Abstract
Viruslike particles which displayed a peculiar wheellike appearance that distinguished them from A-, B- or C-type particles had previously been described in the early mouse embryo. The maximum expression of these so-called epsilon particles was observed in two-cell-stage embryos, followed by their rapid decline at later stages of development and no particles detected at the zygote one-cell stage. Here, we show that these particles are in fact produced by a newly discovered murine endogenous retrovirus (ERV) belonging to the widespread family of mammalian ERV-L elements and named MuERV-L. Using antibodies that we raised against the Gag protein of these elements, Western blot analysis and in toto immunofluorescence studies of the embryos at various stages disclosed the same developmental expression profile as that observed for epsilon particles. Using expression vectors for cloned, full-length, entirely coding MuERV-L copies and cell transfection, direct identification of the epsilon particles was finally achieved by high-resolution electron microscopy.
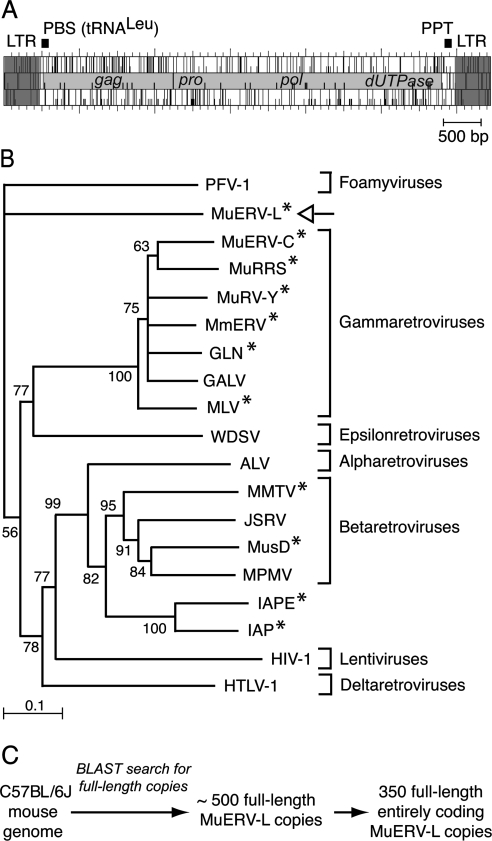
Multiple viruslike particles are generated by mouse tissues under normal and/or pathological conditions. These have been initially characterized according to morphological criteria by electron microscopic examination and classified as type A, B, C, and epsilon particles depending on their site of assembly (i.e., within the cell cytoplasm or at the cell surface) and the structure of the mature particles (3, 24; reviewed in reference 16). These particles were suspected to be generated by so-called endogenous retroviruses (ERVs), as they can be observed in normal animals in the absence of any exogenous infection. Some of these particles were even observed in the developing embryo and, for some of them, at very early stages with, for instance, a peak of occurrence of particles of the epsilon type at the two-cell stage (23, 24). In most cases, the ERVs responsible for the formation of these particles have been identified, thanks to the complete sequencing of the mouse genome (15a), the in silico identification of elements with full coding capacity, and, finally, the cloning of functional copies that could be demonstrated to be responsible for the synthesis of the corresponding particles in appropriate ex vivo assays by electron microscopic analysis. Examples include the highly reiterated intracisternal A-type particles (IAPs) (7; reviewed in reference 12), the Mus musculus type D (MusD) intracytoplasmic elements (18, 19), and the endogenous murine leukemia virus and mouse mammary tumor virus (reviewed in reference 4). Yet, the epsilon particles, first identified more than 25 years ago in mouse embryos (23, 24) and later in some iododeoxyuridine-induced mouse cell lines (13, 14), have up to now remained “orphan.” Actually, these particles have a rather unusual morphology, with a wheel-shaped core disclosing an electron-lucent space surrounded by two concentric electron-dense rings that are separated by a radial array of internal spikes, not found in any present-day infectious retrovirus and at variance with the other types of particles for which similar morphologies can be found among infectious elements of animals. Along this line, we had previously identified a new family of ERVs (ERV-L) in humans (5) that we further demonstrated to be present in all placental mammals and, therefore, to have settled within the genome of a mammalian ancestor more than 70 million years ago (1, 2; see also reference 8). A phylogenetic analysis of their pol genes disclosed a rather unusual position among retroviral elements, with, again, no clear-cut relationship to the major groups of retroviruses (Fig. 1B). Despite its age, this family has most probably maintained some of its elements in an active state, since we could demonstrate relatively recent amplifications of members of this family of elements in the mouse, not observed in the rat, having occurred less than 10 million years ago (1, 2; see also references 6 and 10). Interestingly, in the mouse, most of these MuERV-L elements are full length and have complete open reading frames (ORFs) with identifiable gag and pol genes (but no env) (Fig. 1A and C), and transcripts from these elements have recently been detected in embryos at early developmental stages (9, 11, 20, 21). Altogether, it therefore seems plausible that the epsilon particles could be encoded by members of the MuERV-L family of elements, and experiments were therefore devised to test this hypothesis.
