Abstract
Productive infection of oligodendrocytes, which are responsible for the formation of myelin sheath in the central nervous system, with the human neurotropic virus JC virus (JCV) causes the fatal demyelinating disease progressive multifocal leukoencephalopathy (PML). In addition to encoding T antigen and the capsid proteins, which are produced at the early and late phases of the infection cycle, respectively, JCV encodes a small regulatory protein named agnoprotein that is important for successful completion of the virus life cycle. Here we used bipotential CG-4 cells to examine the impact of agnoprotein on oligodendrocyte differentiation and survival in the absence of JCV lytic infection. We demonstrate that the expression of agnoprotein delayed the formation of complex outgrowth networks of the cells during oligodendrocyte differentiation. These alterations were accompanied by high levels of DNA damage, induction of proapoptotic proteins, and suppression of prosurvival signaling. Accordingly, apoptosis was significantly increased upon the induction of CG-4 cells toward differentiation in cells expressing agnoprotein. These observations provide the first evidence for the possible involvement of agnoprotein, independent from its role in viral replication, in a series of biological events that may contribute to the pathological features seen in PML lesions.
First exposure to the human polyomavirus JC virus (JCV) commonly occurs during childhood and results in a subclinical outcome. Reactivation of the initially latent virus in individuals with impaired immune systems results in the development of the fatal demyelinating disease progressive multifocal leukoencephalopathy (PML) (9, 31, 35). PML manifests clinically with signs of neurological deficits of various degrees, including weakness, visual deficits, and cognitive abnormalities. PML is mainly associated with advanced human immunodeficiency virus (HIV)-induced diseases and AIDS (8, 9), lymphoproliferative or myeloproliferative diseases, malignancies, and rheumatic disorders treated with corticosteroids and cytotoxic drugs (1, 16, 25, 34, 39, 52, 58, 71, 75, 77). Recently, PML has also been diagnosed in patients with multiple sclerosis and Crohn's disease receiving natalizumab (37, 40, 42, 74), a humanized monoclonal antibody that binds to the α4 subunit of α4β1 and α4β7 integrins and inhibits the trafficking of leukocytes across the blood-brain barrier.
The histological hallmarks of white matter from PML patients include the appearance of oligodendrocytes with enlarged hyperchromatic nuclei that contain inclusion bodies shaped by crystalline arrays of JCV particles and astrocytes with bizarre nuclei and mitotic figures (21, 35). The presence of virions has also been demonstrated among lamellae of the myelin sheath of a viable axon (47). In cell culture systems, JCV efficiently infects primary cultures of human brain containing oligodendrocytes and astrocytes. Recent studies have shown that JCV can infect multipotential human central nervous system progenitor cells isolated from human fetal brain tissue (32, 50). It is believed that the demyelination seen in brains of PML patients is a result of the destruction of oligodendroglia due to the reactivation of JCV and its productive replication in these cells (46, 79). As reactivation of the virus occurs mainly in immunocompromised individuals, it is believed that the immune system modulates the state of the latent virus in humans. In support of this notion, previous studies revealed the ability of immune cells to suppress the replication of JCV in a cell culture system derived from human brain (15). Moreover, it remains to be seen whether viral proteins in the absence of the infection cycle can contribute to the pathological features seen in PML brain.
The JCV genome is comprised of a double-stranded circular DNA encoding a family of regulatory proteins, T antigen, and its various splice variants, which are expressed at the early phase of infection prior to DNA replication. The genome also encodes the capsid proteins VP1, VP2, and VP3, which are produced at the late phase of infection, i.e., after DNA replication has been initiated. In addition, the late coding region of JCV expresses a small regulatory protein called agnoprotein encoded in the 5′ leader region (for a review, see reference 36). In PML samples, agnoprotein has been detected in the JCV-infected oligodendrocytes and astrocytes in the area of demyelinated lesions and adjacent myelinated fields. Agnoprotein has been shown to be important for the regulation of viral gene expression and replication. Previous studies demonstrated that the ectopic expression of JCV agnoprotein has an impact on several cellular processes, including cell cycle progression (18, 19). Agnoprotein also sensitizes cells to the cytotoxic effects of the DNA-damaging agent cisplatin, inhibits DNA repair, and disrupts DNA damage-induced cell cycle arrest (19).
Here we utilized bipotential CG-4 progenitor cells and demonstrated that the expression of JCV agnoprotein in these cells impairs the development of mature oligodendrocytes and interferes with the survival of myelin-forming cells. Agnoprotein induces proapoptotic and inhibits prosurvival pathways in cells undergoing oligodendrocytic differentiation. This observation provides important information about mechanisms of destruction of oligodendrocytes in the absence of JCV lytic infection.
MATERIALS AND METHODS
Plasmids and adenovirus vectors.
pLEGFP-C1 agnoprotein was created using a BamH1-Apa1 DNA fragment containing the agnoprotein gene from pcDNA3-Agno (61) and was cloned into the pLEGFPC1 plasmid (Clontech, Palo Alto, CA) after cleavage with BglII and ApaI restriction enzymes. For mutant agnoprotein pLEGFP-C1 agnoprotein*, site-directed mutagenesis was performed using primers (forward, 5′ CGCCAGCTGGCACGTAAGGCTGCTGTGAAAGTTGTTAAAACCTGG 3′; reverse, 5′ CCAGGTTTTAACAACTTTCACAGCAGCCTTACGTGCCAGCTGGCG 3′ [mutated nucleotides underlined]) containing mutations that change amino acids Ser 7 and Ser 11 to Ala and Ser 15 to Val. For the construction of adenoviruses, the genes of interest were first cloned into the adenovirus shuttle plasmid pDC515(IO) (obtained from Microbix, Canada), which contains the murine cytomegalovirus promoter. Adenovirus expressing JCV agnoprotein (Ad-Agno) was generated by PCR amplification of the agnoprotein gene from pcDNA3-Agno (61) using specific primers (forward, 5′-GCTGGCTAGCATGGTTCTTCGCCAGCTGTC-3′ [NheI underlined], and reverse 5′-CTAAGATCTCTATGTAGCTTTTGGTTCAGGCAA-3′ [BglII underlined]) followed by enzymatic digestion of the PCR product with NheI and BglII and subcloning of the resulting fragment into NheI- and BglII-digested adenovirus shuttle plasmid pDC515(IO). After this initial cloning step, the recombinant plasmid clones were cotransfected with the plasmid pBHGfrtΔ E1E3FLP, which provides an adenovirus type 5 genomic backbone deleted in E1 and E3 genes. A site-specific (frt/FLP) recombination results in the generation of recombinant adenovirus. Since the virus is deleted in E1, it can replicate only in HEK 293IQ cells that are capable of providing the missing E1 function in trans. All plasmid constructs were verified by sequence analysis. Adenoviruses generated in this fashion were cloned by limiting dilution and plaque isolation using agarose overlay. Virus was then grown and purified by cesium chloride density gradient centrifugation. Ad-null was generated in a similar fashion using empty shuttle plasmid with no transgene.
Cell culture. (i) CG-4 cells.
The CG-4 (central glia-4) cell line was maintained as described previously (44). Briefly, these cells were propagated on poly-l-ornithine (Sigma, St. Louis, MO)-coated plates in 70% Dulbecco's minimal essential medium (DMEM) containing 2 mM glutamine, N1 supplement (50 μg/ml transferrin, 5 μg/ml insulin, 100 μM putrescine, 20 nM progesterone, and 30 nM selenium), 10 ng/ml biotin, and 30% conditioned medium from the B104 neuroblastoma cell line. Cells were induced to differentiate into oligodendrocytes by withdrawal of mitogens (without the addition of B104-conditioned medium) under serum-free conditions in the presence of 2 mM glutamine, N1 supplement, and insulin for up to 6 days. Withdrawal of the growth factors in the B104-conditioned medium results in the cessation of cell division and the initiation of cell differentiation.
(ii) B104 neuroblastoma cells.
B104 neuroblastoma cells (Interlab cell line, Genoa, Italy) were grown in DMEM containing 10% (vol/vol) heat-inactivated fetal calf serum (Gibco) and 2 mM l-glutamine until 70% confluent and then conditioned with modified Sato medium (see above) for 3 days. This conditioned medium was aspirated, filtered, and frozen for use in the 30% (vol/vol) B104-conditioned medium for maintaining CG-4 cells in the proliferative phase (44). Cells were passaged every 2 to 3 days and harvested using trypsin.
(iii) Stable cell lines.
Stable cell lines were produced by the retroviral transduction method described previously (10). Briefly, the Phoenix retroviral packaging cell line (Orbigen, San Diego, CA) was transfected with pLEGFP-C1 agnoprotein, pLEGFP-C1 agnoprotein*, or pLEGFP-C1 plasmids by use of the calcium phosphate precipitation method as we have previously described (19). Conditioned medium containing virus particles was collected 48 h posttransfection and used to infect CG-4 progenitor cells in the presence of 10 μg/ml Polybrene (Millipore, Billerica, MA). Twenty-four hours posttransduction, cells were subcultured and selected with 700 μg/ml G418 (Gibco/BRL, Invitrogen, Carlsbad, CA). Expression of the transgene was verified by Western blot analysis using anti-agnoprotein antibody or Living Colors full-length polyclonal antibody (BD Biosciences, Clontech), which recognizes enhanced green fluorescent protein (EGFP).
Preparation of protein extracts and immunoblot analysis.
