Abstract
Neutralizing monoclonal antibodies (MAbs) are increasingly being considered for blunting human viral infections. However, whether they can also exert indirect effects on endogenous antiviral immune responses has been essentially overlooked. We have recently shown that a short (several-day) period of immunotherapy with the neutralizing 667 MAb of mouse neonates shortly after infection with the lethal FrCasE retrovirus not only has an immediate effect on the viral load but also permits an endogenous antiviral immunity to emerge. Even though passive immunotherapy was administered during the particular period of immunocompetence acquisition, the endogenous response eventually arising was protective and persisted long (>1 year) after the MAb has disappeared. As very high levels of anti-FrCasE antibodies, predominantly of the immunoglobulin G2a (IgG2a) isotype and showing strong neutralization activity, were found in the sera of MAb-treated mice, it was necessary to address whether this humoral immunity was sufficient on its own to confer full protection against FrCasE or whether a cytotoxic T-lymphocyte (CTL) response was also necessary. Using a variety of in vivo assays in young and adult animals previously infected by FrCasE and treated by 667, we show here that transient 667 immunotherapy is associated with the emergence of a CTL response against virus-infected cells. This cytotoxic activity is indispensable for long-term antiviral protective immunity, as high neutralizing antibody titers, even enhanced in in vivo CD8+ cell depletion experiments, cannot prevent the FrCasE-induced death of infected/treated mice. Our work may have important therapeutic consequences, as it indicates that a short period of MAb-based immunotherapy conducted at a stage where the immune system is still developing can be associated with the mounting of a functional Th1-type immune response characterized by both CTL and IgG2a-type humoral contributions, the cooperation of which is known to be essential for the containment of chronic infections by a variety of viruses.
Neutralizing monoclonal antibodies (MAbs) are increasingly being considered for treating viral infections against which no entirely satisfactory treatment, or no treatment at all, is available (48). Several of these antibodies have already shown in vivo antiviral activity in a variety of preclinical models. These include Ebola virus infection of mice and guinea pigs (53), West Nile virus infection of mice (41) and hamsters (38), H5N1 influenza virus infection of mice (50), hepatitis B virus (HBV) infection of Trimera mice (16) and chimpanzees (16, 33), HCV infection of Trimera mice (17), and human immunodeficiency virus (HIV) and simian-HIV infections of humanized SCID mice (46) and macaques (5, 18-20, 26, 31, 32, 43, 56). In humans, one MAb, pavilizumab, has already been approved for treating individuals infected by respiratory syncytial virus, and others are currently in clinical studies (48). Among them, one can cite MAbs initially shown to be safe, tolerable, and with significant activity in animal models for treating human HBV (22), HCV (23, 49), and HIV (4, 39, 54) infections that figure among the heaviest health burdens worldwide.
To date, antiviral MAbs have essentially been considered for the direct neutralization of viruses to prevent their propagation in infected individuals. However, owing to their effector functions, they can also interact with various components of the immune system, which may provide them with the possibility of affecting endogenous antiviral responses. Indeed, virus-antibody immune complexes may be more readily (or at least differently) taken up by professional antigen-presenting cells than antibody-free virus particles. Should such viral immune complexes help patients mount their own antiviral responses, this may potentially open novel therapeutic perspectives for the treatment of viral diseases. As it is easier to elucidate such fundamental concepts in immunovirology by use of immunocompetent mice than by use of primates, including humans, we recently turned to the ecotropic murine FrCasE infection model (47) to address this point and determine whether short-period MAb-based immunotherapies could favor the emergence of long-term endogenous protective antiviral responses. Upon the inoculation of newborn animals younger than 5 or 6 days of age with a high inoculum (5 × 104 PFU/ml), FrCasE first propagates in peripheral lymphoid organs and then penetrates into the central nervous system (CNS). There, it causes a rapid noninflammatory spongiform degenerative disease primarily involving the motor centers of the brain and the spinal cord (12, 35, 36). This leads to the death of all mice within 1 to 2 months. In contrast, the animals infected at a later age do not develop any neurological illness due to the inability of FrCasE to penetrate the CNS after postnatal day 8. Instead, the virus replicates only in the periphery, where it induces splenomegaly and leukemia within 3 to 6 months postinfection (our unpublished observations). 667 is an in vitro neutralizing immunoglobulin G2a/K (IgG2a/Κ) MAb directed to FrCasE envelope glycoprotein (Env) (37, 45), where it recognizes one of the viral receptor attachment domains (15). It also displays a strong in vivo antiviral activity in passive immunotherapy experiments (24, 25, 44). When newborn viremic mice are briefly treated (>15 days) by 667 shortly after infection (<2 days postinfection), all the animals survive and show signs neither of neurodegeneration nor of leukemia for at least 16 months (end of the experiments) (24). This effect is due not only to an immediate effect on the viral load preventing brain infection but also to the development of a strong protective immune response that is capable of (i) containing viral replication following 667 clearance (which takes approximately 2 weeks) and (ii) conferring on the mice the ability to resist a viral challenge performed 14 months after the primary infection (24). A particularly high and sustained anti-FrCasE humoral response, predominantly of the IgG2a type and showing both in vitro neutralizing antibodies and complement-mediated cell lysis activity, is observed for FrCasE-infected, 667 MAb-treated animals (24, 25). Moreover, maternal transmission to infected neonates of the endogenous anti-FrCasE antibodies appearing after 667 MAb treatment can also favor the mounting of an endogenous anti-FrCasE protective immunity (25). This shows that the latter effect is not restricted to the 667 MAb. Importantly also, the mounting of such an antiviral immune response following short-period immunotherapies may not be a property of the FrCasE model system only. For example, the intensive treatment of juvenile simian immunodeficiency virus-infected macaques with anti-simian immunodeficiency virus hyperimmune serum Igs accelerated the appearance of neutralizing antibodies, as assayed in vitro, in a fraction of the animals (26). Interestingly, this correlated with a delayed onset of the disease in some monkeys (26).
There is strong evidence that cell-mediated immunity is crucial for the control of established chronic infections, such as those by cytomegalovirus (34), Epstein-Barr virus (7), HCV (40), HBV (6), HIV (42), and the Friend retroviral complex (FV) (14, 29). In many of these infections, virus containment is most often associated with Th1-type T-helper-cell responses, which are classically characterized by the presence of both antiviral cytotoxic T lymphocytes (CTLs) and IgG2a antibodies (1). Strikingly, anti-FrCasE antibodies found in FrCasE-infected, 667-treated mice are predominantly of this isotype (24, 25). Moreover, in the context of passive MAb-based immunotherapies reminiscent of those in our study but conducted in adult mice, both CD4+ and CD8+ T-lymphocyte contributions are also required for protection against FV-induced erythroleukemia even in the presence of the therapeutic antibody (27) (see Discussion). Taken together, these observations raised the possibility that protection was not conferred just by high titers of neutralizing anti-FrCasE antibodies (24, 25) in FrCasE-infected, 667 MAb-treated mice but rather by a multipronged immune response. We therefore addressed here whether FrCasE-infected mice receiving the 667 immunotherapy during the neonatal period, a period critical for immune system development and immunocompetence acquisition, also developed a CTL response necessary for long-term antiviral protection.
MATERIALS AND METHODS
MAbs, viral stocks, and virus assay.
The 667 anti-FrCasE Env (37) and the 169.4 anti-CD8 MAb (9) were purified from hybridoma cell culture supernatants and assayed by enzyme-linked immunosorbent assay (ELISA) as previously described (15). Culture supernatants of Mus dunni fibroblasts transfected with the FrCasE proviral clone (47) were used as viral stocks (44). Viral titers of culture supernatants or of blood samples were determined using a focal immunofluorescence assay (51). Briefly, dilutions of virus-containing samples were added to 25%-confluent Mus dunni cell cultures in the presence of 8 μg/ml of Polybrene. Cell-to-cell spread of replication-competent retroviruses was allowed to proceed for 2 days, and focus-forming units (FFU) were visualized by indirect immunofluorescence using the 667 MAb and fluorescein isothiocyanate-conjugated rabbit anti-mouse Ig antiserum.
