Abstract
The definition of plasma neutralizing antibody titers capable of controlling human immunodeficiency virus (HIV) infection in vivo is considered a critical step in vaccine development. Here we provide estimates for effective neutralization titers by assessing samples from a recent passive immunization trial with the neutralizing monoclonal antibodies (MAbs) 2G12, 2F5, and 4E10 using an analytic strategy that dissects the contributions of these MAbs to the total neutralization activity in patient plasma. Assessment of neutralization activities for six responding patients with partial or complete control of viremia during the MAb treatment and for the eight nonresponding patients revealed a significant difference between these groups: Among responders, MAb-mediated activity exceeded the autologous neutralization response by 1 to 2 log units (median difference, 43.3-fold), while in the nonresponder group, the autologous activity prevailed (median difference, 0.63-fold). In order to reach a 50% proportion of the responders in our study cohort, MAb neutralizing titers higher than 1:200 were required based on this analysis. The disease stage appears to have a significant impact on the quantities needed, since titers above 1:1,000 were needed to reach the same effect in chronic infection. Although our analysis is based on very small sample numbers and thus cannot be conclusive, our data provide a first estimate on how in vitro-measured neutralizing antibody activity can relate to in vivo efficacy in controlling HIV infection and may therefore provide valuable information for vaccine development. Interestingly, lower neutralizing antibody levels showed an effect in acute compared to chronic infection, suggesting that in early disease stages, therapeutic vaccination may show promise. Equally, this raises hopes that a preventive vaccine could become effective at comparatively lower neutralizing antibody titers.
Human immunodeficiency virus (HIV) vaccine development faces many obstacles. Most importantly, the type and quantity of immune responses required for protection have not been completely unraveled. The perception that both humoral and cellular immune responses to HIV are essential for successful viral defense receives wide acceptance (5, 13, 17). The specificities of protective antibody and cellular responses, however, remain to be defined, as does the magnitude of these responses. The latter poses a particular conundrum for HIV vaccine design, because efficacy analysis of vaccine candidates must be based largely on preclinical assessment and studies with animal models. Methods that allow data from in vitro analysis of vaccine responses to be related to their in vivo impacts are thus urgently needed. In the present study, we aimed to obtain an estimate for protective neutralization titers (NTs) in vivo by using samples from a recent passive immunization trial with the neutralizing antibodies 2G12, 2F5, and 4E10 (26). Through in-depth analyses of the activities of the monoclonal antibodies (MAbs) in vitro and in patient plasma samples analyzed ex vivo and of their observed in vivo effects, we were able to obtain estimates for in vivo active NTs that could provide guidance for future vaccine studies.
MATERIALS AND METHODS
Clinical specimens.
Patient plasma and virus isolates utilized in this study were obtained during a passive immunization trial recently conducted as described elsewhere (26). Written informed consent was obtained from all 14 individuals enrolled according to the guidelines of the ethics committee of the University Hospital Zurich. The patient demographics and clinical setup have been published previously (26). A brief summary of the patient specificities indicating disease stage (acute/chronic) and response to the passive immunization treatment is provided in Table 1. Prior to participation in the passive immunization trial, acutely and chronically infected patients had to be on successful antiretroviral therapy (viral load < 50 RNA copies/ml) for at least 6 months and 3 months, respectively (26). Patients were stratified into responders and nonresponders as defined previously (26) by classifying those with delayed viral rebound or complete suppression of viremia during the trial as responders and the remaining patients as nonresponders.
TABLE 1.
Ex vivo and in vitro analysis of neutralization activitya
| Patient | Stage | Response | IC70 (μg/ml) of:
|
Mean DPlasma (μg/ml), wk 2-12)b
|
Synergy analysis
|
NT70 of plasma against:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| JR-FL
|
Autologous virus
|
|||||||||||||
| 2G12 | 2F5 | 4E10 | 2G12 | 2F5 | 4E10 | MAb ratioc | Inx70d | Total | MAb | Total | MAb | |||
| NAB01 | C | No | >50 | 1.6 | 0.8 | 406.1 | 85.7 | 144.1 | 20:4:7 | 0.734 | 227 | 241 | 1,125 | 525 |
| NAB02 | C | No | 21.3 | 2.2 | 3.1 | 376.8 | 50.7 | 85.7 | 8:1:2 | 0.380 | 375 | 279 | 281 | 137 |
| NAB03 | C | Yes | 0.6 | 7.0 | 7.9 | 523.9 | 48.4 | 115.1 | 10:1:2.5 | 0.676 | 394 | 490 | 1,022 | 1,691 |
| NAB04 | C | Yes | 3.2 | 0.5 | 1.7 | 640.7 | 97.0 | 161.1 | 14:2:3 | 0.554 | 491 | 539 | 1,109 | 1,349 |
| NAB05 | C | No | >50 | 7.2 | 14.4 | 232.7 | 18.9 | 37.8 | 12:1:2 | 0.621 | 348 | 285 | 112 | 9 |
| NAB06 | C | No | 17.7 | 8.9 | 9.0 | 281.9 | 60.3 | 72.4 | 10:2:2.5 | 0.507 | 878 | 348 | 273 | 76 |
| NAB07 | C | No | >50 | >50 | 9.3 | 463.4 | 46.0 | 98.0 | 10:1:2 | 1.000 | 548 | 624 | 196 | 11 |
| NAB08 | A | Yes | 5.8 | 3.7 | 5.0 | 554.5 | 62.7 | 108.8 | 10:1:2 | 0.524 | 511 | 623 | 308 | 259 |
| NAB09 | C | No | >50 | 1.8 | 14.0 | 271.6 | 47.4 | 70.3 | 7:1:1.5 | 0.769 | 319 | 233 | 2,814 | 58 |
| NAB10 | A | No | >50 | 5.0 | 13.4 | 670.3 | 65.3 | 180.3 | 10:1:3 | 0.634 | 786 | 578 | 181 | 114 |
| NAB11 | A | No | 14.3 | 10.5 | 14.8 | 649.4 | 58.6 | 128.2 | 12:1:2.5 | 0.575 | 334 | 526 | 49 | 162 |
| NAB12 | A | Yes | 9.7 | >50 | 20.0 | 484.4 | 74.6 | 142.3 | 8:1:2 | 0.608 | 513 | 461 | 42 | 125 |
| NAB13e | A | Yes | >50 | 0.2 | 12.8 | 726.2 | 92.7 | 175.7 | 8:1:2 | 1.082 | 351 | 475 | 443 | 742 |
| NAB14e | A | Yes | >50 | 0.3 | 1.7 | 752.2 | 80.6 | 189.8 | 10:1:2.5 | 0.927 | 592 | 612 | 2,508 | 486 |
A, acute HIV infection; C, chronic HIV infection. Response to passive immunization was assessed as described previously (26). IC70s of MAbs against the respective pretreatment patient viruses and mean total or MAb-mediated NT70s of patient plasma samples against JR-FL or autologous viruses were measured in the TZM-bl assay system.