For the preparation of whole-cell extract, cells were lysed for 30 min on ice in LB1 buffer (50 mM HEPES, pH 7.5, 150 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 10% glycerol, 1% Triton X-100) containing 1 μg/ml leupeptin, 1 μg/ml aprotinin, 1 mM phenylmethylsulfonyl fluoride, and 0.2 mM Na-orthovanadate. Cell debris was removed by centrifugation at 14,000 rpm for 15 min at 4°C. The supernatant was assayed for protein content by Bradford analysis (Bio-Rad) and was either used immediately or stored at −80°C. For immunoblots, 50-μg portions of total cell protein were run on sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to a nitrocellulose membrane, and immunoblotted with antibody. Bound antibody was detected using the ECL enhanced chemiluminescence detection kit (Amersham, Arlington Heights, IL) according to the manufacturer's recommendations.
DNA preparation.
Cells were harvested, washed with phosphate-buffered saline (PBS), and resuspended in 700 μl of digestion buffer (50 mM Tris-HCl, pH 8.0, 100 mM EDTA, 100 mM NaCl, 0.5 to 1.0% sodium dodecyl sulfate, 0.5 mg/ml Proteinase K) for 10 min. After incubation at 55°C overnight, 20 μl of 10 mg/ml RNase A (DNase free) was added to the sample, and this mixture was kept at 37°C for 1 to 2 h. The DNA obtained with phenol-chloroform extractions (two times) was resuspended in Tris-EDTA. Electrophoresis was performed with 10 to 15 μg of DNA/sample in a 1.5% agarose gel.
Comet assay.
Comet nucleus formation in progenitor CG-4 cells was analyzed by alkaline comet assay using adenovirus-infected CG-4 progenitor cells (multiplicity of infection, 10) or CG-4 cells constitutively expressing agnoprotein. From two experimental sets, one set was induced to differentiate into oligodendrocytes by the withdrawal of mitogens after infection/transduction, and the second set was left as an uninduced control. Single-cell gel electrophoresis was performed at the third day following induction. The comet assay was performed under alkaline conditions (67). In experiments with H2O2, cells were harvested and half of the cells from each sample were treated with 15 μM H2O2 for 10 min at 37°C. About 105 cells in 250 μl of PBS were mixed with 750 μl of 1.33% low-melting-point agarose, type VII, in PBS (A-4018; Sigma). One hundred microliters of cell suspension was spread on a frosted microscope slide that had been precoated with 1% N-agarose, type I-A in H2O (A-0169; Sigma). Slides were placed in cold lysis solution (2.5 M sodium chloride, 100 mM EDTA, 10 mM Tris [pH 10], and 1% Triton X-100, added freshly before use) for 1 h at 4°C. Slides were incubated for 40 min in alkaline unwinding buffer (300 mM NaOH and 1 mM EDTA, pH >13) in the dark at 4°C. Electrophoresis was conducted under the same conditions for 30 min at 25 V (0.72 V/cm) and 300 mA. Slides were washed with distilled H2O three times for 5 min each, air dried, and stained for analysis with propidium iodide (PI) (2.5 μg/ml) in sodium citrate (pH 8.2) and covered with coverslips. Images of at least 100 cells per sample (50 cells/slide) were evaluated using a fluorescence microscope and Comet 5.0 image analysis software (Kinetic Imaging, Liverpool, United Kingdom). Necrotic and apoptotic cells, identified by their microscopic appearance (comets with no heads and nearly all DNA in the tail), were excluded from the analysis (69). The mean of the olive tail moment (OTM) (the product of the tail length and the fraction of the total DNA in the tail) was calculated as a measure of DNA damage (56).
MTT assay.
For the methylthiazoletetrazolium (MTT) assay, we used a cell proliferation kit (MTT) according to the manufacturer's protocol (Roche). Cells were plated onto poly-l-ornithine-coated 96-well plates in triplicate in two sets at a density of 15,000 cells/well. One set from each cell line was induced to differentiate into oligodendrocytes and the second set was maintained in a proliferative state. After 3 days, 10 μl MTT (5 mg/ml) was added to the wells (final concentration, 0.5 mg/ml) for 4 h, and the reaction was stopped by the addition of 100 μl of solubilization solution. Viable cells with active mitochondria cleave the tetrazolium ring into a visible dark blue formazan reaction product, which was quantified by spectrophotometry in a microplate reader at 570 nm with a reference wavelength of 650 nm. The relative cell viability (percent) for each cell line was determined as the ratio of average absorbance for induced cells to that for uninduced.
Flow cytometric analysis.
Cells were harvested, rinsed with PBS, and fixed in suspension in 73% ethanol in PBS for at least 16 to 20 h at −20°C. After incubation for 24 h at −20°C, the cells were washed with PBS containing 1% bovine serum albumin, stained with PI (10 μg/ml) in PBS containing 250 μg of RNase A/ml, and incubated at 37°C for 30 min in the dark before analysis by fluorescence-activated cell sorting. Cell cycle distribution was analyzed with the GuavaEasy Cyte mini system and using the Guava CytoSoft cell cycle program according to the manufacturer's instructions (Guava Technologies, Hayward, CA). The DNA content determination was based on the intensity of the PI fluorescence.
Caspase-3 assay.
CG-4 cells expressing GFP or GFP-agnoprotein were seeded into 24-well plates in sets of triplicates (50,000 cells/well) and either were uninduced or were induced to differentiate. After 4 days, cells were harvested and processed for the measurement of caspase activity using the Caspase-Glo 3/7 assay kit according to the manufacturer's instructions (Promega, Madison, WI).
JCV infection.
SVGA cells were infected with the JCV Mad-1 strain as we have previously described (20). After infection, cells were fed with DMEM with 2% FBS and maintained at 37°C in a humidified atmosphere with 7% CO2 and processed for protein extract preparation.
Antibodies.
Rabbit polyclonal antibody against JCV agnoprotein was previously described (22). Anti-α-tubulin, clone B512, was obtained from Sigma-Aldrich. Anti-phospho-glycogen synthase kinase 3β (GSK3β) (Ser9) antibody, rabbit polyclonal (#9336), anti-phospho-p44/42 mitogen-activated protein kinase (MAPK) (Thr202/Tyr204) (#4376), anti-p44/42 MAPK, rabbit polyclonal, anti-Bim rabbit polyclonal (#4582), and anti-GRB2 rabbit polyclonal (#3972) were purchased from Cell Signaling (Danvers, MA). Anti-survivin (D-8), mouse monoclonal (sc-17779); anti-p38(C-20), rabbit polyclonal (sc-535); anti-GSK3 β (H-76) rabbit polyclonal (sc-9166); and anti-caspase-3 rabbit polyclonal (sc-7148) were obtained from Santa Cruz (Santa Cruz Biotechnology, Inc., Santa Cruz, CA).
RESULTS
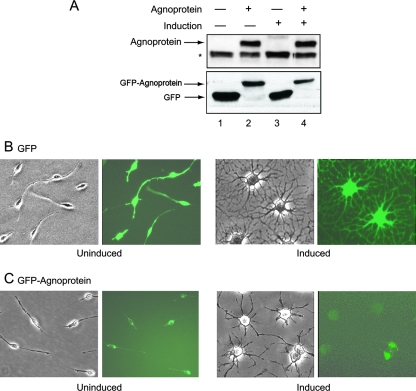
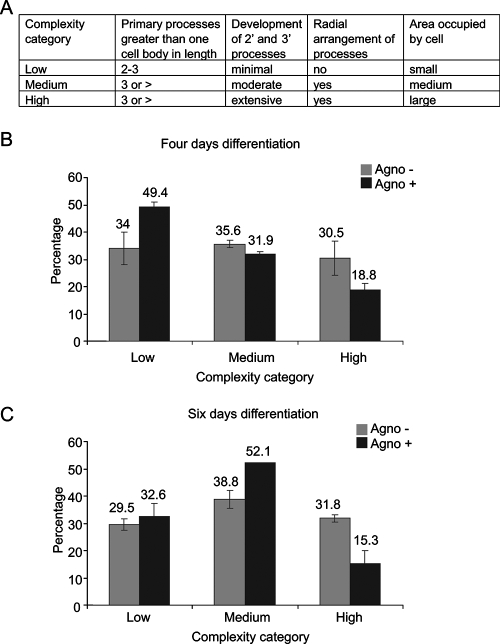
CG-4 is a bipotential cell line that is able to differentiate into either oligodendrocytes or astrocytes under appropriate cell growth conditions (44). These cells are maintained in the progenitor (bipotential) and proliferative state by culturing them in a serum-free medium containing B-104-conditioned medium. Upon mitogen withdrawal, CG-4 cells undergo morphological changes to acquire a multipolar phenotype and express markers of differentiated oligodendrocytes. To investigate the impact of JCV agnoprotein on the process leading to CG-4 cell differentiation toward an oligodendrocyte lineage, CG-4 progenitors were stably transduced with a retroviral vector expressing GFP-agnoprotein. The expression of GFP-agnoprotein in cells that were induced to differentiate into oligodendrocytes by withdrawal of mitogens and in uninduced control cells was determined by Western blot assay (Fig. 1A). Expression of the GFP-agnoprotein (35 kDa) was detected using antibody to agnoprotein (Fig. 1A, top) and using antibody to enhanced GFP (Fig. 1A, bottom), which also detected GFP (27 kDa) expression in the control cells. To examine the effect of agnoprotein on cell morphology, CG-4 cells expressing GFP or GFP-agnoprotein were seeded in duplicate plates at low density in which one set of cells was maintained in the proliferating progenitor state and the second set was induced to differentiate into oligodendrocytes. Similar to control cells expressing GFP, cells with GFP-agnoprotein were predominantly bipolar under proliferation conditions (Fig. 1B and C). Upon removal of the B-104-conditioned medium and replacement of it with differentiation medium, CG-4 cells lost their proliferative capacity and elaborated numerous processes, acquiring morphological characteristics of mature oligodendrocytes. Cell differentiation was evaluated by microscopic observation after 3 days. As shown in Fig. 1B, GFP-expressing CG-4 cells retained their ability to differentiate, as evidenced by extensive process elaboration following mitogen withdrawal. However, GFP-agnoprotein-producing cells demonstrated low morphological complexity upon the induction of differentiation compared to agnoprotein-negative cells, with less arborization of secondary and tertiary processes and a smaller area occupied by single cell (Fig. 1C). We utilized a previously described method (70) for the staging of oligodendrocyte differentiation based on morphological features (Fig. 2A) to further assess the stages of differentiated cells after agnoprotein expression. Individual cells were scored according to their morphological complexity in three categories on the basis of the length and number of primary processes, radial distribution of processes, the development of secondary and tertiary processes and arborization, and the area occupied by the cell, including the process arbor (Fig. 2A). Results from an analysis of 400 cells revealed that at 4 days after induction (Fig. 2B), 49.4% of agnoprotein-producing cells showed low complexity, whereas under similar conditions, only 34% of control cells showed low complexity. The ratio changes when agnoprotein-positive and control cells are evaluated for high complexity, as only 18% of agnoprotein-producing cells showed high complexity compared to 30.5% of the control cells. At the sixth day of differentiation (Fig. 2C), the cell distribution is shifted, with an increase in the number of cells of medium complexity seen for agnoprotein-producing cells reaching 52%. At this time, the number of cells of high complexity was about half in the presence of agnoprotein (15.3%) of that seen for control cells (31.8%). Interestingly, at the sixth day there is even a slight decrease in the high-complexity cell number in agnoprotein-producing cells, possibly because of a loss of differentiated cells.
