Abstract
The molecular mechanisms by which RNA viruses induce apoptosis and apoptosis-associated pathology are not fully understood. Here we show that flock house virus (FHV), one of the simplest RNA viruses (family, Nodaviridae), induces robust apoptosis of permissive Drosophila Line-1 (DL-1) cells. To define the pathway by which FHV triggers apoptosis in this model invertebrate system, we investigated the potential role of Drosophila apoptotic effectors during infection. Suggesting the involvement of host caspases, the pancaspase inhibitor benzyloxycarbonyl-Val-Ala-Asp-fluromethylketone (z-VAD-fmk) prevented FHV-induced cytopathology and prolonged cell survival. RNA interference-mediated ablation of the principal Drosophila effector caspase DrICE or its upstream initiator caspase DRONC prevented FHV-induced apoptosis and demonstrated direct participation of this intrinsic caspase pathway. Prior to the FHV-induced activation of DrICE, the intracellular level of inhibitor-of-apoptosis (IAP) protein DIAP1, the principal caspase regulator in Drosophila melanogaster, was dramatically reduced. DIAP1 was depleted despite z-VAD-fmk-mediated caspase inhibition during infection, suggesting that the loss of DIAP1 was caused by an upstream FHV-induced signal. The RNA interference-mediated knockdown of DIAP1 caused rapid and uniform apoptosis of DL-1 cells and thus indicated that DIAP1 depletion is sufficient to trigger apoptosis. Confirming this conclusion, the elevation of intracellular DIAP1 levels in stable diap1-transfected cells blocked caspase activation and prevented FHV-induced apoptosis. Collectively, our findings suggest that DIAP1 is a critical sensor of virus infection, which upon virus-signaled depletion relieves caspase inhibition, which subsequently executes apoptotic death. Thus, our study supports the hypothesis that altering the level or the activity of cellular IAP proteins is a general mechanism by which RNA viruses trigger apoptosis.
Numerous RNA viruses cause apoptosis of their host cell. As a programmed suicide response widely conserved among vertebrates and invertebrates, apoptosis removes damaged or unwanted cells. These cells undergo chromatin condensation, DNA fragmentation, mitochondrion disruption, and plasma membrane blebbing, which culminate in cytolysis (reviewed in reference 25). Thus, virus-induced apoptosis is associated with cell destruction and tissue damage (14, 21, 37). Because the inhibition of apoptosis can lessen disease severity, virus-induced apoptosis is thought to be a principal contributor to viral pathology (12, 35, 47). Various pathogenic RNA viruses, including the flaviviruses, poliovirus, reovirus, Sindbis virus, human immunodeficiency virus type 1, and others, trigger an apoptotic response in their host (13, 36, 45, 57, 59). Accordingly, diverse stimuli or events, including viral replication factors, disruption of the cell cycle, death receptor engagement, and inhibition of protein synthesis, can induce apoptosis during infection (reviewed in references 43, 53, and 56). However, despite the variety of infection-associated signals, the molecular events that engage death components within the host cell are poorly understood.
Here we report that flock house virus (FHV), one of the simplest animal viruses, induces widespread apoptosis in cultured Drosophila cells. FHV is the best-studied member of the Nodaviridae, a family of small, bipartite RNA viruses infecting insects, fish, and mammals (reviewed in references 1, 17, and 48). The icosahedral FHV virion contains two single-stranded, positive-sense genome RNAs, RNA1 (3.1 kb) and RNA2 (1.4 kb), which encode the RNA-dependent RNA polymerase (RdRp) designated protein A (112 kDa) and capsid precursor α (43 kDa), respectively. FHV RNA replication takes place on the outer mitochondrial membrane (40), and capsid assembly occurs in the nearby cytoplasm (20, 60). During virion maturation, α is cleaved to produce capsid proteins β (38 kDa) and γ (5 kDa) (49). The other major FHV-encoded protein is B2 (11 kDa), which is translated from a subgenomic RNA derived from RNA1. B2 is a potent suppressor of RNA silencing that contributes to FHV multiplication in Drosophila melanogaster (19, 38, 62).
The relative simplicity of FHV, its well-characterized life cycle, and the vigor with which it multiples in Drosophila provide a unique opportunity for investigating the molecular mechanisms by which RNA viruses trigger apoptosis in an organism for which much is known about the host's apoptotic pathways. Here, we define pro- and antiapoptotic factors in Drosophila that participate in FHV-induced apoptosis. Apoptotic execution in Drosophila is strikingly similar to that in mammals, including the required functions of the cysteinyl aspartate-specific proteases known as caspases (reviewed in references 2, 24, 25, and 31). Drosophila encodes seven caspases, including the initiator caspase DRONC and the effector caspase DrICE, which are investigated here. Typical of vertebrate initiator caspases, DRONC proteolytically processes the zymogen proform of DrICE through a series of signal-induced cleavage events to generate an active heterodimeric complex composed of large and small subunits (34, 39, 55). DrICE is an effector caspase that plays a critical role in development- and stress-induced apoptosis (reviewed in reference 24).
Both DRONC and DrICE are subject to negative regulation by Drosophila inhibitor-of-apoptosis (IAP) protein DIAP1. A typical member of the large family of highly conserved IAP proteins (reviewed in references 46 and 54), DIAP1 (48 kDa) is the principal caspase regulator in Drosophila (reviewed in references 24 and 31). DIAP1 binds to effector and initiator caspases through its defining baculovirus IAP repeat motifs. This interaction mediates caspase inhibition or caspase destruction by the ubiquitin proteosome pathway (6, 28, 39, 55, 61). DIAP1 itself is regulated by baculovirus IAP repeat-mediated interactions with proapoptotic effectors, including Reaper, Hid, Grim, Jafrac2, and Sickle. These effectors displace the caspase(s) from DIAP1, liberating the inhibited proteases to execute cell death and destroying DIAP1 (reviewed in references 24 and 58). Recent findings that DIAP1 is destabilized by other unrelated factors, including, for example, Drosophila IκK-related kinase and Drosophila serine protease dOmi (7, 32), confirm the importance of maintaining a critical level of DIAP1 for cell survival. DIAP1 may therefore function as a key sensor of infecting pathogens in which signal-induced destabilization forces cell self-destruction in the face of a viral threat.
We report here that FHV-induced apoptosis in cultured Drosophila Line-1 (DL-1) cells is associated with a dramatic depletion of endogenous DIAP1. The loss of DIAP1 was accompanied by activation of the effector caspase DrICE. Both of the DIAP1-regulated caspases DRONC and DrICE were required for FHV-induced apoptosis. However, caspase activity was not responsible for DIAP1 diminution. Moreover, increasing the intracellular steady-state level of DIAP1 prior to the infection of DL-1 cells was sufficient to prevent FHV-induced apoptosis. Collectively, our findings indicate that DIAP1 is a critical regulator of FHV-triggered cell death. Thus, the invertebrate IAP proteins may function as critical sensors of virus infection capable of executing caspase-dependent suicide as a defense strategy on the part of the infected host.
MATERIALS AND METHODS
Cells and stably transfected cell lines.
DL-1 cells (50) were propagated at 27°C in Schneider's medium (Invitrogen) supplemented with 15% heat-inactivated fetal bovine serum (HyClone). To generate stably transfected cell lines, DL-1 cells were transfected with DNA plasmid pMT-DIAP1HA or pMT-lacZ (see below) by using DOTAP-DOPE {N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium methylsulfate-l-phosphatidylethanolamine, dioleoyl (C18:1, [cis]-9)} and selected by including hygromycin B (Invitrogen) at 300 μg per ml of serum-supplemented Schneider's medium. Drug-resistant cells were pooled and maintained in 300 μg hygromycin B per ml of growth medium. Infections were conducted in the absence of drug selection. Metallothionein promoter-directed gene expression was induced by overlaying stably transfected cells with supplemented Schneider's medium containing 500 μM CuSO4. Where indicated below, infections were conducted in medium containing CuSO4.
Viruses.
FHV (51) was propagated in DL-1 cells with minor modifications from the method described previously (52). FHV stocks were prepared by infecting DL-1 cells at a low multiplicity of infection (MOI) (5 PFU per cell), treating the infected cells and accompanying growth medium with 0.5% NP-40-0.1% β-mercaptoethanol (βME), and purifying released virus by sucrose gradient centrifugation. Virus titers were determined by plaque assay on DL-1 cell monolayers (52) that had been overlaid with 0.6% SeaKem ME agarose containing 62 μg neutral red (Sigma) per ml of Schneider's medium to visualize plaques. Recombinant Autographa californica multicapsid nucleopolyhedrovirus (AcMNPV) vOpIAP (pIE1prmopiapHA/Δ35K/lacZ;Op-iapHA, p35−) was propagated and quantified as described previously (34). DL-1 cell monolayers were inoculated with vOpIAP at an MOI of 20 in TC100 (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum. After 1 h, the inoculum was replaced with supplemented Schneider's medium and the cells were maintained at 27°C.
