Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV) is the causal agent of both KS and primary effusion lymphoma (PEL). Although treatment with paclitaxel has significant antitumor activity in KS, drug resistance represents a major obstacle for improving the overall response and survival of PEL patients. The transcriptional pattern of KSHV is cell/tissue specific, as revealed by the fact that the viral latent protein LANA2 is detected exclusively in B cells. This paper focuses on the mechanism of paclitaxel resistance observed in PEL cells. Here we show that LANA2 protein modulates microtubule dynamics through its direct binding to polymerized microtubules, preventing microtubule stabilization induced by paclitaxel. This is the first demonstration of paclitaxel resistance induced by a viral protein and suggests a link between the expression of LANA2 and the resistance of PEL cells to paclitaxel.
Kaposi's sarcoma-associated herpesvirus (KSHV) is the etiological agent of all forms of KS and lymphoproliferative disorders, such as multicentric Castleman's disease and primary effusion lymphoma (PEL). These disorders show different patterns of viral protein expression, probably due to a cell-specific/tissue-specific control of KSHV gene expression that is likely to be relevant to understanding the pathogenesis of KSHV-related diseases. In this sense, the protein encoded by ORFK10.5, known as LANA2 or vIRF3, is detected exclusively in B cells latently infected with the virus (33). Although LANA2 was initially described as a nuclear protein (33), subsequent studies have demonstrated it to be a nucleocytoplasmic shuttling protein (24, 29). LANA2 has been suggested to play an important role in KSHV-mediated tumorigenesis, due to its ability to inhibit p53 and PKR-dependent apoptosis (10, 33) or the virus-mediated transcriptional activation of the alpha interferon promoter (25). In addition to the differential KSHV gene expression, KS and PEL show differential sensitivity to chemotherapy treatment. PELs, unlike most non-Hodgkin's lymphomas, are relatively resistant to standard cytotoxic chemotherapy, and virtually all PEL patients succumb to the disease, with a median survival of approximately 60 days (20). In contrast, KS has a good response to the chemotherapeutic agent paclitaxel that received approval by the U.S. Food and Drug Administration (FDA) for the treatment of KS (6, 27).
The taxane paclitaxel (Taxol) is a mitotic inhibitor used commonly as a chemotherapeutic agent in the treatment of breast and ovarian cancer or lymphomas. Paclitaxel binds in vitro to the microtubule polymer, enhancing the polymerization of tubulin (26, 31) and artificially stabilizing microtubules (36, 42). Microtubules are dynamic structures involved in many cellular processes, such as cellular division, proliferation, transport, and maintenance of cell shape. Microtubule dynamics are particularly critical during mitosis, as they are responsible for the capture and alignment of chromosomes at metaphase and the subsequent separation of the chromosomes into two daughter cells at anaphase. The disruption of microtubule dynamics by taxanes leads first to mitotic arrest, resulting in a sustained or transient cell cycle block and eventually to cell death caused by an aberrant exit from mitosis (17, 23, 44, 48). PEL cells are relatively resistant to paclitaxel treatment (1). Several potential mechanisms can be proposed to account for the paclitaxel resistance observed in PEL cells: altered metabolism of the drug, decreased sensitivity to death-inducing stimuli, alterations in microtubule dynamics and altered binding of paclitaxel to the microtubule.
Here we show that the KSHV latent protein LANA2 has associated microtubule-depolymerizing activity and inhibits the binding of a fluorescent taxoid to its target, the microtubule. These results (i) suggest that LANA2 is important in the regulation of cytoskeleton organization and (ii) have implications for our understanding of the mechanisms of paclitaxel resistance in PEL cells.
MATERIALS AND METHODS
Cell culture and transfection.
The human breast cancer cell line MCF-7 and the human embryonic kidney cell line HEK293 were grown in Dulbecco's modified Eagle medium (DMEM) (Gibco BRL) supplemented with 10% fetal bovine serum, penicillin (100 units/ml), streptomycin (100 μg/ml), and 2 mM l-glutamine. The NIH-3T3 cell line was grown in DMEM supplemented with antibiotic, glutamine, and 10% calf serum. The BCP-1 PEL cell line (as well as the KSHV-negative human B-cell lymphomas MHH-PREB-1 and Karpas-422) was maintained in RPMI medium plus 20% fetal bovine serum supplemented with antibiotics and glutamine. The 3T3-pcDNA and 3T3-LANA2 cell lines were generated by stable transfection of NIH-3T3 cells with the empty vector pcDNA or the LANA2 expression plasmid pcDNA-LANA2. Transfected cells were selected by growth in the presence of 500 μg ml−1 G418. Stable pools (uncloned mass culture) of transfected cells were maintained in DMEM supplemented with 10% calf serum and 500 μg ml−1 G418. Cells were transfected at 50 to 70% confluence by using the cationic polymer transfection system (FuGENE; Roche) or Lipofectamine (Invitrogen), according to the manufacturer's instructions. In some experiments, cells were treated 24 h after transfection with paclitaxel or Flutax at concentrations between 10 nM and 10 μM.