FIG. 1.
Structure and phylogeny of MuERV-L elements. (A) Genomic organization of an MuERV-L provirus, with the LTRs (in dark gray) flanking two ORFs homologous to the retroviral gag and pol genes (in light gray). The pol gene further discloses domains homologous to retroviral pro and dUTPase genes. A primer binding site (PBS) complementary to tRNALeu and a polypurine track (PPT) can be identified. (B) Phylogeny of retroviruses, based on their reverse transcriptase domain. The tree was constructed by the neighbor-joining method using the seven blocks of conserved residues found in the reverse transcriptase domain of all retroelements and was rooted with non-LTR retrotransposons. All sequences are readily accessible from GenBank and previous reports (e.g., reference 15). Percent bootstrap values obtained from 1,000 replicates are indicated. The retroviruses “endogenized” in the mouse genome are marked (*). The seven retroviral genera are indicated on the right. PFV, primate foamy virus; MuRRS, murine retrovirus-related DNA sequence; MuRV-Y, murine retrovirus Y associated; MmERV, Mus musculus ERV; GLN, murine retrovirus using tRNAGln; GALV, gibbon ape leukemia virus; MLV, murine leukemia virus; WDSV, walleye dermal sarcoma virus; ALV, avian leukosis virus; MMTV, mouse mammary tumor virus; JSRV, jaagsiekte sheep retrovirus; MusD, Mus musculus type D retrovirus; MPMV, Mason-Pfizer monkey virus; IAPE, IAP with an envelope gene; HIV-1, human immunodeficiency virus type 1; HTLV-1, human T-cell leukemia virus type 1. (C) Search for full-length, entirely coding MuERV-L copies in the C57BL/6J genome. Blast search was carried out with the NCBI m36 mouse assembly (April 2006 release) and yielded 489 full-length MuERV-L copies among which 350 contain the two complete gag and pol ORFs.
As a first step, we analyzed the developing embryos for MuERV-L protein expression at the very early stages where epsilon particle formation had been observed. To do so, we produced a recombinant protein, corresponding to the complete Gag polyprotein of a cloned MuERV-L element (GenBank accession no. Y12713), that we used to immunize rabbits and raise specific antibodies. Western blot analyses of cell extracts from Swiss (OF1) mouse embryos using this antiserum (Fig. 2A, left panel) revealed a 66-kDa band most probably corresponding to the complete MuERV-L Gag polyprotein (theoretical molecular mass, 64 kDa), observed as well in the Western blot shown in Fig. 2A, right panel, with cells transfected with an expression vector for MuERV-L (see below). As illustrated in Fig. 2A, this MuERV-L Gag product shows a peak of expression in embryos at the two-cell stage (lane 2c, collected 36 h postfertilization), a much-lower expression level at the eight-cell stage (lane 8c, collected 54 h postfertilization), and no expression in immature oocytes (i.e., in the germinal vesicle) (lane GV) or blastocysts (lane Bl), as previously reported for the epsilon particles. Moreover, immunofluorescence analysis of the embryos with the anti-Gag antiserum (Fig. 2B) revealed an intense intracytoplasmic staining at the two-cell stage (Fig. 2B, panel 2c), possibly at the level of the endoplasmic reticulum (ER), consistent again with the electron microscopic observations made in the past. This staining markedly decreases at the eight-cell stage (panel 8c), is not observed in the immature oocyte (panel GV), is abolished when the antiserum is preincubated with an excess of the Gag recombinant protein, thus confirming the specificity of the signal (Fig. 2B, panel 2c + Gag). Similar observations were made with embryos obtained from AKR mice (data not shown).
