Abstract
The order Mononegavirales (comprised of nonsegmented negative-stranded RNA viruses or NNSVs) contains many important pathogens. Parainfluenza virus 5 (PIV5), formerly known as simian virus 5, is a prototypical paramyxovirus and encodes a V protein, which has a cysteine-rich C terminus that is conserved among all paramyxoviruses. The V protein of PIV5, like that of many other paramyxoviruses, plays an important role in regulating viral RNA synthesis. In this work, we show that V interacts with Akt, a serine/threonine kinase, also known as protein kinase B. Both pharmacological inhibitors and small interfering RNA against Akt1 reduced PIV5 replication, indicating that Akt plays a critical role in PIV5 replication. Furthermore, treatment with Akt inhibitors also reduced the replication of several other paramyxoviruses, as well as vesicular stomatitis virus, the prototypical rhabdovirus, indicating that Akt may play a more universal role in NNSV replication. The phosphoproteins (P proteins) of NNSVs are essential cofactors for the viral RNA polymerase complex and require heavy phosphorylation for their activity. Inhibition of Akt activity reduced the level of P phosphorylation, suggesting that Akt is involved in regulating viral RNA synthesis. In addition, Akt1 phosphorylated a recombinant P protein of PIV5 purified from bacteria. The finding that Akt plays a critical role in replication of NNSV will lead to a better understanding of how these viruses replicate, as well as novel strategies to treat infectious diseases caused by NNSVs.
The single-stranded RNA genomes of the Mononegavirales range from ∼11,000 to 18,000 nucleotides in length and contain a series of tandemly linked genes separated by nontranscribed sequences (32, 34). The viral RNA-dependent RNA polymerase (vRNAP) that transcribes the nucleocapsid protein (NP)-encapsidated RNA into 5′ capped and 3′ polyadenylated mRNAs minimally consists of two proteins, the phosphoprotein (P) and the large polymerase protein (L) (22). The viral RNA polymerase is thought to bind the genomic RNA at a single 3′ entry site and to transcribe the genome by a sequential and polar process. The same vRNAP also replicates viral RNA genome (1, 2, 21, 28). Regulation of the switch between viral RNA transcription and replication is not well understood. It is thought that the phosphorylation status of the P protein may be critical in regulating RNA synthesis (15, 52). For vesicular stomatitis virus (VSV), mutations altering the phosphorylation of the P protein that differentially affect RNA transcription or RNA genome replication have been identified (15, 16). Similarly, mutations in the P protein of respiratory syncytial virus (RSV) that affect viral RNA synthesis have been identified (5, 20, 38). While host kinases are thought to phosphorylate nonsegmented negative-stranded RNA virus (NNSV) P proteins, little is known about the identity of the specific host kinases required. For all paramyxoviruses examined, full activity of RNA synthesis by purified P-L complex with NP-encapsidated RNA genome (3, 17, 40-42, 44, 46, 58) is achieved only when cell extracts are added, indicating that the P-L complex is essential but not sufficient for viral RNA synthesis and host factors are required for viral RNA synthesis. For some of these viruses, such as Sendai virus (SeV) and measles virus (MeV), it is thought that the host protein tubulin is an important component of the transcription initiation complex (42, 45). For RSV, actin and profilin play an essential role in viral RNA synthesis (8, 9). While cytoskeletal proteins may be important for viral RNA synthesis, possibly providing a physical anchor for viral RNA synthesis, these proteins are unlikely to regulate the switch of viral RNA synthesis from mRNA transcription to viral genome replication.
Parainfluenza virus 5 (PIV5), formerly known as simian virus 5, is a prototypical member of the Rubulavirus genus of the family Paramyxoviridae (11, 32). PIV5 has seven genes but encodes eight known viral proteins (32). The V/P gene of PIV5 is transcribed into both the V mRNA and the P mRNA through a process of pseudotemplated addition of nucleotides, commonly called “RNA editing.” The V mRNA is made when the viral RNA polymerase faithfully transcribes the V/P gene. However, during transcription, the viral RNA polymerase complex recognizes a specific RNA sequence in the V/P gene and inserts two nontemplated G residues at the site to generate the P mRNA. As a result, the V/P gene is transcribed at similar levels into two mRNAs that are translated into two proteins, which share identical N termini but are encoded by different C termini (60). The C terminus of the V protein contains a cysteine-rich region that is well conserved among all paramyxovirus V proteins (61). While P is required for the transcription and replication of a PIV5 minigenome, the addition of V inhibits viral RNA synthesis in this system (36). The mechanism by which the V protein regulates viral RNA synthesis is unclear. However, we speculated that a host protein is involved. In this work, we show that V interacts with Akt1, and we have investigated the role of this host protein in viral replication.
Akt, also known as protein kinase B (PKB), was first discovered in retrovirus AKT8 as a viral protooncogene capable of transforming certain cells (6). Identification and cloning of the Akt gene showed that it has high homology to protein kinases A and C, hence the name PKB. Three mammalian Akt genes (Akt1, -2, and -3, also known as PKBα, -β, and -γ, respectively) have been identified, and all have serine/threonine kinase activity. The three Akt isoforms each contain a pleckstrin homology domain, a catalytic domain, and a regulatory domain. There are two major phosphorylation sites within Akt1, amino acid residues Thr308 and Ser473, which are phosphorylated by PDK1 (PI3K-dependent kinase 1) and rictor-mTOR complex, respectively (10, 54). Akt is known to have many downstream targets, including the BCL2 antagonist of cell death, the cyclic AMP response element-binding protein, endothelial nitric oxide synthase, I-κB kinase α, GSK-3, and p21 CIP1 (19). In addition, Akt is a key regulator in the PI3K signaling pathway and plays an important role in many cellular processes, such as cell survival, metabolism, growth, proliferation, and mobility. Akt has been found to be activated in many cancers, and targeting Akt with small molecules has reduced tumor growth in some circumstances (50, 64). While Akt1 and -2 are widely expressed in many organs and cell types, Akt3 is expressed preferentially, but not exclusively, in neuronal cells. All three Akt genes share some redundant functions, but studies using knockout mice and small interfering RNAs (siRNAs) show that each has distinct functions (56). Here, we show that Akt activity is required for optimal replication of a number of paramyxoviruses and that its function may involve regulating the activity of the viral polymerase by phosphorylation of the P protein.
MATERIALS AND METHODS
Plasmids, viruses, and cells.
Plasmids expressing V, P, and green fluorescent protein (GFP) have been described before (36). Genes for Akt1 (GenBank accession number NM_001014432), mitogen-activated protein kinase 1 (MAPK1) (accession number NM_002745), and phosphoinositide-binding protein (PIP3E) (accession number NM_015553) were obtained from Invitrogen (Carlsbad, CA) and OriGene Technologies, Inc. (Rockville, MD) and cloned into expression vector pCAGGS with a FLAG or a hemagglutinin (HA) tag, when necessary, by following standard molecular cloning procedures. Akt1 was cloned into pYFP1-Zip to replace the Zip gene to obtain pYFP1-Akt1, and V was cloned into pYFP2-Zip to replace the Zip gene to obtain pYFP2-V. pYFP1-Zip and pYFP2-Zip were from Stephen Michnick (University of Montreal) (51). Generation of rPIV5-R-Luc, which contains a Renilla luciferase (R-Luc) gene between HN and L of PIV5, was similar to the generation of rPIV5-GFP (26), which contains GFP between HN and L of PIV5. PIV5 and rPIV5-R-Luc were grown in MDBK cells as described before (26). Mumps virus (MuV) and MeV were grown and titrated in Vero cells; RSV was grown in HEp-2 cells and titrated in Vero cells. VSV was grown and titrated in BHK cells. SeV was grown in eggs, and titers were determined by HA assay. RSV A2 was a gift of Brian Murphy (National Institute of Allergy and Infectious Diseases), and rRSV-GFP has been previously described (24). HeLa, MDBK, and Vero cells were maintained in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) with 10% fetal bovine serum (FBS), 100 IU/ml penicillin, and 100 μg/ml streptomycin at 37°C with 5% CO2. BHK cells were maintained in the same medium plus 10% tryptose phosphate. BSR/T7 cells were maintained in the same medium as BHK cells plus 0.4 μg/ml G418 (Geneticin; Invitrogen) to maintain T7 RNA polymerase expression as described by Buchholz et al. (7). After infection, the FBS concentration was reduced to 2%.