Plasmids, antibodies, and reagents.
pcDNA-LANA2, EGFP-LANA2, pcDNA-LANA2 (bp 1 to 430), pcDNA-LANA2 (bp 430 to 1066), pcDNA-LANA2 (bp 1066 to 1321), and pcDNA-LANA2 (bp 1321 to 1706) plasmids were described previously (30, 33). A bacmid containing the wild-type KSHV genome (Bac36) and the green fluorescent protein (GFP) gene cassette was described previously (49). Monoclonal anti-alpha-tubulin antibody was purchased from AbD Serotec, anti-Foxo3a antibody was a gift from Eric W.-F. Lam (Imperial College, London, United Kingdom), and anti-p53 antibody was kindly provided by Manuel Serrano (CNIO, Madrid, Spain). Anti-actin antibody was obtained from ICN. Anti-LANA2 antibody was purchased from Affinity BioReagents. Antibody against alpha-tubulin acetylated on Lys 40 was obtained from Sigma. Paclitaxel and nocodazole were purchased from Sigma. The fluorescent taxoid Flutax was purchased from Molecular Probes.
Western blot analysis and immunofluorescence.
Whole-cell lysates were prepared by resuspending cell pellets in lysis buffer (50 mM Tris-HCl, 5 mM EDTA, 250 mM NaCl, 50 mM NaF, 0.1% Triton, 0.1 mM Na3VO4, and protease inhibitors 1 mM phenylmethylsulfonyl fluoride and 1 μg/ml aprotinin and leupeptin). The lysates were cleared by centrifugation for 15 min at 13,000 × g at 4°C, and total protein extracts were determined. Protein lysates were resolved by separation on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels, electroblotted, and incubated with the antibodies indicated. For immunofluorescence assays, cells were fixed in 2% paraformaldehyde-phosphate-buffered saline (PBS) for 20 min at room temperature, followed by permeabilization in PBS-0.5% Triton X-100 for 30 min at room temperature. After preblocking in 3% bovine serum albumin-PBS for 30 min, cells were incubated for 60 min with the correspondent antibodies and washed three times with PBS. Cells were then incubated with secondary antibodies for 60 min at room temperature and washed three times in PBS. Finally, cells were mounted with Mowiol containing or not containing DAPI (4′,6′-diamidino-2-phenylindole; DAKO). Cell preparations were observed with a Nikon Eclipse TE2000-U microscope or with a Bio-Rad laser scanning confocal microscope.
Cell cycle analysis.
MCF-7 cells in 10-mm dishes were transfected with the indicated plasmids and then incubated in the absence or presence of paclitaxel or vinblastine 24 h after transfection. Cells were fixed and stained by using propidium iodine 48 h after transfection as described previously (30). The distribution of cells in the different phases of the cell cycle was determined by analytical flow cytometry using a CyAn analyzer (DakoCytomation), and data were analyzed by using Summit software (DakoCytomation).
Live-cell Flutax binding studies.
Cells were incubated with Flutax, and at the indicated time, they were imaged using a Nikon Eclipse TE2000-U microscope. In parallel, cells incubated with Flutax were washed in PBS and the fluorescent taxoid content of intact cells, excluding propidium iodide, was determined by flow cytometric analysis using a CyAn analyzer.
In vitro polymerization assay.
In vitro transcription-translation of LANA2 was carried out using the TNT quick coupled transcription/translation system (Promega). Phosphocellulose-purified tubulin was obtained from purified bovine brain tubulin as described previously (46). In vitro tubulin assembly was performed following the method of Vallee (43) in the presence or absence of LANA2.
RESULTS
LANA2 inhibits both G2 arrest and microtubule polymerization in response to paclitaxel.
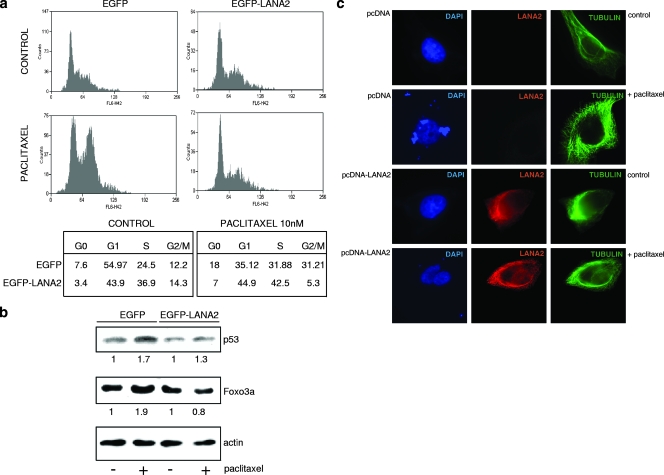
The treatment of some cell lines with low doses of paclitaxel provokes cell cycle arrest in G2/M due to the ability of paclitaxel to induce the stabilization of microtubule and the disruption of the mitotic spindle (15, 17). The paclitaxel-sensitive breast cancer cell line MCF-7 was transfected with enhanced GFP (EGFP)-LANA2 or the empty vector pEGFP. Following treatment of the cells with 10 nM of paclitaxel, a G2/M arrest was observed in the EGFP-expressing cells, whereas no significant arrest was found in the EGFP-LANA2-positive cells (Fig. 1a). Together with the G2/M arrest, an increase in p53 and Foxo3a levels after paclitaxel treatment has been reported previously (14, 35, 40). As shown in Fig. 1b, the treatment of EGFP-transfected cells with 10 nM of paclitaxel induced an increase of p53 and Foxo3a protein levels that was not observed after paclitaxel treatment of the EGFP-LANA2-transfected cells. A high concentration of paclitaxel increases polymer mass and also induces microtubule bundle formation in interphase cells, a phenomenon that becomes a hallmark of paclitaxel binding (37). When MCF-7 cells were treated with concentrations of paclitaxel over 10 μM, we observed the formation of typical microtubule bundles as described previously (Fig. 1c). However, bundle formation in cells transfected with pcDNA-LANA2 was clearly reduced (Fig. 1c).