Viral infection and follow-up of mice.
Inbred 129/Ev mice (H-2b haplotype) were used in this study. Three-day-old pups were infected using 100 μl of a virus suspension containing 5 × 105 FFU/ml as previously described (24, 44). Mice were examined daily for clinical signs of neurodegeneration (reduced weight, ataxia, hind-limb paralysis). Erythroleukemia was assayed by measuring reduction in hematocrit, which is associated with anemia (52), and spleen swelling by direct abdominal palpation and by examination of spleens once animals were sacrificed. Mice were bled at the retro-orbital sinus in order to assay viremia and anti-FrCasE serum Ig concentration. After clotting at room temperature for 15 min, blood samples were centrifuged at 6,000 × g for 15 min and serum aliquots were stored at −20°C until use. For viral challenge experiments, mice were injected intravenously with 300 μl of FrCasE suspension containing 5 × 105 FFU/ml.
ELISA of anti-FrCasE antibodies.
Anti-FrCasE serum antibodies were assayed as described previously (24, 44). Both the 667 MAb used for the standard curve and serum samples were diluted in phosphate-buffered saline (PBS) (0.15 M NaCl, 0.01 M Na phosphate, pH 7) containing 0.1% Tween plus 1% bovine serum albumin. A peroxidase-conjugated anti-mouse IgG γ-chain rabbit antiserum (Sigma) was used as the secondary antibody.
Preparation of splenocytes.
After the sacrifice of mice, spleens were excised and dissociated by repeated pipetting in RPMI 1640 culture medium (2 ml per spleen). Then, dissociated cells were centrifuged and resuspended in the ACK lysis buffer (2 ml per spleen) from Biowhittaker followed by incubation at room temperature for 3 min. Finally, white blood cells were recovered by centrifugation and either washed twice in PBS for flow cytometry analysis or resuspended in RPMI 1640 medium (Sigma) containing 10% fetal calf serum for use as target cells in cytotoxicity experiments.
In vivo depletion of CD8+ T cells.
To deplete CD8+ T cells in vivo, 50 μg of the 169.4 anti-CD8 MAb (9, 28), home-purified from hybridoma cell culture supernatants contained in 0.5 ml of PBS, was administered intraperitoneally to mice twice a week for the periods indicated in the text. CD8+ T-cell depletion efficiency during the 169.4 MAb treatment and CD8+ cell replenishment after termination of the treatment were analyzed by flow cytometry of spleen cells from sacrificed animals (i) 2 weeks after the first injection, (ii) at the end of the depletion period, and (iii) at the end of the experiment. The percentages of CD8+ and CD4+ T-cell depletion in 169.4 MAb-treated mice were calculated in comparison to the fractions of CD8+ and CD4+ cells in spleens from control mice that were not treated with the 169.4 MAb.
In vivo cytotoxicity assays.
Depending on the adoptive transfer experiments, spleens were recovered from 10-day-old 129/Ev neonates that were either noninfected or Fr-CasE infected on day 3 after birth, as previous kinetic infection experiments had shown that the fraction of infected splenocytes is maximal at 7 days postinfection (24). Red blood cell-free splenocytes were prepared as described above and resuspended in RPMI 1640 medium containing 10% fetal calf serum. In experiments whose results are shown below (see Fig. 3), splenocytes from control 15-day-old noninfected mice were labeled with carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes) at a concentration of 0.5 μM in 1 ml of PBS (CFSElow cells) at 37°C for 10 min, whereas splenocytes from 15-day-old mice infected on day 3 after birth were labeled in parallel in a 5 μM CFSE solution under the same conditions (CFSEhigh cells). In these experiments, CFSElow cells were used as a reference to estimate spontaneous cell death, whereas the lysis of CFSEhigh cells was used to measure specific cytotoxic activity. After labeling, cells were washed in ice-cold PBS, centrifuged, resuspended in PBS, counted, and mixed in a 1:1 ratio. One hundred fifty microliters of the mix containing 1 × 107 cells was injected intravenously into each recipient 129/Ev mouse. An aliquot of the mix was analyzed by flow cytometry to quantify the exact proportion of CFSElow versus CFSEhigh cells before adoptive transfer. Sixteen hours after mice received CFSE-labeled cells by intravenous injection in the tail vein, their spleens were excised and splenocytes were prepared as described above for flow cytometry analysis of CFSElow versus CFSEhigh cells. The cytotoxic activity against infected splenocytes was calculated from the ratio of CFSEhigh/CFSElow cells 16 h after transfer corrected by the CFSEhigh/CFSElow ratio assayed before grafting cells into recipient mice. In further experiments whose results are shown below (see Fig. 4), splenocytes prepared from naive mice were pulsed with either the GagL (8) (specific CTL target cells) or the np396-404 control peptide derived from lymphocytic choriomeningitis virus (LCMV) nucleoprotein (NP) (21) (control cells allowing the measurement of spontaneous cell death) at 37°C for 1 h. Peptides were used at a concentration of 1 μg/ml. After being washed in PBS to eliminate excess peptide, splenocytes loaded with the GagL and np396-404 peptides were labeled with 5 μM CFSE (CFSEhigh cells) and 0.5 μM CFSE (CFSElow cells), respectively, at 37°C for 10 min in PBS. Cells were then washed again in ice-cold PBS and resuspended in PBS for adoptive transfer. After being counted, they were mixed in a 1:1 ratio. An aliquot was analyzed by flow cytometry to determine the exact CFSElow/CFSEhigh cell ratio. Cells (107 in 0.2 ml of PBS) of this mixture were injected intravenously in each recipient 129/Ev mouse. Sixteen hours later, mice were sacrificed for flow cytometry quantification of splenic CFSElow and CFSEhigh cells. The CTL activity against GagL-loaded splenocytes was calculated from the ratio of CFSEhigh/CFSElow cells 16 h after adoptive transfer corrected by the CFSEhigh/CFSElow ratio assayed before grafting cells into the recipient mice.
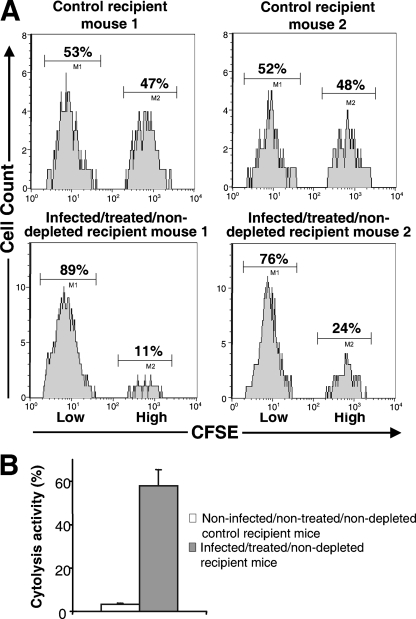
FIG. 3.
In vivo cytolytic activity against FrCasE-infected splenocytes in infected/treated/nondepleted mice. Splenocytes were prepared either from control noninfected mice or from infected mice (see Materials and Methods) and labeled with 1× or 10× CFSE, respectively. They were then mixed in an approximately 1:1 ratio and administered intravenously to either naive (control) or infected/treated/nondepleted mice (2 months old). Sixteen hours later, splenocytes from grafted mice were purified and analyzed by flow cytometry to determine the intensity of CFSE labeling. A reduction of 10× CFSE-labeled cells in infected/treated/nondepleted mice, but not in control mice, is indicative of cytotoxic activity against infected splenocytes. (A) Flow cytometry analysis. Two representative control and two representative infected/treated/nondepleted mice are presented. Splenocytes were analyzed for CFSE fluorescence as described in Materials and Methods. (B) Statistical representation of the data. Each histogram corresponds to an analysis of 12 mice. The cytotoxic activity against infected splenocytes was calculated from the ratio of CFSEhigh/CFSElow cells 16 h after adoptive transfer corrected by the CFSEhigh/CFSElow ratio assayed before grafting cells into recipient mice. Error bars correspond to the standard deviation.