Immunoprecipitation.
To detect protein interactions, cells were lysed and processed for immunoprecipitation as described before (47, 62). The cells transfected with plasmids encoding V, Akt1, or vector were metabolically labeled with [35S]Met and [35S]Cys for 3 h at 24 h posttransfection. The cells were lysed with whole-cell extraction buffer (50 mM Tris-HCl, pH 8; 280 mM NaCl; 0.5% NP-40; 0.2 mM EDTA; 2 mM EGTA; and 10% glycerol), and the lysates were precleared with 40 μl Protein A Sepharose beads and 4 μg mouse immunoglobulin G (Santa Cruz Biotech) for 1 h. The lysates were then precipitated with V-specific antibody (Pk) (49) or anti-Akt1 antibody (Cell Signaling Technology). The precipitated proteins were resolved by 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and visualized using a Storm PhosphorImager (Molecular Dynamics). For 33P labeling, the cells were infected at a multiplicity of infection (MOI) of 5, and at 1 day postinfection, the infected cells were first starved in the medium lacking phosphate for 30 min and then labeled with [33P]orthophosphate for 4 hours in the presence or absence of Akt inhibitor. The cells were then lysed and immunoprecipitated as before.
BiF.
To examine the interaction between V and Akt1, bimolecular fluorescence (BiF) with split yellow fluorescent protein (YFP) molecules was used. Briefly, potentially interacting proteins are fused to either the N-terminal 160 or the C-terminal 61 amino acids of YFP. Interaction between the two proteins of interest brings the two parts of YFP into proximity, resulting in a functional fluorophore. Akt1 and V were inserted into the plasmids YFP1-ZIP and YFP2-ZIP (51), respectively, resulting in plasmids YFP1-AKT1 and YFP-V. HeLa cells at 70% confluence in six-well plates were transfected with 0.8 μg of plasmids encoding YFP1-ZIP, YFP2-ZIP, YFP1-AKT1, YFP2-V, YFP1-ZIP plus YFP2-V, YFP1-ZIP plus YFP2-ZIP, or YFP1-AKT1 plus YFP2-V in triplicate, and empty vector pCAGGS was used to maintain the same total amounts of transfected DNA. The cells were incubated with transfection medium (Opti-MEM) at 37°C overnight. The medium was then changed to normal cell growth medium (DMEM with 10% FBS and 1% penicillin-streptomycin) and incubated at 30°C for 24 h. The cells were collected using trypsin digestion and resuspended in 500 μl of PBS. The YFP fluorescence was examined by flow cytometry.
Detection of viral proteins.
For immunoblotting of viral proteins and Akt1, the cells were mock infected or infected with PIV5 at a MOI of 5 for 1 day and then lysed in 500 μl of protein lysis buffer (2% sodium dodecyl sulfate, 62.5 mM Tris-HCl [pH 6.8], 2% dithiothreitol) and sonicated briefly to shear DNA. Up to 100 μl of the lysate was resolved in 15% SDS-PAGE and subjected to Western blotting with a rabbit anti-PIV5 antiserum or anti-Akt antibody as described above.
To examine the effects of Akt inhibitors on PIV5 replication, cells were infected with rPIV5-R-Luc at a MOI of 1 and incubated with Akt inhibitors. At the indicated times postinfection, the cells were lysed and assayed for luciferase activity by using a Renilla luciferase assay system (Promega), following the manufacturer's instructions.
Minigenome assays.
A minigenome system of PIV5 containing R-Luc (36) was used to examine the effects of Akt inhibitors on viral RNA synthesis. BSR/T7 cells in 24-well plates were transfected with plasmids encoding NP, P, L, and pMG-R-Luc as well as a firefly luciferase gene (F-Luc) under control of a T7 promoter as a transfection control (Promega). The cells were collected, and dual luciferase assays were carried out using a Dual-Luciferase reporter assay system (Promega) according to the manufacturer's protocol. Ratios of R-Luc to F-Luc are expressed as relative luciferase activity. To examine the effects of the Akt protein on the minigenome system, a plasmid encoding Akt1 was transfected into the cells with the minigenome and the V plasmid. The amount of the V plasmid was kept constant, and the pCAGGS-GFP plasmid was used to balance the amount of total transfected DNA.
siRNA and inhibitors.
siRNA against Akt1 along with control siRNA were purchased from Dharmacon. Cells were transfected with 100 nM of siRNA using Oligofectamine (Invitrogen) following the manufacturer's protocol. Briefly, cells in 24-well plates at about 30 to 50% confluence were washed once with Opti-MEM and incubated in 400 μl of Opti-MEM. Five microliters of GL3 siRNA or Akt1 siRNA (10 μM stock) for each well in a 24-well plate was mixed with 95 μl of Opti-MEM for 5 min at room temperature. Meanwhile, 2 μl of Oligofectamine was mixed with 10 μl of Opti-MEM. The mixture was added to the siRNA mixture and incubated for 15 min at room temperature. The mixture of siRNA and Oligofectamine was added to the cells. After 6 h incubation, 250 μl of DMEM with 30% FBS was added to the cells. At 2 days posttransfection, the transfected cells were infected with PIV5 or rPIV5-rLuc at a MOI of 5. The transfected/infected cells were collected for immunoblotting or luciferase assay at 1 day postinfection. Inhibitors against Akt were purchased from Calbiochem, and inhibitors against PI3K (LY294002 and wortmannin) were purchased from Sigma. The compounds were dissolved in dimethyl sulfoxide (DMSO) and used at the concentrations indicated in the figure legends after virus absorption.
Akt kinase assay.
Recombinant PIV5 P protein with a hexahistidine tag at the N terminus was generated by subcloning the P gene into the bacterial expression vector pET15b (Novagen) and transforming this plasmid into BL21(DE3)/pLysS cells. P protein expression was induced with isopropyl-β-d-thiogalactopyranoside (IPTG) after overnight culture, and His-P was purified from cell lysates using a His-Resin (Novagen) column by following the manufacturer's protocol as described previously (27). Recombinant Akt1 (400 ng) (Upstate Biotechnology), which is constitutively active, was incubated with 10 μCi [γ-32P]ATP (Amersham Biosciences) and purified His-P protein in a volume of 30 μl with kinase buffer (25 mM HEPES, 25 mM β-glycerophosphate, 25 mM MgCl2, 2 mM dithiothreitol, and 0.1 mM NaVO3) following a protocol by Powell et al. (48). Reactions were incubated at 30°C for 2 h and terminated by the addition of SDS sample dilution buffer. Proteins were separated by 10% SDS-PAGE, and phosphorylation was visualized by phosphorimagery with a Storm PhosphorImager (Molecular Dynamics).
RESULTS
Interaction between V and Akt.