FIG. 1.
LANA2 inhibits G2/M arrest induced by paclitaxel and prevents paclitaxel-induced microtubule polymerization. (a) MCF-7 cells transfected with EGFP or EGFP-LANA2 were treated with 10 nM paclitaxel for 24 h, stained with propidium iodide for cell cycle analysis, and subjected to flow cytometry. Numbers in lower panels show percentages of cells detected in each cell cycle phase in a representative experiment. (b) Upregulation of p53 or Foxo3a levels after treatment of MCF-7 cells with low doses of paclitaxel (10 nM) for 24 h is not detected in cells expressing LANA2. Cells were transfected and treated with paclitaxel as described above, and whole-cell lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and probed for the proteins indicated. Actin serves as a loading control. The relative intensity of each band was estimated densitometrically by normalizing the values from treated cells (+) to those from untreated cells (−) (relative density, 1) and is displayed below each lane. (c) MCF-7 cells transfected with pcDNA or pcDNA-LANA2 were treated with 10 μM paclitaxel for 24 h and subsequently double-stained with anti-LANA2 and anti-tubulin antibodies and analyzed by fluorescence microscopy.
LANA2 expression impairs the ability of the fluorescent taxoid Flutax to bind microtubules.
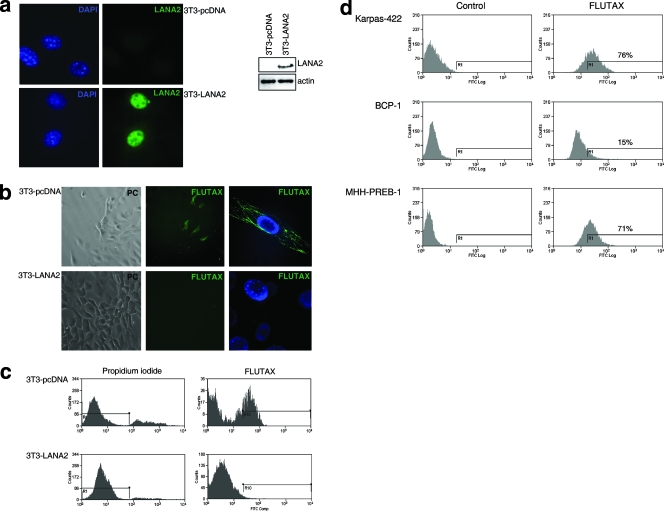
To directly characterize the effect of the expression of LANA2 over paclitaxel action, we employed a paclitaxel derivative conjugated with fluorescein isothiocyanate and Flutax and tested microtubule staining in mouse NIH-3T3 fibroblasts. A derivative 3T3 cell line stably expressing LANA2 was generated by the transfection of NIH-3T3 cells with pcDNA-LANA2 and the selection by culture in medium supplemented with G418. Cells resistant to G418 show the expression of LANA2, as demonstrated by Western blot analysis or immunofluorescence with an anti-LANA2 antibody (Fig. 2a). The incubation of cells in medium supplemented with Flutax (1 μM) revealed stained microtubule cytoskeleton only in 3T3-pcDNA cells, not in 3T3-LANA2 cells (Fig. 2b). The measurement of Flutax incorporation by flow cytometric analysis revealed Flutax labeling in a population of 3T3-pcDNA cells at 60 min after drug exposure, whereas no incorporation was found in the 3T3-LANA2 cells (Fig. 2c). Although PEL cells have previously been demonstrated to be resistant to paclitaxel treatment, the resistance of these cells to bind to the taxoid component has not been reported. In order to determine whether PEL cells are resistant to bind to Flutax, KSHV-positive BCP-1 cells or the KSHV-negative B-cell lymphomas MHH-PREB-1 and Karpas-422 were incubated with the fluorescent taxoid Flutax (1 μM) for 16 h. After washing cells with PBS, we determined the incorporation of Flutax by flow cytometric analysis. More than 70% of the KSHV-negative B cells were positive for Flutax binding (Fig. 2d). However, only 15% of the BCP-1 cells showed staining with the fluorescent compound (Fig. 2d), suggesting that the resistance of PEL cells to paclitaxel is related to the inhibition of paclitaxel binding to these cells.
FIG. 2.
