Abstract
Human immunodeficiency virus type 1 Nef provides immune evasion by decreasing the expression of major histocompatibility complex class I (MHC-I) at the surfaces of infected cells. The endosomal clathrin adaptor protein complex AP-1 is a key cellular cofactor for this activity, and it is recruited to the MHC-I cytoplasmic domain (CD) in the presence of Nef by an uncharacterized mechanism. To determine the molecular basis of this recruitment, we used an MHC-I CD-Nef fusion protein to represent the MHC-I CD/Nef complex during protein interaction assays. The MHC-I CD had no intrinsic ability to bind AP-1, but it conferred binding activity when fused to Nef. This activity was independent of the canonical leucine-based AP-binding motif in Nef; it required residue Y320 in the MHC-I CD and residues E62-65 and P78 in Nef, and it involved the μ but not the γ/σ subunits of AP-1. The impaired binding of mutants encoding substitutions of E62-65 or P78 in Nef was rescued by replacing the Y320SQA sequence in the MHC-I CD with YSQL, suggesting that Nef allows the YSQA sequence to act as if it were a canonical μ-binding motif. These data identify the μ subunit of AP-1 (μ1) as the key target of the MHC-I CD/Nef complex, and they indicate that both Y320 in the MHC-I CD and E62-65 in Nef interact directly with μ1. The data support a cooperative binding model in which Nef functions as a clathrin-associated sorting protein that allows recognition of an incomplete, tyrosine-based μ-binding signal in the MHC-I CD by AP-1.
Nef and its ability to down-regulate major histocompatibility complex class I (MHC-I) are important for the virulence of primate immunodeficiency viruses. In the simian model of AIDS, simian immunodeficiency virus (SIV) is less pathogenic when nef is deleted (28), and in humans, the absence of nef is associated with reduced rates of disease progression (15). The role of Nef in virulence is multifaceted: Nef increases the intrinsic infectivity of viral particles (7), it facilitates the activation of CD4-positive T cells (44), and it contributes to viral evasion of the immune system by removing MHC-I from the surfaces of infected cells (11, 45). In vitro, the extent of Nef's effect on MHC-I is sufficient to hinder antigen presentation, provide escape from the activity of virus-specific cytotoxic T cells, and confer a selective advantage to the virus (11, 53). When monkeys are experimentally infected with nef mutants of SIV that are specifically defective in MHC-I down-regulation, second-site mutations that restore activity occur (46). These observations suggest that the modulation of MHC-I is a significant component of the pathogenetic effect of Nef during primate lentiviral infections.
Nef modulates the expressions of several cellular proteins besides MHC-I, including CD4, the primary receptor for human immunodeficiency virus type 1 (HIV-1) (17, 45). This small, 27-kDa viral protein has no known enzymatic activity. It associates with the cytoplasmic leaflets of cellular membranes via N-terminal myristoylation (26). Common themes in the effects of Nef on cellular proteins are modulation of their trafficking within the endosomal system and the requirement for specific sequences in their cytoplasmic domains (CDs) (1, 32). In the case of MHC-I, Nef induces both internalization from the plasma membrane and retention within the endo-lysosomal system, reducing the level of MHC-I at the cell surface (11, 27, 45). These effects require a tyrosine residue (Y320) in the CD of the MHC-I α chain (9, 32).
The endosomal adaptor complex AP-1 appears to be a key cellular cofactor for the modulation of MHC-I by Nef. In Nef-expressing cells, MHC-I accumulates in a juxtanuclear region that is relatively rich in AP-1 (21), and knockdown of AP-1 interferes with the modulation of MHC-I by Nef (34, 43). AP-1 belongs to a four-member family of related complexes that provide at least two functions: they bind specific sequences in the CDs of transmembrane proteins to recruit the proteins into transport vesicles, and they connect the scaffolding protein clathrin to the nascent vesicle (4, 23). The four AP complex family members (AP-1, −2, −3, and −4) are heterotetramers composed of homologous subunits: a large, specific subunit (γ in AP-1); a large, highly homologous subunit (β1 in AP-1); a medium subunit (μ1 in AP-1); and a small subunit (σ1 in AP-1). The members of the AP complex family have distinct subcellular locations that correlate with specific roles in endosomal trafficking. For example, AP-1 is concentrated on trans-Golgi network (TGN) and juxtanuclear endosomal membranes and appears to mediate transport within the endo-lysosomal system, whereas AP-2 is concentrated along the plasma membrane and mediates endocytosis.
The sequences within the CDs of cellular proteins that are recognized by AP complexes are usually either leucine based or tyrosine based (ΕxxxLφ or Yxxφ motifs, where φ represents a bulky, hydrophobic residue) (29). These distinct sequences bind noncompetitively to AP complexes via different interfaces: leucine-based motifs bind to a “hemicomplex” formed by the large, specific subunit and the small subunit (γ and σ1 in AP-1), whereas tyrosine-based motifs bind to the μ subunit (25, 36-38). The molecular basis of the binding of tyrosine-based motifs to the μ subunit has been defined by X-ray crystallography and involves a “two-prong-in-socket” mechanism (39). One prong is the tyrosine residue, while the other is the bulky, hydrophobic Y+3 residue, usually a leucine or isoleucine. In contrast, the molecular basis for the binding of leucine-based motifs to AP hemicomplexes is unknown.
Nef contains a highly conserved leucine-based AP-binding motif (ExxxLL) within the center of a 30-residue, solvent-exposed, unstructured loop near the C terminus of the protein (5, 12, 20). This sequence is required for the interaction of Nef with intact AP-1 (5, 24), and it binds the γ/σ hemicomplex of AP-1 (10, 25). Although Nef also binds directly to the μ1 subunit of AP-1 (32, 40), this interaction is relatively weak and not completely leucine dependent (14, 16), rendering the role of this subunit in the interaction between Nef and AP-1 unclear. Paradoxically, despite the clear dependence of the Nef/AP-1 interaction on the Nef leucine-motif and the obligatory role of the leucines in the down-regulation of CD4 (5, 12, 20), these residues are dispensable for the modulation of MHC-I by Nef (20, 42).
How does Nef utilize AP-1 to modulate MHC-I, if not through its ExxxLφ motif? The MHC-I molecule and Nef together appear to form a novel, leucine-independent ligand for AP-1 (43). This model derives from the observation that AP-1 coimmunoprecipitates with a chimera in which Nef is fused to the C terminus of the MHC-I α chain (the membrane-distal end of the CD), even when the ExxxLφ motif in Nef is mutated (43). This coimmunoprecipitation required the Y320 residue in the MHC-I CD, which is part of the sequence YSQA. Remarkably, mutation of this sequence to YSQL induces constitutive, Nef-independent removal of MHC-I from the cell surface (31). These observations suggest that the YSQA sequence is very close to a functional AP-binding sequence, and they lead to the hypothesis that Nef provides additional binding avidity that allows the YSQA sequence in the MHC-I CD to bind the canonical binding socket for Yxxφ motifs on the μ subunit. Nevertheless, no data address directly the question of which subunits of AP-1 mediate interaction with the MHC-I/Nef complex.