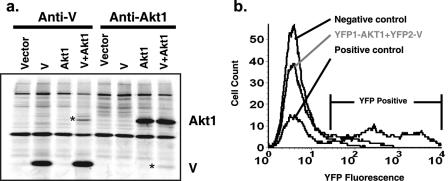
Previously, we found that the V protein of PIV5 inhibits viral RNA synthesis (36). To study the mechanism of the regulation, we used bioinformatics and traditional molecular biology approaches (e.g., yeast two-hybrid screening and affinity copurification) to identify V-interacting proteins. Screening for motifs within V using Scansite (http://scansite.mit.edu) under high-stringency conditions resulted in the identification of three candidate motifs: targeting sites for Akt1, MAPK1, and PIP3E. The Akt1 target site is located at Ser176 (GFHRREYSIGWVGDE [Ser176 is underlined, and surrounding sequences are shown]). Ile83 within the sequence of PKKPRPKIAIVPADD was found to be a potential MAPK1-binding site. Phe170 within the sequence of RGRDTGGFHRRESI was found to be a good PIP3-E binding site. We have mapped the region that is essential and sufficient for the V-mediated inhibition of viral RNA synthesis to residues 120 to 222 of the V protein (data not shown). Interestingly, the target region for Akt1 within V is between residues 169 and 183, which are well conserved in all paramyxovirus V proteins (61). To examine the interactions between V and Akt1, MAPK1, or PIP3E, expression vectors encoding these human proteins were cotransfected with a V expression plasmid into HeLa cells, and coimmunoprecipitation analysis was performed. In cells expressing both V and Akt1 proteins, V-specific antibody immunoprecipitated V, together with a smaller amount of Akt1 (Fig. 1a). Conversely, Akt1, together with a smaller amount of V, was immunoprecipitated with an Akt1-specific antibody. Coimmunoprecipitation of V and Akt1 was readily detected, while no interactions between V and MAPK1 or PIP3E were detected (data not shown). To further confirm the interaction between V and Akt1, BiF using a split YFP system was performed (51). Plasmids encoding YFP1-Akt1 and YFP2-V were transfected into cells, and the fluorescence of transfected cells was examined by flow cytometry. As shown in Fig. 1b, cotransfection of YFP1-Akt1 and YFP2-V gave rise to fluorescence well above background levels, indicating that V and Akt1 interact. As expected, the positive control (YFP1-Zip plus YFP2-Zip) gave rise to significant fluorescence, while transfection of YFP1-ZIP plus YFP2-V did not. These data demonstrate that V protein and Akt1 interact intracellularly, although it should be noted that these experiments do not distinguish between direct and indirect interactions.
FIG. 1.
Interaction of V and Akt1. (a) Coimmunoprecipitation of V and Akt1. HeLa cells were transfected with vector control (empty vector) or plasmids encoding V and/or Akt1. After 24 h, transfected cells were metabolically labeled with [35S]Cys and [35S]Met, lysed, and then subjected to immunoprecipitation with anti-V or anti-Akt1 antibody. The precipitates were resolved by 15% SDS-PAGE, and proteins were visualized by phosphorimagery. Migration of V and Akt1 are indicated on the right, and coprecipitated V or Akt1 are marked with asterisks. (b) BiF analysis of V and Akt1 interaction. HeLa cells were transfected with the following combinations of plasmids: YFP1-Zip and YFP2-V (negative control), YFP1-Zip and YFP2-Zip (positive control), or YFP2-V and YFP1-Akt1. Cells were collected, and fluorescence was measured by flow cytometry. The region considered YFP positive is indicated.
Akt1 played a critical role in viral protein synthesis.
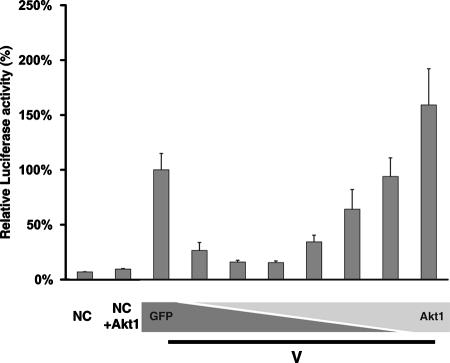
Previously, we have established a vaccinia virus-free minigenome system for PIV5, in which viral RNA synthesis can be studied in the absence of virus infection (36). BSR/T7 cells are transfected with a plasmid containing a reporter gene, R-Luc, flanked with the leader and trailer sequences of PIV5 along with plasmids encoding NP, P, and L of PIV5, resulting in replication and transcription of the minigenome, which can be detected in cell lysates by luciferase assay. Interestingly, the expression of the V protein inhibits viral RNA synthesis in the minigenome system (36). We hypothesized that the interaction of V with cellular Akt1 inhibited the function of Akt in viral RNA synthesis and that overexpression of Akt1 may overcome this inhibition. Alternatively, increased levels of Akt1 binding to V may titrate out the amount of V available to inhibit the vRNAP. Therefore, we cotransfected plasmids encoding Akt1 and V into cells to examine their effect on the minigenome system (Fig. 2). Expression of Akt1 abolished the inhibition of viral RNA synthesis by the V protein in a dose-dependent manner, indicating that Akt1 plays a role in viral RNA synthesis.
FIG. 2.
Effect of Akt1 expression on V-mediated inhibition of the minigenome system. Expression of Akt abrogates the inhibition of viral RNA synthesis by the V protein. Increasing amounts of an Akt1 expression vector were cotransfected together with a fixed amount of plasmid encoding V into a PIV5 minigenome system (36). The total amount of DNA transfected into the cells was maintained constant by using a plasmid expressing GFP. F- and R-Luc activities were detected in cell lysates at 24 h postinfection. Normalized luciferase activity of the positive control (no V or Akt1 plasmid; third bar from left) was set as 100%. NC, negative control.
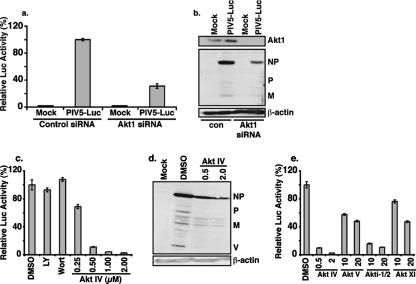
To further confirm the role of Akt1 in viral macromolecule synthesis, siRNA against Akt1 was used to knock down Akt1 expression levels. The effect of the Akt1 siRNA was first examined using a recombinant PIV5 expressing a reporter gene, R-Luc (rPIV5-R-Luc), which was constructed by inserting a R-Luc gene between HN and L of the PIV5 genome; luciferase activity in rPIV5-R-Luc-infected cells thus reflects the level of viral RNA and protein synthesis (data not shown). As shown in Fig. 3a, the siRNA against Akt1 was effective in reducing luciferase activity from rPIV5-R-Luc-infected cells by over 50%. In addition, the effect of siRNA against Akt1 on viral protein expression was examined. As shown in Fig. 3b, siRNA against Akt1 reduced expression of Akt1 and, importantly, PIV5 viral proteins.
FIG. 3.
Role of Akt1 in PIV5 protein expression. (a) Effect of siRNA against Akt1 on PIV5 protein expression. HeLa cells were transfected with siRNA against firefly luciferase (control) or human Akt1 and then infected with rPIV5-R-Luc at a MOI of 5. One day postinfection, luciferase activities (a) and expression levels of Akt1, PIV5 proteins, and actin (b) were determined. Luciferase activity in PIV5-R-Luc cells transfected with control siRNA (con) was set at 100%. (c) Inhibition of luciferase expression from PIV5-R-Luc by Akt inhibitor treatment. HeLa cells were infected with rPIV5-R-Luc at a MOI of 1 and treated with DMSO or increasing concentrations of the AktIV inhibitor (Calbiochem). The cells were collected at 1 day postinfection and assayed for luciferase activity. LY, LY294002 (10 μM); Wort, wortmannin (1 μM). (d) HeLa cells were infected with PIV5 and treated with DMSO or Akt inhibitor. The cells were collected 24 h postinfection and subjected to immunoblotting using anti-PIV5 and anti-actin antibodies. (e) Effects of different Akt inhibitors on PIV5 gene expression. The experiments shown in panel c were repeated using different Akt inhibitors. AktV, Akt1/2, and AktXI from Calbiochem were used in micromolar concentrations as indicated.