To quantify one-step virus production, DL-1 cell monolayers were inoculated with FHV (MOI = 50) for 1 h. The cells were washed with phosphate-buffered saline, pH 6.2, and overlaid with supplemented Schneider's medium with or without 200 μM benzyloxycarbonyl-Val-Ala-Asp-fluromethylketone (z-VAD-fmk) (Calbiochem) dissolved in dimethyl sulfoxide (DMSO). The cells and accompanying growth medium were collected 24 h later, treated on ice with 0.5% NP-40-0.1% βME, and clarified by centrifugation (10,000 × g). Infectious FHV was measured by plaque assay.
Cell survival.
DL-1 cell monolayers (2.5 × 106 cells per plate) were mock infected or infected with FHV (MOI = 50) with or without 200 μM z-VAD-fmk or the vehicle DMSO and photographed 24 h later by using an Axiovert 135TV (Zeiss) microscope with IP Lab Spectrum P software as described previously (34). Surviving cells were readily distinguished from cells undergoing apoptotic blebbing. Values of cell survival shown represent the average numbers (± standard deviations) of surviving cells counted from six evenly distributed, nonoverlapping fields of view in a single plate of infected cells and averaged for three independent plates. The survival of DL-1 cells stably transfected with plasmid pMT-DIAP1HA or pMT-lacZ with or without FHV infection and in the presence of 500 μM CuSO4 was determined similarly. All assays that quantified cell survival were preformed multiple times; the results of a representative experiment are presented in each case.
Antisera and immunoblot analyses.
DIAP1-specific antiserum (anti-DIAP1) was generated by immunization of New Zealand White rabbits (University of Wisconsin Medical School Polyclonal Antibody Service) by using antigen consisting of N-terminally hexahistidine (His6)-tagged DIAP1 produced in Escherichia coli strain BL21(DE3) and purified by Ni2+ affinity chromatography as described previously (3). DRONC-specific antiserum (anti-DRONC) was generated similarly by using antigen consisting of C-terminally His6-tagged DRONC that lacked its 113-residue prodomain and that was produced and purified as described above. For immunoblot analysis, intact cells and apoptotic bodies were collected by centrifugation, lysed in 1% sodium dodecyl sulfate (SDS)-1% βME, and subjected to SDS-polyacrylamide gel electrophoresis (PAGE). Proteins were stained with Bio-Safe Coomassie (Bio-Rad) or transferred to Immobilon-P polyvinyl difluoride (Millipore) or nitrocellulose (Osmonics, Inc.) membranes. Immunodetection was conducted with the following antisera diluted as indicated in parentheses: polyclonal rabbit anti-DrICE (1:5,000) (34), polyclonal rabbit anti-FHV protein A (1:6,000) (40), polyclonal rabbit anti-DIAP1 (1:2,000), mouse monoclonal antiactin (1:5,000) (BD Biosciences), polyclonal rabbit anti-DRONC (1:2,000), and mouse monoclonal antihemagglutinin (anti-HA) (1:1,000) (Covance). The membranes were incubated with goat anti-rabbit or goat anti-mouse immunoglobulin G conjugated to alkaline phosphate (Pierce) and developed using the CDP-Star chemiluminescence detection system (Roche).
Caspase assays.
Infected cells were dislodged, collected by centrifugation, and lysed with caspase activity buffer consisting of 10 mM HEPES (pH 7.0), 2 mM EDTA, 5 mM dithiothreitol, 0.1% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate} (Pierce), and 1× protease inhibitor (Roche). The cell lysates were clarified by centrifugation and caspase activity was measured by using the substrate N-acetyl-Asp-Glu-Val-Asp-7-amido-4-methylcoumarin (DEVD-amc) (Sigma) as previously described (33). Values are reported as the average rates of fluorescent-product accumulation (relative light units [RLU]) ± standard deviations at the times after infection indicated in Fig. 2 and 4 for triplicate plates.
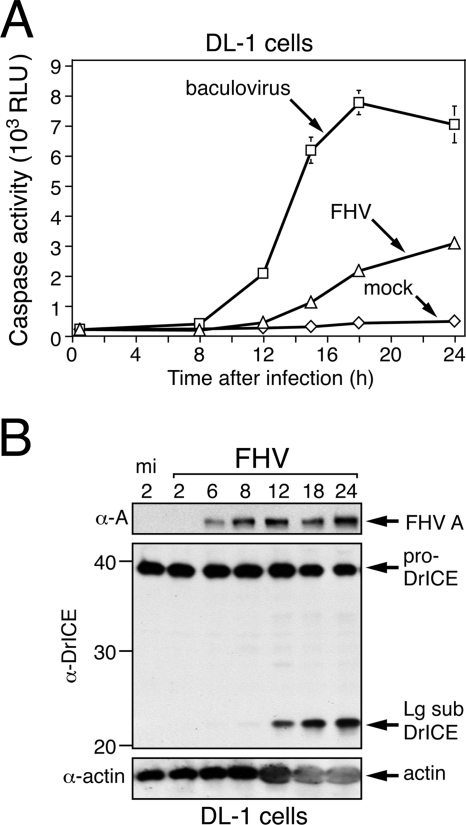
FIG. 2.
FHV induces caspase activation in Drosophila cells. (A) Time course of caspase activity. CHAPS lysates of DL-1 cells prepared at the indicated times (h) after mock infection or infection with FHV (MOI = 50) or the baculovirus vOpIAP (MOI = 20) were assayed for caspase activity by using the tetrapeptide substrate DEVD-amc. Values are shown in RLU and represent the average rates (± standard deviations) of caspase activity determined for triplicate infections. (B) Time course of proDrICE processing. SDS total cell lysates were prepared from DL-1 cells at the indicated times (h) after mock infection (mi) or infection with FHV (MOI = 50) and subjected to immunoblot analysis with anti-protein A (α-A) (top), anti-DrICE (middle), and antiactin (α-actin) (bottom). ProDrICE, large subunit (Lg sub) DrICE, FHV protein A, and Drosophila actin are indicated on the right. Size markers in kilodaltons are indicated on the left. During electrophoresis, FHV capsid protein α migrates with host actin, causing the actin signal to broaden. α, anti.
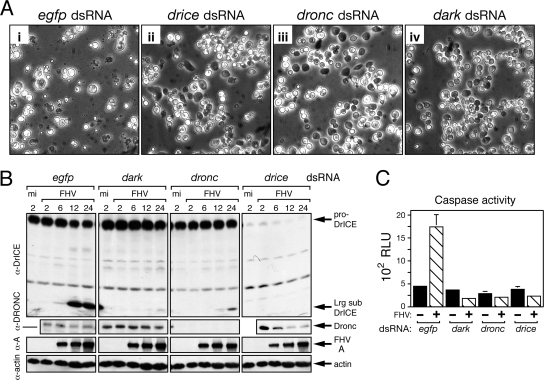
FIG. 4.
RNA silencing of drice, dronc, and dark prevents FHV-induced apoptosis. (A) Cell morphology. DL-1 cells were transfected with egfp-, drice-, dronc-, or dark-specific dsRNA and infected with FHV (MOI = 50) 96 h later. Photographs (magnification, ×500) were taken 24 h after infection. (B) Immunoblot analysis. DL-1 cells were transfected with egfp-, drice-, dronc-, or dark-specific dsRNA and FHV infected as described for panel A. SDS total cell lysates were prepared at the indicated times (h) after mock infection (mi) or FHV infection and subjected to immunoblot analysis by using anti-DrICE, anti-DRONC, anti-protein A, and antiactin (top to bottom, respectively). α, anti; Lg sub, large subunit. (C) Caspase activity. CHAPS lysates of DL-1 cells transfected with the indicated dsRNAs were prepared 24 h after mock infection (−) or FHV infection (+) (MOI = 50) and assayed for intracellular caspase activity by using DEVD-amc as the substrate. Values (RLU) shown represent the average rates of caspase activity (± standard deviations) for triplicate infections. The results of a representative experiment are shown.
Plasmids.
The hygromycin resistance gene (hyg) was inserted into the ie-1 promoter-based expression vector pIE1prm/hr5/PA (5) to create pIE1prm/hr5/hyg/PA. The metallothionein promoter and an AcMNPV polyadenylation (PA) signal were inserted in place of the hr5 element to generate pMTprm/PA/IE1prm/hyg/PA. Lastly, the DIAP1 gene with the influenza virus HA epitope (YPYDVPDYA) inserted at the N terminus of its product (diap1HA) (34) was inserted between the metallothionein promoter and the PA signal to generate plasmid pMTprm/diap1HA/PA/IE1prm/hyg/PA (hereinafter called pMT-DIAP1HA). Similarly, the lacZ gene was inserted to create pMTprm/lacz/PA/IE1prm/hyg/PA (hereinafter called pMT-lacZ). Plasmids used to generate double-stranded RNA (dsRNA) for RNA silencing were created through insertion of all or part of the open reading frames of the genes indicated below into pBS/KS+ (Stratagene). The plasmids used to produce dsRNA specific to egfp (enhanced green fluorescent protein), dark, dronc, and drice have been described previously (34). For diap1-specific dsRNA, a plasmid containing nucleotides 431 to 1795 of diap1 (FlyBase; http://flybase.bio.indiana.edu/) was used (34).