FIG. 2.
Expression of the MuERV-L Gag protein in the early mouse embryo. (A) Western blot analysis with a rabbit anti-Gag MuERV-L antiserum of germinal vesicles (GV); two-cell stage (2c), eight-cell stage (8c), and blastocyst stage (Bl) mouse embryos (left panel); or human 293T cells transfected with the MuERV-L expression vector or a control (ctrl) plasmid (right panel): the lanes in the left panel correspond to an amount of 60 GV or mouse embryos, collected from mated Swiss (OF1) mice and lysed in Laemmli buffer for sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting. (B) Immunofluorescence confocal analysis of germinal vesicles (GV) and two-cell stage (2c) and eight-cell stage (8c) mouse embryos. The samples were fixed in 4% formaldehyde, permeabilized, and stained with the anti-Gag MuERV-L antiserum and a fluorescein isothiocyanate-conjugated antirabbit immunoglobulin G secondary antibody. Preincubation of the antiserum (30 min at 4°C) with an excess of recombinant MuERV-L Gag protein before staining of the two-cell stage embryos was carried out as a control (2c + Gag).
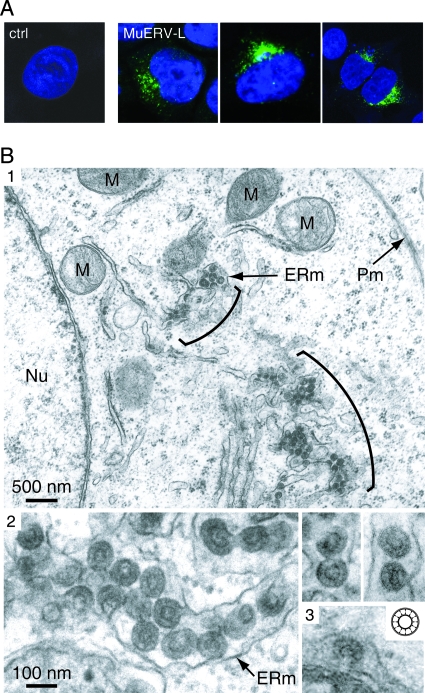
A direct identification of the MuERV-L gene products was then achieved by constructing an expression vector for a full-length, entirely coding MuERV-L element (GenBank accession no. AC084823; nucleotides 186599 to 192994). Since we had previously observed (D. Ribet, unpublished data) that the MuERV-L long terminal repeats (LTRs) contain poorly active promoters, at least in the series of cells in culture commonly used for ex vivo assays, we replaced the U3 domain of the 5′ LTR of the cloned copy with the potent cytomegalovirus promoter. Heterologous human cells were then transfected with this expression vector and analyzed for the MuERV-L gene products. Immunofluorescence analysis of the transfected cells with the anti-Gag antiserum discloses staining in the cell cytoplasm, close to the nucleus, at a location consistent with accumulation within the ER (see also below), not observed with cells transfected with a control plasmid (Fig. 3A). MuERV-L-transfected cells were also analyzed by electron microscopy (Fig. 3B, panels 1 and 2). Forty-eight hours posttransfection, the cells were fixed in 1.6% glutaraldehyde, postfixed in 1% osmium tetroxide, and embedded in Epon 812. Ultrathin sections were stained with uranyl acetate and lead citrate. The cells disclosed intracisternal particles (not observed in cells transfected with a control plasmid) with the canonical epsilon morphology: a central electron-lucent space surrounded by two concentric electron-dense rings separated by a radial array of internal spikes. Budding particles could also be observed at the level of the ER membrane (Fig. 3B, panel 2). Clearly, the structure and site of expression of the observed particles are similar to those of the naturally occurring epsilon particles found in the embryo (Fig. 3B, panel 3) (23). Of note, expression vectors for two other full-length, coding-competent MuERV-L copies (possibly corresponding to distinct MuERV-L burst events within the Mus species; see references 2 and 6) produced particles with an identical morphology.