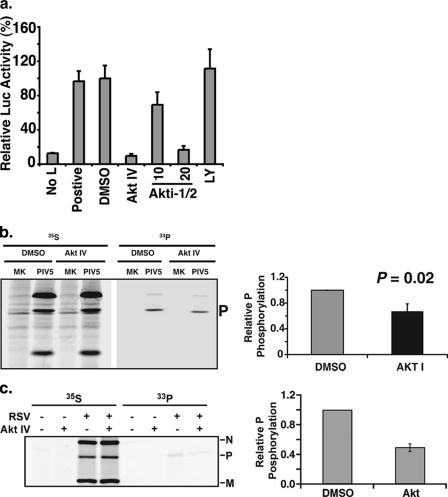
There are three isoforms of Akt: Akt1, Akt2, and Akt3. Thus, it is possible that Akt2 and Akt3 may compensate for the loss of the Akt1 after siRNA knock-down of Akt1 in HeLa cells. Reverse transcription-PCR analysis of mRNA purified from HeLa cells detected Akt1 as well as Akt2 and Akt3, supporting the possibility that Akt2 and Akt3 may compensate for the loss of Akt1 due to Akt1 siRNA in HeLa cells (data not shown). To examine this possibility, a chemical inhibitor of Akt (AktIV) targeting all three Akt isoforms was used. As shown in Fig. 3c, the addition of the inhibitor reduced the luciferase activity in rPIV5-R-Luc-infected cells in a dosage-dependent manner compared to that in control-treated cells. This inhibition was more potent than that seen with the Akt1 siRNA, suggesting that the other isoforms of Akt could indeed compensate for the loss of Akt1 in viral replication. Since Akt is downstream of PI3K, we examined whether PI3K inhibition could also block virus replication. Interestingly, the PI3K inhibitors LY294002 and wortmannin had no effect on PIV5 replication (Fig. 3c), suggesting that Akt activation in the context of viral infection is not PI3K dependent. Treatment with Akt inhibitor showed similar inhibition of viral structural protein production in virus-infected cells, as determined by immunoblotting with anti-PIV5 antibodies (Fig. 3d), compared with luciferase. Because of a general concern over the specificity of small-molecule inhibitors, the effects of three additional Akt inhibitors with different chemical structures and specificities were examined; all were found to be effective at blocking the luciferase expression, confirming Akt's critical role in PIV5 macromolecule synthesis (Fig. 3e).
Akt inhibitor reduced the replication of PIV5, MuV, and MeV.
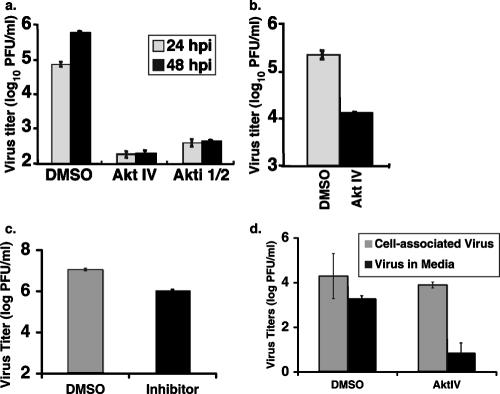
To examine the effect of the Akt inhibitors on virus replication, MDBK cells, the optimal cell type for growing PIV5, were infected with PIV5 at a MOI of 5 and treated with the Akt inhibitor or DMSO. Twenty-four or 48 h postinfection, the supernatants were collected for plaque assays to determine the viral titers. As shown in Fig. 4a, the addition of the Akt inhibitor reduced virus titers over 1,000-fold, indicating that the Akt inhibitor very effectively reduced virus growth. No cell death due to the inhibitor treatment at the inhibitory concentrations was observed by counting trypan blue-stained cells (data not shown). Similar results were obtained when PIV5 growth was analyzed with HeLa cells (Fig. 4b).
FIG. 4.
Akt inhibitor inhibited growth of PIV5, MuV, and MeV. MDBK (a) or HeLa (b) cells were infected with PIV5 at a MOI of 5 and treated with DMSO or Akt inhibitor (0.5 μM AktIV inhibitor or 10 μM Akt 1/2 inhibitor). The media from the cells were collected at the indicated times postinfection. (c) Inhibition of MuV growth. HeLa cells were infected with MuV at a MOI of 3 and treated with DMSO or AktIV inhibitor (0.5 μM). (d) Inhibition of MeV growth. Vero cells were infected with MeV at a MOI of 3 and treated with DMSO or AktIV inhibitor (2 μM). For all samples, virus titers were determined by plaque assay.
MuV is very closely related to PIV5, and the V proteins of PIV5 and MuV are highly homologous (32). To examine whether Akt plays a role in MuV replication, HeLa cells were infected with MuV and treated with the Akt inhibitor. At 24 h postinfection, titers of MuV in the media of infected cells were measured using plaque assays. Treatment with Akt inhibitor reduced MuV replication approximately 10-fold (Fig. 4c). Similarly, the Akt inhibitor reduced both cell-associated and released virus in MeV infection (Fig. 4d), with the released virus being more sensitive to Akt inhibition. The reasons for this difference are not known, though it is possible that the less-dramatic reduction of cell-associated MeV is due to the presence of residual virus from the initial infection. Treatment with the Akt inhibitor also reduced the growth of SeV (data not shown). Together, these data indicate that paramyxoviruses from different subfamilies require the Akt pathway for efficient viral replication.
Inhibition of RSV and VSV replication by the Akt inhibitors.
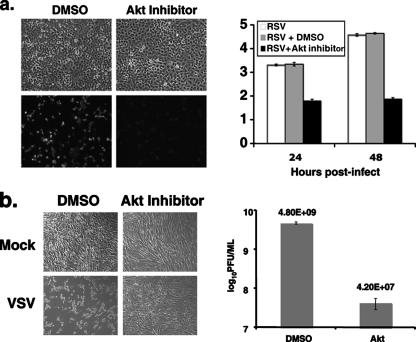
To study whether Akt plays a more general role in paramyxovirus replication, A549 cells, a human lung cell line, were infected with a recombinant RSV containing a GFP gene (rRSV-GFP) and then treated with the Akt inhibitor for 24 h. As shown in Fig. 5a, the Akt inhibitor reduced synthesis of GFP in rRSV-GFP-infected cells, suggesting that Akt plays a role in RSV replication. Furthermore, Akt inhibitor treatment blocked the production of infectious RSV virions, indicating that the decreased GFP expression correlates with viral protein and virion production (Fig. 5a, right panel).
FIG. 5.
Inhibition of RSV and VSV growth by the AktIV inhibitor. (a) Inhibition of RSV gene expression by the Akt inhibitor. Left panel, A549 cells were infected with rRSV-GFP at a MOI of 3 and treated with DMSO or AktIV inhibitor (1 μM). Expression of GFP was monitored by fluorescence microscopy and photographed at 1 day postinfection. Right panel, A549 cells were infected with RSV A2 at a MOI of 3 in triplicate and treated with DMSO or AktIV as described above. The media were collected 1 or 2 days postinfection, and titers of RSV were determined by plaque assay. (b) Inhibition of VSV growth. BHK cells were infected with VSV in triplicate at a MOI of 2 and treated with the AktIV inhibitor (2 μM). The cells were photographed at 18 h postinfection, and titers of virus from media of infected cells were determined by plaque assays. Graphs shown represent a single experiment. Errors bars show standard deviations of the means.
To investigate the role of Akt in the replication of NNSVs other than paramyxoviruses, the effect of the Akt inhibitors on rhabdovirus replication was examined. BHK cells were infected with VSV at a MOI of 2 and treated with the Akt inhibitor. Treatment with the Akt inhibitor reduced VSV titers by 2 to 3 logs (Fig. 5b). Interestingly, the inhibitor effectively blocked the cytopathic effect induced by VSV (Fig. 5b). Additional Akt inhibitors were also tested and found to be effective in blocking VSV replication, with reductions of viral replication ranging from 10- to 20-fold (data not shown).