The modulation of MHC-I by Nef also requires a methionine residue in an α-helical region near the N terminus of Nef (M20), an “acidic cluster” in the N-terminal third of Nef (EEEE62-65), and a single residue in a polyproline helix, P78, which is not required for the SH3-binding activity of Nef (6, 21, 52). The M20 Nef residue is located within the N-terminal domain of Nef involved in binding MHC-I and has also been proposed as required for the binding of the MHC-I/Nef chimera to AP-1 (43, 50, 51). The E62-65 sequence has been suggested to link Nef to PACS-1, an adaptor that links acidic cluster-sorting motifs to AP-1 and is involved in the retrieval of transmembrane proteins to the TGN (41, 49). The role of P78 is unknown, although it has been suggested to enable Nef to block the recycling of internalized MHC-I to the cell surface (6).
These data leave several questions unanswered regarding the molecular basis of the modulation of MHC-I by Nef. What subunit(s) of the AP-1 complex recognizes the MHC-I/Nef complex? What are the roles of specific Nef residues in this interaction? Does the YSQA sequence in the MHC-I CD act as if it were a canonical Yxxφ, μ-binding motif in the presence of Nef? To answer these questions, we used an MHC-I CD-Nef chimeric sequence in a variety of protein interaction assays. The results indicate that the MHC-I CD and Nef bind cooperatively to the μ subunit of AP-1 via a mechanism in which Nef allows the YSQA sequence in the CD to function as it were a tyrosine-based, μ-binding motif.
MATERIALS AND METHODS
Plasmids and constructs.
The pBridge (Clontech) and pGEX 4T-1 plasmids, containing NL4-3 Nef and NL4-3 Nef LL164-165AA, have been described previously (10, 24). The Saccharomyces cerevisiae expression plasmids encoding the TGN38 sequences fused to the GAL4 DNA-binding domain, the μ1 sequence fused to the GAL4 activation domain, or the γ-adaptin sequence fused to the GAL4 activation domain were provided by Juan Bonifacino (25, 37). All other constructs were created using standard overlap PCR mutagenesis and subcloning techniques. Nucleic acid sequences were verified to ensure correct substitutions and to exclude inadvertent mutations.
Cell culture.
SupT1 T cells were obtained through the NIH AIDS Research and Reference Reagent Program (catalog no. 100, submitted by James Hoxie) and were maintained in RPMI medium supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 units of penicillin per ml, and 100 μg of streptomycin per ml. P4R5 cells were obtained from Ned Landau and were derived from CD4-expressing HeLa cells as described previously (8); the cells are a HeLa clone expressing the CD4 receptor and CXCR4 and CCR5 coreceptors and were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 units of penicillin per ml, 100 μg of streptomycin per ml, and 1 μg puromycin per ml.
Flow cytometry.
SupT1 cells (3 × 106) were transfected during exponential growth with 20 μg of the pCI-neo vector (empty or containing Nef sequences) and 2 μg of the pCG-GFP vector (a gift from Jacek Skowronski) as a transfection marker by using Amaxa Cell Kit V, protocol O-17 (Amaxa Systems, Gaithersburg, MD). Cells were incubated for 24 h after transfection and then stained with anti-CD4-allophycocyanin (Becton Dickinson) and anti-HLA-A2-phycoerythrin (BB7.1 clone, a gift from David Camerini, UC Irvine) or with allophycocyanin and phycoerythrin antibody isotype controls. Cells were then fixed and analyzed on a Coulter Elite flow cytometer.
Glutathione S-transferase (GST) pulldowns.
The various proteins were expressed from the pGEX4T-1 plasmid in BL21(DE3) cells. After transformation, single colonies were inoculated into mini-cultures, and a 25-ml culture of LB-ampicillin (100 μg/ml) was grown overnight. The next day, 75 ml of LB-ampicillin was added, and the cells were grown for 3 to 4 h before addition of 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) and growth for an additional 5 hours. The bacterial cells were washed and divided into six aliquots, and pellets were stored at −80°C. For the pulldowns, each bacterial pellet was lysed in 50 mM Tris-HCl (pH 8), 5 mM EDTA, 150 mM NaCl, 10 mM MgCl2, 1 mM dithiothreitol, protease inhibitor cocktail (Sigma), and 1% Triton X-100 for 2 h at 4°C and then incubated with glutathione-conjugated beads (GE Healthsciences) for 30 min at room temperature. After four washes with cold phosphate-buffered saline, aliquots from each preparation were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Bio-Rad) and staining with Coomassie (Bio-Rad) to estimate the relative loadings of protein on the beads. To prepare the cytoplasmic lysates, approximately 2.5 × 106 HeLa P4.R5 cells were pelleted and then lysed in 50 mM Tris-HCl (pH 8), 5 mM EDTA, 150 mM NaCl, 10 mM MgCl2, protease inhibitor cocktail (Sigma), and 1% Triton X-100. Lysates were stored at −80° until used. For the pulldowns, lysates were thawed for 30 min at 4°C and then centrifuged for 10 min at 14,000 rpm to remove cell debris and nuclei. Equal amounts of protein bead complexes were preincubated with cell lysis buffer plus 2 mg/ml bovine serum albumin for 30 min at 4°C. One milliliter of cytoplasmic lysate (derived from 2.5 × 106 HeLa cells) was added and incubated overnight at 4°C. After four washes with lysis buffer, the protein bead complexes were analyzed by SDS-PAGE and Western blotting, as described below. For the pulldowns performed with the μ1 subunit translated in vitro, a coupled transcription/translation kit (Promega) was used, utilizing the circular pTNT vector to express μ1 in the presence of [35S]methionine as recommended by the manufacturer. Each pulldown was performed using 50 μl of the in vitro translation reaction mixture and the same buffers as those for the experiments with the cell lysates. After SDS-PAGE, the gels were stained with Coomassie blue, dried, and autoradiographed using Amplify enhancer (Amersham).
Western blot analysis.
Samples were suspended in loading buffer containing SDS and boiled for 10 min. After resolution on a 12% denaturing polyacrylamide gel (Bio-Rad), the proteins were transferred to a nitrocellulose or polyvinylidene difluoride membrane and blotted with the following antibodies: anti-γ adaptin (clone 100/3, 1:1000; Sigma) and anti-μ1 adaptin (1:500; a gift from L. Traub, University of Pittsburgh). Detection was performed using a goat anti-mouse antibody linked to horseradish peroxidase (Bio-Rad, Hercules, CA) or a goat anti-rabbit antibody linked to horseradish peroxidase (Pierce), followed by development with enhanced chemiluminescence (GE Healthcare, Piscataway, NJ).
Yeast two- and three-hybrid assays.
Yeast cells (strain HF7C) were transformed using lithium acetate as recommended by the manufacturer (BD Biosciences). Transformants were plated on agar containing Leu− Trp− selective medium and grown for 5 to 7 days. For the patch plate assays on solid media, 5 to 10 individual colonies were resuspended in 25 μl of Leu− Trp− media and spotted on Leu− Trp− plates. The next day, the patches were replica plated on Leu− Trp− and Leu− Trp− His− plates and reincubated as indicated. To measure growth rate in liquid media, 5 to 10 individual colonies from the original transformations were pooled and grown overnight in liquid Leu− Trp− media. The next morning, 2 ml Leu− Trp− liquid cultures were inoculated at an optical density at 600 nm (OD600) of 0.200 and grown for 4 to 6 h to an OD600 of 0.4 to 0.6. From this mid-log-phase culture, 5 ml of Leu Trp− His− liquid cultures were inoculated at an OD600 of 0.005. OD600 was measured every 24 h during incubation at 30°C.