FIG. 1.
Expression of agnoprotein in CG-4 cells. (A) (Top) Immunoblot analysis demonstrating the presence of GFP-agnoprotein in uninduced and induced CG-4 cells using an antibody raised against agnoprotein. The position of the GFP-agnoprotein band (35 kDa) is indicated by an arrow. The asterisk depicts a nonspecific band (30 kDa). (Bottom) Immunoblot analysis demonstrating the presence of GFP (27 kDa) and GFP-agnoprotein (35 kDa) in uninduced and induced CG-4 cells by use of an anti-GFP antibody. (B and C) Phase-contrast and fluorescent images of control CG-4 cells expressing GFP- and GFP-agnoprotein-producing cells under uninduced and induced conditions.
FIG. 2.
Morphological complexity seen in CG-4 cells expressing agnoprotein during differentiation. (A) Criteria that were used for evaluation as shown were developed earlier (70). (B and C) Morphological analysis of oligodendrocyte complexity during 4 and 6 days of CG-4 differentiation.
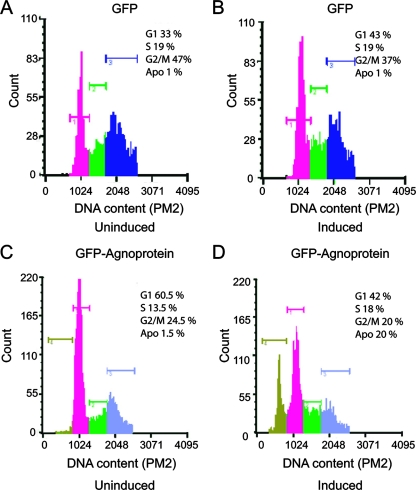
The expression of agnoprotein in NIH 3T3 cells dysregulates cell cycle progression, leading to an accumulation of cells at the G2/M phase, a decline in the activity of cyclins A and B, and the enhancement of p21WAF (18). Here, we performed cell cycle analysis of agnoprotein-producing CG-4 cells to determine whether or not a reduction in the number of mature oligodendrocytes expressing agnoprotein is due to a decrease in cell proliferation and/or an increase in cell death. As before, progenitor CG-4 cells producing agnoprotein and control agnoprotein-negative cells were plated in duplicate with one set maintained in proliferation medium while the second set was induced to differentiate into oligodendrocytes. At the fourth day of induction, cells were harvested and prepared for DNA content analysis by flow cytometry. For each sample, the percentages of cells that were in the G0/G1, S, and G2/M phases of the cell cycle, as well as the subdiploid apoptotic/necrotic cell fractions, were determined. As seen in Fig. 3A and B, the induction of control (agnoprotein-negative) cells slightly increased the accumulation of cells at G1 (from 33% to 43%) and decreased their levels in the G2/M phase (47% to 37%). No change in the number of apoptotic cells was observed. In agnoprotein-producing cells (Fig. 3C and D), the induction of cells toward a progenitor lineage decreased cell accumulation in G1 (60.5% to 42%) and G2/M (24.5% to 20%) and drastically enhanced the appearance of subdiploid cells (1.5% to 20%). These observations suggest that while agnoprotein can dysregulate cell cycle progression, its presence in CG-4 cells promotes signaling events leading to cell death upon its differentiation.
FIG. 3.
Cell cycle distribution of CG-4 cells expressing agnoprotein. Flow cytometric analysis depicting control cells expressing GFP and the agnoprotein-producing cells (GFP-agnoprotein positive) during various stages of the cell cycle. The peaks corresponding to each stage of the cell cycle and the subdiploid peaks defined as the apoptotic (Apo.) fractions were determined. Fluorescein isothiocyanate fluorescence was used to analyze only cells expressing GFP-agnoprotein or GFP.
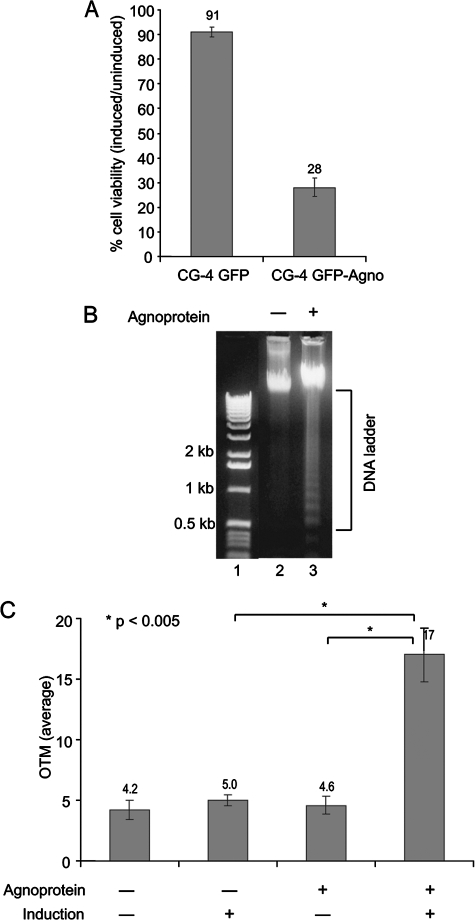
To further investigate cell survival, we employed the MTT assay (53). CG-4 cells expressing agnoprotein and control agno-negative cells were plated on 96-well plates at a density of 15,000 cells/well in triplicate in two sets. One set of each cell line was maintained in proliferation medium, and the second set was induced to differentiate into oligodendrocytes. Cell viability was evaluated at the third day of differentiation. The tetrazolium salt MTT is cleaved to form a formazan dye by mitochondrial reductase enzymes that are present only in viable cells and not in dead cells. The amount of formazan generated is directly proportional to the number of metabolically active cells. The spectrophotometrical absorbance of the undifferentiated and differentiated samples was measured using a microplate (enzyme-linked immunosorbent assay) reader. Relative cell viability (percent) for each sample was determined as the ratio of average absorbance for induced cells to that for uninduced. An assessment of the MTT assay demonstrated that reduction of oligodendrocyte viability is agnoprotein dependent, since when differentiated, only 28% of agnoprotein-producing cells were metabolically active compared to 91% of agnonegative cells (Fig. 4A). All results are based on at least three separate experiments.
FIG. 4.
Survival of CG-4 cells expressing agnoprotein. (A) CG-4 cells expressing JCV agnoprotein and control agnonegative cells were plated equally in triplicate in two sets. One set of each cell line was induced to differentiate into oligodendrocytes, and cell viability was evaluated by MTT assay. The percentage of induced cells relative to that for uninduced was determined for each cell line at the third day of induction. (B) Genomic DNA was prepared from agnopositive and agnonegative CG-4 cells induced to differentiate into oligodendrocytes for 6 days and analyzed on a 1.2% agarose gel. (C) Comet nucleus formation in progenitor CG-4 cells was analyzed by alkaline comet assay. Adenoviral vectors expressing JCV agnoprotein (Ad-Agno) and control adenovirus vector without a transgene (Ad-null) were used for transduction of CG-4 progenitor cells. From two experimental sets, one set was induced to differentiate into oligodendrocytes after infection/transduction and the second set was left as an uninduced control. Single-cell gel electrophoresis was performed at the third day following induction. The average OTM was scored randomly for 100 cells for each condition by use of Comet software.