Mean trough dose of MAb in plasma samples of patients during weeks 2 to 12 of passive immunization (see Trkola et al. [26]).
Averaged MAb ratio (2G12:2F5:4E10) chosen for synergy analysis.
Inx at 70% inhibition, calculated as shown in Table 2. Inx70 was calculated only for MAbs with IC70s of <50 μg/ml.
Week 2 sample unavailable; analysis restricted to samples from weeks 4 to 12.
Antibodies and cell lines.
MAbs 2G12, 2F5, and 4E10 (21, 25, 27, 30) were produced by recombinant expression in CHO cells as immunoglobulin G1(κ) as described previously (1, 24). TZM-bl cells (National Institutes of Health AIDS Research and Reference Reagent Program) (28) and 293T cells were cultivated in Dulbecco's modified Eagle medium containing 10% fetal calf serum.
Full-length-envelope cloning.
The cloning and sequencing of functional viral envelope genes of these patient isolates have been described previously (10). One to three functional envelope clones of each isolate were used to generate Env-pseudotyped HIV particles as described elsewhere (8, 10, 28).
Generation of envelope-pseudotyped HIV particles.
Briefly, 293T cells were transfected with plasmids carrying the reporter gene expressing the virus backbone, pNLluc-AM (20) (a kind gift from A. Marozsan and J. P. Moore), and the functional envelope clone at a ratio of 3:1 by using polyethylenimine (linear 25 kDa; Polysciences) as described elsewhere (10). Viral supernatants were harvested 2 days posttransfection and infectivity determined as described elsewhere (6). To this end, TZM-bl cells were infected with viral supernatants in Dulbecco's modified Eagle medium, 10% heat-inactivated fetal calf serum, and 1% penicillin-streptomycin (BioWhittaker) containing 10 μg/ml DEAE dextran (Amersham Biosciences).
Neutralization assays with Env-pseudotyped reporter gene viruses.
Neutralization activities of MAbs and patient plasma against pseudotyped virus were evaluated on TZM-bl cells essentially as described elsewhere (14). Heat-inactivated plasma samples were centrifuged (10,000 × g) and probed at serial dilutions starting at 1:40. One hundred to 200 50% tissue culture infective doses (TCID50) of the virus were preincubated with serial dilutions of plasma or antibody for 1 h before the infection mixture was transferred to TZM-bl cells. The antibody concentration or plasma dilution causing a 70% reduction in luciferase reporter gene production after 48 h was determined by regression analysis. Two to three independent experiments were performed for each individual envelope clone. Inhibitory doses for a specific patient are presented as means of the values derived against the individual clones. The cutoff in the neutralization assay was a titer of 1:40. For statistical evaluation samples that did not reach 70% neutralization at this dilution, a titer of 1:40 was used.
Pharamacokinetic analyses of 2F5, 4E10, and 2G12 concentrations in plasma.
Plasma 2F5, 4E10, and 2G12 concentrations were quantified previously using established 2F5-, 4E10-, and 2G12-specific double-sandwich enzyme-linked immunosorbent assays (ELISA) (limit of detection, 3 ng/ml) (1, 7, 24, 26). Mean trough (preinfusion) MAb concentrations between weeks 2 and 12 were determined for each patient except for patients NAB13 and -14, for whom week 2 samples were not available and analysis was restricted to weeks 4 to 12 (Table 1).
Determination of synergy in antibody neutralization.
Based on the MAb concentrations determined in plasma for each individual patient and the relative ratios of the three MAbs to each other (Table 1), we assessed the effects of combinations of the MAbs on the inhibition of the patients' viruses by using corresponding Env-pseudotyped viruses with TZM-bl cells and determining the interaction indices (Inx) according to the method of Loewe (2, 3, 9) (Table 1).
Analytic strategy used to dissect neutralization activities in patient sera.
An overview of the strategy used to dissect the neutralization activity is provided in Table 2. As outlined there, MAb doses in plasma (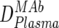 ) were first determined by ELISA, and mean trough concentrations between weeks 2 and 12 were calculated. Then the inhibitory activity of each MAb against the isolate was measured in the TZM-bl assay in vitro, and the individual 70% inhibitory concentrations (IC70s) were determined. Combination effects were assessed via Inx of the MAbs active against the respective patient-specific virus isolate in the TZM-bl assay using a mixture of the MAbs at fixed ratios derived from their mean in vivo concentrations (
) were first determined by ELISA, and mean trough concentrations between weeks 2 and 12 were calculated. Then the inhibitory activity of each MAb against the isolate was measured in the TZM-bl assay in vitro, and the individual 70% inhibitory concentrations (IC70s) were determined. Combination effects were assessed via Inx of the MAbs active against the respective patient-specific virus isolate in the TZM-bl assay using a mixture of the MAbs at fixed ratios derived from their mean in vivo concentrations (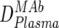 ) (Table 2). If a MAb by itself did not reach an inhibitory activity at concentrations of <50 μg/ml against a specific virus, it was not included in the determination of the Inx against this isolate. We decided on this approach because we found that these MAbs generally do not act antagonistically. Hence, leaving out measurements where IC70 NTs were not achieved is a more conservative approach, since it reduces the chances of overestimating synergy according to formula 4 (Table 2).