RESULTS AND DISCUSSION
Modulation of MHC-I requires M20, the acidic cluster (E62-65), and P78 but not the ExxxLφ motif in Nef.
The molecular determinants of the modulation of MHC-I by Nef were confirmed using a flow cytometric assay in which T cells (SupT1 line) were transfected to transiently express Nef (Fig. 1A). Both cell surface MHC-I-A2 and CD4 were measured. Mutation of the M20 residue, the acidic cluster (E62-65), or the P78 residue impaired the down-regulation of MHC-I by Nef, with minimal or no effect on the down-regulation of CD4. The leucine-based AP-binding motif in the C-terminal flexible loop of Nef was dispensable for the down-regulation of MHC-I, despite its essential role in the modulation of CD4. The expressions of these Nef mutants were confirmed by immunoblot analysis (data not shown).
FIG. 1.
Nef-mediated down-regulation of MHC-I and direct binding to AP-1. (A) Nef-mediated modulation of MHC-I and CD4. T cells (SupT1 line) were transfected with plasmids expressing native Nef and green fluorescent protein (as a transfection marker). The next day, the cells were stained with antibodies to MHC-I-A2 (HLA-A2) and CD4, fixed, and analyzed by three-color flow cytometry. The histograms show cell numbers versus fluorescence intensities for the green fluorescent protein-positive (transfected) cells. “Mock” represents cells transfected with an empty plasmid. Vertical lines represent gates set using an isotype control antibody. The inset numbers are the mean fluorescence intensities of the entire (green fluorescent protein-positive) population. Alanine substitutions are indicated at the left. (B) GST pulldown of intact AP-1 from cytoplasmic lysates. GST proteins were produced in Escherichia coli, bound to glutathione-agarose beads, and quantified by electrophoresis and staining with Coomassie blue. Equal amounts of GST proteins were bound to the beads and incubated with cytoplasmic lysates from HeLa cells; the beads were washed, and the bound proteins were analyzed by SDS-PAGE and immunoblotting using an antibody to the γ subunit of AP-1. The “Input” lane was loaded with an aliquot of cytoplasmic lysate equal to 2% of the amount used in the pulldowns. The blot was also stained with Ponceau red to assess the loading of the GST fusion proteins. (C) Quantification of three independent GST pulldown experiments performed as described for panel B. The bands were quantified using ImageJ; the units are arbitrary. Error bars represent the standard deviations. WT, wild type.
The ExxxLφ motif and to a lesser extent the acidic cluster are required for the binding of native Nef to AP-1.
The major Nef residues involved in the modulation of MHC-I were surveyed for their roles in the binding of Nef to intact AP-1 by using a GST pulldown assay (2, 21, 35, 52) (Fig. 1B and C). Of M20, E62-65, and P78, only the E62-65 sequence contributed directly to the interaction between Nef and AP-1. The decreased binding of NefE62-65A relative to that of wild-type Nef was statistically significant (P < 0.01 by Student's t test). The leucine residues of the ExxxLφ motif were essential for binding. Overall, no consistent relationship was apparent between the roles of these residues in the modulation of MHC-I and in the direct binding of Nef to AP-1. The M20 and LL164-165 sequences were particularly paradoxical; M20 was required for the modulation of MHC-I but dispensable for binding to AP-1, whereas LL164-165 was dispensable for the modulation of MHC-I but required for binding to AP-1.
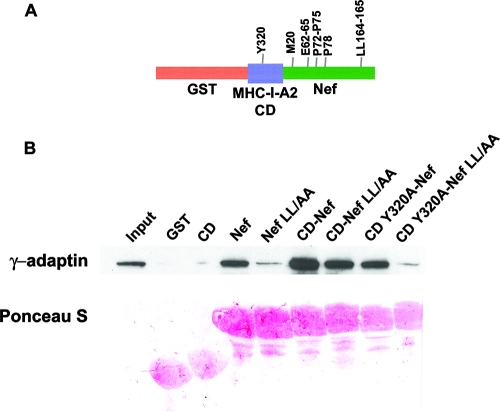
Addition of the MHC-I CD to Nef creates a novel AP-binding activity.
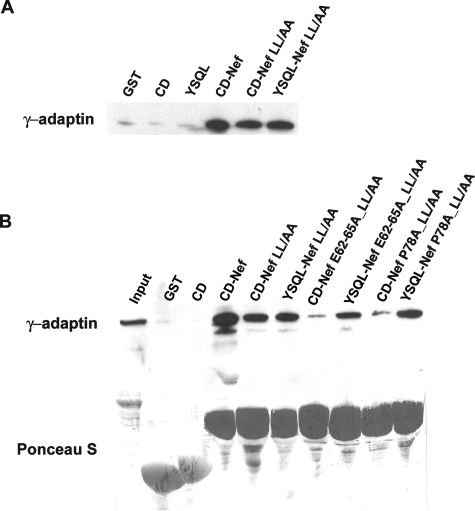
The paradoxical nature of the preceding data can be resolved if the MHC-I CD and Nef together form a novel binding activity that is not characteristic of either protein alone. Such a model was proposed previously based on experiments using a chimeric molecule in which Nef was fused to the cytoplasmic terminus of the MHC-I A2 α chain; AP-1 was coimmunoprecipitated from cell lysates with this molecule independently of the Nef ExxxLφ motif (43). This result was confirmed and extended here using a GST pulldown assay to capture intact AP-1 from cytoplasmic lysates; the key construct is a GST fusion protein in which the MHC-I A2 CD is fused to the N terminus of Nef (Fig. 2A). The MHC-I CD has no intrinsic binding activity for AP-1 (Fig. 2B). However, it increased the efficiency of binding when fused to Nef (Fig. 2B, compare Nef to CD-Nef). Furthermore, the fusion of the MHC-I CD to the N terminus of Nef conferred LL-independent binding (compare Nef LL/AA to CD-Nef LL/AA). Both the increased binding and the LL-independent binding conferred by the MHC-I CD required the tyrosine residue of the YSQA sequence within the CD (Y320) (compare CD-Nef to CD Y320A-Nef and CD-Nef LL/AA to CD Y320A-Nef LL/AA). These data indicate that the MHC-I CD and Nef cooperate to bind AP-1. The data also indicate that this cooperative interaction requires a key determinant of the Nef effect on MHC-I, Y320 in the MHC-I CD.
FIG. 2.
GST pulldown of intact AP-1 from cytoplasmic lysates by use of chimeric proteins in which the MHC-I CD is attached to the N terminus of Nef (CD-Nef). (A) Schematic of the GST-MHC-I CD-Nef fusion protein. Key residues mutated herein are indicated. (B) GST pulldown of intact AP-1 from cytoplasmic lysates of HeLa cells. The assay was performed as described in the legend to Fig. 1. “LL/AA” indicates substitution of alanine for leucines 164 and 165 in the Nef ExxxLφ motif.
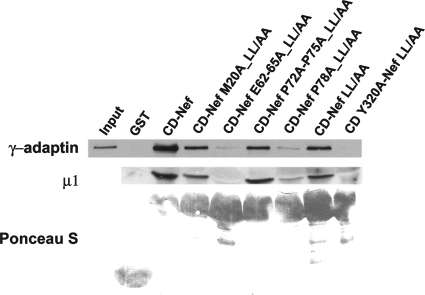
Roles of Nef residues in MHC-I CD-dependent binding to AP-1: requirements for E62-65 and P78.