Cell death by apoptosis is characterized by chromatin condensation and DNA fragmentation accompanied by plasma membrane blebbing and cell shrinkage (81). To ascertain that the observations described above resulted from apoptosis rather than necrosis, DNA samples from differentiated agnoprotein-producing and control cells were analyzed by gel electrophoresis (30). DNA cleavage into oligonucleosome fragments (laddering) was demonstrated in the presence of agnoprotein but not in control cells (Fig. 4B), suggesting that the process of maturation of oligodendrocytes in the presence of agnoprotein is associated with cellular DNA fragmentation, a feature that is characteristic of apoptotic cells.
To further examine DNA fragmentation in these cells, we performed the comet assay (67), in which single-cell gel electrophoresis is used to measure DNA damage. Cells embedded in agarose on a microscope slide are lysed and subjected to electrophoresis, after which intact DNA is revealed as a compact “comet” head and broken DNA as a tail. In the first experiment, we used adenoviral vector expressing JCV agnoprotein (Ad-Agno) and control adenovirus without a transgene (Ad-null) to transduce CG-4 progenitor cells. From two experimental sets, one set was induced to differentiate into oligodendrocytes after transduction and the second set remained in an uninduced condition. At the third day of differentiation, cells were harvested and analyzed by comet assay as described in the Materials and Methods. Images of at least 100 cells per sample (50 cells per slide) were evaluated using a fluorescence microscope. Necrotic and apoptotic cells (comets with no heads and nearly all DNA in the tail) were excluded from analysis (69). The mean of the OTM, representing the product of the tail length and the fraction of the total DNA in the tail and reflecting DNA damage (56), was significantly higher in agnoprotein-producing cells (Fig. 4C). These results suggest that the expression of agnoprotein induces DNA breakage in CG-4 cells upon differentiation toward oligodendrocytes.
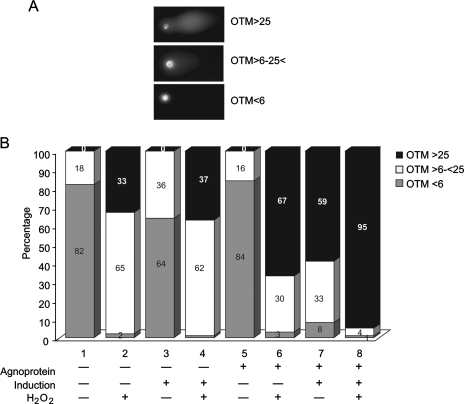
It has been reported that developing oligodendrocytes are vulnerable to oxidant-mediated injury (3). Reactive oxygen species (ROS), such as superoxide (O2•−) and hydrogen peroxide (H2O2), are produced intracellularly as part of normal metabolic reactions (76). In light of this information, we sought to determine whether CG-4 progenitors induced to differentiate into oligodendrocytes show more vulnerability to ROS in the presence of agnoprotein. Agnoprotein-positive and -negative CG-4 cells were plated in two sets. One set was maintained in proliferating conditions while the second set was induced to differentiate into oligodendrocytes. At the fourth day of induction, cells were harvested and half of the cells from each sample were treated with 15 μM H2O2 prior to being placed on agarose slides followed by comet assay. The cell images were arbitrarily classified into three groups according to the OTM values, reflecting the extent of DNA damage as follows: (i) little or no DNA damage (OTM of <6), (ii) moderate DNA damage (OTM of 6 to 25), and (iii) severe DNA damage (OTM of >25) (Fig. 5A). As seen in Fig. 5B, no significant difference was observed for the agnopositive and agnonegative cell lines in the progenitor state (compare lanes 1 and 5). Similar to previous experiments, the induction of progenitors to differentiate into oligodendrocytes caused an increase in the number of comets with high OTM (>25) in the presence of agnoprotein (59%) compared to the OTM in the absence of agnoprotein (0%). Treatment of progenitors with H2O2 caused an increase in DNA breakage in both cell lines, although this effect was more prominent in agnopositive cells, 67% of which had an OTM of >25 compared to 33% in agnonegative cells. Treatment of differentiating cells with H2O2 resulted in massive damage of cells in the presence of agnoprotein; more than 95% of the cells had extensive DNA damage compared to 37% of agnonegative cells. These results suggest that agnoprotein increases the vulnerability of differentiating cells to an exogenous source of ROS, H2O2.
FIG. 5.
Expression of agnoprotein sensitizes CG-4 progenitors differentiating into oligodendrocytes to H2O2. CG-4 progenitors, expressing GFP-agnoprotein and agnonegative (GFP only), were plated in two sets, and one of them was induced to differentiate into an oligodendrocyte lineage. At the fourth day of induction, cells were harvested and half of the cells from each sample was treated with H2O2. (A) The comet assay was performed, and images were classified in three groups according to the extent of DNA damage as follows: OTM of <6, no damage; OTM of 6 to 25, moderate damage; OTM of >25, extensive damage. (B) The percentages of cells with different levels of DNA damage for each sample are presented in bars.
The activation of caspase-3 is considered to be the final executive step in the process of apoptosis. We investigated the level of caspase-3 cleavage during the differentiation of CG-4 progenitor cells. Agnoprotein-producing and control CG-4 cells were plated in two sets. One set was maintained in the proliferating state while the second set was induced to differentiate. Cell lysates were prepared at the third and sixth days following induction. The activation of the effector caspase-3 was assessed using an antibody that recognizes both full-length inactive uncleaved procaspase-3 (35 kDa) and the activated (cleaved) form of the enzyme (17 kDa). A significant decrease in the level of uncleaved procaspase-3 and an increase in the level of cleaved caspase-3 were detected in differentiated CG-4 cells in the presence of agnoprotein at the sixth day of induction (Fig. 6A, top, lane 6). Equal loading of protein was verified by Western blotting with antibody to α-tubulin (Fig. 6, bottom). In another experiment to measure caspase activation, we employed a luminescent enzyme assay based on a proluminescent substrate containing the tetrapeptide DEVD, which is a substrate for caspases 3 and 7. As shown in Fig. 6B, caspase activity was low in cells that were not induced to differentiate and was about the same in cells expressing GFP or GFP-agnoprotein (compare lanes 1 and 3). After induction, the caspase activity in cells expressing GFP or GFP-agnoprotein was induced around 2.5- or 4.5-fold, respectively.
FIG. 6.
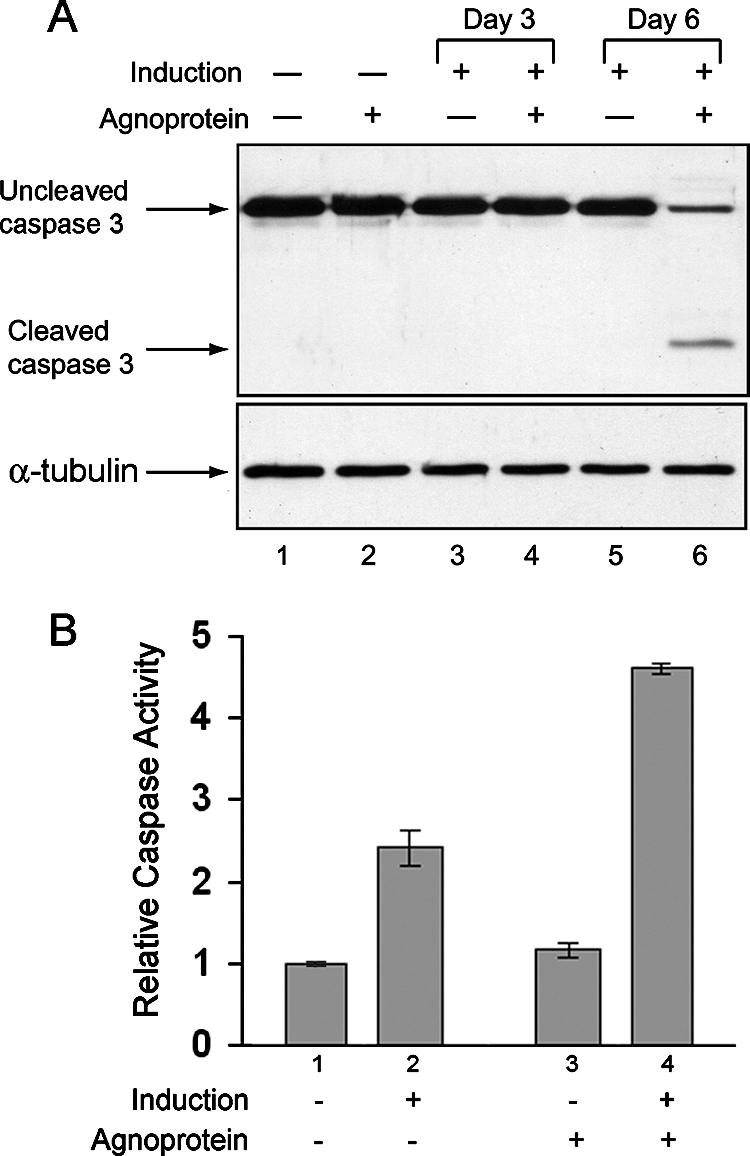
Detection of cleaved caspase-3 in agnopositive cells upon induction as oligodendrocytes. (A) Cell lysates prepared from agnopositive (GFP-agnoprotein) and agnonegative (GFP only) cells at the third and sixth days following the induction of differentiation were subjected to immunoblot analysis for the detection of uncleaved and cleaved caspase-3. Equal loading was verified with anti-α-tubulin antibody. (B) Caspase-3/7 activity was measured for cell lysates prepared from agnopositive (GFP-agnoprotein) and agnonegative (GFP-only) cells at the fourth and sixth days following the induction of differentiation and for uninduced controls.