RNA silencing.
The generation of dsRNA and transfection methods were as described previously (34). DL-1 cells transfected with dark-, dronc-, and drice-specific dsRNA by using DOTAP-DOPE were maintained in suspension for 3 to 4 days at 27°C by continuous shaking and then counted, plated, and inoculated with FHV. Cells transfected with diap1-specific RNA were maintained as monolayers until the times indicated in Fig. 5.
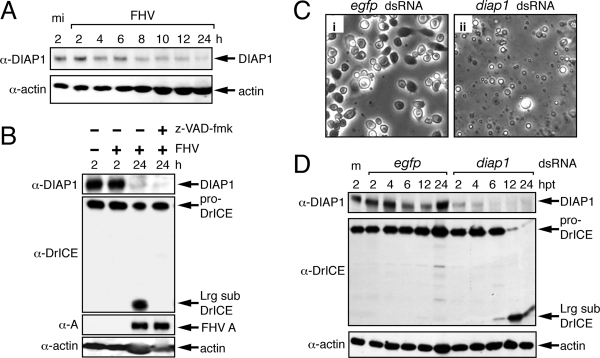
FIG. 5.
FHV depletes endogenous DIAP1. (A) Fate of DIAP1 during infection. SDS total lysates of DL-1 cells prepared at the indicated times (h) after mock infection (mi) or FHV infection (MOI = 50) were subjected to immunoblot analysis with anti-DIAP1 (top) or antiactin (bottom). α, anti. (B) Effect of caspase inhibition on DIAP1 depletion by FHV. SDS total lysates of DL-1 cells prepared at the indicated times (h) after infection with (+) or without (−) FHV in the presence (+) or absence (−) of 200 μM z-VAD-fmk were subjected to immunoblot analysis with anti-DIAP1, anti-DrICE, anti-protein A, and antiactin (top to bottom, respectively). Full-length DIAP1, proDrICE, large-subunit (Lrg sub) DrICE, FHV RdRp protein A, and Drosophila actin are indicated. (C) Cell morphology after RNA silencing. DL-1 cells were transfected with egfp-specific (i) or diap1-specific (ii) dsRNA and photographed (magnification, ×500) 24 h later. (D) Fate of DIAP1 upon RNA silencing. SDS total cell lysates prepared at the indicated times after transfection (hours posttransfection [hpt]) with egfp- or diap1-specific dsRNA were subjected to immunoblot analysis with anti-DIAP1, anti-DrICE, and antiactin (top to bottom, respectively). Lysates of mock-transfected DL-1 cells (m) were included.
Protein radiolabeling.
One hour prior to the times indicated in Fig. 7 after mock or FHV infection, DL cell suspension (2.5 × 106 cells) was withdrawn, collected by centrifugation (284 × g), and suspended in 0.5 ml of phosphate-buffered saline (pH 6.8) containing 100 μCi of Trans 35S Label (1,175 Ci/mmol, ≥70% methionine, ≤15% cysteine; ICN Biomedicals, Inc.) per ml. After a 1-h period of agitation at 27°C, the cells were collected, lysed in 1% SDS-1% βME, and subjected to SDS-PAGE, followed by autoradiography or fluorography. The radiolabeling of host proteins was quantified by using a Typhoon 9400 scanner and ImageQuant software (GE Healthcare Life Sciences). Values for relative levels of host protein synthesis are reported as the average signal intensities (± standard deviations) of host proteins in FHV-infected cells (excluding FHV proteins A, B2, and α) divided by the signal intensities of host proteins in mock-infected cells at 2 h after infection, as determined from duplicate infections.
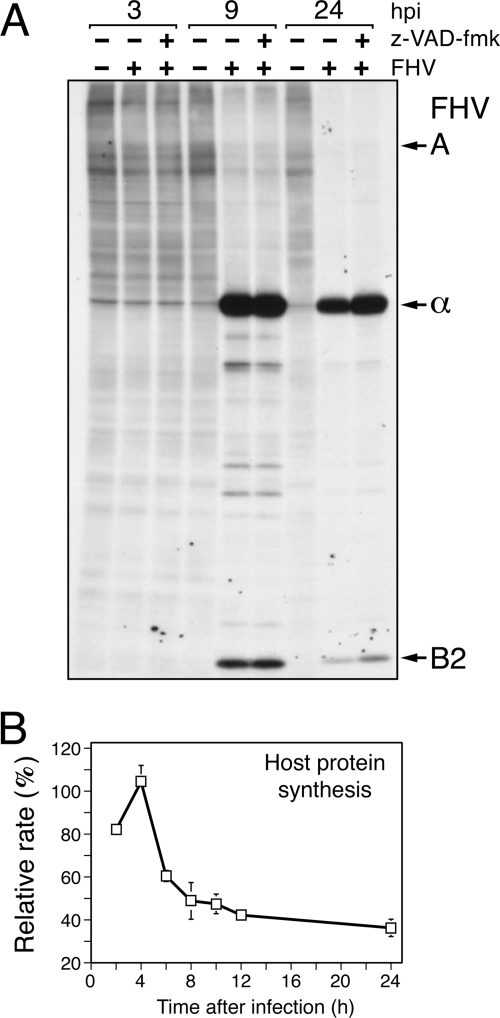
FIG. 7.
FHV inhibits host protein synthesis. (A) Radiolabeling. DL-1 cells were mock infected (−) or FHV infected (+) (MOI = 50) in the presence (+) or absence (−) of 200 μM z-VAD-fmk. The DMSO vehicle was included in each infection mixture. At the indicated times (hours postinfection [hpi]), the cells were incubated for 1 h with [35S]methionine-cysteine, collected, and lysed. Total cell lysates were subjected to SDS-PAGE and fluorography. FHV protein A (112 kDa), capsid α (43 kDa), and B2 (11 kDa) are indicated. (B) Rates of protein synthesis. The levels of all radiolabeled host proteins of mock-infected or FHV-infected DL-1 cells were quantified after electrophoretic analysis by using a Typhoon 9400 scanner. FHV proteins A, α, and B2 were excluded. The values for relative rates of host protein synthesis are reported as percentages of the average signal intensities (± standard deviations) of host proteins radiolabeled at the indicated times after infection with FHV compared to those of host proteins radiolabeled 2 h after mock infection, as determined from duplicate infections. The results of a representative experiment are shown.
RESULTS
FHV induces apoptosis during multiplication in Drosophila cells.
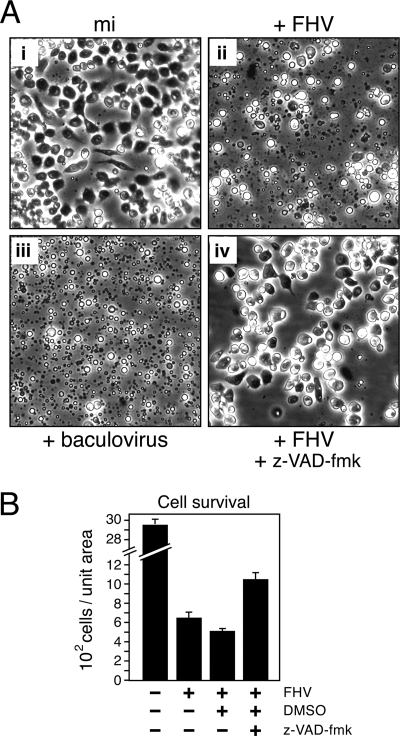
FHV causes extensive cytolysis upon the infection of DL-1 cells (11). The cytopathic morphology includes widespread membrane blebbing and vesicle formation (Fig. 1A, panel ii), which is similar to that caused by inoculation with an apoptosis-inducing baculovirus (Fig. 1A, panel iii). To determine whether apoptosis was the cause of the virus-induced cytolysis, we first tested the effect of caspase inhibition on these morphological effects. When added to DL-1 cells at the time of infection, the cell-permeable pancaspase inhibitor z-VAD-fmk suppressed FHV-induced membrane blebbing and cytolysis, as a majority of these cells remained intact (Fig. 1A, panel iv). The caspase inhibitor also extended the viability of FHV-infected DL-1 cells (Fig. 1B). By 24 h after infection, the survival of z-VAD-fmk-treated cells was twofold higher than that of untreated cells or cells treated with the DMSO vehicle only. Nonetheless, cell growth was slowed or halted since mock-infected cells were two- to threefold more abundant than z-VAD-fmk-treated FHV-infected cells (Fig. 1B). We concluded that FHV-induced cytopathology was caspase dependent.