Inhibition of phosphorylation of P in PIV5-infected cells by Akt inhibitor.
In the experiments described above, the inhibitors were added after the virus was incubated with cells, indicating that the effect of the inhibitors on the virus life cycle was at a postentry step. The fact that the siRNA against Akt and the inhibitors against Akt reduced viral protein expression (Fig. 2 and 3) indicates that Akt likely plays a role in viral RNA synthesis, since PIV5 protein expression is regulated at the level of transcription. To examine this possibility, we utilized the PIV5 minigenome described above (Fig. 2). We found that the Akt inhibitor blocked reporter gene expression from the minigenome system, indicating that, indeed, Akt plays a critical role in viral RNA synthesis (Fig. 6a). Consistent with the previous results (Fig. 3), the PI3K inhibitor LY294002 did not inhibit reporter gene expression from the minigenome.
FIG. 6.
Effect of Akt inhibition on minigenome RNA synthesis and P phosphorylation. (a) Inhibition of reporter gene expression from PIV5 minigenome system (36) by the Akt inhibitors. The minigenome system was as described before, and the inhibitors were added to the cells after transfection. (b) Effect of Akt on the phosphorylation status of the P protein of PIV5. HeLa cells were infected with PIV5 at a MOI of 5, and at 1 day postinfection, they were labeled with 35S-ProMix or [33P]orthophosphate for 4 h. The cells were lysed and immunoprecipitated with anti-P antibody. The average reduction of phosphorylation of the P protein from five experiments was graphed (P = 0.02). (c) A549 cells were infected with RSV at a MOI of 3 and, at 1 day postinfection, they were labeled with 35S-ProMix or [33P]orthophosphate for 4 h. The cells were lysed and immunoprecipitated with anti-RSV antibody. The average reduction of phosphorylation of the P protein from three experiments was graphed (P = 0.01). MK, mock infection.
The fact that Akt inhibitor inhibits viral RNA synthesis in the minigenome system, which does not contain the V protein, indicates that the likely target of Akt is not the V protein itself. In addition, Akt plays a role in the replication of RSV and VSV, which do not encode V protein homologs. Thus, Akt likely targets a component of the viral RNA polymerase complex, which is minimally comprised of the P and L proteins for all NNSVs. It is known that the phosphorylation of the P protein plays a critical role in viral RNA synthesis (38). Since Akt is a protein kinase, we examined the effect of the Akt inhibitor on phosphorylation of P in both PIV5- and RSV-infected cells. HeLa (PIV5) or A549 (RSV) cells were infected for 18 to 24 h and then labeled with either [33P]orthophosphate or [35S]Met/[35S]Cys mix in the presence or absence of the Akt inhibitor. Viral proteins were immunoprecipitated and separated by SDS-PAGE. Treatment with the Akt inhibitor reduced phosphorylation of the P protein of PIV5 by about 30% without affecting viral protein translation during the same labeling period (Fig. 6b). Similarly, the Akt inhibitor reduced phosphorylation of the P protein of RSV by about 50% (Fig. 6c). The partial reduction of phosphorylation of the P proteins suggests that Akt inhibition likely affects some, but not all, of the phosphorylation sites within the P proteins.
Phosphorylation of recombinant P of PIV5 by Akt1 in vitro.
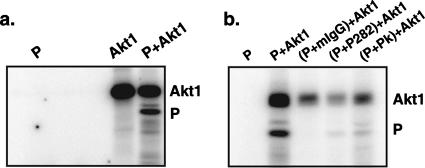
The fact that Akt inhibitor treatment reduced the level of P phosphorylation indicates that Akt plays a role in P phosphorylation in infected cells. However, it is not clear whether this effect is direct or indirect. To test whether Akt can directly phosphorylate P, we first purified recombinant PIV5 P protein with a His tag from Escherichia coli by Ni-affinity chromatography (27) and then performed an in vitro kinase assay using the recombinant P and activated Akt1. We found that Akt1 phosphorylates the recombinant P protein from bacteria (Fig. 7a). To ensure that the protein phosphorylated by Akt is indeed P, not a nonspecific bacteria protein copurified with P that has the same electrophoretic mobility as that which migrated with the P protein, we immunoprecipitated the purified recombinant P protein with P-specific antibodies (P282 or Pk) and carried out the in vitro kinase assay using the precipitates. The result (Fig. 7b) confirms that Akt 1 can directly phosphorylate recombinant P purified from bacteria.
FIG. 7.
Phosphorylation of recombinant P by Akt1. Recombinant PIV5 P with a six-His tag at its N terminus was purified from bacteria as described in Materials and Methods and used in in vitro kinase assays with activated Akt1. (a) Phosphorylation of the recombinant P by Akt1 in vitro. P, Akt1, or P plus Akt1 was mixed with [γ-32P]ATP, and the reaction mixtures were resolved by SDS-PAGE. Phosphorylated proteins were detected by phosphorimagery. Shown on the right are the relative mobilities of Akt1 and P. (b) Phosphorylation of recombinant P immunoprecipitated with P-specific antibody by Akt1. The recombinant P protein purified from bacteria was incubated with P-specific antibody P282, Pk, or control antibody mouse immunoglobulin G and washed with immunoprecipitation buffer as described in Materials and Methods. The precipitated products along with the beads were incubated with activated Akt1 and [γ-32P]ATP. The products were resolved by SDS-PAGE and visualized as described above.
DISCUSSION
We show here that inhibition of Akt by either siRNA or drug treatment results in significant inhibition of PIV5 protein expression and replication. In addition, Akt inhibitor treatment appears to have a broad spectrum of antiviral activity, decreasing the replication of NNSV from the rubulavirus, morbillivirus, pneumovirus, and rhabdovirus families. To the best of our knowledge, ours is the first study to report the potential of Akt inhibition as an antiviral therapeutic intervention. Because of the interest in Akt as a target for cancer therapy, there are many good compounds targeting Akt with low toxicity and high efficacy, and some of them have advanced to clinical trials (13). It is possible that some of these compounds will be effective against infections caused by NNSVs in vivo. The development of a broad-spectrum antiviral against NNSVs would allow for therapeutic intervention in a wide array of human diseases.
We have shown that Akt inhibitors can be effective against RSV, the most important etiologic agent of pediatric viral respiratory infection, which remains a major cause of morbidity and mortality among infants and among immunocompromised subjects and the elderly (reviewed in reference 14). Importantly, there is no vaccine for RSV, nor are there effective curative treatments for severe RSV disease, although aerosolized ribavirin and prophylactic immunoglobulin therapy are used in the clinical setting. However, the high cost of palivizumab prophylaxis due to the need for monthly injections during RSV season raises the question of cost effectiveness relative to health benefits. Therefore, there is a pressing need for safe and effective therapeutic interventions for RSV infection. In addition, Akt inhibitors can block MuV and MeV replication. While MuV and MeV infections have been well controlled in vaccinated population, they still pose a serious health threat in developing counties, where vaccine coverage is poor. VSV, a livestock pathogen, is a rhabdovirus similar to rabies virus, which causes a lethal infection in humans and also lacks an effective antiviral drug. The fact that the Akt inhibitor effectively blocked VSV replication provides evidence that Akt inhibition may be a good strategy for developing anti-rabies virus drugs.