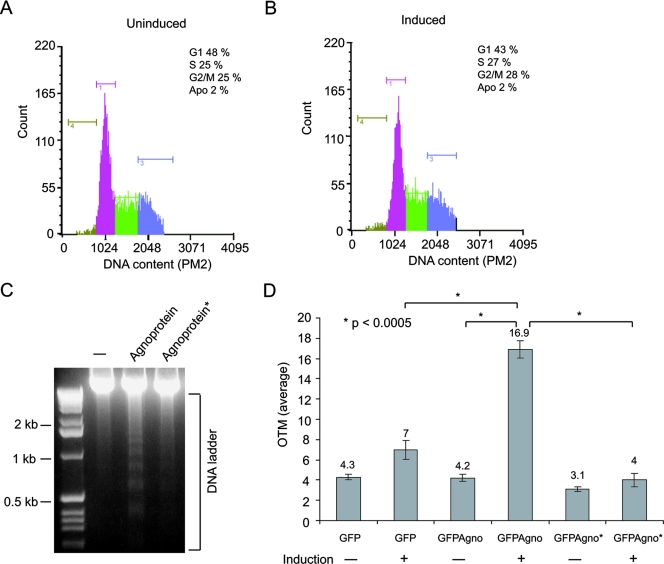
Agnoprotein can be phosphorylated by protein kinase C, and several potential sites including serine at positions 7 and 11 have been implicated experimentally (54, 62). Here, we altered serine 7 and serine 11 to alanine and serine 15 to valine by site-directed mutagenesis and developed CG-4 cells that can constitutively express the mutant protein. First, we examined the cell cycle distribution and DNA breakage of these cells in progenitor and differentiated stages by fluorocytometry and comet assay, respectively. As seen in Fig. 7A and B, unlike that for wild-type agnoprotein, the expression of mutant agnoprotein showed no enhancement in the level of apoptotic cells during differentiation into oligodendrocytes. As before, a small fraction (2%) of apoptotic cells was detected in progenitor cells before and after induction. Results from a DNA fragmentation study did not show a significant level of DNA ladder in cells expressing mutant agnoprotein, suggesting that unlike wild-type agnoprotein, mutant agnoprotein is not capable of inducing apoptosis (Fig. 7C). Finally, this was further confirmed by comet assay. An average OTM seen for wild-type agnoprotein-producing cells induced to differentiate into oligodendrocytes (Fig. 7D) was significantly high compared to that seen for control agnoprotein-negative and mutant agnoprotein*-expressing cells.
FIG. 7.
Effect of mutant agnoprotein on apoptosis of undifferentiated and differentiated CG-4 cells. (A and B) Cell cycle distribution and apoptosis (Apo) of CG-4 cell lines expressing mutant agnoprotein were analyzed by fluorocytometry. (C) DNA-laddering assay from cells expressing wild-type or mutant agnoprotein (GFP-agnoprotein*). (D) DNA fragmentation upon differentiation of cells expressing wild-type agnoprotein (GFP-agno) or mutant agnoprotein (GFP-agno*) was analyzed by comet assay. The measured average OTMs are presented.
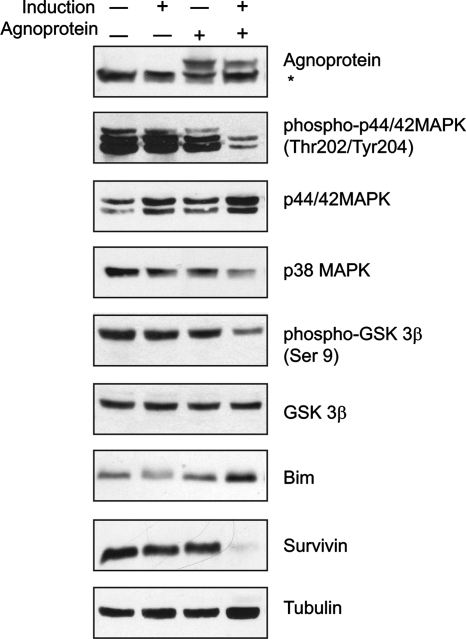
To investigate the molecular basis of the observed changes in progenitors differentiated into oligodendrocytic cells, we further examined the expression pattern of proteins involved in the regulation of cell survival and apoptosis. MAPKs, including the extracellular signal-regulated kinases (ERKs), the stress-activated c-Jun N-terminal kinase (JNK), and the 38-kDa high-osmolarity glycerol response kinase (p38) can all play a central role in oligodendrocyte function, differentiation, cell survival, and cell death (11). We prepared whole-cell protein lysates from proliferating and differentiated agnoprotein-negative and agnoprotein-positive CG-4 cells that were used to analyze the expression of various regulatory proteins by Western blotting (Fig. 8). The differentiation of CG-4 cells in the presence of agnoprotein caused a significant reduction of phospho-p44/p42 MAPK without reducing the total amount of MAPK. While the total amount of GSK3β remained unchanged upon differentiation, mitogen withdrawal caused a decrease in the amount of inactive GSK3β only in cells expressing agnoprotein, which was detected with antibody that recognizes the inhibitory phosphorylation at Ser 9. Examination of β-catenin, one of the targets for GSK3β, showed a decrease in the nuclear appearance of this protein in cells expressing agnoprotein compared to what was seen for the control cells (data not shown). The expression of total GSK3β was low in agnoprotein-producing cells in the progenitor state as well. The level of Bim (Bcl-2-interacting mediator of cell death), which is known to be induced in oligodendrocytes undergoing apoptotic death (7, 65), was increased in the presence of agnoprotein upon differentiation. On the other hand, the level of expression of survivin, which acts through the binding and suppression of caspases, including caspases 3 and 7 (66, 73), was significantly inhibited in agnoprotein-positive cells differentiating into oligodendrocytes. Altogether, these data suggest that by interfering with the level of activation of signaling pathways such as MAPK and anti- and proapoptotic proteins, agnoprotein can promote apoptosis in cells undergoing differentiation toward the oligodendrocyte lineage.
FIG. 8.
Molecular profile of progenitors induced to differentiate into oligodendrocytes in the presence of agnoprotein. Western blot analysis of whole-cell lysates from CG-4 control cells and cells expressing agnoprotein. Extracts were prepared from proliferating cells or cells induced to differentiate into oligodendrocytes over 4 days and Western blotted with primary antibodies as indicated. The asterisk corresponds to a nonspecific band.
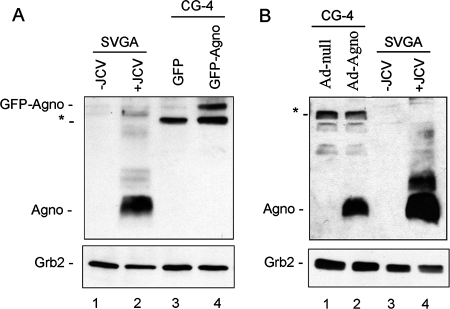
Finally, we were interested in determining how the level of agnoprotein expression occurring in our rat CG-4 model of differentiation compares to the level of agnoprotein that occurs in human glial cells that undergo infection by JCV. To this end, we extracted protein from JCV-infected SVGA cells, which express simian virus 40 T antigen and support active JCV infection in cell culture. Figure 9A shows the expression of agnoprotein (8 kDa) in the JCV-infected cells (lane 2) but not in uninfected control cells (lane 1). GFP-agnoprotein (35 kDa) expressed in CG-4 cells (lane 4) is present at a lower level than agnoprotein in JCV-infected cells (lane 2). A nonspecific band (30 kDa) that is found in CG-4 cells but not SVGA cells is seen. Similarly, agnoprotein expressed in CG-4 cells transduced by adenovirus expressing agnoprotein is present to an extent about the same as or somewhat less than that seen for agnoprotein in JCV-infected cells (Fig. 9B, compare lanes 2 and 4). Importantly, these Western blots indicate that the levels of agnoprotein in our plasmid- and adenovirus-based experimental systems do not exceed the level of agnoprotein that is found during JCV infection.
FIG. 9.
Levels of agnoprotein expression relative to those seen for JCV-infected cells. Human SVGA glial cells were infected with JCV, and agnoprotein expression was compared to that for CG-4 cells expressing GFP-agnoprotein or GFP (negative control) (A) and CG-4 cells transduced with adenovirus expressing agnoprotein (Ad-Agno) or the empty vector Ad-null (negative control) (B). In panel A, a nonspecific band (30 kDa) that is found in CG-4 cells but not SVGA cells is indicated with an asterisk.