) (Table 2). If a MAb by itself did not reach an inhibitory activity at concentrations of <50 μg/ml against a specific virus, it was not included in the determination of the Inx against this isolate. We decided on this approach because we found that these MAbs generally do not act antagonistically. Hence, leaving out measurements where IC70 NTs were not achieved is a more conservative approach, since it reduces the chances of overestimating synergy according to formula 4 (Table 2).
TABLE 2.
Analytic strategy used to dissect MAb and autologous neutralization activities in patient plasma
| Analysis | Description | Analysis type | Formula/symbols |
|---|---|---|---|
| 1 | Trough MAb dose in plasma (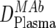 ) ) |
ELISA, ex vivo analysis of plasma samples |
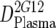 or or 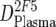 or or 
|
| 2 | Inhibitory dose of MAb against the isolate in vitro (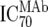 ) ) |
TZM-bl neutralization assay, in vitro analysis of MAbs against viruses; 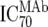 (μg/ml) determined by linear regression (μg/ml) determined by linear regression |
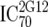 or or 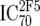 or or 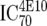
|
| 3 | Inhibitory doses of the MAbs in combination at fixed ratios of MAbs reflecting in vivo concentrations against the isolate in vitro (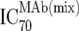 ) ) |
TZM-bl neutralization assay, in vitro analysis of MAb combinations against viruses; 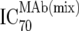 (μg/ml) determined by linear regression (μg/ml) determined by linear regression |
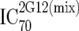 or or 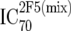 or or 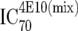
|
| 4 | Combination effects of the MAbs were assessed as interaction indices (Inx) according to the work of Loewe (9) using fixed ratios of MAbs reflecting in vivo concentrations | Inx determined according to the method of Loewe at 70% inhibition in TZM-bl neutralization assay in vitro | Inx70 = IC702G12(mix)/IC702G12 + IC702F5(mix)/IC702F5 + IC704E10(mix)/IC704E10 |
| 5 | Calculate expected MAb NTs |
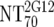 , , 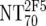 , and , and 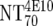 calculated from corresponding inhibitory doses, Inx70, and the measured plasma MAb level calculated from corresponding inhibitory doses, Inx70, and the measured plasma MAb level |
NT70MAb = (1/Inx70) · (DPlasma2G12/IC702G12 + DPlasma2F5/IC702F5 + DPlasma4E10/IC704E10) |
| 6 | Total plasma neutralization activity (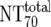 ) ) |
Ex vivo analysis of patient plasma in TZM-bl neutralization assay; reciprocal titer (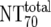 ) determined by linear regression ) determined by linear regression |
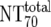 |
| 7 | Autologous plasma neutralization activity (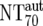 ) ) |
Calculation | 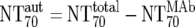 |
The expected 70% MAb NTs (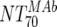 ) were then calculated from their respective inhibitory doses and Inx and the measured plasma MAb level using the formula given for analysis 5 in Table 2. Total plasma neutralization activity (
) were then calculated from their respective inhibitory doses and Inx and the measured plasma MAb level using the formula given for analysis 5 in Table 2. Total plasma neutralization activity (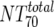 ), which consists of plasma antibody- and MAb-mediated activities, was determined experimentally in the TZM-bl assay by assessing the reciprocal 70% inhibitory titers in patient plasma against the corresponding virus throughout the trial. From these data, autologous plasma neutralization activity (
), which consists of plasma antibody- and MAb-mediated activities, was determined experimentally in the TZM-bl assay by assessing the reciprocal 70% inhibitory titers in patient plasma against the corresponding virus throughout the trial. From these data, autologous plasma neutralization activity (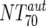 ) was deduced by subtracting the MAb-mediated NT from the respective total NT (analysis 7). In cases where
) was deduced by subtracting the MAb-mediated NT from the respective total NT (analysis 7). In cases where 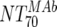 was ≥
was ≥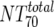 , a lower cutoff of 10 was used for
, a lower cutoff of 10 was used for 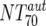 . Data used for statistical analysis are means of NTs measured between weeks 2 and 12.
. Data used for statistical analysis are means of NTs measured between weeks 2 and 12.
Data analysis.
Data analyses were performed using Prism, version 4.03 for Windows (GraphPad Software, San Diego, CA) and Stata SE, version 10 for Windows (Stata Corporation, College Station, TX). Nonparametric methods were employed for testing of group differences (the Mann-Whitney U test for unpaired comparisons and the Wilcoxon signed-rank test for paired comparisons). Linear regression with robust standard errors to account for the potential clustering of data points from the same individual was used for assessing the relationship between total NTs and MAb titers. All tests of significance were two-tailed, and the level of significance was set at 0.05. No adjustments for multiple testing were made.
Nucleotide sequence accession numbers.