The AP-1 binding activity of the MHC-I CD-Nef molecule required Y320 in the CD of MHC-I but not an intact ExxxLφ sequence in Nef; these results support a correlation between the interaction with AP-1 in vitro and the modulation of MHC-I in living cells. To test further the correlation between binding and function, we examined how mutations of the major Nef residues involved in the modulation of MHC-I affect the interaction of CD-Nef with AP-1 (Fig. 3). Mutations of the N-terminal α-helix (M20), acidic cluster, and polyproline helix were studied in the context of the CD-Nef LL/AA chimera to test their effects on the abilities of the MHC-I CD and Nef to constitute a leucine-independent binding activity for AP-1. The data indicate that this interaction requires E62-65 and P78 in Nef but not M20 or P72/75.
FIG. 3.
GST pulldown of intact AP-1 from cytoplasmic lysates by use of mutated MHC-I CD-Nef chimeras. Nef residues were mutated in the context of the LL164/165AA sequence to reveal their role in the LL-independent AP-1 binding activity of the MHC-I CD/Nef complex. The assay was performed as described in the legend to Fig. 1, except that the blot was probed with antibodies to both the γ and μ subunits of AP-1 to confirm that the interactions detected are with the intact AP-1 complex.
These determinants of MHC-I CD-dependent binding to intact AP-1 observed in vitro correlated with the determinants of modulation of native MHC-I by Nef in living cells, with one exception: Nef-M20. Intracellular modulation requires the M20, E62-65, and P78 residues in Nef (Fig. 1A) (2, 21, 52). Notably, the importance of P72 and P75 is controversial. These central residues in the polyproline helix, which are key to the SH3-binding activity of Nef, have been variously reported as either required, moderately contributory, or contributory only because of their influence on protein stability (6, 13, 21, 42). Here, E62-65 and P78 were each required for the leucine-independent binding of the MHC-I CD/Nef complex to AP-1 in vitro, consistent with their critical roles in intracellular modulation. P72 and P75 were dispensable for binding in vitro, consistent with some of the published data on intracellular modulation. Nef-M20 was also dispensable for the leucine-independent binding of the MHC-I CD/Nef complex to AP-1 in vitro, despite its noncontroversial importance to modulation of MHC-I in cells (Fig. 1A) (2). To explain this, we hypothesize that M20 is required for the presumed interaction of Nef with the MHC-I CD; the determinants of this interaction should be obviated by the covalent attachment of the MHC-I CD to Nef in the CD-Nef molecule.
These results partly corroborate the conclusions drawn from experiments in which Nef was appended to the C terminus of the entire MHC-I-A2 α chain (43). Coimmunoprecipitation of AP-1 with this chimeric transmembrane protein required Y320 in the MHC-I CD, like the binding of the MHC-I CD-Nef chimera to AP-1 in vitro as shown here. However, differences are apparent regarding the roles of Nef residues. Coimmunoprecipitation of AP-1 reportedly required M20 but not E62-65 or P78 in Nef, results that are the opposite of those herein. Consequently, the two data sets suggest distinct models for the roles of residues in Nef: either E62-65 and P78 bind AP-1 while M20 binds the MHC-I CD (data herein) or the reverse occurs (43). In potential support of the data herein, the exposed surface of the μ subunit has a positive electrostatic potential that could bind the Nef E62-65 sequence (39); in contrast, the 33-residue MHC-I CD contains no positive charge clusters that might bind the Nef E62-65 sequence, with the exception of the RRK sequence that is juxtaposed to the inner leaflet of the membrane and likely interacts with negatively charged phospholipid head groups. In partial corroboration of the results herein, unpublished data indicate that roles for the E62-65 and polyproline regions in the binding of the complete MHC-I α chain-Nef chimera to AP-1 can be revealed by coimmunoprecipitation (Kathleen Collins, personal communication).
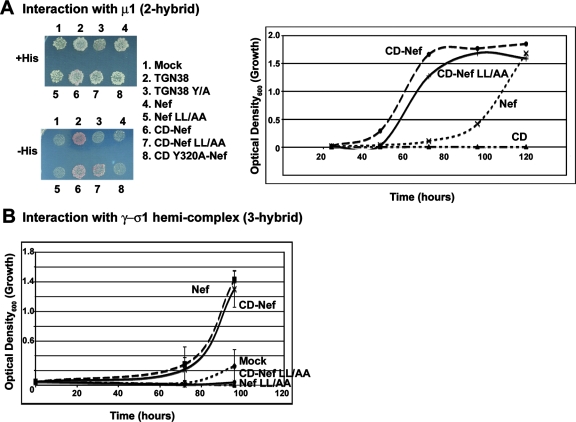
The tyrosine-dependent binding of the MHC-I CD-Nef chimera to AP-1 is based on an interaction with the μ subunit.
We used yeast two-and three-hybrid assays as well as GST pulldown of in vitro-translated protein to determine which subunits of AP-1 recognize the MHC-I CD-Nef chimera. Yeast two-hybrid assays indicated that the MHC-I CD markedly increased the affinity of Nef for the μ1 subunit (Fig. 4A). This increase was dependent on the Y320 residue in the MHC-I CD (Fig. 4A). As expected, the MHC-I CD alone had no μ1-binding activity. These data are consistent with the hypothesis that the tyrosine-dependent binding activity created by adding the MHC-I CD to the N terminus of Nef recognizes the μ subunit of AP-1. To exclude a role for the γ/σ hemicomplex, which recognizes ExxxLφ motifs, we used the yeast three-hybrid assay (Fig. 4C). This assay indicated that the MHC-I CD had no effect on the affinity of Nef for the γ/σ1 hemicomplex and that it did not confer leucine-independent binding. These results are consistent with the additive nature of the MHC-I CD-dependent and Nef-leucine-dependent binding activities shown in Fig. 2; these two activities recognize distinct, noncompetitive sites on the AP-1 complex.
FIG. 4.
Interaction of Nef and the MHC-I CD-Nef chimera with subunits of AP-1 detected using yeast two- and three-hybrid assays. (A) Two-hybrid assay detecting interaction with μ1. Yeast cells were cotransformed with plasmids expressing the indicated proteins fused to the GAL4 DNA-binding domain and a plasmid expressing μ1 fused to the GAL4 activation domain. Cotransformed colonies were selected on plates lacking leucine and tryptophan, pooled, and either patch plated onto solid media containing histidine, followed by replica plating onto solid media containing (+His) or lacking (−His) histidine, or inoculated into liquid media lacking histidine. In each assay format, growth in media lacking histidine reflects an interaction between the indicated protein and μ1. “TGN38” contains a canonical Yxxφ sequence (SDYQRL); “TGN38Y/A” encodes a substitution of alanine for the tyrosine. Replica plates (left) were incubated at 30°C for 4 days. Growth in liquid media (right) was measured as OD600. (B) Three-hybrid assay detecting interaction with the γ/σ1 hemicomplex of AP-1. Yeast cells were cotransformed with plasmids expressing the indicated proteins fused to the GAL4 DNA-binding domain, along with σ1 as a “bridge” protein, and a plasmid expressing γ-adaptin fused to the GAL4 activation domain. Cotransformed colonies were selected on plates lacking leucine and tryptophan, pooled, and inoculated into liquid media lacking histidine. Growth in media lacking histidine reflects an interaction between the indicated protein and the γ/σ hemicomplex of AP-1.