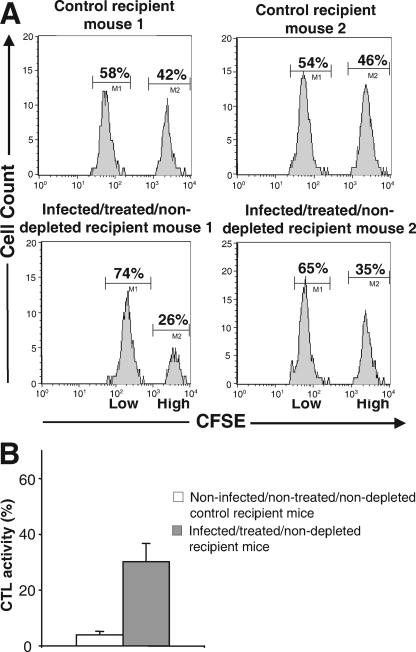
FIG. 4.
In vivo CTL activity against GagL peptide-presenting splenocytes in infected/treated/nondepleted mice. Splenocytes were prepared from noninfected mice and either (i) loaded with the GagL peptide and labeled with 10× CFSE or (ii) loaded with the np396-404 LCMV NP-derived peptide and labeled with 1× CFSE. The two populations were then mixed in an approximately 1:1 ratio and administered intravenously to either naive (control) or infected/treated/nondepleted 2-month-old mice. Sixteen hours later, splenocytes from grafted mice were purified and analyzed by flow cytometry for CFSE fluorescence. A reduction of 10× CFSE-labeled cells in infected/treated/nondepleted mice but not in control mice is indicative of CTL activity against infected splenocytes. (A) Flow cytometry analysis. Two representative control and two representative infected/treated/nondepleted mice are presented. Splenocytes were analyzed for CFSE fluorescence as described in Materials and Methods. (B) Statistical representation of data. The histograms correspond to the analyses of 12 mice each. The CTL activity against GagL-loaded splenocytes was calculated from the ratio of CFSEhigh/CFSElow cells 16 h after adoptive transfer corrected by the CFSEhigh/CFSElow ratio assayed before grafting cells into the recipient mice. Error bars correspond to the standard deviation.
Flow cytometry analysis.
For quantification of CD4+ and CD8+ splenocytes (Fig. 1), cells were labeled with 1/500 dilutions of the anti-CD8 α-fluorescein isothiocyanate and anti CD4-PE antibodies from BD PharMingen in PBS containing 0.1% bovine serum albumin. The incubation was at 4°C for 30 min. Then, splenocytes were washed once in PBS and analyzed with a FACSCalibur flow cytometer using the CELLQuest software. For the quantification of the ratio of CFSElow to CFSEhigh cells (see Fig. 3 and 4 below), splenocytes were simply washed and resuspended in PBS before flow cytometry analysis.
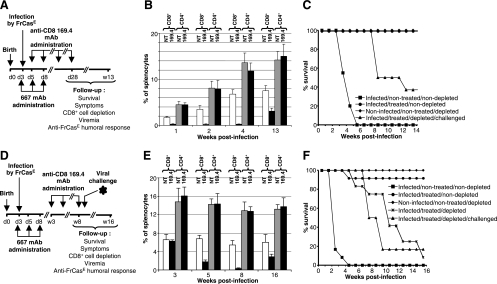
FIG. 1.
Leukemia development in FrCasE-infected, 667-treated 129/Ev mice depleted of CD8+ cells. (A) Design of the experiments conducted with young mice. The timing of infection with FrCasE and those of 667 and 169.4 MAb administrations are indicated in the figure (d, day; w, week). Four groups of 12 mice each were followed up using the indicated criteria. These groups were as follows: infected/nontreated/nondepleted mice, infected/treated/nondepleted mice, infected/treated/depleted mice, and noninfected/nontreated/depleted mice. “Infected” refers to infection with FrCasE, “treated” to 667 MAb administration, and “depleted” to 169.4 MAb administration (see text). (B) Splenic CD4+ and CD8+ cells. Mice treated as described for panel A were sacrificed at various times for flow cytometry assay of the fractions of CD4+ and CD8+ splenocytes. (C) Survival of mice treated as indicated in panel A. (D) Design of the experiments conducted with adult mice. In addition to the four groups of mice shown in panel A, another group (infected/treated/depleted/challenged) of 12 mice was challenged with FrCasE on week 8. (E) Splenic CD4+ and CD8+ cells. Mice treated with the 169.4 MAb as for panel D, or not treated, were sacrificed at various times for flow cytometry assay of the fractions of CD4+ and CD8+ splenocytes. (F) Survival of mice treated as indicated in panel A. Error bars in panels B and D indicate standard deviations.
RESULTS
CD8+ cell depletion in young FrCasE-infected, 667-treated animals leads to the development of leukemia.
Neonatal T cells can develop into effector CD8+ CTLs, even though significant quantitative and qualitative differences distinguish them from their adult counterparts (2). We therefore began our study by addressing whether CD8+ cells may contribute to protection against FrCasE-induced retroviral diseases in young infected 667-treated animals by ablating them through injection of an anti-CD8 MAb (169.4) (9, 28) during the period immediately following infection and immunotherapy. The following four different groups of 12 mice each were followed up for 13 weeks: (i) mice only infected with FrCasE (infected/nontreated/nondepleted); (ii) mice infected with FrCasE and treated with 667 (infected/treated/nondepleted); (iii) mice infected with FrCasE, treated with 667, and depleted of CD8+ cells (infected/treated/depleted); and (iv) control mice neither infected nor treated by 667 but depleted of CD8+ cells (noninfected/nontreated/depleted). Infections and 667 treatments were carried out as previously described (24, 25).: FrCasE was inoculated on day 3 after birth and 667 was administered first 1 hour later, a time sufficient to allow infection to proceed, and then another two times on days 5 and 8 after birth (Fig. 1A). As the present study required mouse-to-mouse cell transfer (see below), we had to resort to inbred animals instead of the outbred Swiss Webster mice used in our previous studies (24, 25). 129/Ev (H-2b) mice were selected, as after neonatal infection, they turned out to behave like Swiss Webster mice in 667-based immunotherapy experiments (not shown).
The 169.4 MAb treatment lasted from day 5 to day 28 after birth (Fig. 1A), i.e., it began during the neonatal period (ending with weaning by day 18 to 20 after birth for 129/Ev mice) and ended just before sexual maturity (occurring by day 28 to 30 after birth for 129/Ev mice). As shown in Fig. 1B, this period is characterized by an intense development of the mouse immune system with a steady accumulation of CD4+ and CD8+ cells in the spleen. CD8+ cell depletion was quick and efficient (98% reduction) for the duration of the treatment. Thereafter, partial replenishment of this cell compartment occurred with 40% recovery by week 13 (Fig. 1B). The anti-CD8 MAb treatment depleted CD4+ T lymphocytes by, at the very most, 3 to 6%, demonstrating the specificity of the effect (Fig. 1B). Moreover, no particular pathological or behavioral manifestation was detected for control noninfected/nontreated/depleted mice for the 13 weeks of the follow-up, indicating the innocuousness of the CD8+ cell depletion per se under the experimental conditions used. As expected, we observed the following results (Fig. 1C). (i) All infected/nontreated/nondepleted mice died before week 5 postinfection after having shown the characteristic symptoms of the FrCasE-induced neurodegeneration (reduced weight, ataxia, and hind-limb paralysis were already detectable by day 17 postinfection) and high plasma viremia (105 to 106 FFU/ml from day 7 to death) quantified in focal immunofluorescence assays (see Materials and Methods). (ii) Infected/treated/nondepleted animals remained healthy with signs of neither neurodegeneration nor leukemia. Moreover, the latter animals showed no detectable viremia (limit of detection of 102 FFU/ml) for the 13 weeks of the experiment. In contrast, although all infected/treated/depleted mice were still alive by week 7 postinfection without displaying any neuropathological signs, they all began to develop leukemia thereafter and two-thirds of them were dead by week 13 (Fig. 1C). In addition, the animals still alive at that time showed signs of severe and irreversible splenomegaly, which would have led to death within a few days. In these experiments, disease onset and progression were classically followed up by regular control of spleen swelling, easily detectable by abdominal palpation, and hematocrit, which decreased (by approximately 25%, when splenomegaly became detectable, to 60% of the normal red blood cell count at the time of death) due to erythroleukemia-associated anemia (52). No viremia was detected for infected/treated/depleted animals after CD8+ cell depletion was initiated. This excluded a dramatic viral rebound (not shown) while not ruling out the possibility of a residual production of viruses (possibly neutralized by circulating anti-FrCasE antibodies) below the detection threshold of the assay. Thus, starting the depletion of CD8+ cells as early as 5 days after birth does not lead to neurodegeneration in infected/treated/depleted mice. However, the presence of CD8+ cells during the first month after infection and MAb treatment are essential for preventing leukemia at a later stage.