DISCUSSION
Human pathogenic viruses usually contain one or more regulatory proteins that, in addition to their role in viral replication, may also dysregulate cellular function, thus contributing to the development and progression of disease, in either the absence or the presence of viral lytic infection. With respect to polyomaviruses, including JCV, much attention has focused on the early protein T antigen, which has a regulatory impact on viral gene expression and replication and derails various biological events in its host cells (17, 38). The agnoprotein of JCV, another nonstructural protein encoded by the late genome of the virus, has captured our attention because its deletion abrogates viral replication and its expression impacts on host cell cycle progression and response to DNA damage (18, 19, 36). In this study, we employed a bipotential CG-4 cell line that can be differentiated to cells with mature oligodendrocyte characteristics (44, 45, 55). Our results show that expression of agnoprotein in the absence of JCV simplifies the complexity of the network processes seen upon the differentiation of CG-4 cells into oligodendrocytes. Moreover, the survival of differentiated cells expressing agnoprotein was diminished compared to what was seen for control agnoprotein-negative cells as determined by MTT assay, the appearance of oligonucleosome-size fragments by DNA laddering and comet assays. Together, these data suggest that these cells undergo active programmed cell death. Analysis of the molecular profiles of progenitors differentiating into oligodendrocytes revealed activation of proapoptotic and suppression of prosurvival pathways in oligodendrocytes expressing agnoprotein. It has previously been shown that the inactivation of ERKs together with the activation of JNK and/or p38 may be critical for apoptosis, and an increase in ERK activity has been correlated to the increased survival of CG-4 cells differentiated into oligodendrocytic cells (29) and neuronal cell survival (82), whereas an increase of JNK or p38 activity may trigger apoptosis (82). Mitogen withdrawal that induces differentiation of CG-4 into multipolar oligodendrocytic cells, at an early (30-min) stage, is associated with a reduction of CREB and p44/42 MAPK (ERK1/2) phosphorylation (48, 72). We observed an inhibition of ERK activity by agnoprotein at late stages (4 days) of differentiation. Inactivation of ERKs in these cells was associated with inhibition of p38 MAPK and an increase in the expression of Bim. In contrast to what was seen in studies with neurons (7), the expression of Bim in CG-4 agnopositive cells induced to differentiate into oligodendrocytes occurred in the absence of p38 MAPK and ERK1/2 activation. Interestingly, Weston et al. (80) demonstrated that the serum withdrawal-induced expression of Bim is JNK independent and is repressed by ERK. Other studies have implicated p38 MAPK in oligodendrocyte differentiation and demonstrated that the long-term inhibition of p38 MAPK promoted the apoptosis of primary oligodendrocytes and differentiated CG-4 cells and suggested that a sustained p38 MAPK activation could be an important factor in oligodendrocyte survival (11, 26). In agnoprotein-producing cells differentiated into oligodendrocytes, inactive GSK3β was detected at significantly low levels compared to what was seen for controls. Active GSK3β phosphorylates β-catenin at the N-terminal region and enhances β-catenin degradation (51, 84), thereby (i) preventing the association of β-catenin with nuclear transcription factors and activation of target gene expression such as cyclin D1 and c-myc (24, 28) and (ii) binding the intracellular domain of N-cadherin and α-catenin, an actin binding protein, to stabilize the cytoskeleton during process outgrowth (4, 85). Our primary observations on the low level of β-catenin in the nuclear fraction of agnopositive cells (data not shown) together with the activation of GSK3β and the decrease in activity of ERK further support our hypothesis of the involvement of agnoprotein in the pathways that regulate the survival and differentiation of oligodendrocytes.
Another important observation emerged from the experiment where cells were stressed by treatment with H2O2. It has been shown that oligodendrocytes are vulnerable to endogenous oxidative stress (68). The free radical injury to the developing oligodendrocytes plays a key role in the pathogenesis of periventricular leukomalacia (2, 27) and multiple sclerosis (49). We demonstrate that the presence of agnoprotein sensitizes differentiating oligodendrocyte progenitors to an exogenous source of ROS, since the treatment of CG-4 cells with the H2O2 oxidant resulted in extensive DNA breakage in agnopositive cells. One of the important histological features of PML lesions is the presence of microglial nodules (21). Activated microglia release ROS that cause neurotoxicity and white matter injury (12, 41). The presence of agnoprotein may augment the direct cytotoxic effects of ROS, produced both endogenously and exogenously, on oligodendrocytes, thereby contributing to demyelination. Whereas oligodendrocyte death can occur during normal brain development (5, 6), it is evident that under pathological conditions such as multiple sclerosis and PML, cells can undergo apoptosis (13, 31, 35, 60, 63). Although apoptosis has been implicated in several virally induced diseases of the brain, there are conflicting reports regarding the role of apoptosis in JCV infection. Richardson-Burns et al. (59) demonstrated that apoptosis occurs in brain tissue of patients with both HIV-associated and non-HIV-associated PML, and apoptotic changes were limited almost exclusively to oligodendrocytes associated with demyelinated lesions. Another group (83), based on findings of the overexpression of Bax and the down-expression of Bcl-2 in JCV-infected oligodendroglial cells in paraffin-embedded PML specimens from HIV-positive, non-Hodgkin's lymphoma, and systemic lupus erythematosus patients, suggested that oligodendrocytes may be undergoing apoptosis, which may contribute to the demyelination in PML. On the contrary, Seth et al. (64) considered nonapoptotic cell death and necrosis as the mechanism of oligodendrocyte destruction in PML, although their conclusions were based on in vitro studies where a human central nervous system progenitor-derived astrocyte culture was utilized. However, increasing evidence suggests the coexistence of the two mechanisms of cell death in many pathological situations (43). Recently, Piña-Oviedo et al. (57) reported induction of expression of the inhibitor-of-apoptosis protein survivin in JCV-infected glial cell cultures and in PML clinical samples (57). This event may be a part of a defensive mechanism and an early response to viral invasion and replication. Interestingly, the expression of survivin was detected at high levels at the early phase of JCV infection of primary oligodendrocytes and astrocytes and reduced at later stages of infection, when production of the late viral proteins, including agnoprotein, increased. We have recently reported moderate apoptosis in JCV-infected primary astrocytes, in an experimental setting similar to that of Piña-Oviedo et al. (57), late in infection (20). Since we detect an increase of the expression of proapoptotic Bim and a decrease of the apoptosis inhibitor survivin in differentiated CG-4 agnoprotein-producing cells, this suggests that the expression of the late protein agnoprotein inhibits prosurvival and/or activates proapoptotic signaling in differentiating oligodendrocytes.
Many studies have demonstrated that the development and survival of oligodendrocytes depend strongly on axons and axon-derived signals (5, 14, 23, 33). Further studies are under way to determine if agnoprotein may induce the death of oligodendrocytes in the presence of neurons (in coculture with neurons or mixed brain cell culture) in the same way as it does in a monocultural (oligodendroglial) system. The demonstration of the agnoprotein-mediated inhibition of the differentiation and survival of oligodendrocytic cells that we describe here implies a new role for agnoprotein of JCV in the pathogenesis of PML.
Acknowledgments
We thank past and present members of the Department of Neuroscience and the Center for Neurovirology for their continued support, insightful discussions, and sharing of reagents and ideas. We also thank C. Schriver for editorial assistance.
This work was made possible by grants awarded by the NIH to K.K. and S.A.
Footnotes
Published ahead of print on 7 November 2007.
REFERENCES
- 1.Arbusow, V., M. Strupp, W. Samtleben, H. Hatz, H. Bruckmann, and T. Brandt. 1999. Progressive multifocal leukoencephalopathy as a result of immunosuppressive therapy. Dtsch. Med. Wochenschr. 124653-656. [DOI] [PubMed] [Google Scholar]
- 2.Back, S. A. 2006. Perinatal white matter injury: the changing spectrum of pathology and emerging insights into pathogenetic mechanisms. Ment. Retard. Dev. Disabil. Res. Rev. 12129-140. [DOI] [PubMed] [Google Scholar]
- 3.Back, S. A., N. L. Luo, N. S. Borenstein, J. J. Volpe, and H. C. Kinney. 2002. Arrested oligodendrocyte lineage progression during human cerebral white matter development: dissociation between the timing of progenitor differentiation and myelinogenesis. J. Neuropathol. Exp. Neurol. 61197-211. [DOI] [PubMed] [Google Scholar]
- 4.Bamji, S. X. 2005. Cadherins: actin with the cytoskeleton to form synapses. Neuron 47175-178. [DOI] [PubMed] [Google Scholar]
- 5.Barres, B. A., and M. C. Raff. 1999. Axonal control of oligodendrocyte development. J. Cell Biol. 1471123-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barres, B. A., I. K. Hart, H. S. Coles, J. F. Burne, J. T. Voyvodic, W. D. Richardson, and M. C. Raff. 1992. Cell death and control of cell survival in the oligodendrocyte lineage. Cell 7031-46. [DOI] [PubMed] [Google Scholar]
- 7.Becker, E. B., and A. Bonni. 2004. Cell cycle regulation of neuronal apoptosis in development and disease. Prog. Neurobiol. 721-25. [DOI] [PubMed] [Google Scholar]
- 8.Berger, J. R., and S. Houff. 2006. Progressive multifocal leukoencephalopathy: lessons from AIDS and natalizumab. Neurol. Res. 28299-305. [DOI] [PubMed] [Google Scholar]
- 9.Berger, J. R., L. Pall, D. Lanska, and M. Whiteman. 1998. Progressive multifocal leukoencephalopathy in patients with HIV infection. J. Neurovirol. 459-68. [DOI] [PubMed] [Google Scholar]
- 10.Bergonzini, V., S. Delbue, J. Y. Wang, K. Reiss, M. Prisco, S. Amini, K. Khalili, and F. Peruzzi. 2004. HIV-Tat promotes cellular proliferation and inhibits NGF-induced differentiation through mechanisms involving Id1 regulation. Oncogene 237701-7711. [DOI] [PubMed] [Google Scholar]
- 11.Bhat, N. R., P. Zhange, and S. B. Mohanty. 2007. p38 MAP kinase regulation of oligodendrocyte differentiation with CREB as a potential target. Neurochem. Res. 32293-302. [DOI] [PubMed] [Google Scholar]
- 12.Block, M. L., L. Zecca, and J. S. Hong. 2007. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat. Rev. Neurosci. 857-69. [DOI] [PubMed] [Google Scholar]
- 13.Bonetti, B., C. Stegagno, B. Cannella, N. Rizzuto, G. Moretto, and C. S. Raine. 1999. Activation of NF-kappaB and c-jun transcription factors in multiple sclerosis lesions: implications for oligodendrocyte pathology. Am. J. Pathol. 1551433-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bozzali, M., and L. Wrabetz. 2004. Axonal signals and oligodendrocyte differentiation. Neurochem. Res. 29979-988. [DOI] [PubMed] [Google Scholar]
- 15.Chang, C.-F., J. Otte, D. A. Kerr, M. Valkkila, C. E. Calkins, and K. Khalili. 1996. Evidence that the soluble factors secreted by activated immune cells suppress replication of human neurotropic JC virus DNA in glial cells. Virology 221226-231. [DOI] [PubMed] [Google Scholar]
- 16.Choy, D. S., A. Weiss, and P. T. Li. 1992. Progressive multifocal leukoencephalopathy following treatment for Wegener's granulomatosis. JAMA 268600-601. [DOI] [PubMed] [Google Scholar]
- 17.Cole, C. N. 1996. Polyoma virinae: the viruses and their replication, p. 917-946. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fundamental virology, 3rd ed. Lippincott, Williams & Wilkins, Philadelphia, PA.
