberg, H. 1942. The anaemia of the flexed-tail mice (Mus musculus L.) II. Siderocytes. J. Genet. 44246-271. [Google Scholar]
- 20.Gruneberg, H. 1942. The anaemia of the flexed-tail mouse (Mus musculus L.) I. Static and dynamic haematology. J. Genet. 4345-68. [Google Scholar]
- 21.Hepker, J., R. K. Blackman, and R. Holmgren. 1999. Cubitus interruptus is necessary but not sufficient for direct activation of a wing-specific decapentaplegic enhancer. Development 1263669-3677. [DOI] [PubMed] [Google Scholar]
- 22.Hoatlin, M. E., and D. Kabat. 1995. Host-range control of a retroviral disease: Friend erythroleukemia. Trends Microbiol. 351-57. [DOI] [PubMed] [Google Scholar]
- 23.Hunt, H., and D. Premar. 1928. Flexed-tail a mutation in the house mouse. Anat. Rec. 41117. [Google Scholar]
- 24.Hursh, D. A., R. W. Padgett, and W. M. Gelbart. 1993. Cross regulation of decapentaplegic and Ultrabithorax transcription in the embryonic visceral mesoderm of Drosophila. Development 1171211-1222. [DOI] [PubMed] [Google Scholar]
- 25.Ikeda, H., and H. Sugimura. 1989. Fv-4 resistance gene: a truncated endogenous murine leukemia virus with ecotropic interference properties. J. Virol. 635405-5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kost, T. A., M. J. Koury, W. D. Hankins, and S. B. Krantz. 1979. Target cells for Friend virus-induced erythroid bursts in vitro. Cell 18145-152. [DOI] [PubMed] [Google Scholar]
- 27.Lenox, L., J. Perry, and R. Paulson. 2005. BMP4 and Madh5 regulate the erythroid response to acute anemia. Blood 1052741-2748. [DOI] [PubMed] [Google Scholar]
- 28.Lilly, F. 1970. Fv2: identification and location of a second gene governing the spleen focus response to Friend leukemia virus in mice. J. Natl. Cancer Inst. 45163-169. [PubMed] [Google Scholar]
- 29.McGarry, M., and E. Mirand. 1973. Incidence of Friend virus-induced polycythemia in splenectomized mice. Proc. Soc. Exp. Biol. Med. 142538-541. [DOI] [PubMed] [Google Scholar]
- 30.Mikkola, H. K., Y. Fujiwara, T. M. Schlaeger, D. Traver, and S. H. Orkin. 2003. Expression of CD41 marks the initiation of definitive hematopoiesis in the mouse embryo. Blood 101508-516. [DOI] [PubMed] [Google Scholar]
- 31.Mirand, E., J. Hoffman, J. T. Grace, Jr., and P. Trudel. 1961. Modification of the Friend virus disease by splenectomy. Proc. Soc. Exp. Biol. Med. 107824-829. [DOI] [PubMed] [Google Scholar]
- 32.Mitjavila-Garcia, M. T., M. Cailleret, I. Godin, M. M. Nogueira, K. Cohen-Solal, V. Schiavon, Y. Lecluse, F. Le Pesteur, A. H. Lagrue, and W. Vainchenker. 2002. Expression of CD41 on hematopoietic progenitors derived from embryonic hematopoietic cells. Development 1292003-2013. [DOI] [PubMed] [Google Scholar]
- 33.Moreau-Gachelin, F., A. Tavitian, and P. Tambourin. 1988. Spi-1 is a putative oncogene in virally induced murine erythroleukaemias. Nature 331277-280. [DOI] [PubMed] [Google Scholar]
- 34.Mowat, M., A. Cheng, N. Kimura, A. Bernstein, and S. Benchimol. 1985. Rearrangements of the cellular p53 gene in erythroleukaemic cells transformed by Friend virus. Nature 314633-636. [DOI] [PubMed] [Google Scholar]
- 35.Ney, P., and A. D'Andrea. 2000. Friend erythroleukemia revisited. Blood 963675-3680. [PubMed] [Google Scholar]
- 36.Nishigaki, K., D. Thompson, C. Hanson, T. Yugawa, and S. Ruscetti. 2001. The envelope glycoprotein of friend spleen focus-forming virus covalently interacts with and constitutively activates a truncated form of the receptor tyrosine kinase Stk. J. Virol. 757893-7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perry, J. M., O. F. Harandi, and R. F. Paulson. 2007. BMP4, SCF, and hypoxia cooperatively regulate the expansion of murine stress erythroid progenitors. Blood 1094494-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Persons, D. A., R. F. Paulson, M. R. Loyd, M. T. Herley, S. M. Bodner, A. Bernstein, P. H. Correll, and P. A. Ney. 1999. Fv2 encodes a truncated form of the Stk receptor tyrosine kinase. Nat. Genet. 23159-165. [DOI] [PubMed] [Google Scholar]
- 39.Spiro, C., B. Gliniak, and D. Kabat. 1988. A tagged helper-free Friend virus causes clonal erythroblast immortality by specific proviral integration in the cellular genome. J. Virol. 624129-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steeves, R., M. Bennett, E. Mirand, and G. Cudkowicz. 1968. Genetic control by the W locus of susceptibility to (Friend) spleen focus forming virus. Nature 218372-374. [DOI] [PubMed] [Google Scholar]
- 41.Steeves, R., J. Bubbers, F. Plata, and F. Lilly. 1978. Origin of spleen colonies generated by Friend virus-infected cells in mice. Cancer Res. 382729-2733. [PubMed] [Google Scholar]
- 42.Steeves, R., and F. Lilly. 1977. Interactions between host and viral genomes in mouse leukemia. Annu. Rev. Genet. 11277-296. [DOI] [PubMed] [Google Scholar]
- 43.Steinheider, G., I. Bertoncello, and H. Seidel. 1980. Characterization of early leukemic cells in spleen and bone marrow of Friend virus infected mice. Exp. Hematol. 8779-787. [PubMed] [Google Scholar]
- 44.Steinheider, G., and R. Steeves. 1978. A Target cell assay for Friend virus. Leukemia Res. 2197-200. [Google Scholar]
- 45.Subramanian, A., H. E. Teal, P. H. Correll, and R. F. Paulson. 2005. Resistance to Friend virus-induced erythroleukemia in W/Wv mice is caused by a spleen-specific defect which results in a severe reduction in target cells and a lack of Sf-Stk expression. J. Virol. 7914586-14594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolff, L., and S. Ruscetti. 1985. Malignant transformation of erythroid cells in vivo by introduction of a nonreplicating retrovirus vector. Science 2281549-1552. [DOI] [PubMed] [Google Scholar]
- 47.Wolff, L., P. Tambourin, and S. Ruscetti. 1986. Induction of the autonomous stage of transformation in erythroid cells infected with SFFV: helper virus is not required. Virology 152272-276. [DOI] [PubMed] [Google Scholar]
- 48.Zhang, J., M. S. Randall, M. R. Loyd, W. Li, R. L. Schweers, D. A. Persons, J. E. Rehg, C. T. Noguchi, J. N. Ihle, and P. A. Ney. 2006. Role of erythropoietin receptor signaling in Friend virus-induced erythroblastosis and polycythemia. Blood 10773-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zsebo, K. M., D. A. Williams, E. N. Geissler, V. C. Broudy, F. H. Martin, H. L. Atkins, R. Y. Hsu, N. C. Birkett, K. H. Okino, D. C. Murdock, et al. 1990. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell 63213-224. [DOI] [PubMed] [Google Scholar]