CD8+ cells during adulthood are crucial for preventing FrCasE-induced leukemia.
New infection/immunotherapy/depletion experiments were conducted to address whether CD8+ cells contribute to the protection of FrCasE-infected and 667-treated mice during adulthood. The same four groups of animals as shown in Fig. 1A to C were considered, except that the treatment for CD8+ cell depletion began on week 3 after birth and continued until week 8 (Fig. 1D). As in the case of young animals, CD4+ cells were weakly affected by the 169.4 MAb treatment (Fig. 1E). In contrast, CD8+ cell depletion was efficient (95% reduction by the end of the 169.4 MAb treatment) and was followed by a progressive and partial reconstitution, reaching 45% by week 16 postinfection (Fig. 1F). Moreover, no particular pathological or behavioral manifestations were detected for control noninfected/nontreated/depleted mice. As expected, all infected/nontreated/nondepleted mice died within 5 weeks and all noninfected/nontreated/depleted and infected/treated/nondepleted animals remained healthy for the duration of the experiments, except for one mouse of the latter category, which died on week 6 for an unknown reason that involved neither neurodegeneration nor precocious leukemia (Fig. 1F). In contrast, CD8+ cell depletion led to the induction of leukemia in infected/treated/depleted mice as follows: (i) all of them showed a reduced hematocrit (30% of the normal red blood cell count) by week 6 after primary infection; (ii) most of them died between weeks 8 and 13 after primary infection; and (iii) the few animals still alive on week 16 (termination of the experiment) presented signs of severe and irreversible splenomegaly, which would have led to death within a few days (Fig. 1F). Thus, CD8+ cells are required during adulthood to prevent mice from developing leukemia.
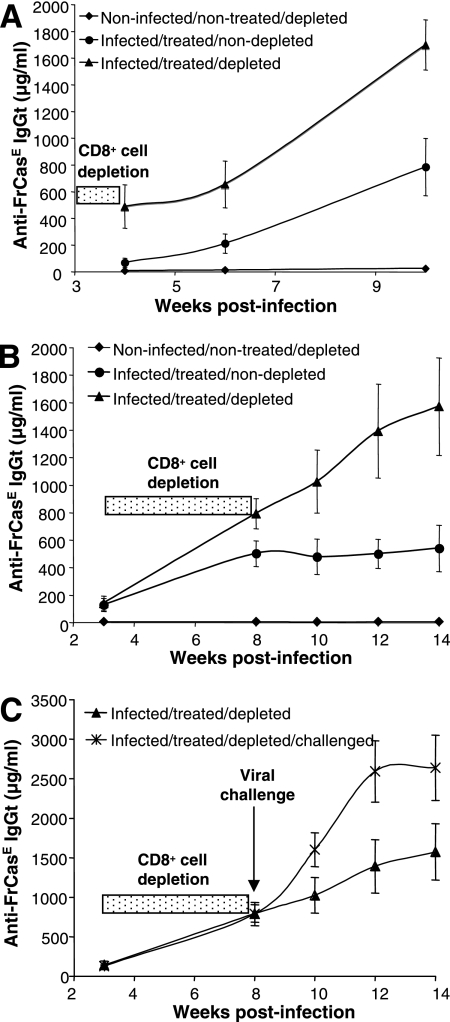
The endogenous anti-FrCasE humoral response is higher in CD8+ cell-depleted mice but is not sufficient for long-term antiviral protection.
We next investigated whether the anti-FrCasE humoral response could be significantly affected by CD8+ cell depletion. As 667 MAb clearance from the plasma of young animals takes approximately 15 days after the end of the treatment (24, 25), endogenous anti-FrCasE antibodies were assayed by ELISA at later time points for animals from the experiments described in the legend to Fig. 1. In mice of Fig. 1A to C, no endogenous anti-FrCasE humoral response was seen for noninfected/nontreated/nondepleted controls (Fig. 2A). In contrast, such a response became detectable in infected/treated/nondepleted animals by week 4 postinfection and dramatically increased after week 6, whether CD8+ cells were depleted or not (Fig. 2A). Strikingly, however, CD8+ cell elimination resulted in a stronger humoral immune response, as anti-FrCasE antibodies were at least twice as abundant in infected/treated/depleted mice as in infected/treated/nondepleted animals. Our data were very similar for the mice used for Fig. 1D to F, as shown in Fig. 2B. Using the neutralization assay described in the work of Gros et al. (24), we also showed that serum neutralization activities were comparable in infected/treated/depleted mice and in infected/treated/nondepleted animals on week 14 (not shown). Although we have no explanation for the fact that neutralization did not increase correlatively with antibody titers in infected/treated/nondepleted mice, these data ruled out that leukemia developing in these animals might be due to a loss in the antiviral activity of the anti-FrCasE humoral response in the absence of CD8+ cells. In our previous work (24), we have shown that challenging infected/treated/nondepleted mice with FrCasE long after the 667 has disappeared induces a strong antiviral humoral memory response. We therefore asked whether the humoral response could be altered by CD8+ cell depletion at an adult age. FrCasE-infected, 667-treated mice were consequently treated with the 169.4 MAb from weeks 3 to 8 after birth and reinfected by FrCasE just at the end of the treatment (Fig. 1D). Despite an enhanced stimulation (twofold) of the anti-FrCasE humoral response (Fig. 2C), this treatment led to fast death of these mice compared to what was seen for nonchallenged infected/treated/depleted animals (Fig. 1F), further strengthening the role of CD8+ cells in antiviral protection.
FIG. 2.
Anti-FrCasE humoral response in FrCasE-infected, 667-treated mice depleted of CD8+ cells. (A) Anti-FrCasE antibodies in young CD8+ cell-depleted mice. Serum anti-FrCasE antibodies from the mice of Fig. 1A to C were assayed by ELISA. (B) Anti-FrCasE antibodies in adult CD8+ cell-depleted mice. Serum anti-FrCasE antibodies from the mice of Fig. 1D to F were assayed by ELISA. (C) Anti-FrCasE antibodies in adult CD8+ cell-depleted mice after viral challenge. Infected/treated mice depleted of CD8+ cells at the adult age as indicated in Fig. 2B were challenged with FrCasE. Serum concentrations of anti-FrCasE antibodies were compared to those of infected/treated/depleted mice that were not challenged. Error bars indicate standard deviations.
In vivo cytotoxic activity against FrCasE-infected splenocytes in infected/treated/nondepleted mice.