The clonal envelope sequences generated in this study have been deposited in the GenBank database (http://www.ncbi.nlm.nih.gov/GenBank/index.html) (10). The assigned accession numbers are as follows: for NAB1pre-cl_12, EU023916; for NAB1pre-cl_30, EU023917; for NAB1pre-cl_39x, EU023918; for NAB2pre-cl_3, EU023919; for NAB3pre-cl_7, EU023920; for NAB3pre-cl_43, EU023921; for NAB4pre-cl_1, EU023922; for NAB5pre-cl_1, EU023923; for NAB6pre-cl_8, EU023924; for NAB6pre-cl_11, EU023925; for NAB7pre-cl_20, EU023926; for NAB8pre-cl_11, EU023927; for NAB9pre-cl_106, EU023928; for NAB10pre-cl_2, EU023929; for NAB10pre-cl_11, EU023930; for NAB11pre-cl_11, EU023931; for NAB11pre-cl_18, EU023932; for NAB12pre-cl_7, EU023933; for NAB12pre-cl_10, EU023934; for NAB12pre-cl_12, EU023935; for NAb13pre-cl_1, EU023936; for NAB13pre-cl_9, EU023937; for NAB14pre-cl_15, EU023938; and for NAB14pre-cl_7, EU023939.
RESULTS
Dissecting the contribution of MAbs and autologous antibodies to neutralization activity ex vivo.
The first aim of our study was to define if and to what extent the passively transferred MAbs 2G12, 2F5, and 4E10 can contribute to the neutralization activity in patient plasma. Using samples from a recently conducted passive immunization trial with these MAbs (7, 26), we first assessed the activities of the MAbs and patients' plasma antibodies against the heterologous virus JR-FL. For this analysis, a neutralization assay system based on the infection of the genetically engineered reporter cell line TZM-bl with an envelope-pseudotyped reporter gene virus was chosen; owing to its robustness, sensitivity, and high reproducibility, this assay allows for a detailed mathematical analysis (8, 23, 28). To derive information on the individual contributions of the MAbs and the autologous patient antibodies, we established an iterative analysis strategy that took into account the individual MAb concentration in patient plasma, the sensitivity of the viral strain to the MAbs alone and in combination, and potential synergistic and antagonistic effects (Table 1). Using this strategy (outlined in detail in Table 2 and Materials and Methods), we were able to define the theoretical contributions of the MAbs to the overall neutralization activity and, in turn, the autologous neutralization activity during the passive immunization period.
Passively transferred MAbs 2G12, 2F5, and 4E10 are active in patient plasma.
We first assessed neutralization activity in patient plasma against the heterologous virus JR-FL in order to determine if any of the MAbs were active in patient plasma during passive immunization and, if so, which of the three MAbs were active. Longitudinal plasma samples collected before passive immunization (pretreatment time point), during treatment (preimmunization time points at weeks 2, 4, 6, 8, 10, and 12), and during the follow-up period (weeks 14, 16, 18, 20, 22, and 24) were analyzed for neutralization activity (Fig. 1A). Activities against JR-FL before and after passive immunization were markedly lower for all patients than the activities measured during MAb treatment, indicating that the MAbs were active in the patient plasma (Fig. 1A and B; Table 3, analyses I and II). Altogether, the examination of the neutralization activity against JR-FL provided further evidence that all three MAbs retained their neutralization capacities in patient plasma and contributed to the MAb-induced inhibitory activity in plasma, confirming that no interference with binding to cellular proteins in plasma occurred.
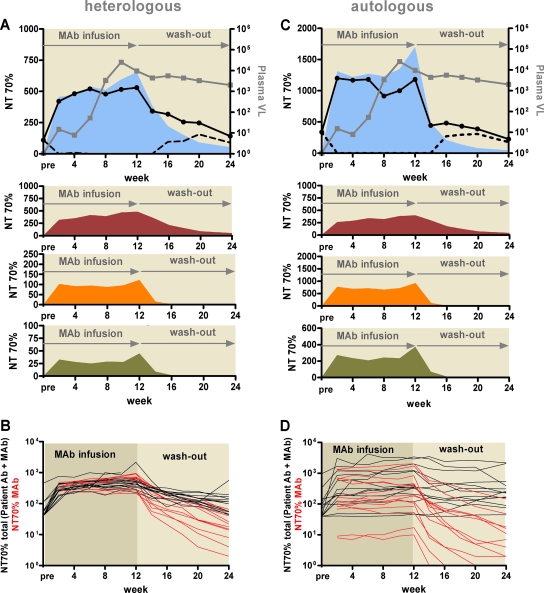
FIG. 1.
Dissection of MAb contributions to neutralization activity. Total, MAb-mediated, and patients' (autologous) antibody (Ab)-mediated neutralization activities (70% NT) against the heterologous virus JR-FL (A and B) and the autologous viruses (C and D) were evaluated for all 14 patients. Data are means from two to four independent experiments. (A and C) Representative profiles of neutralization activities of plasma from patient NAB04 against JR-FL (A) and the autologous virus of patient NAB04 (C), analyzed throughout the trial. The MAb infusion period and off-treatment phase (MAb washout) are indicated. Gray squares, viral load (VL; expressed as copies of HIV RNA/ml of patient plasma); black circles with solid lines, total neutralization activity in plasma (combined autologous-Ab and MAb activities); dashed lines, autologous-Ab activity. The calculated MAb NTs are shown in separate panels as dark red, orange, and green areas for 2G12, 2F5, and 4E10, respectively. The sum of the activities of all three MAbs is shown as a light blue area in the top panel. (B and D) Summary of total neutralization activity (black) and MAb-mediated neutralization activity (red) for each of the 14 individuals during the trial against JR-FL (B) and autologous viruses (D).
TABLE 3.