FIG. 1.
FHV induces an apoptosis-like cytopathology in DL-1 cells. (A) Cell morphology. Plated DL-1 cells were mock infected (mi) (i), inoculated with FHV (MOI = 50) (ii and iv), or inoculated with the baculovirus vOpIAP (MOI = 20) (iii). Infected cells were incubated in the absence (i, ii, and iii) or presence (iv) of the pancaspase inhibitor z-VAD-fmk (200 μM) and photographed (magnification, ×500) 24 h later. The DMSO vehicle was included in all infection mixtures (i to iv). (B) Cell survival. Intact DL-1 cells that were inoculated 24 h earlier with (+) or without (−) FHV (MOI = 50) in the presence (+) or absence (−) of z-VAD-fmk or the DMSO vehicle were counted by using computer-aided microscopy. Values shown are the average numbers (± standard deviations) of surviving cells counted from six evenly distributed, nonoverlapping fields of view in a single plate of infected cells and averaged for three independent plates. The results of a representative experiment are shown.
To verify the involvement of host Drosophila caspases in FHV-induced cytopathic effects, we monitored caspase activity during the course of infection. By using DEVD-amc as a substrate, effector caspase activity was detected in cytoplasmic extracts of DL-1 cells by 12 h after infection (Fig. 2A), the time at which cell membrane blebbing was initiated. By 24 h, caspase activity was sixfold higher than that in mock-infected cells but lower than that in cells inoculated with an apoptosis-inducing baculovirus (Fig. 2A). The rise in FHV-induced caspase activity paralleled the proteolytic processing of the Drosophila effector caspase DrICE (Fig. 2B). Activation of DrICE requires processing of its inactive proform to large and small subunits (16). In DL-1 cells, the large subunit of DrICE was detected between 8 and 12 h after infection and its level increased through 24 h after infection (Fig. 2B). Thus, caspase activity and the accumulation of DrICE subunits coincided with FHV-induced cytopathology. We concluded that FHV induces a caspase-dependent apoptotic response in DL-1 cells.
FHV multiplication is not affected by apoptosis.
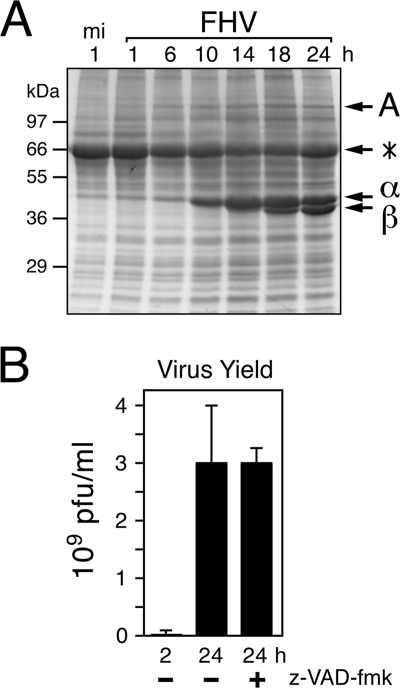
It has been postulated that apoptosis represents an antivirus response, because premature suicide of the infected host cell often prevents or reduces virus replication (reviewed in references 43 and 56). Despite the widespread apoptosis and cytolysis in DL-1 cells, FHV multiplication was vigorous, as indicated by the high-level intracellular accumulation of FHV proteins A, α, and β during the course of infection (Fig. 3A). Nonetheless, to determine the possible effects of apoptosis on virus replication, we compared yields of infectious progeny in the presence and absence of the pancaspase inhibitor z-VAD-fmk. As determined by plaque assay, caspase inhibition had little effect on virus production at 24 h after infection (Fig. 3B). We concluded that apoptosis has no effect on FHV multiplication in DL-1 cells. Also, caspase activity is not required for FHV replication or the maturation of infectious virus.
FIG. 3.
Suppression of apoptosis has no effect on FHV yields. (A) Time course of FHV protein accumulation. SDS total cell lysates prepared from mock-infected (mi) or FHV-infected DL-1 cells at the indicated times (h) after infection were subjected to SDS-PAGE and stained with Coomassie blue. FHV RdRp A, procapsid α, and mature capsid β are indicated on the right, along with bovine serum albumin (*) residing in the culture medium. (B) Production of infectious virus. Virus yields at the indicated times (h) after FHV infection in the presence (+) or absence (−) of 200 μM z-VAD-fmk were determined by plaque assay using DL-1 cells. Values are reported in PFU per ml and represent the averages (± standard deviations) obtained for triplicate infections. The results of a representative experiment are shown.
DrICE, DRONC, and Dark are required for FHV-induced apoptosis.
To investigate the mechanism by which FHV triggers apoptosis in its host cell, we next identified components in the Drosophila cell death pathway that are required for FHV-induced apoptosis. Because DrICE is a central component in the Drosophila apoptotic pathway (reviewed in reference 24) and DrICE was processed (Fig. 2B), we first tested the involvement of this effector caspase in FHV-induced apoptosis. To this end, we used RNA silencing to deplete endogenous DrICE and then assessed the effect on the sensitivity of DL-1 cells to FHV-induced apoptosis. As a control for effects of dsRNA transfection, we used a dsRNA specific to the nonhomologous egfp gene. Upon the transfection of egfp-specific dsRNA, DL-1 cells remained susceptible to UV-induced apoptosis, whereas transfection with drice-specific dsRNA conferred resistance to UV-induced cell death (data not shown). Thus, functional inactivation of DrICE by RNA silencing was confirmed. Upon FHV inoculation, egfp-silenced cells underwent apoptosis but drice-silenced cells did not (Fig. 4A, compare panels i and ii). Immunoblot analysis indicated that proDrICE was depleted in drice-silenced cells and that no DrICE processing was detected (Fig. 4B). Confirming the absence of active DrICE, FHV failed to increase intracellular caspase activity in drice-silenced cells as measured by DEVD-amc cleavage in cytoplasmic extracts (Fig. 4C). In contrast, FHV triggered normal DrICE processing in egfp-silenced cells and intracellular caspase activity was three- to fourfold higher than that of uninfected cells (Fig. 4B and C). Nonetheless, the accumulation of FHV RdRp protein A in drice-silenced cells was comparable to that in the control cells (Fig. 4B). This finding indicated that RNA silencing did not affect FHV entry or replication and suggested that virus-induced apoptotic signaling was normal. Since drice-specific dsRNA eliminated endogenous DrICE, reduced caspase activity, and prolonged virus-infected cell survival, we concluded that DrICE is a host component required for FHV-induced apoptosis.
In many Drosophila tissues, DrICE proteolytic processing and activation requires initiator caspase DRONC and its cofactor Dark (2, 24, 31). Thus, to determine the role of DRONC and Dark during infection, we treated DL-1 cells with dronc- or dark-specific dsRNA and tested for sensitivity to FHV-induced apoptosis. Demonstrating that dronc and dark were functionally inactivated, transfection with either dronc- or dark-specific dsRNA conferred resistance to UV-induced apoptosis (data not shown). Furthermore, dronc-specific dsRNA depleted DRONC below the levels of immunoblot detection (Fig. 4B). Upon FHV infection, dronc- and dark-silenced cells failed to undergo apoptosis (Fig. 4A, panels iii and iv). Levels of accumulation of FHV protein A were comparable in all dsRNA-transfected cells, indicating that virus entry and replication was normal (Fig. 4B). Unlike in egfp-specific-dsRNA-treated cells, the processing of proDrICE was blocked upon the silencing of dronc and dark. Confirming this finding, intracellular caspase activity in FHV-infected cells silenced for dronc and dark was as low as that of uninfected cells (Fig. 4C). We concluded that DRONC and Dark are also required for FHV-induced apoptosis through their role as activators of effector caspases, including DrICE. Thus, the mechanism by which FHV triggers apoptosis likely involves upstream effectors that regulate the activation of these Drosophila caspases.
FHV depletes endogenous levels of DIAP1.
In Drosophila, DIAP1 is essential for cell survival through the negative regulation of caspases, including DRONC and DrICE (6, 28, 39, 55, 63, 64). Our finding that DrICE, DRONC, and Dark participate in FHV-induced apoptosis suggested that DIAP1 is also involved. To test this possibility, we first monitored the levels of endogenous DIAP1 during the course of FHV infection. Immunoblot analysis using our affinity-purified polyclonal anti-DIAP1 readily detected DIAP1 in uninfected DL-1 cells (Fig. 5A). However, as infection progressed, the levels of DIAP1 declined markedly; actin levels demonstrated equivalent protein loadings (Fig. 5A). By 24 h after infection, DIAP1 was at or below the level of immunoblot detection. To determine if DIAP1 depletion was caspase mediated and thus was an indirect result of FHV-induced apoptosis, we tested the effect of caspase inhibition on DIAP1 levels. DIAP1 depletion occurred in the presence or absence of z-VAD-fmk (Fig. 5B). The absence of processed DrICE (large subunit) in z-VAD-fmk-treated cells (Fig. 5B) demonstrated that caspase inhibition was accomplished. DIAP1 levels also declined in FHV-infected DL-1 cells silenced for dronc and dark (data not shown). Collectively, these findings indicated that FHV depletes endogenous DIAP1 by a caspase-independent mechanism.