FIG. 3.
Localization and morphology of MuERV-L-associated viruslike particles. (A) Confocal images of human cells transfected with the MuERV-L vector or a control plasmid (ctrl). Forty-eight hours posttransfection, cells were fixed, permeabilized, and stained with the anti-Gag MuERV-L antiserum and an Alexa fluor 488-conjugated antirabbit immunoglobulin G secondary antibody (in green). Nuclei were stained with TO-PRO-3 iodide (in blue). (B) Electron microscopy of mouse epsilon particles. (1) Representative low-magnification image of 293T cells transfected with MuERV-L disclosing particles accumulated in the cisternae of the ER (brackets). No particles can be observed at the plasma membrane (Pm). ERm, ER membrane; Nu, nucleus; M; mitochondria. (2) High-magnification images of 293T cells transfected with MuERV-L disclosing particles within or budding into the ER. (3) High-magnification image of a naturally occurring epsilon particle budding into the ER of a mouse embryo, and schematic structure of an epsilon particle (inset, adapted with permission from reference 13).
In conclusion, the present study unambiguously demonstrates that the epsilon particles are indeed generated by the MuERV-L endogenous elements. This family of ERVs is therefore the most ancient “parasite” of mammals identified to date still able to generate viruslike particles. Their morphology—not recovered in any present-day infectious retrovirus—is rather unique, and a remaining question to be answered now concerns the possible role of these particles. In particular, the intracisternal localization of the generated epsilon particles is similar to that of the still-functional IAPs, which are also actively transcribed at early stages of mouse embryonic development, albeit with a peak of expression occurring later, at the 8- to 16-cell stages (24), and which transpose at a high rate. Experiments should now be devised to determine whether some present-day MuERV-L elements are still competent for autonomous retrotransposition or whether, as suggested by a recent report, they are mainly used by their host as “early” promoters dispersed throughout the genome (17).
Acknowledgments
We thank E. Pichard for technical assistance and C. Lavialle for critical reading of the manuscript.
This work was supported by the CNRS and by a grant from the Ligue Nationale contre le Cancer (Equipe Labellisée) and a fellowship (from the Association pour la Recherche sur le Cancer) to D.R.
Footnotes
Published ahead of print on 28 November 2007.
REFERENCES
- 1.Bénit, L., N. de Parseval, J.-F. Casella, I. Callebaut, A. Cordonnier, and T. Heidmann. 1997. Cloning of a new murine endogenous retrovirus, MuERV-L, with strong similarity to the human HERV-L element and with a gag coding sequence closely related to the Fv1 restriction gene. J. Virol. 715652-5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bénit, L., J. B. Lallemand, J. F. Casella, H. Philippe, and T. Heidmann. 1999. ERV-L elements: a family of endogenous retrovirus-like elements active throughout the evolution of mammals. J. Virol. 733301-3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernhard, W. 1958. Electron microscopy of tumor cells and tumor viruses; a review. Cancer Res. 18491-509. [PubMed] [Google Scholar]
- 4.Boeke, J. D., and J. P. Stoye. 1997. Retrotransposons, endogenous retroviruses, and the evolution of retroelements, p. 343-435. In J. M. Coffin, S. H. Hughes, and H. E. Varmus (ed.), Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [PubMed]
- 5.Cordonnier, A., J.-F. Casella, and T. Heidmann. 1995. Isolation of novel human endogenous retrovirus-like elements with foamy virus-related pol sequence. J. Virol. 695890-5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costas, J. 2003. Molecular characterization of the recent intragenomic spread of the murine endogenous retrovirus MuERV-L. J. Mol. Evol. 56181-186. [DOI] [PubMed] [Google Scholar]