Expression of LANA2 inhibits the binding of Flutax. (a) NIH-3T3 cells stably expressing LANA2 were generated as described in Materials and Methods. 3T3-pcDNA and pcDNA-LANA2 cells were then stained with the anti-LANA2 antibody (left panel) or subjected to Western blot analysis with the same antibody (right panel). (b) 3T3-pcDNA and pcDNA-LANA2 cells were incubated in medium supplemented with Flutax (10 μM) for 60 min. The incorporation of Flutax was then analyzed in vivo by fluorescence microscopy (phase contrast image). A more detailed image after the fixation and staining of the cells with DAPI cells is shown in the right panels. (c) Cells stably expressing LANA2 were incubated with Flutax as described above and then analyzed for microtubule staining by flow cytometric analysis. (d) KSHV-negative (Karpas-422 and MHH-PREB-1) and KSHV-positive BCP-1 cell lines were incubated in medium supplemented with 500 nM Flutax, and after 16 h, cells were analyzed for Flutax incorporation by flow cytometric analysis. FITC, fluorescein isothiocyanate.
LANA2 binds to polymerized microtubules.
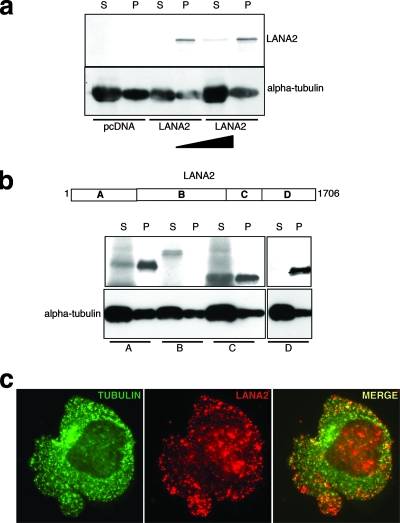
To test whether LANA2 can bind to microtubules, we performed an in vitro microtubule cosedimentation assay with purified tubulin in the presence of in vitro-translated LANA2 protein. After the polymerization of tubulin, LANA2 was detected in the pellet fraction associated with polymerized microtubules (Fig. 3a). To determine whether there is a discrete region within the primary structure of LANA2 that mediates microtubule binding, we tested the abilities of different LANA2 deletion constructs to associate with polymerized microtubules. Although only the C terminus of LANA2 was detected exclusively in the pellet fraction, two other LANA2 deletion constructs were detected in the polymerized microtubule fraction, suggesting that LANA2 binding is not restricted to a specific region (Fig. 3b). Finally, two-color fluorescence microscopy with the anti-LANA2 antibody and a monoclonal antibody to alpha-tubulin revealed that the LANA2 dots label microtubules in BCP-1 cells, indicating the colocalization of LANA2 and tubulin in PEL cells (Fig. 3c).
FIG. 3.
LANA2 protein interacts with tubulin. (a) In vitro-translated [35S]methionine LANA2 protein was incubated with purified tubulin and subjected to an in vitro polymerization assay. Assembled microtubules were separated from unpolymerized tubulin by centrifugation. Shown are LANA2 and tubulin detected in supernatants (S [unpolymerized tubulin]) and pellets (P [polymerized tubulin]). (b) In vitro-translated [35S]methionine LANA2 deletion constructs were incubated with purified tubulin and subjected to an in vitro polymerization assay. Shown are LANA2 deletion constructs and tubulin in supernatants (S) and pellets (P). (c) LANA2 colocalizes with tubulin in KSHV-infected BCP-1 cells. Cytospins of BCP-1 cells were double-stained with anti LANA2 and anti-alpha-tubulin antibody. The colocalization of both proteins is shown in yellow. Areas A, B, C, and D in panel b correspond to areas designated for bp 1 to 1706.
LANA2 expression decreases microtubule stability.
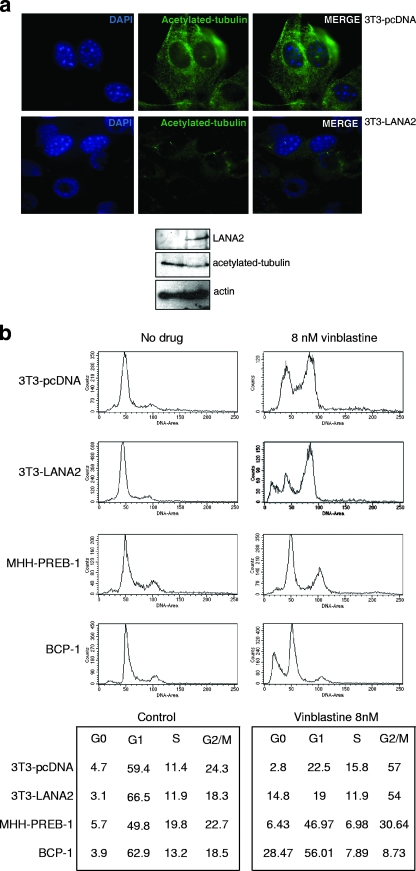
Acetylated tubulin is an indicator of stable microtubules; therefore, we next examined the distribution of acetylated tubulin in the LANA2-expressing cells. As shown in Fig. 4a, 3T3-LANA2 cells show diminished acetylated tubulin staining in comparison with that of the 3T3-pcDNA cells, suggesting that the presence of LANA2 decreases microtubule stability. In addition, since vinblastine is known to destabilize microtubules (16, 19), cell cycle progression in the different cell lines following exposure to vinblastine was also evaluated. As expected, control 3T3-pcDNA cells accumulated in the G2/M phase of the cell cycle after exposure to 8 nM vinblastine (Fig. 4b). Interestingly, the treatment of 3T3-LANA2 cells with this depolymerizing agent resulted in a higher fraction of cells accumulated in the G2/M phase and an accumulation of cells in the hypodiploid region of the histograms. An increased sensitivity to vinblastine by cells expressing LANA2 was also observed for B cells. KSHV-negative MHH-PREB-1 cells show a mild degree of G2/M arrest after incubation with 8 nM vinblastine for 24 h. However, a large fraction of hypodiploid cells was seen in BCP-1 PEL cells following vinblastine exposure (Fig. 4b).