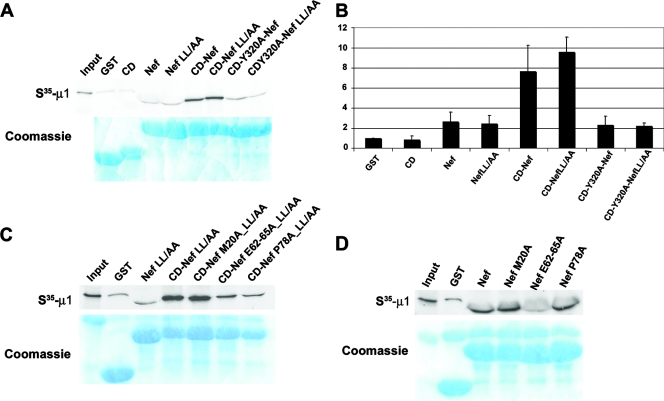
We confirmed that the μ subunit is the basis of the CD-dependent interaction of the MHC-I CD-Nef chimera with AP-1 by using GST pulldown of μ1 translated in vitro (Fig. 5). The data of Fig. 5A and B confirmed the results of the two-hybrid experiments whose results are shown in Fig. 4: the CD-Nef molecule bound μ1 much more avidly than Nef alone, and the MHC-I CD alone had virtually no binding activity. In addition, the binding of CD-Nef to μ1 required Y320 in the MHC-I CD and was independent of the ExxxLφ sequence in Nef.
FIG. 5.
GST pulldown of μ1 translated in vitro using MHC-I CD-Nef chimeras. RNA encoding μ1 was transcribed using T7 RNA polymerase and translated using rabbit reticulocyte lysates in the presence of [35S]methionine. GST pulldowns were performed as described in the legend to Fig. 1, except that the gels were stained with Coomassie blue and dried before autoradiography. The “Input” lanes were loaded with an aliquot of in vitro translation reaction product equal to approximately 2% of the amount used in the pulldowns. (A) The GST constructs used are the same as those used for Fig. 2. (B) Quantification of three independent GST pulldown experiments performed as described for panel A. The bands were quantified using ImageJ and normalized to an arbitrary value of 1.0 for GST only. Error bars represent the standard deviations. (C) Nef residues were mutated in the context of the LL164/165AA sequence to reveal their role in the LL-independent μ1-binding activity of the MHC-I CD/Nef complex. (D) GST fusion proteins contain Nef alone (without the MHC-I CD) to assess the role of residues in the interaction between Nef and μ1.
Next, we examined the roles of Nef residues in the MHC-I CD-dependent binding of Nef to μ1 (Fig. 5C). GST pulldown of μ1 translated in vitro indicated that the MHC-I CD-dependent binding required the E62-65 sequence, whereas M20 was not required. Notably, repeated experiments indicated that the role of P78 is less dramatic than suggested in Fig. 5C, in which the CD-NefP78A protein is slightly underloaded. With the possible exception of P78, the leucine-independent determinants within the MHC-I CD-Nef chimera for binding to μ1 are the same as the determinants for binding to intact AP-1 (compare Fig. 3 and 5), supporting the hypothesis that the μ subunit is the basis of the interaction with the complex. The data also supported a role for the Nef acidic cluster (E62-65) in binding directly to AP-1 via the μ1 subunit: GST pulldown assays using Nef without the MHC-I CD indicated a direct involvement of the E62-65 sequence, but not P78, in the binding of Nef itself to μ1 (Fig. 5D).
A YSQA-to-YSQL substitution in the MHC-I CD rescues the defects in AP-1 binding activity caused by mutation of Nef-E62-65 or -P78.
The data so far fit a model in which Nef enables the MHC-I CD to bind the μ subunit of AP-1 as if it contained a canonical Yxxφ motif. As noted, the critical Y320 residue in the MHC-I CD is within the sequence YSQA, which is missing the bulky, hydrophobic Y+3 residue found in canonical sequences. To test the hypothesis that Nef provides the binding activity “lost” by the presence of an alanine at the Y+3 position of the MHC-I CD, we replaced the YSQA sequence with YSQL (Fig. 6). Surprisingly, the MHC-I CD containing the YSQL sequence (“YSQL”) was insufficient to bind AP-1 (Fig. 6A). Nevertheless, this sequence rescued the defects in AP-1 binding caused by mutations of Nef residue E62-65 or P78 (Fig. 6B). These data support a model in which the E62-65 and P78 residues bind AP-1 in a manner that compensates for the absence of a bulky, hydrophobic residue at the Y+3 position in the MHC-I CD. In the context of the MHC-I CD-Nef chimera, a leucine at the Y+3 position in the MHC-I CD is functionally equivalent to Nef residue E62-65 or P78 with respect to binding AP-1.
FIG. 6.
YSQA-to-YSQL mutation in the MHC-I CD rescues defects in AP-1 binding activity caused by mutation of Nef-E62-65 and -P78. GST pulldown assays using cytoplasmic lysates of HeLa cells were performed as described in the legend to Fig. 1. The blots were probed with an antibody to the γ subunit of AP-1.
A cooperative binding model in which Nef acts as a virally encoded clathrin-associated sorting protein (CLASP).
We have shown that when the CD of the MHC-I α chain is attached to the N terminus of HIV-1 Nef, a novel binding activity that recognizes AP-1 via the μ1 subunit is created. This binding activity is distinct from that provided by the canonical ExxxLφ motif in Nef, which recognizes the γ/σ1 hemicomplex of AP-1. Consequently, this interaction can explain how the modulation of MHC-I by Nef is mediated by AP-1 and yet is independent of the ExxxLφ sequence. Since neither the MHC-I CD nor Nef without its leucine-based motif has intrinsic binding activity for AP-1, the activity revealed when the two sequences are joined reflects a synergistic or cooperative interaction. A similar cooperative interaction occurs between the CD of the ζ chain of CD3, SIV-Nef, and the AP-2 complex (47).
The observations that the modulation of MHC-I by Nef requires a tyrosine in the MHC-I α chain CD and that Nef binds μ1 were made almost 10 years ago (1, 32). Nevertheless, until now, no data have demonstrated either that the target of the MHC-I/Nef complex is the μ subunit of AP-1 or that such binding is dependent on the key tyrosine in the MHC-I CD. Furthermore, until now, no residues in Nef that are required for both the modulation of MHC-I and the binding of Nef to μ1 have been identified; data herein suggest that the E62-65 sequence fits this role.
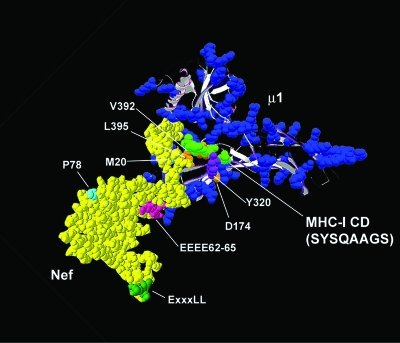
How exactly do the MHC-I CD and Nef cooperate to bind μ1 (and AP-1)? The data so far are consistent with the model depicted in Fig. 7. In this model, the YSQA sequence in the MHC-I CD behaves as an incomplete Yxxφ motif, binding via Y320 to the socket on μ1 for Yxxφ motifs. Nef provides a bridging and stabilizing function, allowing the YSQA sequence to bind even though it is missing the φ residue at position Y+3. Nef residues E62-65 and P78 are essential for this bridging and stabilizing function. For E62-65, this function is provided by a direct interaction with μ1, but for P78, the interaction may be elsewhere on the complex. Finally, since the M20 residue is not required for the binding of the MHC-I CD-Nef chimera to either intact AP-1 or the μ1 subunit yet is required for the modulation of the MHC-I α chain by Nef, we suggest that this residue is involved in the binding between Nef and the MHC-I CD. Altogether, we propose three interfaces in the ternary interaction under native conditions: (i) an interface between the MHC-1 CD and μ1, mediated by MHC-I-Y320 and the tyrosine binding socket on μ1; (ii) an interface between Nef and the MHC-I CD, mediated by Nef-M20; and (iii) an interface between Nef and AP-1, mediated by Nef-E62-65 and -P78 and based partly on a direct interaction between E62-65 and μ1.