To next determine if the loss of endogenous DIAP1 is sufficient to trigger apoptosis in DL-1 cells, we monitored caspase activation and apoptotic cytolysis as a function of time after the RNA silencing-mediated depletion of DIAP1. When transfected with diap1-specific dsRNA, DL-1 cells underwent apoptosis that encompassed all cells by 24 h after transfection (Fig. 5C). As expected, endogenous DIAP1 levels were significantly reduced in diap1-silenced cells compared to levels in control egfp-silenced cells (Fig. 5D, top). Moreover, proDrICE was processed to its active large subunit (Fig. 5D, middle), which coincided with the onset of apoptosis in the diap1-silenced cells. As expected, neither DrICE processing nor apoptosis occurred in cells transfected with egfp dsRNA. We concluded that DIAP1 depletion is sufficient to induce apoptosis. Thus, like DIAP1 in cultured Drosophila S2 cells (27, 41, 66), DIAP1 in DL-1 cells appears to repress a constitutive proapoptotic signal. This finding is consistent with a mechanism in which the FHV-mediated reduction of DIAP1 activates apoptosis in host Drosophila cells.
Overexpression of DIAP1 inhibits FHV-induced apoptosis.
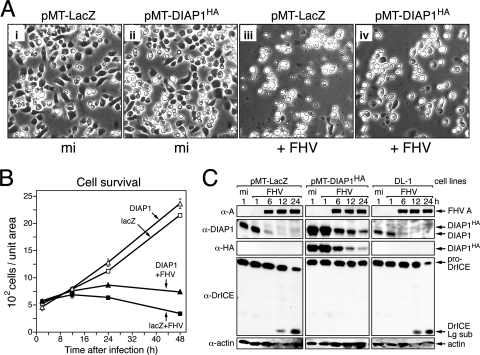
If the FHV-induced depletion of DIAP1 is the trigger for apoptosis, we reasoned that increasing the intracellular levels of DIAP1 by overexpression would compensate for the virus-mediated loss of DIAP1 and thereby eliminate apoptosis. To test this possibility, we generated stably transfected, hygromycin B-resistant DL-1 cell lines that used the metallothionein promoter (pMT) to direct the inducible expression of diap1 or lacZ, as a control. An N-terminal epitope tag (HA) was used to distinguish overexpressed DIAP1HA from endogenous DIAP1. The functionality of overexpressed DIAP1HA was verified by the increased resistance of pMT-DIAP1HA cells to UV radiation-induced apoptosis compared to that of pMT-LacZ cells (data not shown). Neither DIAP1HA nor β-galactosidase had any obvious effects on growth rates or the morphology of the stably transfected DL-1 cells (Fig. 6A, panels i and ii).
FIG. 6.
Overexpression of DIAP1HA prevents FHV-induced apoptosis. (A) Effect on cell morphology. Plated pMT-LacZ (i and iii) and pMT-DIAP1HA (ii and iv) stably transfected DL-1 cell lines were treated with Cu2+ to induce gene expression and either mock infected (mi) (i and ii) or infected with FHV (MOI = 50) (iii and iv). Photographs (magnification, ×500) were taken 24 h later. (B) Cell survival. Cells stably transfected with pMT-DIAP1HA (triangles) or pMT-LacZ (squares) were mock infected (open symbols) or infected (filled symbols) with FHV (MOI = 50). Intact, surviving cells were quantified at the indicated times (h) after infection as described in the legend to Fig. 1B. Values reported are the average numbers (± standard deviations) of cells counted from six evenly distributed, nonoverlapping fields of view in a single plate of infected cells and averaged for three independent plates. The results of a representative experiment are shown. (C) Fate of DIAP1 and DrICE. pMT-LacZ, pMT-DIAP1HA, and parental DL-1 cell lines were mock infected or infected with FHV (MOI = 50) in the presence of Cu2+. SDS total cell lysates prepared at the indicated times (h) after infection were subjected to immunoblot analysis with anti-protein A, anti-DIAP1, anti-HA, anti-DrICE, and antiactin (top to bottom, respectively). α, anti; Lg sub, large subunit.
To determine the effect of DIAP1HA overexpression on FHV-induced apoptosis, the stable cell lines were treated with Cu2+ to induce gene expression and inoculated 24 h later with FHV. The pMT-DIAP1HA cells failed to exhibit the typical cytolysis and membrane blebbing that was widespread among pMT-LacZ cells (Fig. 6A, panels iii and iv). DIAP1HA overexpression extended the viability of FHV-infected DL-1 cells (Fig. 6B). By 48 h, the survival of infected pMT-LacZ control cells was reduced to less than 50% of that of infected pMT-DIAP1HA cells, which were protected from apoptosis. These data also showed that FHV halts host cell growth since mock-infected cell numbers continued to increase over the 48-h period, whereas FHV-infected pMT-DIAP1HA cells remained relatively constant (Fig. 6B). Indicating that pMT-DIAP1HA cells supported FHV replication, the level and timing of FHV protein A synthesis were comparable to those of pMT-LacZ and parental DL-1 cells (Fig. 6C). In addition, the 48-h yield of infectious FHV from DIAP1HA-overproducing cells ([7.7 ± 1.3] × 109 PFU/ml) was comparable to that in control pMT-LacZ cells ([7.8 ± 3.5] × 109 PFU/ml). Consistent with the lack of apoptosis, proDrICE was not processed in pMT-DIAP1HA cells but was processed in apoptotic pMT-LacZ and parental cells (Fig. 6C). As expected, DIAP1 levels in the pMT-DIAP1HA cells were significantly higher than the level in the control cells. Although DIAP1 levels declined in each cell line during infection, the 24-h level of DIAP1 in pMT-DIAP1HA cells was significantly higher than those in the two cell lines undergoing apoptosis (Fig. 6C). We concluded that the overexpression of DIAP1 conferred resistance to FHV-induced apoptosis. Thus, our findings are consistent with a mechanism involving the FHV-mediated depletion of DIAP1, which leads to effector caspase activation and subsequent apoptotic death.
FHV inhibits host protein synthesis in Drosophila cells.
Our findings suggested that DIAP1 disappearance is the result of an upstream, virus-mediated signaling event. Here, we considered the simple possibility that FHV depletes DIAP1 indirectly by inhibiting host protein synthesis. Thus, by virtue of its short (30- to 45-min) half-life, DIAP1 would turn over faster than it is synthesized, thereby reducing the intracellular pool of DIAP1 (63, 65). To determine the extent to which FHV affects host protein synthesis, we first measured rates of synthesis by radiolabeling DL-1 cells at intervals after infection with [35S]methionine-cysteine. Whereas FHV α and B2 were the most prominent proteins synthesized, radiolabeling of host protein declined markedly through infection (Fig. 7A). The decrease in host protein synthesis was most pronounced between 4 and 8 h after infection, as indicated by a quantitative analysis of radiolabeled host proteins (Fig. 7B). To determine if caspase activity, and thus apoptosis, was responsible for this virus-induced translational arrest, we compared the radiolabeling of host proteins in the presence of the caspase inhibitor z-VAD-fmk with that in its absence. Caspase inhibition had no effect on the FHV-mediated inhibition of host protein synthesis (Fig. 7A). Thus, the observed shutoff of host protein synthesis was caspase independent. We concluded that FHV inhibits host protein synthesis and that this shutoff may contribute to DIAP1 depletion.
DISCUSSION
RNA viruses induce apoptosis, which often accounts for the viral pathogenesis exhibited by an infected host. However, the molecular mechanisms by which these viruses trigger apoptosis by engaging the host cell's death machinery are only now being defined. We have used the experimentally advantageous model system of Drosophila to identify host cell components that participate in apoptosis induced by a relatively simple but prolific RNA virus, FHV. We report here that FHV induces apoptosis in cultured Drosophila cells by activating a prototypical initiator-effector caspase pathway. Our findings indicated that cellular DIAP1 negatively regulates this caspase pathway and suggested that the FHV-induced destruction of DIAP1 is the trigger for apoptosis. Thus, our study is consistent with the hypothesis that DIAP1 functions as a critical sensor of virus infection that can initiate host cell suicide in an attempt to eliminate the virus threat. Moreover, the cellular-IAP-regulated activation of caspases may represent a general mechanism by which host cells respond to virus infections.