Group comparisons of measured parametersa
| Parameter | Analysis | Unit of analysis | Subgroup 1
|
Subgroup 2
|
Test | P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subgroup name | n | Median | IQR | Subgroup name | n | Median | IQR | |||||
Total plasma NT70 (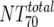 ) ) |
I | Heterologous virus NT70 | Pretreatment | 14 | 40 | 40-84 | Wk 12 | 14 | 482 | 342-578 | W | 0.0001 |
| II | Heterologous virus NT70 | Wk 12 | 13b | 436 | 342-551 | Wk 24 | 13 | 113 | 55-154 | W | 0.0046 | |
| III | Heterologous virus NT70 | Responders | 6 | 501 | 373-553 | Nonresponders | 8 | 361 | 327-667 | M | 0.491 | |
| IV | Autologous virus NT70 | Responders | 6 | 732 | 175-1,808 | Nonresponders | 8 | 234 | 147-703 | M | 0.414 | |
MAb NT70 (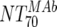 ) ) |
V | Heterologous virus NT70 | 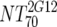 |
14 | 350 | 205-455 | 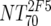 |
14 | 78 | 40-122 | M | <0.0001 |
| VI | Heterologous virus NT70 | 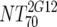 |
14 | 350 | 205-455 | 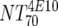 |
14 | 30 | 18-44 | M | <0.0001 | |
| VII | Heterologous virus NT70 | Responders | 6 | 514 | 468-618 | Nonresponders | 8 | 317 | 260-552 | M | 0.181 | |
| VIII | Autologous virus NT70 | Responders | 6 | 614 | 192-1,520 | Nonresponders | 8 | 95 | 35-149 | M | 0.0127 | |
| IX | Autologous virus NT70; pretreatment | Acute | 6 | 40 | 40-40 | Chronic | 8 | 110 | 93-243 | NAc | ||
| X | Autologous virus NT70; wk 24 | Acute | 6 | 168 | 51-455 | Chronic | 7 | 178 | 160-2,062 | M | 0.366 | |
Autologous-Ab NT70 (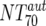 ) ) |
XI | Autologous virus NT70 | Responders | 6 | 10 | 10-1,036 | Nonresponders | 8 | 165 | 85-399 | M | 0.0813 |
| Fold difference | XII | 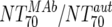 |
Responders | 6 | 43.3 | 2.74-152 | Nonresponders | 8 | 0.63 | 0.07-16.2 | M | 0.0293 |
| XIII | 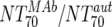 |
Responders without NAB14d | 5 | 74.2 | 8.9-152 | Nonresponders | 8 | 0.63 | 0.07-16.2 | M | 0.0062 | |
IQR, interquartile range; W, Wilcoxon signed rank test; M, Mann-Whitney U test; NA, not applicable; Ab, antibody.
Patient NAB06 reinititated antiretroviral therapy after week 12; thus, data from only 13 patients are available for group comparison of weeks 12 and 24.
Group comparison not applicable, because at the pretreatment time point, no neutralization activity was detectable in acute infection; the NT range for chronic infection was 71 to 965.
Patient NAB14 was excluded from the analysis.
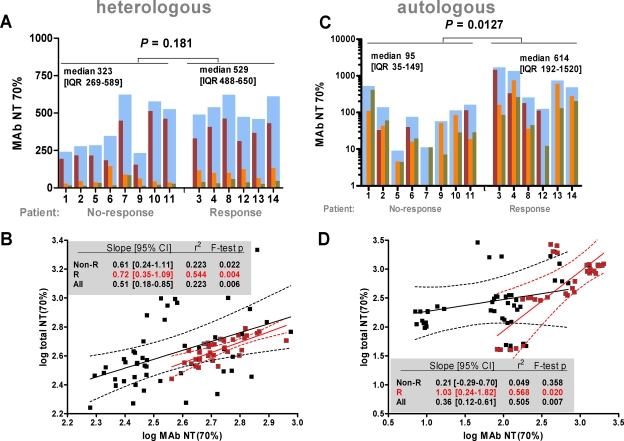
In agreement with our previous analyses (7, 10, 26), we found that 2G12 was the most potent of the three MAbs, reflecting the higher plasma concentrations 2G12 achieved (Fig. 2A; Table 3, analyses V and VI). The cross-neutralization activity measured in total plasma against the heterologous strain JR-FL associated to similar extents with the calculated MAb-mediated activity in responding and in nonresponding patients (Fig. 2B), also suggesting that the MAbs might have an impact on in vivo neutralization activity. Nonetheless, neither the total neutralization activity in plasma measured against the heterologous strain JR-FL nor the MAb-induced activity against this virus was indicative of a patient's response to the passive immunization treatment (Table 3, analyses III and VII).
FIG. 2.
Dissection of MAb contribution to neutralization activity. (A and C) Comparison of mean MAb-mediated NT70s between weeks 2 and 12 for nonresponding and responding patients against the heterologous virus JR-FL (A) and the autologous viruses (C). Dark red, orange, and green bars represent 2G12-, 2F5-, and 4E10-mediated activities, respectively. Cumulative MAb NTs (sum of all three MAb NTs) (blue bars) for each patient were used to compare the effects of MAb neutralization in responders and nonresponders. Median (interquartile range [IQR]) MAb NTs and P values by the Mann-Whitney U test are provided. (B and D) Simple linear regression analysis of interdependency between total and MAb-mediated neutralization activities against the heterologous virus JR-FL (B) and autologous viruses (D) using robust methods of variance estimation to adjust for intrapatient clustering of measurements. For regression model output and measures of goodness of fit, see insets. Note that confidence intervals (CI) not encompassing zero indicate statistically significant slopes. Black and red symbols represent nonresponders and responders, respectively. Data are means from two to four independent experiments.
Evaluating the contributions of MAbs and plasma antibody to inhibition of the autologous patient virus.