FIG. 7.
Hypothetical model of the MHC-I CD/μ/Nef complex. Nef is yellow (18, 30); the μ subunit of AP-1 is shown as a ribbon structure, with blue indicating basic, positively charged residues (22); and residues SYSQAAS of the MHC-I CD are green. Key residues in Nef, the MHC-I CD, and μ1 are indicated. Y320 in the MHC-I CD presumably interacts with D174 in μ1, as in canonical Yxxφ motifs, whereas the hydrophobic pocket formed by μ1 residues V392 and L395, which typically accept the bulky, hydrophobic residue at position Y+3, is likely unoccupied. Nef-P78 and -E62-65 compensate for the lack of the latter interaction. In the case of the E62-65 sequence, binding activity is presumably based on an electrostatic interaction with a positive charge cluster on μ1. The precise role of Nef-P78 is unknown, but it may bind to another subunit of the complex. Structures were generated using DeepView (Swiss-Prot database).
The concept that the acidic cluster in Nef is involved in a direct interaction with μ1 is novel, although a similar suggestion has been made to explain the AP-binding activities of acidic residues in the CD of the mannose-6-phosphate receptor (19). In support of this concept, the exposed surface of μ is markedly positively charged (39), so the Nef acidic cluster may bind via a simple, electrostatic interaction. Interestingly, the E62-65 sequence contributes modestly but significantly to the binding between Nef and AP-1 (Fig. 1B and C), though only in the context of an intact ExxxLφ motif. That this contribution is via the μ subunit is supported by GST pulldown of μ1 translated in vitro (Fig. 5D) and by the yeast two-hybrid assay (data not shown). This model is distinct from the reported role of the E62-65 sequence as a ligand for phosphofurin acidic cluster sorting protein-1 (PACS-1), a sorting adaptor discovered in a two-hybrid screen for proteins able to bind the acidic cluster motif in furin (49). PACS-1 binds both acidic cluster motifs and AP-1 and has been proposed to link the E62-65 sequence in Nef to AP-1 (41). Despite substantial evidence in support of this model, the GST pulldown experiments using μ1 translated in vitro herein (Fig. 5) support the hypothesis that the E62-65 sequence mediates a direct interaction with the μ subunit of AP-1, obviating a bridging protein such as PACS-1. In accordance with this conclusion, recent data indicate that knockdown of PACS-1 by use of RNA interference does not interfere with the Nef-mediated modulation of MHC-I (34).
The proposed role of Nef-M20 in binding the MHC-I CD is the most speculative aspect of this model. This role fits the data herein, but alternative models are possible. For example, Nef-M20 could be required for trafficking events that bring the MHC-I/Nef complex into proximity with AP-1 in vivo; the proposed M20-dependent block to recycling of MHC-I could fit this role (3). This scenario would allow for the observation that Nef-M20 is required for the coimmunoprecipitation of AP-1 from cells expressing an MHC-I α chain-Nef chimera, a finding with which the model of Fig. 7 is inconsistent (43). Despite these uncertainties, the in vitro binding data herein indicate conclusively that Nef-M20 is not required for the direct interaction between the MHC-I CD-Nef chimera and either AP-1 or its μ subunit.
Why the ExxxLφ motif in Nef does not contribute to the modulation of MHC-I remains unclear. Nef interacts via its ExxxLφ sequence directly with AP hemicomplexes that contain the large, specific subunit and the small subunit of the heterotetramer, such as the γ/σ hemicomplex of AP-1 (Fig. 5B) (10, 25). The data herein confirm that this mode of interaction of Nef with AP complexes is specifically important for the modulation of CD4 but further indicate that the interaction with the μ subunit of AP-1 is important for the modulation of MHC-I. The data herein also indicate that ExxxLφ-mediated binding is additive with binding mediated by the MHC-I CD in concert with Nef (Fig. 2). Consequently, the lack of contribution by the ExxxLφ motif to the modulation of MHC-I remains paradoxical. This paradox might be resolved if the MHC-I CD induces a conformational change in Nef that renders the ExxxLφ motif inactive. If alternate conformers of Nef exist in the GST pulldown assays described herein, then the additive nature of the ExxxLφ-dependent and MHC-I CD-dependent interactions may represent the signals obtained from alternatively folded proteins, one displaying an AP-1 binding interface formed by the MHC-I CD and Nef-E62-65 and -P78 and the other displaying an AP-1 binding interface formed by the ExxxLφ motif. This model can account for the perplexing observation that coimmunoprecipitation of AP-1 with the MHC-I α chain-Nef chimera was dependent on Y320A in the MHC-I CD even when the Nef ExxxLφ motif was intact (43); by immunoprecipitating MHC-I, the conformation in which the ExxxLφ motif is inactive may have been selected.
Overall, the data herein suggest that with respect to its influence on endosomal trafficking, Nef may be viewed as a virally encoded CLASP (48). CLASPs are a category of cellular proteins that bind to specific sequences in target proteins and in some cases to members of the AP complex family, extending the breadth and specificity of molecular sorting in the endosomal system. In the example herein, Nef presumably interacts with the MHC-I CD to allow this target protein to be recognized by the clathrin adaptor AP-1. Interestingly, the Y320 residue in the MHC-I CD is required not only for modulation by Nef but also for the “cross-presentation” by MHC-I of antigens internalized from the extracellular space, a key event in the priming of naïve CD8 T cells by professional antigen-presenting cells during the adaptive immune response (33). At least some of the loading of extracellular antigen onto MHC-I appears to take place within late endosomes. If a physiologic mechanism for directing MHC-I to this compartment exists, then it becomes interesting to speculate that such trafficking requires AP-1 and a cellular CLASP that is to some extent reminiscent of HIV-1 Nef. Whereas canonical sorting motifs cause the CDs of transmembrane proteins to be recognized constitutively by the endosomal sorting machinery, Nef and the MHC-I α chain may exemplify how an “incomplete” sorting motif enables the transport of a cargo protein to be regulated by a specific CLASP-like molecule that stabilizes its interaction with the coat components of endosomal vesicles.
Acknowledgments
This work was supported by grant D05-VMRF-404 from the Universitywide AIDS Research Program of the University of California, by grants AI038201 and AI076040 from the National Institutes of Health, by the UCSD Center for AIDS Research (NIH AI 36214), and by the Research Center for AIDS and HIV Infection of the San Diego Veterans Healthcare System.
We thank Ricardo Madrid, Cecile Servant, Jerome Bouchet, Judy Nordberg, Caroline Ignacio, Parris Jordan, Sean Kandel, and Mary Pacold for technical assistance; David Camerini and Linton Traub for antisera; Juan Bonifacino for expression vectors used during the two- and three-hybrid assays; and Kathleen Collins for sharing unpublished results and helpful discussion.