FHV-induced activation of the Drosophila DRONC→DrICE pathway.
We have shown that FHV triggers caspase-mediated apoptosis in cultured DL-1 cells. RNA-mediated gene silencing indicated that the effector caspase DrICE is necessary for this suicide response (Fig. 4). DrICE is also required for the apoptosis that is induced in DL-1 cells by inoculation with the baculovirus AcMNPV (34), a large DNA virus infecting lepidopteran hosts (moths and butterflies). Thus, DrICE mediates not only development- and stress-induced apoptosis in Drosophila (24, 31) but also apoptosis triggered by either RNA or DNA viruses. The observed requirement of DRONC and Dark (Fig. 4), the apical caspase and its cofactor involved in normal development and stress responses in Drosophila (24), indicated that FHV triggers the activation of the classical DRONC/Dark→DrICE pathway, which executes cell death (Fig. 8). It is possible that other Drosophila caspases, including effector caspase DCP-1, function in this FHV-induced pathway.
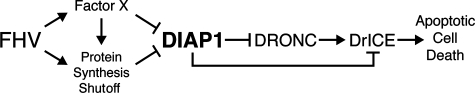
FIG. 8.
Model for induction of apoptosis by FHV. During infection, intracellular levels of DIAP1 are reduced indirectly by virus-mediated inhibition of host protein synthesis or directly through the activity of an unidentified DIAP1 antagonist, designated factor X. Factor X is a virus or host factor that inactivates and destabilizes DIAP1 or does so by contributing to host protein synthesis shutoff that also depletes DIAP1 because of DIAP1's short half-life. The loss of DIAP1 releases the inhibition of the initiator caspase DRONC, which subsequently activates the effector caspase DrICE. Both caspases and possibly others are required for FHV-induced apoptosis. DIAP1 plays a central role in arbitrating virus-induced cell death since an increase or decrease in the steady-state level of DIAP1 prevents or triggers apoptosis, respectively.
FHV-induced apoptosis by DIAP1 depletion.
The participation of DRONC and its involvement in DrICE activation (Fig. 4) implicated DIAP1 in FHV-induced apoptosis. By virtue of its negative regulation of caspases, DIAP1 is a critical survival factor in Drosophila (24, 31, 58). In particular, the steady-state level of intracellular DIAP1 is a key determinant of cell survival (27, 41, 66). As such, DIAP1 is subject to multiple levels of regulation that affect its concentration and anticaspase activities (see below). FHV infection reduced DIAP1 levels at a rate inversely proportional to caspase activation (Fig. 5 and 6). Although DIAP1 binds and inhibits active DrICE and inhibits DRONC by binding and stimulating DRONC degradation, DIAP1 is also cleaved by caspases (6, 15, 28, 39, 42, 55, 63, 64). However, the possibility that active caspases depleted DIAP1 during infection was ruled out by the finding that the pancaspase inhibitor z-VAD-fmk had no effect on the virus-induced loss of DIAP1 (Fig. 5). We concluded that DIAP1 depletion is mediated by an event occurring upstream of caspase activation.
Several of our experimental observations suggested that the loss of DIAP1 was responsible for FHV-induced caspase activation. First, when RNA silencing was used to deplete DIAP1, DL-1 cells underwent rapid and uniform apoptosis with concomitant DrICE activation (Fig. 5C and D). Thus, DIAP1 depletion is sufficient to trigger apoptosis, suggesting that DIAP1 inhibits a constitutive apoptotic signal in DL-1 cells, like that of Drosophila S2 cells (27, 41, 65, 66). Second, when the intracellular level of DIAP1 was raised through inducible diap1HA expression, DL-1 cells were protected from FHV-induced apoptosis (Fig. 6). Interestingly, the level of overexpressed DIAP1 was also reduced during FHV infection but never below the level of detection. This observation suggested that overexpressed DIAP1 was subject to the same mechanism of depletion, but because DIAP1 levels were sufficiently high, caspase activation was blocked. Collectively, these findings indicated that DIAP1 regulates the caspases responsible for FHV-induced apoptosis (Fig. 8).
Mechanism of FHV-mediated depletion of DIAP1.
Considering the short half-life (30 to 45 min) of DIAP1, a straightforward explanation for the observed loss of DIAP1 during infection involves the virus-induced inhibition of host protein synthesis. In a pattern typical of many RNA viruses, FHV caused a rapid inhibition of host protein synthesis despite accelerated virus protein synthesis (Fig. 7). The shutdown of protein synthesis coincided with DIAP1 depletion and DrICE activation. Because DRONC and DrICE are significantly more stable than DIAP1 (65; E. Settles, E. Lannan, and P. Friesen, unpublished data), virus-mediated inhibition of DIAP1 synthesis depletes DIAP1 more rapidly than the caspases, thereby relieving the DIAP1-mediated inhibition of DRONC and allowing caspase activation (Fig. 8).
The inhibition of host protein synthesis is associated with the onset of apoptosis by several RNA viruses, including arthropod-vectored vesicular stomatitis virus and bunyavirus (8, 30). The bunyavirus nonstructural protein NSs is a host protein synthesis inhibitor that bears sequence similarity to the Drosophila prodeath protein Reaper and exhibits analogous proapoptotic activity (8). NSs, like Reaper and the Drosophila prodeath factor Grim, contribute to apoptotic induction by blocking the synthesis of short-lived survival factors like DIAP1 (8, 26, 65). The proapoptotic activities of Reaper and Grim are also attributed to direct the antagonism of DIAP1 through binding and destabilization (24, 58). Similarly, Drosophila prodeath HID, Sickle, Jafrac2, and other factors, including Omi, D. melanogaster, IκKɛ, and Hippo (7, 23, 32, 44), degrade DIAP1 or regulate its activity. Thus, these IAP antagonists are additional host factor candidates that may contribute to FHV-induced DIAP1 depletion (Fig. 8).
Black beetle virus, an alphanodavirus closely related to FHV, inhibits host protein synthesis by a mechanism involving a direct competition between viral and host mRNAs for limited translational machinery (18). Due to the relative abundance of FHV messenger-active RNA (1, 48), an FHV shutdown of host protein synthesis could involve a similar competition. Greasy grouper nervous necrosis virus, a fish betanodavirus, also induces apoptosis (22). The proapoptotic activity of greasy grouper nervous necrosis virus is linked to capsid precursor α. Thus, a comparison of nodavirus gene products and their capacities to affect host protein synthesis or cellular IAP levels should provide insight into the mechanisms promoting nodavirus-induced apoptosis.
Invertebrate IAP proteins as sensors of virus infection.
Our study suggests that DIAP1 functions as a critical sensor of FHV replication. By virtue of its short half-life, instability, and sensitivity to inactivation by diverse mechanisms, DIAP1 is well adapted to function as a general sentinel for virus infection. Moreover, DIAP1 has the regulatory activity necessary to initiate suicide in an attempt to eliminate the virus pathogen. Consistent with this role in host security, preliminary experiments indicated that the infection of Drosophila cells with the DNA baculovirus AcMNPV also depletes intracellular DIAP1 (K. Schultz, E. Settles, and P. Friesen, unpublished results). This depletion correlated with widespread apoptosis, which was suppressed by increasing intracellular levels of DIAP1. It will be of interest to assess the in vivo role of DIAP1 in affecting virus infection in Drosophila larvae and adults and to determine whether IAP proteins of other insects function similarly. It is relevant in this regard that apoptosis induced by mammalian reovirus is also associated with the depletion of cellular IAP proteins, including the caspase inhibitor XIAP (29). Like that of FHV, the reovirus-mediated depletion of IAP proteins is accompanied by the activation of human initiator caspase-9, a DRONC homologue, and human effector caspase-3, a DrICE homologue. This interesting finding raises the possibility that IAP protein depletion may be a general mechanism for virus-induced apoptosis that extends to vertebrates.
Role of apoptosis in FHV multiplication and pathology.
FHV represents the first alphanodavirus demonstrated to induce apoptosis in permissive cells. For some viruses, the premature loss of cell viability due to apoptosis severely limits virus yields (reviewed in references 43 and 56). Surprisingly, apoptosis had little or no effect on FHV multiplication in DL-1 cells (Fig. 3). Although the caspase inhibitor z-VAD-fmk and DIAP1 overexpression prolonged the survival of infected cells, there was no effect on the yield of infectious FHV within the first 24 to 48 h of infection (Fig. 1B, 3B, and 6B). Thus, caspase activity was not required for FHV multiplication. Indeed, significant virion maturation (49) indicated by the capsid cleavage of α to β was observed (Fig. 3A) prior to the morphological signs of apoptosis, indicating that the virus' life cycle is completed before the onset of cell death. Thus, apoptosis has little impact on virus replication events in cultured DL-1 cells. Interestingly, FHV also caused caspase-mediated apoptosis upon the inoculation of Schneider Line 2 (S2) cells (E. Settles and P. Friesen, unpublished observations), which are commonly used for studies of Drosophila apoptosis (16, 41, 42, 63, 66). However, despite comparable levels of FHV production, S2 cells exhibited limited morphological changes, lower levels of intracellular caspase activity, and less DrICE processing than DL-1 cells (data not shown). The molecular mechanism responsible for this reduced response to FHV requires additional study.