FIG. 4.
Expression of LANA2 decreases microtubule stability. (a) Expression of LANA2 diminishes acetylated tubulin staining. 3T3-pcDNA and 3T3-LANA2 cells were stained with anti-alpha-tubulin acetylated on Lys 40 antibody and visualized by fluorescence microscopy (upper panel) or subjected to Western blot analysis with the indicated antibodies (lower panel). (b) Cell cycle progression in the presence of vinblastine of 3T3 and B cells with or without expressing LANA2. 3T3-pcDNA, 3T3-LANA2, MHH-PREB-1, and BCP-1 cells were treated for 24 h with 8 nM vinblastine, and subsequently, cell cycle analysis was carried out as described in Materials and Methods. Numbers in the lower panels show percentages of cells detected in each cell cycle phase in a representative experiment.
Coexpression of LANA2 with the KSHV latent proteins expressed in 293 cells prevents microtubule polymerization in response to paclitaxel.
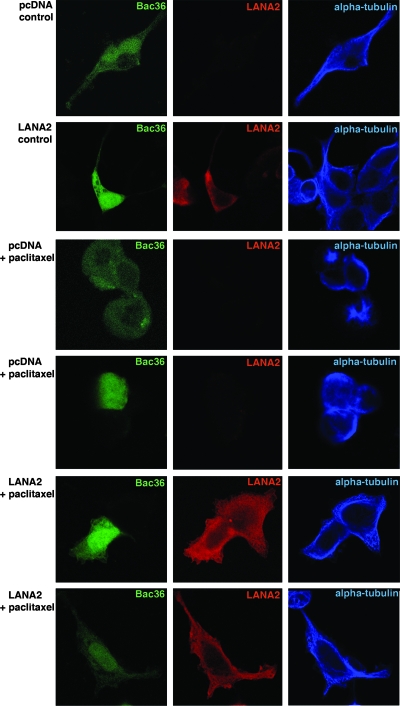
In order to determine whether the resistance of PEL cells to paclitaxel could be due to the expression of other KSHV latent genes, HEK293 cells were cotransfected with the bacmid containing the KSHV genome and GFP (Bac36) DNA, together with the empty vector pcDNA or the pcDNA-LANA2 expression plasmid, and their responses to paclitaxel were analyzed. As expected, HEK293 cells cotransfected with Bac36 DNA and the empty vector pcDNA did not show LANA2 expression, and only those cells cotransfected with the pcDNA-LANA2 plasmid showed positive labeling for both Bac36 and LANA2 (Fig. 5). In addition, and as shown in Fig. 5, aster-like microtubule changes induced by paclitaxel treatment were detected only in cells cotransfected with Bac36 DNA and pcDNA (not in cells expressing LANA2), suggesting LANA2 as the main mediator of the paclitaxel resistance observed in PEL cells.
FIG. 5.
Cotransfection of pcDNA-LANA2 and Bac36 DNA decreases the microtubule polymerization induced by paclitaxel. HEK293 cells were transfected with Bac36 DNA, together with empty pcDNA or pcDNA-LANA2, and then treated with 10 μM paclitaxel for 24 h. Cells were then fixed and stained with anti-LANA2 and anti-alpha-tubulin antibodies. Cells expressing GFP, that is, those containing Bac36, and not expressing LANA2 show aster-like microtubule changes or altered tubulin structures induced by paclitaxel treatment, while those positive for GFP (green), and thus carrying Bac36, together with LANA2 (red), did not show the effects of paclitaxel.
DISCUSSION
Since its approval by the FDA in 1992 for the treatment of ovarian cancer, the use of paclitaxel in cancer chemotherapy has increased dramatically. The antiproliferative action of paclitaxel relates to its abilities to bind tubulin, promote microtubule assembly, and stabilize microtubules by bundle formation (22, 36, 37). These effects of the drug are correlated with the arrest of cells in the G2/M phase of the cell cycle as well as cellular toxicity (15, 34, 36). In this sense, those proteins that regulate microtubule dynamics by interacting with tubulin dimers or polymerized microtubules clearly have the potential to modulate the sensitivity of a cell toward paclitaxel. The good response to paclitaxel exhibited by KS resulted in the approval of this drug by the FDA for its use in the treatment of this disease (6, 27). However, there are no current therapies effective against PEL; it remains a fatal disease, with a median life expectancy of approximately 2 months (20). The results in this report demonstrate that the latent protein LANA2, expressed exclusively in KSHV-infected B cells, was able to inhibit G2/M cell cycle arrest and reduced bundle formation induced by the chemotherapeutic agent paclitaxel in the MCF-7-sensitive cell line. In addition, LANA2 impaired the ability of the fluorescent taxoid Flutax to bind and decorate microtubules. Since Flutax competes with paclitaxel for the same microtubule binding site, these results suggest that LANA2 interferes with the taxoid binding to microtubules. A predominant interaction of Flutax with sites at which tubulin is newly polymerized was suggested (11). The results obtained after an in vitro tubulin polymerization assay demonstrated that LANA2 was able to interact with polymerized tubulin, thus reinforcing our hypothesis.