Footnotes
Published ahead of print on 5 December 2007.
REFERENCES
- 1.Aiken, C., J. Konner, N. R. Landau, M. Lenburg, and D. Trono. 1994. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell 76853-864. [DOI] [PubMed] [Google Scholar]
- 2.Akari, H., S. Arold, T. Fukumori, T. Okazaki, K. Strebel, and A. Adachi. 2000. Nef-induced major histocompatibility complex class I down-regulation is functionally dissociated from its virion incorporation, enhancement of viral infectivity, and CD4 down-regulation. J. Virol. 742907-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blagoveshchenskaya, A. D., L. Thomas, S. F. Feliciangeli, C.-H. Hung, and G. Thomas. 2002. HIV-1 Nef downregulates MHC-I by a PACS-1- and PI3K-regulated ARF6 endocytic pathway. Cell 111853-866. [DOI] [PubMed] [Google Scholar]
- 4.Boehm, M., and J. S. Bonifacino. 2001. Adaptins: the final recount. Mol. Biol. Cell 122907-2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bresnahan, P. A., W. Yonemoto, S. S. Ferrell, D. G. R. Williams-Herman, and W. C. Greene. 1998. A dileucine motif in HIV-1 Nef acts as an internalization signal for CD4 downregulation and binds the AP-1 clathrin adaptor. Curr. Biol. 81235-1238. [DOI] [PubMed] [Google Scholar]
- 6.Casartelli, N., G. Giolo, F. Neri, C. Haller, M. Potesta, P. Rossi, O. T. Fackler, and M. Doria. 2006. The Pro78 residue regulates the capacity of the human immunodeficiency virus type 1 Nef protein to inhibit recycling of major histocompatibility complex class I molecules in an SH3-independent manner. J. Gen. Virol. 872291-2296. [DOI] [PubMed] [Google Scholar]
- 7.Chowers, M. Y., C. A. Spina, T. J. Kwoh, N. J. S. Fitch, D. D. Richman, and J. C. Guatelli. 1994. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J. Virol. 682906-2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clavel, F., and P. Charneau. 1994. Fusion from without directed by human immunodeficiency virus particles. J. Virol. 681179-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen, G. B., R. T. Gandhi, D. M. Davis, O. Mandelboim, B. K. Chen, J. L. Strominger, and D. Baltimore. 1999. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity 10661-671. [DOI] [PubMed] [Google Scholar]
- 10.Coleman, S. H., N. Van Damme, J. R. Day, C. M. Noviello, D. Hitchin, R. Madrid, S. Benichou, and J. C. Guatelli. 2005. Leucine-specific, functional interactions between human immunodeficiency virus type 1 Nef and adaptor protein complexes. J. Virol. 792066-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins, K. L., B. K. Chen, S. A. Kalams, B. D. Walker, and D. Baltimore. 1998. HIV-1 nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 391397-400. [DOI] [PubMed] [Google Scholar]
- 12.Craig, H. M., M. W. Pandori, and J. C. Guatelli. 1998. Interaction of HIV-1 Nef with the cellular dileucine-based sorting pathway is required for CD4 down-regulation and optimal viral infectivity. Proc. Natl. Acad. Sci. USA 9511229-11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craig, H. M., M. W. Pandori, N. L. Riggs, D. D. Richman, and J. C. Guatelli. 1999. Analysis of the SH3-binding region of HIV-1 nef: partial functional defects introduced by mutations in the polyproline helix and the hydrophobic pocket. Virology 26255-63. [DOI] [PubMed] [Google Scholar]
- 14.Craig, H. M., T. R. Reddy, N. L. Riggs, P. P. Dao, and J. Guatelli. 2000. Interactions of HIV-1 Nef with the μ subunits of adaptor protein complexes 1, 2, and 3: role of the dileucine-based sorting motif. Virology 2719-17. [DOI] [PubMed] [Google Scholar]
- 15.Deacon, N. J., A. Tsykin, A. Solomon, K. Smith, M. Ludford-Menting, D. J. Hooker, D. A. McPhee, A. L. Greenway, A. Ellett, C. Chatfield, V. A. Lawson, S. Crowe, A. Maerz, S. Sonza, J. Learmont, J. S. Sullivan, A. Cunningham, D. Dwyer, D. Dowton, and J. Mills. 1995. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 270988-991. [DOI] [PubMed] [Google Scholar]
- 16.Erdtmann, L., K. Janvier, G. Raposo, H. M. Craig, P. Benaroch, C. Berlioz-Torrent, J. C. Guatelli, R. Bernarous, and S. Benichou. 2000. Two independent regions of HIV-1 nef are required for connection with the endocytic pathway through binding to the μ1 chain of AP1 complex. Traffic 1871-883. [DOI] [PubMed] [Google Scholar]
- 17.Garcia, J. V., and A. D. Miller. 1991. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature 350508-511. [DOI] [PubMed] [Google Scholar]
- 18.Geyer, M., C. E. Munte, J. Schorr, R. Kellner, and H. R. Kalbitzer. 1999. Structure of the anchor-domain of myristoylated and non-myristoylated HIV-1 Nef protein. J. Mol. Biol. 289123-138. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh, P., and S. Kornfeld. 2004. The cytoplasmic tail of the cation-independent mannose 6-phosphate receptor contains four binding sites for AP-1. Arch. Biochem. Biophys. 426225-230. [DOI] [PubMed] [Google Scholar]
- 20.Greenberg, M., L. DeTulleo, I. Rapoport, J. Skowronski, and T. Kirchhausen. 1998. A dileucine motif in HIV-1 Nef is essential for sorting into clathrin-coated pits and for downregulation of CD4. Curr. Biol. 81239-1242. [DOI] [PubMed] [Google Scholar]
- 21.Greenberg, M. E., A. J. Iafrate, and J. Skowronski. 1998. The SH3 domain-binding surface and an acidic motif in HIV-1 Nef regulate trafficking of class I MHC complexes. EMBO J. 172777-2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heldwein, E. E., E. Macia, J. Wang, H. L. Yin, T. Kirchhausen, and S. C. Harrison. 2004. Crystal structure of the clathrin adaptor protein 1 core. Proc. Natl. Acad. Sci. USA 10114108-14113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirst, J., and M. S. Robinson. 1998. Clathrin and adaptors. Biochim. Biophys. Acta 1404173-193. [DOI] [PubMed] [Google Scholar]
- 24.Janvier, K., H. Craig, D. Hitchin, R. Madrid, N. Sol-Foulon, L. Renault, J. Cherfils, D. Cassel, S. Benichou, and J. Guatelli. 2003. HIV-1 Nef stabilizes the association of adaptor protein complexes with membranes. J. Biol. Chem. 2788725-8732. [DOI] [PubMed] [Google Scholar]
- 25.Janvier, K., Y. Kato, M. Boehm, J. R. Rose, J. A. Martina, B. Y. Kim, S. Venkatesan, and J. S. Bonifacino. 2003. Recognition of dileucine-based sorting signals from HIV-1 Nef and LIMP-II by the AP-1 gamma-sigma1 and AP-3 delta-sigma3 hemicomplexes. J. Cell Biol. 1631281-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaminchik, J., N. Bashan, A. Itach, N. Sarver, M. Gorecki, and A. Panet. 1991. Genetic characterization of human immunodeficiency virus type 1 nef gene products translated in vitro and expressed in mammalian cells. J. Virol. 65583-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasper, M. R., and K. L. Collins. 2003. Nef-mediated disruption of HLA-A2 transport to the cell surface in T cells. J. Virol. 773041-3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kestler, H. W., III, D. J. Ringler, K. Mori, D. L. Panicali, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65651-662. [DOI] [PubMed] [Google Scholar]