A reasonable explanation for leukemia induction in the CD8+ cell depletion experiments presented in Fig. 1D to F is the elimination of CTL activity against FrCasE-infected cells. However, various cell types other than CTLs also express CD8 at their surfaces. These include (i) certain T-cell precursors, (ii) regulatory T cells (10), (iii) subsets of natural killers (NK) and natural killer T cells (55, 57), and (iv) subsets of dendritic cells (58, 59). As a first step to establish a role for CTLs in antiviral protection, we addressed whether infected/treated/nondepleted mice showed a cytotoxic activity specifically directed against FrCasE-infected splenocytes, as the spleen is a major organ for FrCasE replication. Since the infection of splenocytes by FrCasE is maximal at 7 days postinfection, with 50 to 60% of them expressing retroviral Env at their surfaces (24), such infected spleen cells were used as targets in in vivo cytoxicity assays in 2-month-old mice, as described in Materials and Methods and in the legend to Fig. 3. Three experiments including four mice each were conducted. Typical flow cytometry analyses for 2 control and 2 infected/treated/nondepleted mice are presented in Fig. 3A, and the means of lytic activities, estimated from the percentage of lysed cells in the 12 animals of each group, are shown in Fig. 3B. A basal cytolytic activity against FrCasE-infected splenocytes was observed for control animals. This basal activity is visible in the mice of Fig. 3A, as the CFSEhigh splenocytes represented 47 and 48% of the remaining grafted splenocytes in animals 1 and 2, respectively, in an experiment where the ratio of the adoptively transferred CFSEhigh versus CFSElow cells was 46:54 before grafting. The mean cytolytic activity, as measured by the fraction of infected cells disappearing during the experiment, was 3% ± 0.5% in this group of mice. In contrast, cytolysis was much more efficient in infected/treated/nondepleted mice, as (i) the CFSEhigh splenocytes represented only 11 and 24% of the remaining grafted splenocytes in infected/treated/nondepleted recipient mice 1 and 2 of Fig. 3A, respectively, and (ii) the mean of infected cell cytolysis was 57.8% ± 7.7% in this group of animals (Fig. 3B). Thus, infected/treated/nondepleted animals show a clear cytolytic activity specifically directed against FrCasE-infected cells.
In vivo CTL activity against GagL epitope-presenting splenocytes in infected/treated/nondepleted mice.
We then had to demonstrate formally the contribution of CTLs to the in vivo cytolysis of infected splenocytes in infected/treated/nondepleted mice. CTLs recognize their cognate antigenic peptides presented by major histocompatibility complex (MHC) class I molecules at the surfaces of target cells via their T-cell receptors. We therefore assessed whether splenocytes taken from naive mice and loaded in vitro with an MHC class I-restricted murine leukemia virus (MuLV)-specific antigenic peptide would be killed more efficiently after grafting into infected infected/treated/nondepleted mice than after grafting into naive mice. To our knowledge, the GagL CTL epitope is the only H-2Db-restricted antigenic peptide that has been described in terms of its ability to drive the cytolysis of cells infected by a variety of MuLVs (8). It is derived from the leader region of the glycosylated transmembrane form of the Gag polyprotein (gPr80gag) expressed by infected cells but poorly incorporated (if at all) into viral particles. gPr80gag has no reported discernible function for MuLV replication but is necessary for the efficient spreading and pathogenesis of MuLVs in vivo (11). Specific in vivo CTL activity was assayed as described in Materials and Methods and in the legend for Fig. 4 after the grafting of a mixture of splenocytes from a naive mouse previously loaded with either the GagL peptide or a control peptide (np306-404, which is derived from the LCMV NP and also binds H-2Db MHC class I molecules [21]) to infected/treated/nondepleted and infected/treated/depleted mice. Three experiments including four mice each were conducted. Typical analyses for two control and two infected/treated/nondepleted mice are presented in Fig. 4A. The means of lytic activities, estimated from the percentages of lysed cells in the 12 animals of each group, are shown in Fig. 4B. An average basal 4% ± 1% cytolytic activity (Fig. 4B) against splenocytes loaded with the GagL peptide was measured for control noninfected/nontreated/nondepleted mice (6 and 1% in the two control mice for which results are presented in Fig. 4A). The fraction of the same cells that was lysed in infected/treated/nondepleted animals was dramatically higher (26 and 41% in the two infected/treated/nondepleted mice of Fig. 4A) with an average cytolysis activity of 30.2% ± 6.6% (Fig. 4B). Thus, FrCasE-infected, 667-treated mice display a strong CTL activity directed against cells presenting the H-2Db-restricted GagL antigenic peptide.
DISCUSSION
Previously, we described the emergence of a strong long-lasting endogenous neutralizing humoral response that follows a short period of 667 immunotherapy of mice infected neonatally with a high FrCasE inoculum (24, 25). Here, we report that this antibody response is complemented by a CTL activity that is necessary for the long-term protection of mice against FrCasE-induced leukemia.
CD8+ cell ablation in infected/treated neonates does not affect protection against neurodegeneration but leads to the development of leukemia.
If FrCasE is inoculated into neonates older than 5 or 6 days, no neurodegeneration occurs (12; unpublished data). Moreover, the intracranial implantation of infected neuroglial cells later than day 10 after birth induces neuropathological effects (36). This indicates that the resistance of the CNS to infection after day 5 or 6 lies essentially at the blood-brain barrier. In turn, this suggests that diminishing viral propagation by 667 should be sufficient on its own to maintain a viremia below the critical level necessary for the entry of FrCasE into the brain and prevent neurodegeneration. Our data in Fig. 1A to C are consistent with this hypothesis, as the efficient depletion of CD8+ cells in infected/treated neonates did not lead to any neurodegeneration. Strikingly, all infected/treated neonates depleted of CD8+ cells shortly after infection developed the characteristic FrCasE-induced leukemia. However, it is not clear whether this pathology developed because of the absence of CD8+ cells during the neonatal period, during adulthood, or during both periods, as the CD8+ cell depletion extended far beyond the period of anti-CD8 MAb administration. Several nonexclusive explanations may account for leukemia development. First, the lower numbers of CD8+ cells at adulthood might be associated with low CTL activity, which would prevent the elimination of infected and leukemic cells (also see below). Second, CD8 is not expressed just on CTLs but also on other immune cells. These include dendritic cells, which are at the origin of any adaptive immune response, and CD4+/CD8+ double-positive thymocytes, which are the precursors of both helper T cells and CTLs. This must be taken into consideration because the anti-CD8 MAb administration was performed during the most intense period of immune system development (also see below). However, should these two cell populations be affected, it is clear that they are not totally ablated, as (i) CD4+ cell development is not detectably affected, as shown by cytometry analysis (Fig. 1B); and (ii) a humoral anti-FrCasE immune response can develop in infected/treated/depleted mice (Fig. 2).
An essential cytotoxic activity contributes to antiviral protection in adult infected/treated animals.
The administration of an anti-CD8 MAb to infected/treated mice at adult age (i.e., after the endogenous anti-FrCasE immunity has been mounted and after 667 has been cleared due to its limited life span) was accompanied by the efficient depletion of CD8+ T cells and led to the development of leukemia in all cases (Fig. 1D to F). Importantly, neutralization activity was maintained in infected/treated/depleted mice, and total anti-FrCasE antibody levels were even increased. Why neutralization activity did not increase correlatively with antibody titers is unclear. Whatever the mechanisms involved, this indicated that leukemogenesis cannot be explained by a reduction in the activity of anti-FrCasE humoral response. Moreover, adult infected/treated/nondepleted animals showed a strong cytolytic activity against splenocytes either infected by FrCasE or loaded with the MHC class I-restricted GagL antigenic peptide. Altogether, these data suggest that a CTL activity against FrCasE-infected cells plays a crucial role in the long-term antiviral protection that develops in neonatally infected/treated mice.
Even though our data demonstrate a CTL activity against FrCasE-infected cells in adult infected/treated mice, they do not rule out the possibility that other cells displaying CD8 might also contribute to long-term antiviral protection. It is noteworthy that the efficiency of elimination of infected splenocytes was double that of GagL peptide-loaded splenocytes in our in vivo cytolysis assays (see Fig. 3 and 4). It therefore would be interesting to investigate whether NK cells also play a role in the antiviral protection observed for infected/treated mice. As (i) Env is expressed by infected cells and (ii) high titers of anti-Env antibodies are found for infected/treated animals, antibody-dependent T-cell cytolysis would constitute a plausible mechanism, although antibody-independent contributions might be possible as well.