Several mechanisms have been proposed to account for the resistance observed in human tumors or cell lines to paclitaxel: overexpression of the multidrug transporter P-glycoprotein (13), altered metabolism of the drug, decreased sensitivity to death-inducing stimuli (2), alterations in microtubule dynamics, and altered binding of paclitaxel to its cellular target, the microtubule (8, 9). Since the paclitaxel-binding site is present only on polymerized tubulin, not on tubulin dimers (31), the selection of a less stable polymer, that is, a polymer with increased microtubule dynamics, could potentially offer a survival advantage for a tumor challenged with a microtubule-stabilizing drug such as paclitaxel. According to Cabral and coworkers, paclitaxel-resistant cell lines contain “hypostable” microtubules in which the equilibrium between the dimer and polymer is shifted toward the former (4, 5, 28). We wanted to determine whether the binding of LANA2 to the tubulin might alter the microtubule dynamics. It has been shown that a minor population of microtubules is stabilized in cultured cells, and they are enriched in acetylated and/or detyrosinated tubulin (3, 21, 32, 38, 39, 41, 45). In the present study, we have shown that the level of acetylated tubulin was clearly reduced in the 3T3 cells expressing LANA2, suggesting that LANA2 expression is responsible for a less stable polymer. These results are also in agreement with the observation that in the paclitaxel-resistant cell lines, the equilibrium between weakly and highly dynamic microtubules has been shifted toward the latter (7, 12, 18, 47). Moreover, since vinblastine is known to destabilize microtubules (16, 19), LANA2 expression may result in increased susceptibility to the mitotic effects of vinblastine. The stronger perturbation in cell cycle progression observed in the LANA2-expressing cells indicates that the expression of LANA2 results in increased cell susceptibility to vinblastine.
The interference of LANA2 expression in KSHV-infected B cells leads to a rapid induction of cell death (our unpublished observations), thus precluding a direct demonstration of the role of LANA2 in the paclitaxel resistance of PEL cells. However, in order to determine whether the resistance of PEL cells to paclitaxel could be due to the expression of other KSHV latent genes, HEK293 cells were cotransfected with the bacmid containing the KSHV genome (Bac36). Cells transfected with Bac36 DNA did not show any resistance to paclitaxel, pointing to LANA2 as the main factor that mediates PEL resistance.
Together, these results demonstrate that LANA2 increases cell resistance to paclitaxel by binding to polymerized tubulin and by the modulation of microtubule dynamics. The identification of LANA2 as a negative microtubule regulator sheds new light on its role in PEL resistance to paclitaxel.
Acknowledgments
We are very grateful to M. A. Piris for supplying the MHH-PREB-1 and Karpas-422 cell lines.
This work was supported by a grant from Ministerio de Ciencia y Tecnologia de España (BIO2005-02417) and Fundación Mutua Madrileña. L.M.-V. was supported by Comunidad de Madrid.
Footnotes
Published ahead of print on 21 November 2007.
REFERENCES
- 1.An, J., Y. Sun, M. Fisher, and M. B. Rettig. 2004. Antitumor effects of bortezomib (PS-341) on primary effusion lymphomas. Leukemia 181699-1704. [DOI] [PubMed] [Google Scholar]
- 2.Blagosklonny, M. V., and T. Fojo. 1999. Molecular effects of paclitaxel: myths and reality (a critical review). Int. J. Cancer 83151-156. [DOI] [PubMed] [Google Scholar]
- 3.Bulinski, J. C., J. E. Richards, and G. Piperno. 1988. Posttranslational modifications of alpha tubulin: detyrosination and acetylation differentiate populations of interphase microtubules in cultured cells. J. Cell Biol. 1061213-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cabral, F., and S. B. Barlow. 1989. Mechanisms by which mammalian cells acquire resistance to drugs that affect microtubule assembly. FASEB J. 31593-1599. [DOI] [PubMed] [Google Scholar]
- 5.Cabral, F. R., R. C. Brady, and M. J. Schibler. 1986. A mechanism of cellular resistance to drugs that interfere with microtubule assembly. Ann. N. Y. Acad. Sci. 466745-756. [DOI] [PubMed] [Google Scholar]
- 6.Cattelan, A. M., M. Trevenzoli, and S. M. Aversa. 2002. Recent advances in the treatment of AIDS-related Kaposi's sarcoma. Am. J. Clin. Dermatol. 3451-462. [DOI] [PubMed] [Google Scholar]
- 7.Derry, W. B., L. Wilson, and M. A. Jordan. 1995. Substoichiometric binding of Taxol suppresses microtubule dynamics. Biochemistry 342203-2211. [DOI] [PubMed] [Google Scholar]