- 29.Kirchhausen, T., J. S. Bonifacino, and H. Riezman. 1997. Linking cargo to vesicle formation: receptor tail interactons with coat proteins. Curr. Opin. Cell Biol. 9488-495. [DOI] [PubMed] [Google Scholar]
- 30.Lee, C. H., K. Saksela, U. A. Mirza, B. T. Chait, and J. Kuriyan. 1996. Crystal structure of the conversed core of HIV-1 Nef complexed with a Src family SH3 domain. Cell 85931-942. [DOI] [PubMed] [Google Scholar]
- 31.Le Gall, S., F. Buseyne, A. Trocha, B. D. Walker, J. M. Heard, and O. Schwartz. 2000. Distinct trafficking pathways mediate Nef-induced and clathrin-dependent major histocompatibility complex class I down-regulation. J. Virol. 749256-9266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Gall, S., L. Erdtmann, S. Benichou, C. Berlioz-Torrent, L. Liu, R. Benarous, J.-M. Heard, and O. Schwartz. 1998. Nef interacts with the μ subunit of clathrin adaptor complexes and reveals a cryptic sorting signal in MHC 1 molecules. Immunity 8483-495. [DOI] [PubMed] [Google Scholar]
- 33.Lizee, G., G. Basha, J. Tiong, J. P. Julien, M. Tian, K. E. Biron, and W. A. Jefferies. 2003. Control of dendritic cell cross-presentation by the major histocompatibility complex class I cytoplasmic domain. Nat. Immunol. 41065-1073. [DOI] [PubMed] [Google Scholar]
- 34.Lubben, N. B., D. A. Sahlender, A. M. Motley, P. J. Lehner, P. Benaroch, and M. S. Robinson. 2007. HIV-1 Nef-induced down-regulation of MHC class I requires AP-1 and clathrin but Not PACS-1 and is impeded by AP-2. Mol. Biol. Cell 183351-3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mangasarian, A., V. Piguet, J. K. Wang, Y. L. Chen, and D. Trono. 1999. Nef-induced CD4 and major histocompatibility complex class I (MHC-I) down-regulation are governed by distinct determinants: N-terminal alpha helix and proline repeat of Nef selectively regulate MHC-I trafficking. J. Virol. 731964-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marks, M. S., L. Woodruff, H. Ohno, and J. S. Bonifacino. 1996. Protein targeting by tyrosine- and di-leucine-based signals: evidence for distinct saturable components. J. Cell Biol. 135341-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohno, H., M. C. Fournier, G. Poy, and J. S. Bonifacino. 1996. Structural determinants of interaction of tyrosine-based sorting signals with the adaptor medium chains. J. Biol. Chem. 27129009-29015. [DOI] [PubMed] [Google Scholar]
- 38.Ohno, H., J. Stewart, M. C. Fournier, H. Bosshart, I. Rhee, S. Miyatake, T. Saito, A. Gallusser, T. Kirchhausen, and J. S. Bonifacino. 1995. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science 2691872-1875. [DOI] [PubMed] [Google Scholar]
- 39.Owen, D. J., and P. R. Evans. 1998. A structural explanation for the recognition of tyrosine-based endocytotic signals. Science 2821327-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piguet, V., Y.-L. Chen, A. Mangasarian, M. Foti, J.-L. Carpentier, and D. Trono. 1998. Mechanism of Nef-induced CD4 endocytosis: Nef connects CD4 with the μ chain of adaptor complexes. EMBO J. 172472-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piguet, V., L. Wan, C. Borel, A. Mangasarian, N. Demaurex, G. Thomas, and D. Trono. 2000. HIV-1 nef protein binds to the cellular protein PACS-1 to downregulate class I major histocompatibility complexes. Nat. Cell Biol. 2163-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riggs, N. L., H. M. Craig, M. W. Pandori, and J. Guatelli. 1999. The dileucine-based sorting motif in HIV-1 nef is not required for down-regulation of class I MHC. Virology 258203-207. [DOI] [PubMed] [Google Scholar]
- 43.Roeth, J. F., M. Williams, M. R. Kasper, T. M. Filzen, and K. L. Collins. 2004. HIV-1 Nef disrupts MHC-I trafficking by recruiting AP-1 to the MHC-I cytoplasmic tail. J. Cell Biol. 167903-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schrager, J. A., and J. W. Marsh. 1999. HIV-1 nef increases T cell activation in a stimulus-dependent manner. Proc. Natl. Acad. Sci. USA 968167-8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwartz, O., V. Marechal, S. Le Gall, F. Lemonnier, and J.-M. Heard. 1996. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat. Med. 2338-342. [DOI] [PubMed] [Google Scholar]
- 46.Swigut, T., L. Alexander, J. Morgan, J. Lifson, K. G. Mansfield, S. Lang, R. P. Johnson, J. Skowronski, and R. Desrosiers. 2004. Impact of Nef-mediated downregulation of major histocompatibility complex class I on immune response to simian immunodeficiency virus. J. Virol. 7813335-13344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swigut, T., M. Greenberg, and J. Skowronski. 2003. Cooperative interactions of simian immunodeficiency virus Nef, AP-2, and CD3-ζ mediate the selective induction of T-cell receptor-CD3 endocytosis. J. Virol. 778116-8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Traub, L. M. 2005. Common principles in clathrin-mediated sorting at the Golgi and the plasma membrane. Biochim. Biophys. Acta 1744415-437. [DOI] [PubMed] [Google Scholar]
- 49.Wan, L., S. S. Molloy, L. Thomas, G. Liu, Y. Xiang, S. L. Rybak, and G. Thomas. 1998. PACS-1 defines a novel gene family of cytosolic sorting proteins required for trans-Golgi network localization. Cell 94205-216. [DOI] [PubMed] [Google Scholar]
- 50.Williams, M., J. F. Roeth, M. R. Kasper, T. M. Filzen, and K. L. Collins. 2005. Human immunodeficiency virus type 1 Nef domains required for disruption of major histocompatibility complex class I trafficking are also necessary for coprecipitation of Nef with HLA-A2. J. Virol. 79632-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams, M., J. F. Roeth, M. R. Kasper, R. I. Fleis, C. G. Przybycin, and K. L. Collins. 2002. Direct binding of human immunodeficiency virus type 1 Nef to the major histocompatibility complex class I (MHC-I) cytoplasmic tail disrupts MHC-I trafficking. J. Virol. 7612173-12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamada, T., N. Kaji, T. Odawara, J. Chiba, A. Iwamoto, and Y. Kitamura. 2003. Proline 78 is crucial for human immunodeficiency virus type 1 Nef to down-regulate class I human leukocyte antigen. J. Virol. 771589-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang, O. O., P. T. Nguyen, S. A. Kalams, T. Dorfman, H. G. Gottlinger, S. Stewart, I. S. Chen, S. Threlkeld, and B. D. Walker. 2002. Nef-mediated resistance of human immunodeficiency virus type 1 to antiviral cytotoxic T lymphocytes. J. Virol. 761626-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]