One potential drawback is that the effects of the Akt inhibitors were variable in different cells for different viruses. For instance, in MDBK cells, a bovine cell line that is optimal for PIV5 growth, the Akt inhibitor had a dramatic effect on virus growth, reducing virus titers by 2 to 3 logs, while the same inhibitor reduced the PIV5 growth by only 1 to 2 logs in HeLa cells. We speculate that the differences are a reflection of how effective the inhibitors are in various cell lines, based on their tissue of origin and level of Akt isoform expression. Further optimization of Akt inhibitor treatment in primary cell culture and animal models of viral disease will enhance their potency for use in humans for therapy of infectious diseases caused by NNSV. While we have not tested the effects of Akt inhibitors on DNA virus replication, we do not expect the inhibitors to be effective against DNA virus. The AktIV inhibitor was originally identified as an inhibitor of the FOXO protein nuclear translocation, in which FOXO was expressed from a recombinant adenovirus (29). In the report, the inhibitor did not appear to inhibit adenovirus gene expression, suggesting that Akt activation is not necessary for adenovirus replication.
Akt, a serine/threonine kinase, is activated through phosphorylation at different sites within the protein (6). Since some of the inhibitors we used (e.g., the AktIV inhibitor) are known to inhibit phosphorylation of Akt (29), it is likely that phosphorylation of Akt plays a role in viral RNA synthesis. Which kinase activates Akt in virus-infected cells and how it is activated itself are not known. It is well established that Akt is one of the major downstream targets for PI3K (6). However, two well-known PI3K inhibitors, wortmannin and LY29002, had no effect on PIV5 replication (Fig. 3a), indicating that PI3K activation is not required for Akt activation in virus-infected cells. Therefore, we propose that a kinase downstream of PI3K, or potentially a novel kinase, is responsible for activating Akt in virus-infected cells.
While factors required for its activation remain unclear, our studies provide insight as to the mechanism by which Akt regulates PIV5 RNA synthesis. Chemical inhibition of Akt results in a significant decrease in phosphorylation of the viral P protein. In addition, Akt can phosphorylate P in vitro. The phosphorylation status of P is thought to be a key regulator of the switch by vRNAP from viral mRNA synthesis (transcription) to a viral RNA replication (12, 15, 55). Previous studies have shown that the P proteins of various NNSV are phosphorylated by host kinases (33). For VSV, there are two regions (N terminus region, domain I, and C terminus region, domain II) within the P protein that are phosphorylated. One of the host kinases known to phosphorylate the N-terminal region of VSV P is casein kinase II (CKII) (4). While the host kinase for the C-terminal region has not been identified (16), there is some circumstantial evidence that the host kinase may be Akt. Kim et al. first reported that K-252a, a broad nonspecific kinase inhibitor (isolated from Nocardiopsis sp.), and its derivative molecule KT5926 have anti-VSV activities in BHK cells (31). KT5926 does not inhibit CKII (30) but has been shown to target a host kinase that matches the size of Akt (59). In addition, staurosporine inhibits VSV RNA synthesis (53) and has been shown to target Akt in addition to protein kinases A and C (27).
Like other P proteins of paramyxoviruses, RSV P is a major cofactor for vRNAP and plays an essential role in viral RNA synthesis. Phosphorylation of P plays an essential role in its functions (20). There are five major phosphorylation sites within the P protein (43). Mutating these five phosphorylation sites resulted in a recombinant RSV that is severely attenuated in animals and is reduced in growth in some tissue culture cells (38). Interestingly, this mutant P protein was still phosphorylated in infected cells, albeit at much lower levels than wild-type P (38). The P protein purified from bacteria can be phosphorylated by casein kinase II, and this phosphorylated P is as active as the P protein treated with cell extract (presumably containing the host kinase that phosphorylates P) in an in vitro viral RNA synthesis assay, indicating that CKII can phosphorylate P (39, 63). However, CKII has not been shown to phosphorylate P in infected cells. We found that inhibition of Akt reduced the phosphorylation of the P protein, indicating that Akt plays a critical role in the phosphorylation of the P protein in RSV-infected cells.
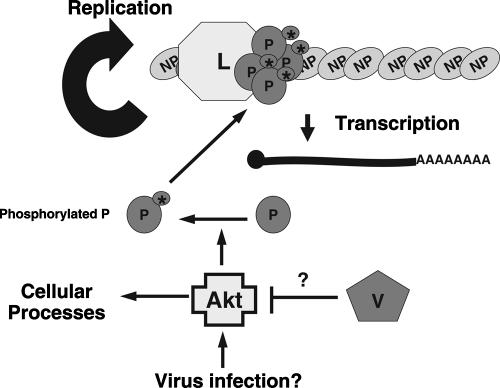
Our data lead us to a specific model for the role of Akt in the replication of NNSV (Fig. 8). Infection of cells by NNSV results in the activation of Akt. While the mechanism by which Akt is activated is unclear, PI3K is unlikely involved, as treatment with PI3K inhibitors has no effect on viral replication. Activated Akt then phosphorylates the viral P protein, which is an essential cofactor for vRNAP, regulating viral RNA synthesis. Since Akt inhibition causes a significant decrease in viral protein production, it is possible that phosphorylation of P by Akt is responsible for regulating the switch between transcription and RNA replication by the vRNAP in favor of transcription. Increased transcription would then allow for increased viral protein production and, subsequently, genome replication and virion morphogenesis. Inhibition of Akt thus blocks viral replication at an early stage postentry. In the paramyxoviruses which encode V proteins or other accessory proteins, such as W or C, we propose that these proteins regulate viral RNA synthesis by interacting with Akt and inhibiting its function, either by decreasing its kinase activity or by sequestering Akt itself. Thus, we propose that Akt plays a critical role in viral replication by regulating RNA synthesis through the phosphorylation of the P protein in infected cells.
FIG. 8.
A model for the involvement of Akt in viral RNA synthesis. The replication of viral RNA and synthesis of viral mRNA require P and L complex. The phosphorylation status of the P protein plays a critical in viral RNA synthesis. Akt phosphorylates P, thus leading to the activation of P. For viruses encoding a V protein, the V protein can regulate viral RNA synthesis via its interaction with Akt through an unknown mechanism. The V-Akt1 interaction may also contribute to regulation of innate immune responses by the V protein. Activation of Akt1 is often through the PI3K pathway. However, it is not clear what activates Akt1 in virus-infected cells, since PI3K inhibitors had no effect on virus replication.
The studies presented in this paper arose from our hypothesis that the V protein of PIV5 exerts its numerous activities by interacting with host proteins. We initially used a computational method to predict potential V-interacting proteins and then determined empirically that V binds specifically to one of the predicted interactors, Akt1/PKB. This interaction has functional consequences in that Akt overexpression alleviates V-mediated inhibition of PIV5 RNA synthesis in a minigenome system. Beyond this effect on the viral polymerase activity, it is possible that the V-Akt interaction plays additional roles in viral infection. Akt plays an important role in many cellular signaling pathways, such as those for apoptosis and interferon signaling and production (23). The V protein of PIV5 is known to inhibit apoptosis induced by viral infection (57). Therefore, the interaction between V and Akt is consistent with the role of V in apoptosis. Furthermore, V has also been shown to block interferon production and interferon signaling as well as to block interleukin-6 expression (18, 25, 37). It will be interesting to examine whether the interaction between V and Akt plays a role in these processes. Finally, PIV5 infection slows down the cell cycle, and the V protein of PIV5 is thought to play a critical in the process (35). It is tempting to speculate that the interaction of V and Akt may contribute to the slowing down of the cell cycle by PIV5, since Akt signaling also affects cell cycle control (6). Thus, Akt activation, and interaction with V, may play multiple roles in the replication and pathogenesis of NNSV.
Acknowledgments
We are grateful to Robert A. Lamb for providing SeV Z strain and VSV, Richard Randall for providing antibodies, Stephen Michnick for the YFP system, and Anthony Schmitt for critical readings of the manuscript. We thank the members of Biao He's laboratory for helpful discussion and technical assistance. We also thank Kim Tran for assistance with generating some of the figures.
The work was supported by grants from the National Institute of Allergy and Infectious Disease to B.H. (AI051372 and K02 AI65795).
Footnotes
Published ahead of print on 24 October 2007.