Infection by FV is the most extensively studied model for the analysis of antiretroviral immune responses in adult mice, including in immunotherapeutic and vaccination contexts (see references 14, 29, and 30). Even though FV is a retroviral complex and is more aggressive than FrCasE, it is worth comparing the two models. Interestingly, Hasenkrug et al. have reported that a CTL response is indispensable for protection against FV-induced leukemia in mice treated by passive MAb-based immunotherapy (27). However, there were significant biological and technical differences between their setting and ours. The first important difference relates to the immunological status of the animals used. Our experiments were conducted in the context of the developing immune system, whereas in the FV system, experiments were carried out in adult animals with an already mature immune system. Moreover, in the FV model, mice were unable to mount an antibody response due to a genetic defect. Therefore, although the MAb was administered for a long period (several weeks versus several days in our case) and provided humoral protection, that work did not address whether an endogenous polyclonal humoral response could compensate for the loss of CTL activity upon CD8+ cell depletion. In contrast, we clearly show here that high antibody titers previously shown to display high neutralization and complement-dependent cytolysis activities (24, 25) were not sufficient to protect infected/treated mice against FrCasE-induced leukemia. The second important difference lies in the design of the experiments. First, in the FV experiments, passive immunotherapy was started later (10 days postinfection versus several hours for FrCasE up to a maximum of 2 days postinfection [see reference 24]). This implies that viremia could reach high titers for at least a few days in the FV model, whereas it was contained to a moderate level and was rapidly blocked below the detection threshold in the case of FrCasE (24). Consequently, in the initial phase of the FV experiments, the immune system of the mice was stimulated by much higher levels of viral antigens under conditions for which virus-antibody complexes cannot form (also see below) due to the lack of a cognate antibody. Second, CD8+ cell depletion was started before infection in the case of FV infection, whereas in our experiments, depletion was conducted after antiviral immunity had been installed in FrCasE-infected, 667-treated mice. Immune cell homeostases in the two experimental settings were therefore different at the moments of both infection and administration of passive immunotherapy. Thus, the results of the FV and FrCasE studies give complementary indications in terms of the potential therapeutic applications of MAb-based immunotherapy. The former study indicates that the development of a CTL response must not be impeded in viremic individuals that will be subjected to passive immunotherapy. In contrast, the latter study indicates that the CTL response that has been established after immunotherapy must not be abrogated even though treated individuals have mounted a strong and sustained antiviral polyclonal humoral response. As the conditions of induction of the CTL responses were different in many respects in the two experimental systems, it will now be important to compare them in terms of strength, antigenic specificity, and duration.
Even though the immediate and major action of 667 is clearly the prevention of neurodegeneration by diminishing viral spread in the early stages of the immunotherapy, an important question is whether this MAb may also have other actions in FrCasE-infected, 667-treated neonates, as these animals develop a long-lasting antiviral response persisting long after 667 has disappeared. A first possibility is that reduced viremia may give sufficient time for the endogenous immune system of treated mice to react against FrCasE, whereas that of nontreated mice would be overwhelmed. Secondly, there is increasing evidence that tolerance is not an intrinsic property of the newborn immune system. Depending on (i) the antigen, (ii) the antigen-presenting cells involved, and (iii) the experimental conditions, neonates may acquire immunity against pathogens (3). It is therefore possible that 667, through the formation of immune complexes with FrCasE, accelerates and/or strengthens the antiviral response via better stimulation of professional antigen-presenting cells. Supporting this idea, cross-linked immune complexes dramatically improve antigen presentation after binding to signal-inducing Fc receptors compared to what is seen for other modes of antigen capture (13). Experiments are currently under way to test this hypothesis with infected/treated mice.
Finally, it is important to underline that the FrCasE infection model used here is reminiscent of perinatal or breastfeeding contamination of human children by mothers infected by viruses such as HIV, as initial virus propagation occurs during the period of immunocompetence acquisition. Therefore, our work potentially opens new therapeutic perspectives, as it indicates that short-period neutralizing MAb-based immunotherapies given shortly after the infection of young individuals may be associated not only with the emergence of neutralizing antiviral antibodies (24, 25) but also with that of CTLs specific for virus-infected cells.
Acknowledgments
M. Piechaczyk's laboratory is an “Equipe labelisée” funded by the Ligue Nationale contre le Cancer. This work has also been supported by grants from the ARC.
We are grateful to K. Hasenkrug for the kind gift of the anti-CD8 MAb 169.4 hybridoma and to E. Jouffre and M. Plays for skillful technical assistance. We are also indebted to I. Robbins and V. Kalatzis for critical reading of the manuscript.
Footnotes
Published ahead of print on 21 November 2007.
REFERENCES
- 1.Adkins, B. 2000. Development of neonatal Th1/Th2 function. Int. Rev. Immunol. 19157-171. [DOI] [PubMed] [Google Scholar]
- 2.Adkins, B. 1999. T-cell function in newborn mice and humans. Immunol. Today 20330-335. [DOI] [PubMed] [Google Scholar]
- 3.Adkins, B., C. Leclerc, and S. Marshall-Clarke. 2004. Neonatal adaptive immunity comes of age. Nat. Rev. Immunol. 4553-564. [DOI] [PubMed] [Google Scholar]
- 4.Armbruster, C., G. M. Stiegler, B. A. Vcelar, W. Jager, N. L. Michael, N. Vetter, and H. W. Katinger. 2002. A phase I trial with two human monoclonal antibodies (hMAb 2F5, 2G12) against HIV-1. AIDS 16227-233. [DOI] [PubMed] [Google Scholar]
- 5.Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T. C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6200-206. [DOI] [PubMed] [Google Scholar]
- 6.Boni, C., P. Fisicaro, C. Valdatta, B. Amadei, P. Di Vincenzo, T. Giuberti, D. Laccabue, A. Zerbini, A. Cavalli, G. Missale, A. Bertoletti, and C. Ferrari. 2007. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J. Virol. 814215-4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Callan, M. F. 2003. The evolution of antigen-specific CD8+ T cell responses after natural primary infection of humans with Epstein-Barr virus. Viral Immunol. 163-16. [DOI] [PubMed] [Google Scholar]
- 8.Chen, W., H. Qin, B. Chesebro, and M. A. Cheever. 1996. Identification of a gag-encoded cytotoxic T-lymphocyte epitope from FBL-3 leukemia shared by Friend, Moloney, and Rauscher murine leukemia virus-induced tumors. J. Virol. 707773-7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cobbold, S. P., A. Jayasuriya, A. Nash, T. D. Prospero, and H. Waldmann. 1984. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature 312548-551. [DOI] [PubMed] [Google Scholar]