In order to evaluate the MAb responses against the patient's autologous isolates, we cloned the envelope genes from all 14 patient viruses before the start of passive immunization therapy. All derived replication-competent envelope genes used for this analysis were sequenced to verify that they were representative for the individual patients (see Materials and Methods) (10). In most cases we found good agreement between the sensitivities of the isolates that had been determined previously using a peripheral blood mononuclear cell-based assay system (10, 26) and the inhibitory activities of the MAbs against the cloned Env-pseudotyped viruses in the TZM-bl assay (Table 1). The virus from patient NAB14 was known to be insensitive to 2G12 (10, 26). Notably though, 6 out of the 13 prestudy viruses that were inhibited by 2G12 at relatively high doses in the peripheral blood mononuclear cell assay were not inhibited by 2G12 at concentrations below 50 μg/ml in the TZM-bl assay (Table 1) (10). Equally, 2F5 at 50 μg/ml did not effect 50% inhibition of two isolates (from NAB07 and NAB12) in the TZM-bl assay. In all these cases, mutations within the core epitopes of the MAbs were present both in the original virus isolate and in the cloned envelope, suggesting that the TZM-bl system is more prone to detect such epitope mismatches (10, 26; P. Rusert et al., unpublished data). Nevertheless, we observed trends similar to those described in our initial studies, which indicated a higher neutralization activity of 2G12, but not of 2F5 or 4E10, among responding patients (Fig. 2A and C). Total plasma neutralization activity (consisting of the activities of autologous plasma antibody and passively administered MAbs) against the autologous virus before, during, and after passive immunization was defined as described above. This activity differed substantially among patients (Fig. 1C and D). As expected, the autologous neutralization activity was markedly higher in chronic than in acute infection at the pretreatment time point but was also increased in acutely HIV infected individuals with resuming viral replication (Table 3, analyses IX and X). At the completion of the trial, no significant difference in neutralization activity between acute and chronic infection was observed (Table 3, analysis X). We noted a wider range for the neutralization activity mediated by the triple MAb combination in plasma against the patients' autologous viruses (NT70 range, 7 to 2,025 [Fig. 1D]) than for the activity measured against the heterologous virus JR-FL (NT70 range, 190 to 946 [Fig. 1B]). The latter was to be expected, since heterologous neutralization activity is notoriously low in HIV infection, and therefore the inhibitory activity against JR-FL depends predominantly on MAb levels in patient plasma, while the autologous plasma antibodies, the MAb doses, and the sensitivity of the specific viral strain to the MAbs influence the NT against the autologous virus. Dissection of the homologous activity (plasma antibody- and MAb-mediated inhibition of the autologous virus) indicated that all three MAbs were active against these patient viruses and contributed to the total plasma neutralization activity (Fig. 2C). Of note, for five patients (NAB03, NAB04, NAB11, NAB12, and NAB13), the predicted MAb neutralization activity was higher than the observed total neutralization activity. The cause of this discrepancy is not clear, because we were unable to detect antagonistic effects between antibodies in patient plasma and the three MAbs (data not shown). However, overall we observed a high degree of association between MAb-mediated activity and total neutralization activity in responders but not in nonresponding patients, underlining further that among nonresponders the MAbs had a low impact on the neutralization activity (Fig. 2D).
Effect analysis.
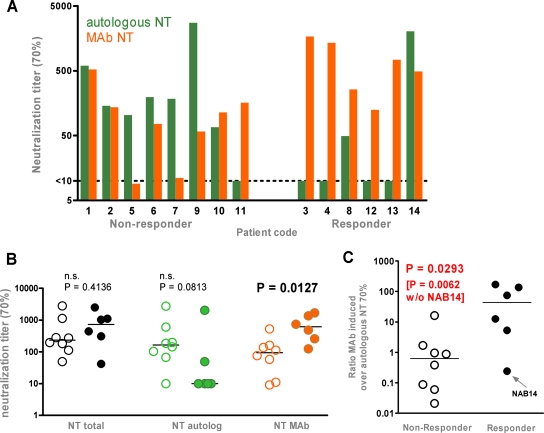
Side-by-side comparison of the neutralization activity of the autologous antibody response and the MAb-mediated activity against each patient's isolate (Fig. 3A) revealed a marked difference between the patient groups: With one exception (NAB14), MAb activity exceeded the patients' autologous antibody NTs in responding patients, while in nonresponders the autologous activity was dominant. To what extent the viremia control of patient NAB14, who controlled viremia to undetectable levels up to the last available sampling time point (2 years of follow-up), was mediated by monoclonal or autologous antibody activity cannot be assessed conclusively. For the other five responders, MAb NTs exceeded autologous-antibody titers by approximately 1 to 2 log units (Fig. 3C; Table 3, analyses XII and XIII). Of particular interest, the MAb-mediated neutralization activity was significantly higher in responders, whereas no difference in total or autologous neutralization activity was observed between the patient groups, thus confirming the influence of the MAb treatment on the trial outcome (Fig. 3B; Table 3, analyses VIII, IV, and XI). We found no association with treatment success when we analyzed total or autologous neutralization activity. Overall, the result of this investigation was strikingly explicit: in general, responses to passive immunization were observed for those individuals for whom the activity of the MAb exceeded the patient's own neutralization response.
FIG. 3.
Influence of MAb-mediated neutralization activity on treatment outcome. Shown are analyses of mean NTs between weeks 2 and 12. (A) Cumulative MAb NT70s (orange bars) and autologous neutralization activity (green bars) against autologous viruses in nonresponders and responders. (B) Comparison of total (black), autologous-antibody-mediated (green), and MAb-mediated (orange) NT70s against autologous viruses in nonresponding (open symbols) and responding (solid symbols) patients. Group comparisons were performed using the Mann-Whitney test. (C) Ratio of MAb-induced responses to autologous responses in responding (solid circles) versus nonresponding (open circles) patients. Group comparisons were performed using the Mann-Whitney test.
Prediction of protective in vivo titers.