It is likely that apoptosis plays a role in FHV-mediated pathology in infected insects. FHV is a potent pathogen of both Drosophila melanogaster and Anopheles gambiae adults, causing morbidity and mortality of from 50 to 100% in a dose-dependent manner (10, 19, 62). FHV induces morphological defects during infection and is disseminated throughout the insect to diverse tissues, including the fat body, the midgut, muscle, and the head (9, 19). These observations raise the interesting possibility that apoptosis facilitates FHV cell-to-cell spread, perhaps by the transport of FHV through apoptotic vesicles in a manner analogous to that of the invertebrate ascoviruses (4). Further studies of nodavirus infections in flies and mosquitoes should provide important insight into the role of apoptosis in viral pathology and virus spread in insects that vector medically relevant diseases in humans.
Acknowledgments
We thank Bruce Hay, Sharad Kumar, Paul Ahlquist, and Annette Schneemann for reagents used in this study. We also thank Diccon Fiore, Melinda Brady-Osborne, Erica Lannan, and Duy Tran for assistance with cell line generation and the preparation of the DIAP1 and DRONC antisera.
This work was supported in part by Public Health Service grants AI40482 and AI25557 from the National Institute of Allergy and Infectious Diseases (P.D.F.).
Footnotes
Published ahead of print on 7 November 2007.
REFERENCES
- 1.Ball, L. A., and K. L. Johnson. 1998. Nodaviruses of insects, p. 225-267. In L. K. Miller and L. A. Ball (ed.), The insect viruses. Plenum Press, New York, NY.
- 2.Bao, Q., and Y. Shi. 2007. Apoptosome: a platform for the activation of initiator caspases. Cell Death Differ. 1456-65. [DOI] [PubMed] [Google Scholar]
- 3.Bertin, J., S. M. Mendrysa, D. J. LaCount, S. Gaur, J. F. Krebs, R. C. Armstrong, K. J. Tomaselli, and P. D. Friesen. 1996. Apoptotic suppression by baculovirus P35 involves cleavage by and inhibition of a virus-induced CED-3/ICE-like protease. J. Virol. 706251-6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bideshi, D. K., Y. Tan, Y. Bigot, and B. A. Federici. 2005. A viral caspase contributes to modified apoptosis for virus transmission. Genes Dev. 191416-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cartier, J. L., P. A. Hershberger, and P. D. Friesen. 1994. Suppression of apoptosis in insect cells stably transfected with baculovirus p35: dominant interference by N-terminal sequences p35(1-76). J. Virol. 687728-7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chai, J., N. Yan, J. R. Huh, J. W. Wu, W. Li, B. A. Hay, and Y. Shi. 2003. Molecular mechanism of Reaper-Grim-Hid-mediated suppression of DIAP1-dependent Dronc ubiquitination. Nat. Struct. Biol. 10892-898. [DOI] [PubMed] [Google Scholar]
- 7.Challa, M., S. Malladi, B. J. Pellock, D. Dresnek, S. Varadarajan, Y. W. Yin, K. White, and S. B. Bratton. 2007. Drosophila Omi, a mitochondrial-localized IAP antagonist and proapoptotic serine protease. EMBO J. 263144-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colon-Ramos, D. A., P. M. Irusta, E. C. Gan, M. R. Olson, J. Song, R. I. Morimoto, R. M. Elliott, M. Lombard, R. Hollingsworth, J. M. Hardwick, G. K. Smith, and S. Kornbluth. 2003. Inhibition of translation and induction of apoptosis by Bunyaviral nonstructural proteins bearing sequence similarity to reaper. Mol. Biol. Cell 144162-4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dasgupta, R., L. L. Cheng, L. C. Bartholomay, and B. M. Christensen. 2003. Flock house virus replicates and expresses green fluorescent protein in mosquitoes. J. Gen. Virol. 841789-1797. [DOI] [PubMed] [Google Scholar]
- 10.Dasgupta, R., H. M. Free, S. L. Zietlow, S. M. Paskewitz, S. Aksoy, L. Shi, J. Fuchs, C. Hu, and B. M. Christensen. 2007. Replication of flock house virus in three genera of medically important insects. J. Med. Entomol. 44102-110. [DOI] [PubMed] [Google Scholar]
- 11.Dasgupta, R., B. Selling, and R. Rueckert. 1994. Flock house virus: a simple model for studying persistent infection in cultured Drosophila cells. Arch. Virol. Suppl. 9121-132. [DOI] [PubMed] [Google Scholar]
- 12.DeBiasi, R. L., C. L. Edelstein, B. Sherry, and K. L. Tyler. 2001. Calpain inhibition protects against virus-induced apoptotic myocardial injury. J. Virol. 75351-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Despres, P., M. Flamand, P. E. Ceccaldi, and V. Deubel. 1996. Human isolates of dengue type 1 virus induce apoptosis in mouse neuroblastoma cells. J. Virol. 704090-4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Despres, P., M. P. Frenkiel, P. E. Ceccaldi, C. Duarte Dos Santos, and V. Deubel. 1998. Apoptosis in the mouse central nervous system in response to infection with mouse-neurovirulent dengue viruses. J. Virol. 72823-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ditzel, M., R. Wilson, T. Tenev, A. Zachariou, A. Paul, E. Deas, and P. Meier. 2003. Degradation of DIAP1 by the N-end rule pathway is essential for regulating apoptosis. Nat. Cell Biol. 5467-473. [DOI] [PubMed] [Google Scholar]
- 16.Fraser, A. G., N. J. McCarthy, and G. I. Evan. 1997. drICE is an essential caspase required for apoptotic activity in Drosophila cells. EMBO J. 166192-6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friesen, P. D. 2007. Insect viruses, p. 725-727. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5th ed., vol. 1. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 18.Friesen, P. D., and R. R. Rueckert. 1984. Early and late functions in a bipartite RNA virus: evidence for translational control by competition between viral mRNAs. J. Virol. 49116-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galiana-Arnoux, D., C. Dostert, A. Schneemann, J. A. Hoffmann, and J. L. Imler. 2006. Essential function in vivo for Dicer-2 in host defense against RNA viruses in Drosophila. Nat. Immunol. 7590-597. [DOI] [PubMed] [Google Scholar]
- 20.Gallagher, T. M., and R. R. Rueckert. 1988. Assembly-dependent maturation cleavage in provirions of a small icosahedral insect ribovirus. J. Virol. 623399-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Girard, S., T. Couderc, J. Destombes, D. Thiesson, F. Delpeyroux, and B. Blondel. 1999. Poliovirus induces apoptosis in the mouse central nervous system. J. Virol. 736066-6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo, Y. X., T. Wei, K. Dallmann, and J. Kwang. 2003. Induction of caspase-dependent apoptosis by betanodaviruses GGNNV and demonstration of protein alpha as an apoptosis inducer. Virology 30874-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harvey, K. F., C. M. Pfleger, and I. K. Hariharan. 2003. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell 114457-467. [DOI] [PubMed] [Google Scholar]
- 24.Hay, B. A., and M. Guo. 2006. Caspase-dependent cell death in Drosophila. Annu. Rev. Cell Dev. Biol. 22623-650. [DOI] [PubMed] [Google Scholar]
- 25.Hengartner, M. O. 2000. The biochemistry of apoptosis. Nature 407770-776. [DOI] [PubMed] [Google Scholar]
- 26.Holley, C. L., M. R. Olson, D. A. Colon-Ramos, and S. Kornbluth. 2002. Reaper eliminates IAP proteins through stimulated IAP degradation and generalized translational inhibition. Nat. Cell Biol. 4439-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Igaki, T., Y. Yamamoto-Goto, N. Tokushige, H. Kanda, and M. Miura. 2002. Down-regulation of DIAP1 triggers a novel Drosophila cell death pathway mediated by Dark and DRONC. J. Biol. Chem. 27723103-23106. [DOI] [PubMed] [Google Scholar]
- 28.Kaiser, W. J., D. Vucic, and L. K. Miller. 1998. The Drosophila inhibitor of apoptosis D-IAP1 suppresses cell death induced by the caspase drICE. FEBS Lett. 440243-248. [DOI] [PubMed] [Google Scholar]