- 18.Darbinyan, A., N. Darbinian, M. Safak, S. Radhakrishnan, A. Giordano, and K. Khalili. 2002. Evidence for dysregulation of cell cycle by human polyomavirus, JCV, late auxiliary protein. Oncogene 215574-5581. [DOI] [PubMed] [Google Scholar]
- 19.Darbinyan, A., K. M. Siddiqui, D. Slonina, N. Darbinian, S. Amini, M. K. White, and K. Khalili. 2004. Role of JC virus agnoprotein in DNA repair. J. Virol. 788593-8600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darbinyan, A., M. K. White, S. Akan, S. Radhakrishnan, L. Del Valle, S. Amini, and K. Khalili. 2007. Alterations of DNA damage repair pathways resulting from JCV infection. Virology 36473-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Del Valle, L., and S. Piña-Oviedo. 2006. HIV disorders of the brain: pathology and pathogenesis. Front. Biosci. 11718-732. [DOI] [PubMed] [Google Scholar]
- 22.Del Valle, L., J. Gordon, S. Enam, S. Delbue, S. Croul, S. Abraham, S. Radhakrishnan, M. Assimakopoulou, C. D. Katsetos, and K. Khalili. 2002. Expression of human neurotropic polyomavirus JCV late gene product agnoprotein in human medulloblastoma. J. Natl. Cancer Inst. 94267-273. [DOI] [PubMed] [Google Scholar]
- 23.Fields, R. D., and B. Stevens-Graham. 2002. New insights into neuron-glia communication. Science 298556-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galceran, J., I. Farinas, M. J. Depew, H. Clevers, and R. Grosschedl. 1999. Wnt3a−/−-like phenotype and limb deficiency in Lef1(−/−)Tcf1(−/−) mice. Genes Dev. 13709-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gentile, S., I. Sacerdote, D. Roccatello, and M. T. Giordana. 1996. Progressive multifocal leukoencephalopathy during cyclosporine treatment. A case report. Ital. J. Neurol. Sci. 17363-366. [DOI] [PubMed] [Google Scholar]
- 26.Hamanoue, M., K. Sato, and K. Takamatsu. 2007. Inhibition of p38 mitogen-activated protein kinase-induced apoptosis in cultured mature oligodendrocytes using SB202190 and SB203580. Neurochem. Int. 5116-24. [DOI] [PubMed] [Google Scholar]
- 27.Haynes, R. L., O. Baud, J. Li, H. C. Kinney, J. J. Volpe, and D. R. Folkerth. 2005. Oxidative and nitrative injury in periventricular leukomalacia: a review. Brain Pathol. 15225-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He, T. C., A. B. Sparks, C. Rago, H. Hermeking, L. Zawel, L. T. da Costa, P. J. Morin, B. Vogelstein, and K. W. Kinzler. 1998. Identification of c-MYC as a target of the APC pathway. Science 2811509-1512. [DOI] [PubMed] [Google Scholar]
- 29.Hiraiwa, M., E. M. Taylor, W. M. Campana, S. J. Darin, and J. S. O'Brien. 1997. Cell death prevention, MAP kinase stimulation, and enhanced sulfatide synthesis in Schwann cell and oligodendrocyte. Proc. Natl. Acad. Sci. USA 944778-4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hockenbery, D., G. Nuñez, C. Millman, R. D. Schreiber, and S. J. Korsmeyer. 1990. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature 348334-336. [DOI] [PubMed] [Google Scholar]
- 31.Hou, J., and E. O. Major. 2000. Progressive multifocal leukoencephalopathy: JC virus induced demyelination in the immune compromised host. J. Neurovirol. 6S98-S100. [PubMed] [Google Scholar]
- 32.Hou, J., P. Seth, and E. O. Major. 2006. JC virus can infect human immune and nervous system progenitor cells: implications for pathogenesis. Adv. Exp. Med. Biol. 577266-273. [DOI] [PubMed] [Google Scholar]
- 33.Hu, Q. D., Q. H. Ma, G. Gennarini, and Z. C. Xiao. 2006. Cross-talk between F3/contactin and Notch at axoglial interface: a role in oligodendrocyte development. Dev. Neurosci. 2825-33. [DOI] [PubMed] [Google Scholar]
- 34.Kappers, M. H., A. J. Swaak, D. M. Zuidgeest, and A. Dees. 2006. Progressive multifocal leukoencephalopathy in a patient following long-term immunosuppressive therapy for systemic lupus erythematosus. Ned. Tijdschr. Geneeskd. 150387-392. [PubMed] [Google Scholar]
- 35.Khalili, K., and M. K. White. 2006. Human demyelinating disease and the polyomavirus JCV. Mult. Scler. 12133-142. [DOI] [PubMed] [Google Scholar]
- 36.Khalili, K., M. K. White, H. Sawa, K. Nagashima, and M. Safak. 2005. The agnoprotein of polyomaviruses: a multifunctional auxiliary protein. J. Cell. Physiol. 2041-7. [DOI] [PubMed] [Google Scholar]
- 37.Khalili, K., M. K. White, F. Lublin, P. Ferrante, and J. R. Berger. 2007. Reactivation of JC virus and development of PML in patients with multiple sclerosis. Neurology 68985-990. [DOI] [PubMed] [Google Scholar]
- 38.Kim, H.-S., J. W. Henson, and R. J. Frisque. 2001. Transcription and replication in the human polyomaviruses, p. 73-126. In K. Khalili and G. L. Stoner (ed.), Human polyomaviruses: molecular and clinical perspective. Wiley-Liss Inc., New York, NY.