- 10.Cone, R. E., X. Li, R. Sharafieh, J. O'Rourke, and A. T. Vella. 2007. The suppression of delayed-type hypersensitivity by CD8+ regulatory T cells requires interferon-gamma. Immunology 120112-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corbin, A., A. C. Prats, J. L. Darlix, and M. Sitbon. 1994. A nonstructural gag-encoded glycoprotein precursor is necessary for efficient spreading and pathogenesis of murine leukemia viruses. J. Virol. 683857-3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Czub, S., W. P. Lynch, M. Czub, and J. L. Portis. 1994. Kinetic analysis of spongiform neurodegenerative disease induced by a highly virulent murine retrovirus. Lab. Investig. 70711-723. [PubMed] [Google Scholar]
- 13.Dhodapkar, K. M., and M. V. Dhodapkar. 2005. Recruiting dendritic cells to improve antibody therapy of cancer. Proc. Natl. Acad. Sci. USA 1026243-6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dittmer, U., and K. J. Hasenkrug. 2001. Cellular and molecular mechanisms of vaccine-induced protection against retroviral infections. Curr. Mol. Med. 1431-436. [DOI] [PubMed] [Google Scholar]
- 15.Dreja, H., L. Gros, S. Villard, E. Bachrach, A. Oates, C. Granier, T. Chardes, J. C. Mani, M. Piechaczyk, and M. Pelegrin. 2003. Monoclonal antibody 667 recognizes the variable region A motif of the ecotropic retrovirus CasBrE envelope glycoprotein and inhibits Env binding to the viral receptor. J. Virol. 7710984-10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eren, R., E. Ilan, O. Nussbaum, I. Lubin, D. Terkieltaub, Y. Arazi, O. Ben-Moshe, A. Kitchinzky, S. Berr, J. Gopher, A. Zauberman, E. Galun, D. Shouval, N. Daudi, A. Eid, O. Jurim, L. O. Magnius, B. Hammas, Y. Reisner, and S. Dagan. 2000. Preclinical evaluation of two human anti-hepatitis B virus (HBV) monoclonal antibodies in the HBV-trimera mouse model and in HBV chronic carrier chimpanzees. Hepatology 32588-596. [DOI] [PubMed] [Google Scholar]
- 17.Eren, R., D. Landstein, D. Terkieltaub, O. Nussbaum, A. Zauberman, J. Ben-Porath, J. Gopher, R. Buchnick, R. Kovjazin, Z. Rosenthal-Galili, S. Aviel, E. Ilan, Y. Shoshany, L. Neville, T. Waisman, O. Ben-Moshe, A. Kischitsky, S. K. Foung, Z. Y. Keck, O. Pappo, A. Eid, O. Jurim, G. Zamir, E. Galun, and S. Dagan. 2006. Preclinical evaluation of two neutralizing human monoclonal antibodies against hepatitis C virus (HCV): a potential treatment to prevent HCV reinfection in liver transplant patients. J. Virol. 802654-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrantelli, F., K. A. Buckley, R. A. Rasmussen, A. Chalmers, T. Wang, P. L. Li, A. L. Williams, R. Hofmann-Lehmann, D. C. Montefiori, L. A. Cavacini, H. Katinger, G. Stiegler, D. C. Anderson, H. M. McClure, and R. M. Ruprecht. 2007. Time dependence of protective post-exposure prophylaxis with human monoclonal antibodies against pathogenic SHIV challenge in newborn macaques. Virology 35869-78. [DOI] [PubMed] [Google Scholar]
- 19.Ferrantelli, F., R. Hofmann-Lehmann, R. A. Rasmussen, T. Wang, W. Xu, P. L. Li, D. C. Montefiori, L. A. Cavacini, H. Katinger, G. Stiegler, D. C. Anderson, H. M. McClure, and R. M. Ruprecht. 2003. Post-exposure prophylaxis with human monoclonal antibodies prevented SHIV89.6P infection or disease in neonatal macaques. AIDS 17301-309. [DOI] [PubMed] [Google Scholar]
- 20.Ferrantelli, F., R. A. Rasmussen, K. A. Buckley, P. L. Li, T. Wang, D. C. Montefiori, H. Katinger, G. Stiegler, D. C. Anderson, H. M. McClure, and R. M. Ruprecht. 2004. Complete protection of neonatal rhesus macaques against oral exposure to pathogenic simian-human immunodeficiency virus by human anti-HIV monoclonal antibodies. J. Infect. Dis. 1892167-2173. [DOI] [PubMed] [Google Scholar]
- 21.Gairin, J. E., H. Mazarguil, D. Hudrisier, and M. B. Oldstone. 1995. Optimal lymphocytic choriomeningitis virus sequences restricted by H-2Db major histocompatibility complex class I molecules and presented to cytotoxic T lymphocytes. J. Virol. 692297-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galun, E., R. Eren, R. Safadi, Y. Ashour, N. Terrault, E. B. Keeffe, E. Matot, S. Mizrachi, D. Terkieltaub, M. Zohar, I. Lubin, J. Gopher, D. Shouval, and S. Dagan. 2002. Clinical evaluation (phase I) of a combination of two human monoclonal antibodies to HBV: safety and antiviral properties. Hepatology 35673-679. [DOI] [PubMed] [Google Scholar]
- 23.Galun, E., N. A. Terrault, R. Eren, A. Zauberman, O. Nussbaum, D. Terkieltaub, M. Zohar, R. Buchnik, Z. Ackerman, R. Safadi, Y. Ashur, S. Misrachi, Y. Liberman, L. Rivkin, and S. Dagan. 2007. Clinical evaluation (phase I) of a human monoclonal antibody against hepatitis C virus: safety and antiviral activity. J. Hepatol. 4637-44. [DOI] [PubMed] [Google Scholar]
- 24.Gros, L., H. Dreja, A. L. Fiser, M. Plays, M. Pelegrin, and M. Piechaczyk. 2005. Induction of long-term protective antiviral endogenous immune response by short neutralizing monoclonal antibody treatment. J. Virol. 796272-6280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gros, L., M. Pelegrin, M. Plays, and M. Piechaczyk. 2006. Efficient mother-to-child transfer of antiretroviral immunity in the context of preclinical monoclonal antibody-based immunotherapy. J. Virol. 8010191-10200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haigwood, N. L., D. C. Montefiori, W. F. Sutton, J. McClure, A. J. Watson, G. Voss, V. M. Hirsch, B. A. Richardson, N. L. Letvin, S. L. Hu, and P. R. Johnson. 2004. Passive immunotherapy in simian immunodeficiency virus-infected macaques accelerates the development of neutralizing antibodies. J. Virol. 785983-5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hasenkrug, K. J., D. M. Brooks, and B. Chesebro. 1995. Passive immunotherapy for retroviral disease: influence of major histocompatibility complex type and T-cell responsiveness. Proc. Natl. Acad. Sci. USA 9210492-10495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasenkrug, K. J., D. M. Brooks, and U. Dittmer. 1998. Critical role for CD4+ T cells in controlling retrovirus replication and spread in persistently infected mice. J. Virol. 726559-6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasenkrug, K. J., and U. Dittmer. 2007. Immune control and prevention of chronic Friend retrovirus infection. Front. Biosci. 121544-1551. [DOI] [PubMed] [Google Scholar]
- 30.Hasenkrug, K. J., and U. Dittmer. 2000. The role of CD4 and CD8 T cells in recovery and protection from retroviral infection: lessons from the Friend virus model. Virology 272244-249. [DOI] [PubMed] [Google Scholar]
- 31.Hofmann-Lehmann, R., J. Vlasak, R. A. Rasmussen, S. Jiang, P. L. Li, T. W. Baba, D. C. Montefiori, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, H. Katinger, G. Stiegler, L. A. Cavacini, M. R. Posner, and R. M. Ruprecht. 2002. Postnatal pre- and postexposure passive immunization strategies: protection of neonatal macaques against oral simian-human immunodeficiency virus challenge. J. Med. Primatol. 31109-119. [DOI] [PubMed] [Google Scholar]