- 29.Kominsky, D. J., R. J. Bickel, and K. L. Tyler. 2002. Reovirus-induced apoptosis requires mitochondrial release of Smac/DIABLO and involves reduction of cellular inhibitor of apoptosis protein levels. J. Virol. 7611414-11424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kopecky, S. A., and D. S. Lyles. 2003. Contrasting effects of matrix protein on apoptosis in HeLa and BHK cells infected with vesicular stomatitis virus are due to inhibition of host gene expression. J. Virol. 774658-4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kornbluth, S., and K. White. 2005. Apoptosis in Drosophila: neither fish nor fowl (nor man, nor worm). J. Cell Sci. 1181779-1787. [DOI] [PubMed] [Google Scholar]
- 32.Kuranaga, E., H. Kanuka, A. Tonoki, K. Takemoto, T. Tomioka, M. Kobayashi, S. Hayashi, and M. Miura. 2006. Drosophila IKK-related kinase regulates nonapoptotic function of caspases via degradation of IAPs. Cell 126583-596. [DOI] [PubMed] [Google Scholar]
- 33.LaCount, D. J., S. F. Hanson, C. L. Schneider, and P. D. Friesen. 2000. Caspase inhibitor P35 and inhibitor of apoptosis Op-IAP block in vivo proteolytic activation of an effector caspase at different steps. J. Biol. Chem. 27515657-15664. [DOI] [PubMed] [Google Scholar]
- 34.Lannan, E., R. Vandergaast, and P. D. Friesen. 2007. Baculovirus caspase inhibitors P49 and P35 block virus-induced apoptosis downstream of effector caspase DrICE activation in Drosophila melanogaster cells. J. Virol. 819319-9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levine, B., J. E. Goldman, H. H. Jiang, D. E. Griffin, and J. M. Hardwick. 1996. Bc1-2 protects mice against fatal alphavirus encephalitis. Proc. Natl. Acad. Sci. USA 934810-4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levine, B., Q. Huang, J. T. Isaacs, J. C. Reed, D. E. Griffin, and J. M. Hardwick. 1993. Conversion of lytic to persistent alphavirus infection by the bcl-2 cellular oncogene. Nature 361739-742. [DOI] [PubMed] [Google Scholar]
- 37.Lewis, J., S. L. Wesselingh, D. E. Griffin, and J. M. Hardwick. 1996. Alphavirus-induced apoptosis in mouse brains correlates with neurovirulence. J. Virol. 701828-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li, H., W. X. Li, and S. W. Ding. 2002. Induction and suppression of RNA silencing by an animal virus. Science 2961319-1321. [DOI] [PubMed] [Google Scholar]
- 39.Meier, P., J. Silke, S. J. Leevers, and G. I. Evan. 2000. The Drosophila caspase DRONC is regulated by DIAP1. EMBO J. 19598-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller, D. J., M. D. Schwartz, and P. Ahlquist. 2001. Flock house virus RNA replicates on outer mitochondrial membranes in Drosophila cells. J. Virol. 7511664-11676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muro, I., B. A. Hay, and R. J. Clem. 2002. The Drosophila DIAP1 protein is required to prevent accumulation of a continuously generated, processed form of the apical caspase DRONC. J. Biol. Chem. 27749644-49650. [DOI] [PubMed] [Google Scholar]
- 42.Muro, I., J. C. Means, and R. J. Clem. 2005. Cleavage of the apoptosis inhibitor DIAP1 by the apical caspase DRONC in both normal and apoptotic Drosophila cells. J. Biol. Chem. 28018683-18688. [DOI] [PubMed] [Google Scholar]
- 43.O'Brien, V. 1998. Viruses and apoptosis. J. Gen. Virol. 791833-1845. [DOI] [PubMed] [Google Scholar]
- 44.Pantalacci, S., N. Tapon, and P. Leopold. 2003. The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat. Cell Biol. 5921-927. [DOI] [PubMed] [Google Scholar]
- 45.Parquet, M. C., A. Kumatori, F. Hasebe, K. Morita, and A. Igarashi. 2001. West Nile virus-induced bax-dependent apoptosis. FEBS Lett. 50017-24. [DOI] [PubMed] [Google Scholar]
- 46.Salvesen, G. S., and C. S. Duckett. 2002. IAP proteins: blocking the road to death's door. Nat. Rev. Mol. Cell Biol. 3401-410. [DOI] [PubMed] [Google Scholar]
- 47.Samuel, M. A., J. D. Morrey, and M. S. Diamond. 2007. Caspase 3-dependent cell death of neurons contributes to the pathogenesis of West Nile virus encephalitis. J. Virol. 812614-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schneemann, A., V. Reddy, and J. E. Johnson. 1998. The structure and function of nodavirus particles: a paradigm for understanding chemical biology. Adv. Virus Res. 50381-446. [DOI] [PubMed] [Google Scholar]
- 49.Schneemann, A., W. Zhong, T. M. Gallagher, and R. R. Rueckert. 1992. Maturation cleavage required for infectivity of a nodavirus. J. Virol. 666728-6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schneider, I. 1972. Cell lines derived from late embryonic stages of Drosophila melanogaster. J. Embryol. Exp. Morphol. 27353-365. [PubMed] [Google Scholar]
- 51.Scotti, P. D., S. Dearing, and D. W. Mossop. 1983. Flock house virus: a nodavirus isolated from Costelytra zealandica (White) (Coleoptera: Scarabaeidae). Arch. Virol. 75181-189. [DOI] [PubMed] [Google Scholar]
- 52.Selling, B. H., and R. R. Rueckert. 1984. Plaque assay for black beetle virus. J. Virol. 51251-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shen, Y., and T. E. Shenk. 1995. Viruses and apoptosis. Curr. Opin. Genet. Dev. 5105-111. [DOI] [PubMed] [Google Scholar]
- 54.Shi, Y. 2004. Caspase activation, inhibition, and reactivation: a mechanistic view. Protein Sci. 131979-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tenev, T., A. Zachariou, R. Wilson, M. Ditzel, and P. Meier. 2005. IAPs are functionally non-equivalent and regulate effector caspases through distinct mechanisms. Nat. Cell Biol. 770-77. [DOI] [PubMed] [Google Scholar]
- 56.Teodoro, J. G., and P. E. Branton. 1997. Regulation of apoptosis by viral gene products. J. Virol. 711739-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Terai, C., R. S. Kornbluth, C. D. Pauza, D. D. Richman, and D. A. Carson. 1991. Apoptosis as a mechanism of cell death in cultured T lymphoblasts acutely infected with HIV-1. J. Clin. Investig. 871710-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thomenius, M., and S. Kornbluth. 2006. Multifunctional reaper: sixty-five amino acids of fury. Cell Death Differ. 131305-1309. [DOI] [PubMed] [Google Scholar]
- 59.Tyler, K. L., M. K. Squier, S. E. Rodgers, B. E. Schneider, S. M. Oberhaus, T. A. Grdina, J. J. Cohen, and T. S. Dermody. 1995. Differences in the capacity of reovirus strains to induce apoptosis are determined by the viral attachment protein sigma 1. J. Virol. 696972-6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Venter, P. A., N. K. Krishna, and A. Schneemann. 2005. Capsid protein synthesis from replicating RNA directs specific packaging of the genome of a multipartite, positive-strand RNA virus. J. Virol. 796239-6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang, S. L., C. J. Hawkins, S. J. Yoo, H. A. Muller, and B. A. Hay. 1999. The Drosophila caspase inhibitor DIAP1 is essential for cell survival and is negatively regulated by HID. Cell 98453-463. [DOI] [PubMed] [Google Scholar]
- 62.Wang, X. H., R. Aliyari, W. X. Li, H. W. Li, K. Kim, R. Carthew, P. Atkinson, and S. W. Ding. 2006. RNA interference directs innate immunity against viruses in adult Drosophila. Science 312452-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilson, R., L. Goyal, M. Ditzel, A. Zachariou, D. A. Baker, J. Agapite, H. Steller, and P. Meier. 2002. The DIAP1 RING finger mediates ubiquitination of Dronc and is indispensable for regulating apoptosis. Nat. Cell Biol. 4445-450. [DOI] [PubMed] [Google Scholar]
- 64.Yan, N., J. W. Wu, J. Chai, W. Li, and Y. Shi. 2004. Molecular mechanisms of DrICE inhibition by DIAP1 and removal of inhibition by Reaper, Hid and Grim. Nat. Struct. Mol. Biol. 11420-428. [DOI] [PubMed] [Google Scholar]
- 65.Yoo, S. J., J. R. Huh, I. Muro, H. Yu, L. Wang, S. L. Wang, R. M. Feldman, R. J. Clem, H. A. Muller, and B. A. Hay. 2002. Hid, Rpr and Grim negatively regulate DIAP1 levels through distinct mechanisms. Nat. Cell Biol. 4416-424. [DOI] [PubMed] [Google Scholar]
- 66.Zimmermann, K. C., J. E. Ricci, N. M. Droin, and D. R. Green. 2002. The role of ARK in stress-induced apoptosis in Drosophila cells. J. Cell Biol. 1561077-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]