- 8.Drukman, S., and M. Kavallaris. 2002. Microtubule alterations and resistance to tubulin-binding agents (review). Int. J. Oncol. 21621-628. [PubMed] [Google Scholar]
- 9.Dumontet, C., and B. I. Sikic. 1999. Mechanisms of action of and resistance to antitubulin agents: microtubule dynamics, drug transport, and cell death. J. Clin. Oncol. 171061-1070. [DOI] [PubMed] [Google Scholar]
- 10.Esteban, M., M. A. Garcia, E. Domingo-Gil, J. Arroyo, C. Nombela, and C. Rivas. 2003. The latency protein LANA2 from Kaposi's sarcoma-associated herpesvirus inhibits apoptosis induced by dsRNA-activated protein kinase but not RNase L activation. J. Gen. Virol. 841463-1470. [DOI] [PubMed] [Google Scholar]
- 11.Evangelio, J. A., M. Abal, I. Barasoain, A. A. Souto, M. P. Lillo, A. U. Acuna, F. Amat-Guerri, and J. M. Andreu. 1998. Fluorescent taxoids as probes of the microtubule cytoskeleton. Cell Motil. Cytoskelet. 3973-90. [DOI] [PubMed] [Google Scholar]
- 12.Goncalves, A., D. Braguer, K. Kamath, L. Martello, C. Briand, S. Horwitz, L. Wilson, and M. A. Jordan. 2001. Resistance to Taxol in lung cancer cells associated with increased microtubule dynamics. Proc. Natl. Acad. Sci. USA 9811737-11742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gottesman, M. M. 2002. Mechanisms of cancer drug resistance. Annu. Rev. Med. 53615-627. [DOI] [PubMed] [Google Scholar]
- 14.Heliez, C., L. Baricault, N. Barboule, and A. Valette. 2003. Paclitaxel increases p21 synthesis and accumulation of its AKT-phosphorylated form in the cytoplasm of cancer cells. Oncogene 223260-3268. [DOI] [PubMed] [Google Scholar]
- 15.Horwitz, S. B. 1992. Mechanism of action of Taxol. Trends Pharmacol. Sci. 13134-136. [DOI] [PubMed] [Google Scholar]
- 16.Jordan, M. A., D. Thrower, and L. Wilson. 1991. Mechanism of inhibition of cell proliferation by Vinca alkaloids. Cancer Res. 512212-2222. [PubMed] [Google Scholar]
- 17.Jordan, M. A., K. Wendell, S. Gardiner, W. B. Derry, H. Copp, and L. Wilson. 1996. Mitotic block induced in HeLa cells by low concentrations of paclitaxel (Taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res. 56816-825. [PubMed] [Google Scholar]
- 18.Jordan, M. A., and L. Wilson. 1998. Microtubules and actin filaments: dynamic targets for cancer chemotherapy. Curr. Opin. Cell Biol. 10123-130. [DOI] [PubMed] [Google Scholar]
- 19.Jordan, M. A., and L. Wilson. 1999. The use and action of drugs in analyzing mitosis. Methods Cell Biol. 61267-295. [DOI] [PubMed] [Google Scholar]
- 20.Komanduri, K. V., J. A. Luce, M. S. McGrath, B. G. Herndier, and V. L. Ng. 1996. The natural history and molecular heterogeneity of HIV-associated primary malignant lymphomatous effusions. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 13215-226. [DOI] [PubMed] [Google Scholar]
- 21.Kreis, T. E. 1987. Microtubules containing detyrosinated tubulin are less dynamic. EMBO J. 62597-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar, N. 1981. Taxol-induced polymerization of purified tubulin. Mechanism of action. J. Biol. Chem. 25610435-10441. [PubMed] [Google Scholar]
- 23.Kung, A. L., S. W. Sherwood, and R. T. Schimke. 1990. Cell line-specific differences in the control of cell cycle progression in the absence of mitosis. Proc. Natl. Acad. Sci. USA 879553-9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lubyova, B., M. J. Kellum, A. J. Frisancho, and P. M. Pitha. 2004. Kaposi's sarcoma-associated herpesvirus-encoded vIRF-3 stimulates the transcriptional activity of cellular IRF-3 and IRF-7. J. Biol. Chem. 2797643-7654. [DOI] [PubMed] [Google Scholar]
- 25.Lubyova, B., and P. M. Pitha. 2000. Characterization of a novel human herpesvirus 8-encoded protein, vIRF-3, that shows homology to viral and cellular interferon regulatory factors. J. Virol. 748194-8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manfredi, J. J., J. Parness, and S. B. Horwitz. 1982. Taxol binds to cellular microtubules. J. Cell Biol. 94688-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGarvey, M. E., A. Tulpule, J. Cai, T. Zheng, R. Masood, B. Espina, N. Arora, D. L. Smith, and P. S. Gill. 1998. Emerging treatments for epidemic (AIDS-related) Kaposi's sarcoma. Curr. Opin. Oncol. 10413-421. [DOI] [PubMed] [Google Scholar]
- 28.Minotti, A. M., S. B. Barlow, and F. Cabral. 1991. Resistance to antimitotic drugs in Chinese hamster ovary cells correlates with changes in the level of polymerized tubulin. J. Biol. Chem. 2663987-3994. [PubMed] [Google Scholar]