- 7.Dewannieux, M., A. Dupressoir, F. Harper, G. Pierron, and T. Heidmann. 2004. Identification of autonomous IAP LTR retrotransposons mobile in mammalian cells. Nat. Genet. 36534-539. [DOI] [PubMed] [Google Scholar]
- 8.Greenwood, A. D., C. C. Englbrecht, and R. D. MacPhee. 2004. Characterization of an endogenous retrovirus class in elephants and their relatives. BMC Evol. Biol. 438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kageyama, S., M. Nagata, and F. Aoki. 2004. Isolation of nascent messenger RNA from mouse preimplantation embryos. Biol. Reprod. 711948-1955. [DOI] [PubMed] [Google Scholar]
- 10.Khier, H., S. Bartl, B. Schuettengruber, and C. Seiser. 1999. Molecular cloning and characterization of the mouse histone deacetylase 1 gene: integration of a retrovirus in 129SV mice. Biochim. Biophys. Acta 1489365-373. [DOI] [PubMed] [Google Scholar]
- 11.Kigami, D., N. Minami, H. Takayama, and H. Imai. 2003. MuERV-L is one of the earliest transcribed genes in mouse one-cell embryos. Biol. Reprod. 68651-654. [DOI] [PubMed] [Google Scholar]
- 12.Kuff, E. L., and K. K. Lueders. 1988. The intracisternal A-particle gene family: structure and functional aspects. Adv. Cancer Res. 51183-276. [DOI] [PubMed] [Google Scholar]
- 13.Lasneret, J., M. Canivet, P. Bittoun, and J. Peries. 1981. IdUr induction of a new type of retrovirus-like particle (epsilon-particle) in transformed fibroblastic mouse cells. Ann. Virol. Inst. Pasteur 132151-159. [Google Scholar]
- 14.Lasneret, J., L. Dianoux, J. Lesser, J. Peries, and M. Canivet. 1990. In vitro induction of early mouse embryo intracisternal particles (epsilon particles) in cultured cell lines. Biol. Cell 69205-210. [DOI] [PubMed] [Google Scholar]
- 15.Malik, H. S., and T. H. Eickbush. 2001. Phylogenetic analysis of ribonuclease H domains suggests a late, chimeric origin of LTR retrotransposable elements and retroviruses. Genome Res. 111187-1197. [DOI] [PubMed] [Google Scholar]
- 15a.Mouse Genome Sequencing Consortium. 2002. Initial sequencing and comparative analysis of the mouse genome. Nature 420520-562. [DOI] [PubMed] [Google Scholar]
- 16.Nermut, M. V., and D. J. Hockley. 1996. Comparative morphology and structural classification of retroviruses. Curr. Top. Microbiol. Immunol. 2141-24. [DOI] [PubMed] [Google Scholar]
- 17.Peaston, A. E., A. V. Evsikov, J. H. Graber, W. N. de Vries, A. E. Holbrook, D. Solter, and B. B. Knowles. 2004. Retrotransposons regulate host genes in mouse oocytes and preimplantation embryos. Dev. Cell 7597-606. [DOI] [PubMed] [Google Scholar]
- 18.Ribet, D., M. Dewannieux, and T. Heidmann. 2004. An active murine transposon family pair: retrotransposition of “master” MusD copies and ETn trans-mobilization. Genome Res. 142261-2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ribet, D., F. Harper, M. Dewannieux, G. Pierron, and T. Heidmann. 2007. Murine MusD retrotransposon: structure and molecular evolution of an “intracellularized” retrovirus. J. Virol. 811888-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Svoboda, P., P. Stein, M. Anger, E. Bernstein, G. J. Hannon, and R. M. Schultz. 2004. RNAi and expression of retrotransposons MuERV-L and IAP in preimplantation mouse embryos. Dev. Biol. 269276-285. [DOI] [PubMed] [Google Scholar]
- 21.Wang, Q., Y. G. Chung, W. N. deVries, M. Struwe, and K. E. Latham. 2001. Role of protein synthesis in the development of a transcriptionally permissive state in one-cell stage mouse embryos. Biol. Reprod. 65748-754. [DOI] [PubMed] [Google Scholar]
- 22.Reference deleted.
- 23.Yotsuyanagi, Y., and D. Szollosi. 1980. Embryo development and intracisternal particles in the mouse. Biol. Cell. 39201-204. [Google Scholar]
- 24.Yotsuyanagi, Y., and D. Szöllösi. 1981. Early mouse embryo intracisternal particles: fourth type of retrovirus-like particles associated with the mouse. JNCI 67677-683. [PubMed] [Google Scholar]