- 32.Hofmann-Lehmann, R., J. Vlasak, R. A. Rasmussen, B. A. Smith, T. W. Baba, V. Liska, F. Ferrantelli, D. C. Montefiori, H. M. McClure, D. C. Anderson, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, H. Katinger, G. Stiegler, L. A. Cavacini, M. R. Posner, T. C. Chou, J. Andersen, and R. M. Ruprecht. 2001. Postnatal passive immunization of neonatal macaques with a triple combination of human monoclonal antibodies against oral simian-human immunodeficiency virus challenge. J. Virol. 757470-7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hong, H. J., C. J. Ryu, H. Hur, S. Kim, H. K. Oh, M. S. Oh, and S. Y. Park. 2004. In vivo neutralization of hepatitis B virus infection by an anti-preS1 humanized antibody in chimpanzees. Virology 318134-141. [DOI] [PubMed] [Google Scholar]
- 34.Khan, N., N. Shariff, M. Cobbold, R. Bruton, J. A. Ainsworth, A. J. Sinclair, L. Nayak, and P. A. Moss. 2002. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J. Immunol. 1691984-1992. [DOI] [PubMed] [Google Scholar]
- 35.Lynch, W. P., S. Czub, F. J. McAtee, S. F. Hayes, and J. L. Portis. 1991. Murine retrovirus-induced spongiform encephalopathy: productive infection of microglia and cerebellar neurons in accelerated CNS disease. Neuron 7365-379. [DOI] [PubMed] [Google Scholar]
- 36.Lynch, W. P., S. J. Robertson, and J. L. Portis. 1995. Induction of focal spongiform neurodegeneration in developmentally resistant mice by implantation of murine retrovirus-infected microglia. J. Virol. 691408-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McAtee, F. J., and J. L. Portis. 1985. Monoclonal antibodies specific for wild mouse neurotropic retrovirus: detection of comparable levels of virus replication in mouse strains susceptible and resistant to paralytic disease. J. Virol. 561018-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morrey, J. D., V. Siddharthan, A. L. Olsen, G. Y. Roper, H. Wang, T. J. Baldwin, S. Koenig, S. Johnson, J. L. Nordstrom, and M. S. Diamond. 2006. Humanized monoclonal antibody against West Nile virus envelope protein administered after neuronal infection protects against lethal encephalitis in hamsters. J. Infect. Dis. 1941300-1308. [DOI] [PubMed] [Google Scholar]
- 39.Nakowitsch, S., H. Quendler, H. Fekete, R. Kunert, H. Katinger, and G. Stiegler. 2005. HIV-1 mutants escaping neutralization by the human antibodies 2F5, 2G12, and 4E10: in vitro experiments versus clinical studies. AIDS 191957-1966. [DOI] [PubMed] [Google Scholar]
- 40.Neumann-Haefelin, C., H. E. Blum, F. V. Chisari, and R. Thimme. 2005. T cell response in hepatitis C virus infection. J. Clin. Virol. 3275-85. [DOI] [PubMed] [Google Scholar]
- 41.Oliphant, T., M. Engle, G. E. Nybakken, C. Doane, S. Johnson, L. Huang, S. Gorlatov, E. Mehlhop, A. Marri, K. M. Chung, G. D. Ebel, L. D. Kramer, D. H. Fremont, and M. S. Diamond. 2005. Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nat. Med. 11522-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pantaleo, G., and R. A. Koup. 2004. Correlates of immune protection in HIV-1 infection: what we know, what we don't know, what we should know. Nat. Med. 10806-810. [DOI] [PubMed] [Google Scholar]
- 43.Parren, P. W., P. A. Marx, A. J. Hessell, A. Luckay, J. Harouse, C. Cheng-Mayer, J. P. Moore, and D. R. Burton. 2001. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 758340-8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pelegrin, M., M. Marin, A. Oates, D. Noel, R. Saller, B. Salmons, and M. Piechaczyk. 2000. Immunotherapy of a viral disease by in vivo production of therapeutic monoclonal antibodies. Hum. Gene Ther. 111407-1415. [DOI] [PubMed] [Google Scholar]
- 45.Pincus, S. H., R. Cole, R. Ireland, F. McAtee, R. Fujisawa, and J. Portis. 1995. Protective efficacy of nonneutralizing monoclonal antibodies in acute infection with murine leukemia virus. J. Virol. 697152-7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poignard, P., R. Sabbe, G. R. Picchio, M. Wang, R. J. Gulizia, H. Katinger, P. W. Parren, D. E. Mosier, and D. R. Burton. 1999. Neutralizing antibodies have limited effects on the control of established HIV-1 infection in vivo. Immunity 10431-438. [DOI] [PubMed] [Google Scholar]
- 47.Portis, J. L., S. Czub, C. F. Garon, and F. J. McAtee. 1990. Neurodegenerative disease induced by the wild mouse ecotropic retrovirus is markedly accelerated by long terminal repeat and gag-pol sequences from nondefective Friend murine leukemia virus. J. Virol. 641648-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reichert, J. M. 2007. Trends in the development and approval of monoclonal antibodies for viral infections. BioDrugs 211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schiano, T. D., M. Charlton, Z. Younossi, E. Galun, T. Pruett, R. Tur-Kaspa, R. Eren, S. Dagan, N. Graham, P. V. Williams, and J. Andrews. 2006. Monoclonal antibody HCV-AbXTL68 in patients undergoing liver transplantation for HCV: results of a phase 2 randomized study. Liver Transplant. 121381-1389. [DOI] [PubMed] [Google Scholar]
- 50.Simmons, C. P., N. L. Bernasconi, A. L. Suguitan, K. Mills, J. M. Ward, N. V. Chau, T. T. Hien, F. Sallusto, Q. Ha Do, J. Farrar, M. D. de Jong, A. Lanzavecchia, and K. Subbarao. 2007. Prophylactic and therapeutic efficacy of human monoclonal antibodies against H5N1 influenza. PLoS Med. 4e178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sitbon, M., J. Nishio, K. Wehrly, D. Lodmell, and B. Chesebro. 1985. Use of a focal immunofluorescence assay on live cells for quantitation of retroviruses: distinction of host range classes in virus mixtures and biological cloning of dual-tropic murine leukemia viruses. Virology 141110-118. [DOI] [PubMed] [Google Scholar]
- 52.Sitbon, M., B. Sola, L. Evans, J. Nishio, S. F. Hayes, K. Nathanson, C. F. Garon, and B. Chesebro. 1986. Hemolytic anemia and erythroleukemia, two distinct pathogenic effects of Friend MuLV: mapping of the effects to different regions of the viral genome. Cell 47851-859. [DOI] [PubMed] [Google Scholar]
- 53.Takada, A., H. Ebihara, S. Jones, H. Feldmann, and Y. Kawaoka. 2007. Protective efficacy of neutralizing antibodies against Ebola virus infection. Vaccine 25993-999. [DOI] [PubMed] [Google Scholar]
- 54.Trkola, A., H. Kuster, P. Rusert, B. Joos, M. Fischer, C. Leemann, A. Manrique, M. Huber, M. Rehr, A. Oxenius, R. Weber, G. Stiegler, B. Vcelar, H. Katinger, L. Aceto, and H. F. Gunthard. 2005. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat. Med. 11615-622. [DOI] [PubMed] [Google Scholar]
- 55.Tsujimura, K., Y. Obata, Y. Matsudaira, K. Nishida, Y. Akatsuka, Y. Ito, A. Demachi-Okamura, K. Kuzushima, and T. Takahashi. 2006. Characterization of murine CD160+ CD8+ T lymphocytes. Immunol. Lett. 10648-56. [DOI] [PubMed] [Google Scholar]
- 56.Veazey, R. S., R. J. Shattock, M. Pope, J. C. Kirijan, J. Jones, Q. Hu, T. Ketas, P. A. Marx, P. J. Klasse, D. R. Burton, and J. P. Moore. 2003. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat. Med. 9343-346. [DOI] [PubMed] [Google Scholar]
- 57.Vokurkova, D., J. Sinkora, J. Vavrova, M. Rezacova, J. Knizek, and J. Ostereicher. 2006. CD8+ natural killer cells have a potential of a sensitive and reliable biodosimetric marker in vitro. Physiol. Res. 55689-698. [DOI] [PubMed] [Google Scholar]
- 58.Vremec, D., M. Zorbas, R. Scollay, D. J. Saunders, C. F. Ardavin, L. Wu, and K. Shortman. 1992. The surface phenotype of dendritic cells purified from mouse thymus and spleen: investigation of the CD8 expression by a subpopulation of dendritic cells. J. Exp. Med. 17647-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu, L., D. Vremec, C. Ardavin, K. Winkel, G. Suss, H. Georgiou, E. Maraskovsky, W. Cook, and K. Shortman. 1995. Mouse thymus dendritic cells: kinetics of development and changes in surface markers during maturation. Eur. J. Immunol. 25418-425. [DOI] [PubMed] [Google Scholar]