- 39.Kinoshita, M., K. Iwana, H. Shinoura, S. Aotsuka, and M. Sumiya. 1998. Progressive multifocal leukoencephalopathy resembling central nervous system systemic lupus erythematosus. Clin. Exp. Rheumatol. 16313-315. [PubMed] [Google Scholar]
- 40.Kleinschmidt-DeMasters, B. K., and K. L. Tyler. 2005. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N. Engl. J. Med. 353369-374. [DOI] [PubMed] [Google Scholar]
- 41.Kutzelnigg, A., C. F. Lucchinetti, C. Stadelmann, W. Bruck, H. Rauschka, M. Bergmann, M. Schmidbauer, J. E. Parisi, and H. Lassmann. 2005. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 1282705-2712. [DOI] [PubMed] [Google Scholar]
- 42.Langer-Gould, A., S. W. Atlas, A. J. Green, A. W. Bollen, and D. Pelletier. 2005. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N. Engl. J. Med. 353375-381. [DOI] [PubMed] [Google Scholar]
- 43.Leist, M., and P. Nicotera. 1997. The shape of cell death. Biochem. Biophys. Res. Commun. 2361-9. [DOI] [PubMed] [Google Scholar]
- 44.Louis, J. C., E. Magal, D. Muir, M. Manthorpe, and S. Varon. 1992. CG-4, a new bipotential glial cell line from rat brain, is capable of differentiating in vitro into either mature oligodendrocytes or type-2 astrocytes. J. Neurosci. Res. 31193-204. [DOI] [PubMed] [Google Scholar]
- 45.Louis, J. C., D. Muir, and S. Varon. 1992. Autocrine inhibition of mitotic activity in cultured oligodendrocyte-type-2 astrocyte (O-2A) precursor cells. Glia 630-38. [DOI] [PubMed] [Google Scholar]
- 46.Major, E. O., K. Amemiya, C. S. Tornatore, S. A. Houff, and J. R. Berger. 1992. Pathogenesis and molecular biology of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin. Microbiol. Rev. 549-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mazlo, M., and I. Tariska. 1980. Morphological demonstration of the first phase of polyomavirus replication in oligodendroglia cells of human brain in progressive multifocal leukoencephalopathy (PML). Acta Neuropathol. (Berlin) 49133-143. [DOI] [PubMed] [Google Scholar]
- 48.McNulty, S., M. Crouch, D. Smart, and M. Rumsby. 2001. Differentiation of bipolar CG-4 line oligodendrocytes is associated with regulation of CREB, MAP kinase and PKC signaling pathways. Neurosci. Res. 41217-226. [DOI] [PubMed] [Google Scholar]
- 49.Merrill, J. E., and E. N. Benveniste. 1996. Cytokines in inflammatory brain lesions: helpful and harmful. Trends Neurosci. 19331-338. [DOI] [PubMed] [Google Scholar]
- 50.Messam, C. A., J. Hou, R. M. Gronostajski, and E. O. Major. 2003. Lineage pathway of human brain progenitor cells identified by JC virus susceptibility. Ann. Neurol. 53636-646. [DOI] [PubMed] [Google Scholar]
- 51.Miller, J. R., and R. T. Moon. 1997. Analysis of the signaling activities of localization mutants of beta-catenin during axis specification in Xenopus. J. Cell Biol. 139229-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morgenstern, L. B., and C. A. Pardo. 1995. Progressive multifocal leukoencephalopathy complicating treatment for Wegener's granulomatosis. J. Rheumatol. 221593-1595. [PubMed] [Google Scholar]
- 53.Mosmann, T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 6555-63. [DOI] [PubMed] [Google Scholar]
- 54.Okada, Y., S. Endo, H. Takahashi, H. Sawa, T. Umemura, and K. Nagashima. 2001. Distribution and function of JCV agnoprotein. J. Neurovirol. 7302-306. [DOI] [PubMed] [Google Scholar]
- 55.Olby, N. J., and W. F. Blakemore. 1996. Reconstruction of the glial environment of a photochemically induced lesion in the rat spinal cord by transplantation of mixed glial cells. J. Neurocytol. 25481-498. [DOI] [PubMed] [Google Scholar]
- 56.Olive, P. L., J. P. Banath, and R. E. Durand. 1990. Heterogeneity in radiation-induced DNA damage and repair in tumor and normal cells measured using the “comet” assay. Radiat. Res. 12286-94. [PubMed] [Google Scholar]
- 57.Piña-Oviedo, S., K. Urbanska, S. Radhakrishnan, T. Sweet, K. Reiss, K. Khalili, and L. Del Valle. 2007. Effects of JC virus infection on anti-apoptotic protein survivin in progressive multifocal leukoencephalopathy. Am. J. Pathol. 1701291-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rankin, E., and F. Scaravilli. 1995. Progressive multifocal leukoencephalopathy in a patient with rheumatoid arthritis and polymyositis. J. Rheumatol. 22777-779. [PubMed] [Google Scholar]
- 59.Richardson-Burns, S. M., B. K. Kleinschmidt-DeMasters, R. L. DeBiasi, and K. L. Tyler. 2002. Progressive multifocal leukoencephalopathy and apoptosis of infected oligodendrocytes in the central nervous system of patients with and without AIDS. Arch. Neurol. 591930-1936. [DOI] [PubMed] [Google Scholar]
- 60.Rodriguez, M., and C. F. Lucchinetti. 1999. Is apoptotic death of the oligodendrocyte a critical event in the pathogenesis of multiple sclerosis? Neurology 531615-1616. [DOI] [PubMed] [Google Scholar]
- 61.Safak, M., R. Barrucco, A. Darbinyan, Y. Okada, K. Nagashima, and K. Khalili. 2001. Interaction of JC virus agnoprotein with T antigen modulates transcription and replication of the viral genome in glial cells. J. Virol. 751476-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sariyer, I. K., I. Akan, V. Palermo, J. Gordon, K. Khalili, and M. Safak. 2006. Phosphorylation mutants of JC virus agnoprotein are unable to sustain the viral infection cycle. J. Virol. 803893-3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Segal, B. M., and A. H. Cross. 2000. Fas(t) track to apoptosis in MS: TNF receptors may suppress or potentiate CNS demyelination. Neurology 55906-907. [DOI] [PubMed] [Google Scholar]
- 64.Seth, P., F. Diaz, J. H. Tao-Cheng, and E. O. Major. 2004. JC virus induces nonapoptotic cell death of human central nervous system progenitor cell-derived astrocytes. J. Virol. 784884-4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shibata, M., H. Hattori, T. Sasaki, J. Gotoh, J. Hamada, and Y. Fukuuchi. 2002. Temporal profiles of the subcellular localization of Bim, a BH3-only protein, during middle cerebral artery occlusion in mice. J. Cereb. Blood Flow Metab. 22810-820. [DOI] [PubMed] [Google Scholar]
- 66.Shin, S., B. J. Sung, Y. S. Cho, H. J. Kim, N. C. Ha, J. I. Hwang, C. W. Chung, Y. K. Jung, and B. H. Oh. 2001. An anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and -7. Biochemistry 401117-1123. [DOI] [PubMed] [Google Scholar]
- 67.Singh, N. P., M. T. McCoy, R. R. Tice, and E. L. Schneider. 1988. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 175184-191. [DOI] [PubMed] [Google Scholar]
- 68.Smith, K. J., R. Kapoor, and P. A. Felts. 1999. Demyelination: the role of reactive oxygen and nitrogen species. Brain Pathol. 969-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Speit, G. H., and A. Hartmann. 1999. The comet assay (single-cell gel test). A sensitive genotoxicity test for the detection of DNA damage and repair. Methods Mol. Biol. 113203-212. [DOI] [PubMed] [Google Scholar]
- 70.Sperber, B. R., and F. A. McMorris. 2001. Fyn tyrosine kinase regulates oligodendroglial cell development but is not required for morphological differentiation of oligodendrocytes. J. Neurosci. Res. 63303-312. [DOI] [PubMed] [Google Scholar]
- 71.Sponzilli, E. E., J. K. Smith, N. Malamud, and J. R. McCulloch. 1975. Progressive multifocal leukoencephalopathy: a complication of immunosuppressive treatment. Neurology 25664-668. [DOI] [PubMed] [Google Scholar]
- 72.Stariha, R. L., and S. U. Kim. 2001. Mitogen-activated protein kinase signaling in oligodendrocytes: a comparison of primary cultures and CG-4. Int. J. Dev. Neurosci. 19427-437. [DOI] [PubMed] [Google Scholar]
- 73.Tamm, I., Y. Wang, E. Sausville, D. A. Scudiero, N. Vigna, T. Oltersdorf, and J. C. Reed. 1998. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 585315-5320. [PubMed] [Google Scholar]
- 74.Van Assche, G., M. Van Ranst, R. Sciot, B. Dubois, S. Vermeire, M. Noman, J. Verbeeck, K. Geboes, W. Robberecht, and P. Rutgeerts. 2005. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn's disease. N. Engl. J. Med. 353362-368. 625. [DOI] [PubMed] [Google Scholar]
- 75.Viallard, J. F., E. Lazaro, E. Ellie S. Eimer, F. Camou, O. Caubet, M. E. Lafon, H. Fleury, and J. L. Pellegrin. 2007. Improvement of progressive multifocal leukoencephalopathy after cidofovir therapy in a patient with a destructive polyarthritis. Infection 3533-36. [DOI] [PubMed] [Google Scholar]
- 76.Vignais, P. V. 2002. The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell. Mol. Life Sci. 591428-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Warnatz, K., H. H. Peter, M. Schumacher, L. Wiese, A. Prasse, F. Petschner, P. Vaith, B. Volk, and S. M. Weiner. 2003. Infectious CNS disease as a differential diagnosis in systemic rheumatic diseases: three case reports and a review of the literature. Ann. Rheum. Dis. 6250-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reference deleted.
- 79.Weiner, L. P., R. M. Herndon, O. Narayan, R. T. Johnson, K. Shah, L. J. Rubinstein, T. J. Preziosi, and F. K. Conley. 1972. Isolation of virus related to SV40 from patients with progressive multifocal leukoencephalopathy. N. Engl. J. Med. 286385-390. [DOI] [PubMed] [Google Scholar]
- 80.Weston, C. R., K. Balmanno, C. Chalmers, K. Hadfield, S. A. Molton, R. Ley, E. F. Wagner, and S. J. Cook. 2003. Activation of ERK1/2 by deltaRaf-1:ER* represses Bim expression independently of the JNK or PI3K pathways. Oncogene 221281-1293. [DOI] [PubMed] [Google Scholar]
- 81.Wyllie, A. H. 1980. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 284555-556. [DOI] [PubMed] [Google Scholar]
- 82.Xia, Z., M. Dickens, J. Raingeaud, R. J. Davis, and M. E. Greenberg. 1995. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 2701326-1331. [DOI] [PubMed] [Google Scholar]
- 83.Yang, B., and R. A. Prayson. 2000. Expression of Bax, Bcl-2, and P53 in progressive multifocal leukoencephalopathy. Mod. Pathol. 131115-1120. [DOI] [PubMed] [Google Scholar]
- 84.Yost, C., M. Torres, J. R. Miller, E. Huang, D. Kimelman, and R. T. Moon. 1996. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 101443-1454. [DOI] [PubMed] [Google Scholar]
- 85.Yu, X., and R. C. Malenka. 2003. Beta-catenin is critical for dendritic morphogenesis. Nat. Neurosci. 61169-1177. [DOI] [PubMed] [Google Scholar]