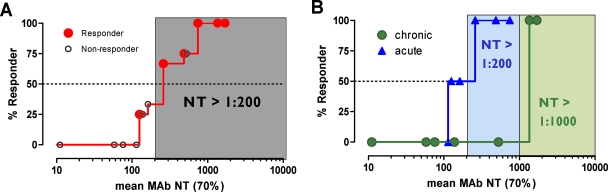
The main and final intent of the present study was to define ex vivo plasma NTs of the MAbs that show in vivo impact. With this we hoped to derive directives on the magnitude of plasma NTs that must be evoked by successful vaccines. To address this, we employed the method of Reed and Muench (15, 22) (Fig. 4A), which allowed us to estimate NTs required to initiate response or to reach a specific proportion of responding patients (e.g., 50 or 100%). In our cohort, response minimally required a MAb-mediated NT of >1:100 (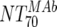 of the responder with the lowest activity). To achieve a 50% proportion of all responders, the MAb-mediated NTs had to be above 1:200 (Fig. 4A). Above an
of the responder with the lowest activity). To achieve a 50% proportion of all responders, the MAb-mediated NTs had to be above 1:200 (Fig. 4A). Above an 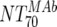 of 525, all patients were responders. Notably, when patients were stratified into acute- and chronic-phase patients, it became evident that considerably higher MAb NTs were required for protection in chronic infection (NT, >1,000) than in acute infection (NT, >200) to achieve the 50% proportion of responders (Fig. 4B).
of 525, all patients were responders. Notably, when patients were stratified into acute- and chronic-phase patients, it became evident that considerably higher MAb NTs were required for protection in chronic infection (NT, >1,000) than in acute infection (NT, >200) to achieve the 50% proportion of responders (Fig. 4B).
FIG. 4.
Prediction of MAb NTs required for protection. (A) Estimated MAb NTs against the autologous viruses required to achieve 50% protection among responding patients according to the method of Reed and Muench (15, 22). The gray shaded area represents the threshold of MAb NT activity required to achieve a 50% proportion of responders. (B) Analysis as described for panel A of a patient cohort subgrouped into acute and chronic individuals. Blue and green shaded areas represent the thresholds of MAb NT activity required to achieve a 50% proportion of responders among acute and chronic individuals, respectively.
DISCUSSION
HIV vaccine development has to overcome a multitude of hurdles, including the definition of the type and quantity of cellular and humoral responses required for protection (5, 13, 17). The failure to create vaccines based on humoral immunity, despite concentrated efforts over the past 2 decades, has made obvious that vaccination strategies that can elicit potent HIV neutralizing antibody responses will require not only new concepts in immunogen design but also the installment of appropriate assay systems that allow, at the preclinical stage, assessment of the induced responses and their potential in vivo impacts (8, 23, 28). In the present study, we focused on the latter topic. By performing a retrospective analysis of NTs in plasma evoked by passive immunization with the neutralizing MAbs 2G12, 2F5, and 4E10 (26), we aimed to define in vitro plasma NTs that were associated with in vivo effects. Samples from 14 patients who participated in the passive immunization trial with the three MAbs were available for this analysis. As outlined, we defined the theoretical contributions of the MAbs to the overall neutralization activity in patient plasma by taking into consideration MAb concentrations, the sensitivities of the viral strains to the MAbs, synergistic and antagonistic effects, and autologous-antibody activity. The highly standardizable neutralization assay system employing Env-pseudotyped reporter gene viruses on TZM-bl cells is recommended for assessment of vaccine responses, due to its robustness and low inter- and intralaboratory variation (8, 11). Use of this system allowed us to perform an in-depth analysis combining experimental and arithmetic approaches. Deriving the theoretical neutralization activity of the passively administered MAbs during the trial enabled us to relate their in vitro neutralization activities in patient plasma to the in vivo effects recorded for the same individuals. The latter analysis provided a strikingly clear result: Generally, irrespective of the disease stage, MAb-induced activity had to exceed the patient's own antibody activity by 1 to 2 log units in order to show an in vivo effect. More specifically, in order to achieve a 50% proportion of the responders in our trial, we estimated that in vitro MAb NTs (NT70s) of >1:200 were required. Notably, the two responding patients with chronic infection had very low autologous neutralization activity. Nevertheless, a distinct difference between the acutely and chronically infected patients in the quantities required for this response was evident: response in chronic infection depended on MAb NTs of >1:1,000. Our analysis is based on very small patient numbers and therefore has to be interpreted with caution. Nevertheless, the estimates of protective titers we derived from this analysis are intriguing: While during passive immunization high MAb doses had to be delivered in order to achieve such neutralization activities in patient plasma, these titers are in a reasonable range compared to the autologous neutralizing antibody responses that can be observed in HIV infection using this assay system (8, 23, 28). Provided that immunogens can be developed that match in vivo responses, effective vaccines may thus come in reach.
Our clinical study was not specifically designed to evaluate the origin of viral escape mutants that emerge upon MAb treatment. It can be reasoned, however, that the greater viral diversity in chronic infection and the ensuing greater pool of viral variants that can give rise to escape mutations may have led to the lower efficacy of the MAbs at this clinical stage. Equally, chances that MAb-resistant viral variants already exist and will be reactivated from latently infected cells are likely higher in chronic infection. A lower frequency of latently infected cells during acute infection may have further impacted MAb activity, since the number of infected cells will influence the viral burden upon treatment interruption (4, 29). Altogether, the higher efficacy of the MAb intervention in acute infection may bear several important practical implications for vaccination. Since a lower antibody activity showed an effect early in infection, therapeutic vaccination is more likely to eventually become feasible in this setting. Extrapolating this further, one could speculate that the relatively low virus quantities encountered during transmission may require even less active antibody to induce protection, and thus even lower NTs might be effective in the setting of protective vaccination. Nishimura et al. have suggested that NTs in plasma of 1:38 or greater would confer almost complete protection against a virus challenge of 75 TCID50 based on studies of simian/HIV challenge in pig-tailed macaques (15), and similar plasma NTs have been found to confer protection in other animal studies (12, 16, 18, 19). Considerably more work will need to be done to determine exactly how high the bar for neutralizing titers is in the diverse treatment and intervention settings in human infection. Our study provides a first estimate of the range of NTs required for in vivo activity. However, these estimates need to be carefully evaluated, since they are based on small sample numbers. More data linking in vitro assessment and clinical impact are needed to establish a greater degree of accuracy in this matter. Nevertheless, our study leaves room for cautious optimism with respect to the development of effective vaccines, because it provides evidence that the neutralization response required for in vivo activity is within the range of responses that can be mounted during natural infection.