REFERENCES
- 1.Abraham, G., and A. K. Banerjee. 1976. Sequential transcription of the genes of vesicular stomatitis virus. Proc. Natl. Acad. Sci. USA 731504-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ball, L. A., and C. N. White. 1976. Order of transcription of genes of vesicular stomatitis virus. Proc. Natl. Acad. Sci. USA 73442-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barik, S. 1992. Transcription of human respiratory syncytial virus genome RNA in vitro: requirement of cellular factor(s). J. Virol. 666813-6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barik, S., and A. K. Banerjee. 1992. Phosphorylation by cellular casein kinase II is essential for transcriptional activity of vesicular stomatitis virus phosphoprotein P. Proc. Natl. Acad. Sci. USA. 896570-6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barik, S., T. McLean, and L. C. Dupuy. 1995. Phosphorylation of Ser232 directly regulates the transcriptional activity of the P protein of human respiratory syncytial virus: phosphorylation of Ser237 may play an accessory role. Virology 213405-412. [DOI] [PubMed] [Google Scholar]
- 6.Brazil, D. P., and B. A. Hemmings. 2001. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem. Sci. 26657-664. [DOI] [PubMed] [Google Scholar]
- 7.Buchholz, U. J., S. Finke, and K. K. Conzelmann. 1999. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J. Virol. 73251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burke, E., L. Dupuy, C. Wall, and S. Barik. 1998. Role of cellular actin in the gene expression and morphogenesis of human respiratory syncytial virus. Virology 252137-148. [DOI] [PubMed] [Google Scholar]
- 9.Burke, E., N. M. Mahoney, S. C. Almo, and S. Barik. 2000. Profilin is required for optimal actin-dependent transcription of respiratory syncytial virus genome RNA. J. Virol. 74669-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan, T. O., S. E. Rittenhouse, and P. N. Tsichlis. 1999. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu. Rev. Biochem. 68965-1014. [DOI] [PubMed] [Google Scholar]
- 11.Chatziandreou, N., N. Stock, D. Young, J. Andrejeva, K. Hagmaier, D. J. McGeoch, and R. E. Randall. 2004. Relationships and host range of human, canine, simian and porcine isolates of simian virus 5 (parainfluenza virus 5). J Gen. Virol. 853007-3016. [DOI] [PubMed] [Google Scholar]
- 12.Chen, J. L., T. Das, and A. K. Banerjee. 1997. Phosphorylated states of vesicular stomatitis virus P protein in vitro and in vivo. Virology 228200-212. [DOI] [PubMed] [Google Scholar]
- 13.Cheng, J. Q., C. W. Lindsley, G. Z. Cheng, H. Yang, and S. V. Nicosia. 2005. The Akt/PKB pathway: molecular target for cancer drug discovery. Oncogene 247482-7492. [DOI] [PubMed] [Google Scholar]
- 14.Collins, P. L., R. M. Chanock, and B. R. Murphy. 2001. Respiratory syncytial virus, p. 1443-1485. In D. M. Knipe and P. M. Howley (ed.), Virology, 4th ed. Raven Press, New York, NY.
- 15.Das, S. C., and A. K. Pattnaik. 2004. Phosphorylation of vesicular stomatitis virus phosphoprotein P is indispensable for virus growth. J. Virol. 786420-6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das, S. C., and A. K. Pattnaik. 2005. Role of the hypervariable hinge region of phosphoprotein P of vesicular stomatitis virus in viral RNA synthesis and assembly of infectious virus particles. J. Virol. 798101-8112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De, B. P., and A. K. Banerjee. 1999. Involvement of actin microfilaments in the transcription/replication of human parainfluenza virus type 3: possible role of actin in other viruses. Microsc. Res. Tech. 47114-123. [DOI] [PubMed] [Google Scholar]
- 18.Didcock, L., D. F. Young, S. Goodbourn, and R. E. Randall. 1999. The V protein of simian virus 5 inhibits interferon signalling by targeting STAT1 for proteasome-mediated degradation. J. Virol. 739928-9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du, K., and P. N. Tsichlis. 2005. Regulation of the Akt kinase by interacting proteins. Oncogene 247401-7409. [DOI] [PubMed] [Google Scholar]
- 20.Dupuy, L. C., S. Dobson, V. Bitko, and S. Barik. 1999. Casein kinase 2-mediated phosphorylation of respiratory syncytial virus phosphoprotein P is essential for the transcription elongation activity of the viral polymerase; phosphorylation by casein kinase 1 occurs mainly at Ser215 and is without effect. J. Virol. 738384-8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emerson, S. U. 1982. Reconstitution studies detect a single polymerase entry site on the vesicular stomatitis virus genome. Cell 31635-642. [DOI] [PubMed] [Google Scholar]
- 22.Emerson, S. U., and Y.-H. Yu. 1975. Both NS and L proteins are required for in vitro RNA synthesis by vesicular stomatitis virus. J. Virol. 151348-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franke, T. F., C. P. Hornik, L. Segev, G. A. Shostak, and C. Sugimoto. 2003. PI3K/Akt and apoptosis: size matters. Oncogene 228983-8998. [DOI] [PubMed] [Google Scholar]
- 24.Fuentes, S., K. C. Tran, P. Luthra, M. N. Teng, and B. He. 2007. Function of the respiratory syncytial virus small hydrophobic protein. J. Virol. 818361-8366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He, B., R. G. Paterson, N. Stock, J. E. Durbin, R. K. Durbin, S. Goodbourn, R. E. Randall, and R. A. Lamb. 2002. Recovery of paramyxovirus simian virus 5 with a V protein lacking the conserved cysteine-rich domain: the multifunctional V protein blocks both interferon-beta induction and interferon signaling. Virology 30315-32. [DOI] [PubMed] [Google Scholar]
- 26.He, B., R. G. Paterson, C. D. Ward, and R. A. Lamb. 1997. Recovery of infectious SV5 from cloned DNA and expression of a foreign gene. Virology 237249-260. [DOI] [PubMed] [Google Scholar]
- 27.He, B., M. Rong, D. Lyakhov, H. Gartenstein, G. Diaz, R. Castagna, W. T. McAllister, and R. K. Durbin. 1997. Rapid mutagenesis and purification of phage RNA polymerases. Protein Expr. Purif. 9142-151. [DOI] [PubMed] [Google Scholar]
- 28.Iverson, L. E., and J. K. Rose. 1982. Sequential synthesis of 5′-proximal vesicular stomatitis virus mRNA sequences. J. Virol. 44356-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kau, T. R., F. Schroeder, S. Ramaswamy, C. L. Wojciechowski, J. J. Zhao, T. M. Roberts, J. Clardy, W. R. Sellers, and P. A. Silver. 2003. A chemical genetic screen identifies inhibitors of regulated nuclear export of a Forkhead transcription factor in PTEN-deficient tumor cells. Cancer Cell 4463-476. [DOI] [PubMed] [Google Scholar]
- 30.Kim, Y. S., and A. Kawai. 1998. Studies on the antiviral mechanisms of protein kinase inhibitors K-252a and KT5926 against the replication of vesicular stomatitis virus. Biol. Pharm. Bull. 21498-505. [DOI] [PubMed] [Google Scholar]
- 31.Kim, Y. S., J. Sagara, and A. Kawai. 1995. Studies on the antiviral activity of protein kinase inhibitors against the replication of vesicular stomatitis virus. Biol. Pharm. Bull. 18895-899. [DOI] [PubMed] [Google Scholar]