- 29.Munoz-Fontela, C., M. Collado, E. Rodriguez, M. A. Garcia, A. Alvarez-Barrientos, J. Arroyo, C. Nombela, and C. Rivas. 2005. Identification of a nuclear export signal in the KSHV latent protein LANA2 mediating its export from the nucleus. Exp. Cell Res. 31196-105. [DOI] [PubMed] [Google Scholar]
- 30.Munoz-Fontela, C., E. Rodriguez, C. Nombela, J. Arroyo, and C. Rivas. 2003. Characterization of the bipartite nuclear localization signal of protein LANA2 from Kaposi's sarcoma-associated herpesvirus. Biochem. J. 374545-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parness, J., and S. B. Horwitz. 1981. Taxol binds to polymerized tubulin in vitro. J. Cell Biol. 91479-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piperno, G., M. LeDizet, and X. J. Chang. 1987. Microtubules containing acetylated alpha-tubulin in mammalian cells in culture. J. Cell Biol. 104289-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rivas, C., A. E. Thlick, C. Parravicini, P. S. Moore, and Y. Chang. 2001. Kaposi's sarcoma-associated herpesvirus LANA2 is a B-cell-specific latent viral protein that inhibits p53. J. Virol. 75429-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rowinsky, E. K., R. C. Donehower, R. J. Jones, and R. W. Tucker. 1988. Microtubule changes and cytotoxicity in leukemic cell lines treated with Taxol. Cancer Res. 484093-4100. [PubMed] [Google Scholar]
- 35.Saito, Y., C. G. Milross, W. N. Hittelman, D. Li, T. Jibu, L. J. Peters, and L. Milas. 1997. Effect of radiation and paclitaxel on p53 expression in murine tumors sensitive or resistant to apoptosis induction. Int. J. Radiat. Oncol. Biol. Phys. 38623-631. [DOI] [PubMed] [Google Scholar]
- 36.Schiff, P. B., J. Fant, and S. B. Horwitz. 1979. Promotion of microtubule assembly in vitro by Taxol. Nature 277665-667. [DOI] [PubMed] [Google Scholar]
- 37.Schiff, P. B., and S. B. Horwitz. 1980. Taxol stabilizes microtubules in mouse fibroblast cells. Proc. Natl. Acad. Sci. USA 771561-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schulze, E., D. J. Asai, J. C. Bulinski, and M. Kirschner. 1987. Posttranslational modification and microtubule stability. J. Cell Biol. 1052167-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schulze, E., and M. Kirschner. 1987. Dynamic and stable populations of microtubules in cells. J. Cell Biol. 104277-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sunters, A., S. Fernandez de Mattos, M. Stahl, J. J. Brosens, G. Zoumpoulidou, C. A. Saunders, P. J. Coffer, R. H. Medema, R. C. Coombes, and E. W. Lam. 2003. FoxO3a transcriptional regulation of Bim controls apoptosis in paclitaxel-treated breast cancer cell lines. J. Biol. Chem. 27849795-49805. [DOI] [PubMed] [Google Scholar]
- 41.Thompson, W. C., D. J. Asai, and D. H. Carney. 1984. Heterogeneity among microtubules of the cytoplasmic microtubule complex detected by a monoclonal antibody to alpha tubulin. J. Cell Biol. 981017-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turner, P. F., and R. L. Margolis. 1984. Taxol-induced bundling of brain-derived microtubules. J. Cell Biol. 99940-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vallee, R. B. 1986. Reversible assembly purification of microtubules without assembly-promoting agents and further purification of tubulin, microtubule-associated proteins, and MAP fragments. Methods Enzymol. 13489-104. [DOI] [PubMed] [Google Scholar]
- 44.Wang, T. H., H. S. Wang, and Y. K. Soong. 2000. Paclitaxel-induced cell death: where the cell cycle and apoptosis come together. Cancer 882619-2628. [DOI] [PubMed] [Google Scholar]
- 45.Webster, D. R., G. G. Gundersen, J. C. Bulinski, and G. G. Borisy. 1987. Differential turnover of tyrosinated and detyrosinated microtubules. Proc. Natl. Acad. Sci. USA 849040-9044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weingarten, M. D., A. H. Lockwood, S. Y. Hwo, and M. W. Kirschner. 1975. A protein factor essential for microtubule assembly. Proc. Natl. Acad. Sci. USA 721858-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson, L., and M. A. Jordan. 1995. Microtubule dynamics: taking aim at a moving target. Chem. Biol. 2569-573. [DOI] [PubMed] [Google Scholar]
- 48.Woods, C. M., J. Zhu, P. A. McQueney, D. Bollag, and E. Lazarides. 1995. Taxol-induced mitotic block triggers rapid onset of a p53-independent apoptotic pathway. Mol. Med. 1506-526. [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou, F. C., Y. J. Zhang, J. H. Deng, X. P. Wang, H. Y. Pan, E. Hettler, and S. J. Gao. 2002. Efficient infection by a recombinant KSHV cloned in a bacterial artificial chromosome: application for genetic analysis. J. Virol. 766185-6196. [DOI] [PMC free article] [PubMed] [Google Scholar]