- 32.Lamb, R. A., and D. Kolakofsky. 2001. Paramyxoviridae: the viruses and their replication. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 33.Lenard, J. 1999. Host cell protein kinases in nonsegmented negative-strand virus (Mononegavirales) infection. Pharmacol. Ther. 8339-48. [DOI] [PubMed] [Google Scholar]
- 34.Li, Z., M. Yu, H. Zhang, D. E. Magoffin, P. J. Jack, A. Hyatt, H. Y. Wang, and L. F. Wang. 2006. Beilong virus, a novel paramyxovirus with the largest genome of non-segmented negative-stranded RNA viruses. Virology 346219-228. [DOI] [PubMed] [Google Scholar]
- 35.Lin, G. Y., and R. A. Lamb. 2000. The paramyxovirus simian virus 5 V protein slows progression of the cell cycle. J. Virol. 749152-9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin, Y., F. Horvath, J. A. Aligo, R. Wilson, and B. He. 2005. The role of simian virus 5 V protein on viral RNA synthesis. Virology 338270-280. [DOI] [PubMed] [Google Scholar]
- 37.Lin, Y., M. Sun, S. M. Fuentes, C. D. Keim, T. Rothermel, and B. He. 2007. Inhibition of interleukin-6 expression by the V protein of parainfluenza virus 5. Virology 368262-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu, B., C. H. Ma, R. Brazas, and H. Jin. 2002. The major phosphorylation sites of the respiratory syncytial virus phosphoprotein are dispensable for virus replication in vitro. J. Virol. 7610776-10784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mazumder, B., G. Adhikary, and S. Barik. 1994. Bacterial expression of human respiratory syncytial viral phosphoprotein P and identification of Ser237 as the site of phosphorylation by cellular casein kinase II. Virology 20593-103. [DOI] [PubMed] [Google Scholar]
- 40.Moscona, A., and R. W. Peluso. 1996. Analysis of human parainfluenza virus 3 receptor binding variants: evidence for the use of a specific sialic acid-containing receptor. Microb. Pathog. 20179-184. [DOI] [PubMed] [Google Scholar]
- 41.Moyer, S. A., S. C. Baker, and S. M. Horikami. 1990. Host cell proteins required for measles virus reproduction. J. Gen. Virol. 71775-783. [DOI] [PubMed] [Google Scholar]
- 42.Moyer, S. A., S. C. Baker, and J. L. Lessard. 1986. Tubulin: a factor necessary for the synthesis of both Sendai virus and vesicular stomatitis virus RNAs. Proc. Natl. Acad. Sci. USA 835405-5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Navarro, J., C. Lopez-Otin, and N. Villanueva. 1991. Location of phosphorylated residues in human respiratory syncytial virus phosphoprotein. J. Gen. Virol. 721455-1459. [DOI] [PubMed] [Google Scholar]
- 44.Ogino, T., M. Iwama, J. Kinouchi, Y. Shibagaki, T. Tsukamoto, and K. Mizumoto. 1999. Involvement of a cellular glycolytic enzyme, phosphoglycerate kinase, in Sendai virus transcription. J. Biol. Chem. 27435999-36008. [DOI] [PubMed] [Google Scholar]
- 45.Ogino, T., M. Iwama, Y. Ohsawa, and K. Mizumoto. 2003. Interaction of cellular tubulin with Sendai virus M protein regulates transcription of viral genome. Biochem. Biophys. Res. Commun. 311283-293. [DOI] [PubMed] [Google Scholar]
- 46.Ogino, T., T. Yamadera, T. Nonaka, S. Imajoh-Ohmi, and K. Mizumoto. 2001. Enolase, a cellular glycolytic enzyme, is required for efficient transcription of Sendai virus genome. Biochem. Biophys. Res. Commun. 285447-455. [DOI] [PubMed] [Google Scholar]
- 47.Paterson, R. G., and R. A. Lamb. 1993. The molecular biology of influenza viruses and paramyxoviruses, p. 35-73. In A. Davidson and R. M. Elliott (ed.), Molecular virology: a practical approach. IRL Oxford University Press, Oxford, United Kingdom.
- 48.Powell, D. W., M. J. Rane, Q. Chen, S. Singh, and K. R. McLeish. 2002. Identification of 14-3-3ζ as a protein kinase B/Akt substrate. J. Biol. Chem. 27721639-21642. [DOI] [PubMed] [Google Scholar]
- 49.Randall, R. E., and D. F. Young. 1988. Comparison between parainfluenza virus type 2 and simian virus 5: monoclonal antibodies reveal major antigenic differences. J. Gen. Virol. 692051-2060. [DOI] [PubMed] [Google Scholar]
- 50.Redaelli, C., F. Granucci, L. De Gioia, and L. Cipolla. 2006. Synthesis and biological activity of Akt/PI3K inhibitors. Mini Rev. Med. Chem. 61127-1136. [DOI] [PubMed] [Google Scholar]
- 51.Remy, I., and S. W. Michnick. 2004. A cDNA library functional screening strategy based on fluorescent protein complementation assays to identify novel components of signaling pathways. Methods 32381-388. [DOI] [PubMed] [Google Scholar]
- 52.Richardson, J. C., and R. W. Peluso. 1996. Inhibition of VSV genome RNA replication but not transcription by monoclonal antibodies specific for the viral P protein. Virology 21626-34. [DOI] [PubMed] [Google Scholar]
- 53.Rigaut, K. D., Y. Gao, and J. Lenard. 1993. Effects of staurosporine on transcription by vesicular stomatitis virus. Virology 194433-440. [DOI] [PubMed] [Google Scholar]
- 54.Sarbassov, D. D., D. A. Guertin, S. M. Ali, and D. M. Sabatini. 2005. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 3071098-1101. [DOI] [PubMed] [Google Scholar]
- 55.Spadafora, D., D. M. Canter, R. L. Jackson, and J. Perrault. 1996. Constitutive phosphorylation of the vesicular stomatitis virus P protein modulates polymerase complex formation but is not essential for transcription or replication. J. Virol. 704538-4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stambolic, V., and J. R. Woodgett. 2006. Functional distinctions of protein kinase B/Akt isoforms defined by their influence on cell migration. Trends Cell Biol. 16461-466. [DOI] [PubMed] [Google Scholar]
- 57.Sun, M., T. A. Rothermel, L. Shuman, J. A. Aligo, S. Xu, Y. Lin, R. A. Lamb, and B. He. 2004. Conserved cysteine-rich domain of paramyxovirus simian virus 5 V protein plays an important role in blocking apoptosis. J. Virol. 785068-5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takeuchi, K., K. Tanabayashi, K. Okazaki, M. Hiahiyama, and A. Yamada. 1993. In vitro transcription and replication of the mumps virus genome. Arch. Virol. 128177-183. [DOI] [PubMed] [Google Scholar]
- 59.Teng, K. K., and L. A. Greene. 1994. KT5926 selectively inhibits nerve growth factor-dependent neurite elongation. J. Neurosci. 142624-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thomas, S. M., R. A. Lamb, and R. G. Paterson. 1988. Two mRNAs that differ by two nontemplated nucleotides encode the amino coterminal proteins P and V of the paramyxovirus SV5. Cell 54891-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tidona, C. A., H. W. Kurz, H. R. Gelderblom, and G. Darai. 1999. Isolation and molecular characterization of a novel cytopathogenic paramyxovirus from tree shrews. Virology 258425-434. [DOI] [PubMed] [Google Scholar]
- 62.Ulane, C. M., and C. M. Horvath. 2002. Paramyxoviruses SV5 and HPIV2 assemble STAT protein ubiquitin ligase complexes from cellular components. Virology 304160-166. [DOI] [PubMed] [Google Scholar]
- 63.Villanueva, N., J. Navarro, E. Mendez, and I. Garcia-Albert. 1994. Identification of a protein kinase involved in the phosphorylation of the C-terminal region of human respiratory syncytial virus P protein. J. Gen. Virol. 75555-565. [DOI] [PubMed] [Google Scholar]
- 64.Yoeli-Lerner, M., and A. Toker. 2006. Akt/PKB signaling in cancer: a function in cell motility and invasion. Cell Cycle 5603-605. [DOI] [PubMed] [Google Scholar]