Acknowledgments
We thank our patients for their commitment; Leonardo Aceto, Mike Winiger, Ursi Berberat, and Christina Grube for excellent patient care; Barbara Niederöst and Amapola Manrique for assisting with sequencing; Ingrid Nievergelt, Erika Gremlich, and Christine Vögtli for administrative assistance; and Marek Fischer for helpful discussions.
Support was provided by the Swiss National Science Foundation (PP00B-102647 to A.T. and 3100A0-103748 to H.F.G. and A.T.), by research grants from the Gebert-Rüf Foundation (P-041/02), by the Union Bank of Switzerland AG in the name of a donor to A.T., and by a research grant of the Kanton Zürich. A.T. is an Elizabeth Glaser Scientist supported by the Elizabeth Glaser Pediatric AIDS Foundation.
Footnotes
Published ahead of print on 21 November 2007.
REFERENCES
- 1.Armbruster, C., G. M. Stiegler, B. A. Vcelar, W. Jager, U. Koller, R. Jilch, C. G. Ammann, M. Pruenster, H. Stoiber, and H. W. Katinger. 2004. Passive immunization with the anti-HIV-1 human monoclonal antibody (hMAb) 4E10 and the hMAb combination 4E10/2F5/2G12. J. Antimicrob. Chemother. 54915-920. [DOI] [PubMed] [Google Scholar]
- 2.Berenbaum, M. C. 1978. A method for testing for synergy with any number of agents. J. Infect. Dis. 137122-130. [DOI] [PubMed] [Google Scholar]
- 3.Berenbaum, M. C. 1989. What is synergy? Pharmacol. Rev. 4193-141. [PubMed] [Google Scholar]
- 4.Fischer, M., B. Joos, B. Hirschel, G. Bleiber, R. Weber, and H. F. Gunthard. 2004. Cellular viral rebound after cessation of potent antiretroviral therapy predicted by levels of multiply spliced HIV-1 RNA encoding nef. J. Infect. Dis. 1901979-1988. [DOI] [PubMed] [Google Scholar]
- 5.Haynes, B. F., and D. C. Montefiori. 2006. Aiming to induce broadly reactive neutralizing antibody responses with HIV-1 vaccine candidates. Expert Rev. Vaccines 5579-595. [DOI] [PubMed] [Google Scholar]
- 6.Huber, M., M. Fischer, B. Misselwitz, A. Manrique, H. Kuster, B. Niederost, R. Weber, V. von Wyl, H. F. Gunthard, and A. Trkola. 2006. Complement lysis activity in autologous plasma is associated with lower viral loads during the acute phase of HIV-1 infection. PLoS Med. 3e441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joos, B., A. Trkola, H. Kuster, L. Aceto, M. Fischer, G. Stiegler, C. Armbruster, B. Vcelar, H. Katinger, and H. F. Gunthard. 2006. Long-term multiple-dose pharmacokinetics of human monoclonal antibodies (MAbs) against human immunodeficiency virus type 1 envelope gp120 (MAb 2G12) and gp41 (MAbs 4E10 and 2F5). Antimicrob. Agents Chemother. 501773-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li, M., F. Gao, J. R. Mascola, L. Stamatatos, V. R. Polonis, M. Koutsoukos, G. Voss, P. Goepfert, P. Gilbert, K. M. Greene, M. Bilska, D. L. Kothe, J. F. Salazar-Gonzalez, X. Wei, J. M. Decker, B. H. Hahn, and D. C. Montefiori. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 7910108-10125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loewe, S. 1928. Die quantitativen Probleme der Pharmakologie. Ergebn. Physiol. 2747-187. [Google Scholar]
- 10.Manrique, A., P. Rusert, B. Joos, M. Fischer, H. Kuster, C. Leemann, B. Niederost, R. Weber, G. Stiegler, H. Katinger, H. F. Gunthard, and A. Trkola. 2007. In vivo and in vitro escape from neutralizing antibodies 2G12, 2F5, and 4E10. J. Virol. 818793-8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mascola, J. R., P. D'Souza, P. Gilbert, B. H. Hahn, N. L. Haigwood, L. Morris, C. J. Petropoulos, V. R. Polonis, M. Sarzotti, and D. C. Montefiori. 2005. Recommendations for the design and use of standard virus panels to assess neutralizing antibody responses elicited by candidate human immunodeficiency virus type 1 vaccines. J. Virol. 7910103-10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mascola, J. R., G. Stiegler, T. C. VanCott, H. Katinger, C. B. Carpenter, C. E. Hanson, H. Beary, D. Hayes, S. S. Frankel, D. L. Birx, and M. G. Lewis. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6207-210. [DOI] [PubMed] [Google Scholar]
- 13.McMichael, A. J. 2006. HIV vaccines. Annu. Rev. Immunol. 24227-255. [DOI] [PubMed] [Google Scholar]
- 14.Montefiori, D. C. 2004. Evaluating neutralizing antibodies against HIV, SIV, and SHIV in luciferase reporter gene assays, p. 12.11.1-12.11.17. In J. E. Coligan, A. M. Kruisbeek, D. H. Margulies, E. M. Shevach, and W. Strober (ed.), Current protocols in immunology. John Wiley and Sons, Inc., New York, NY. [DOI] [PubMed]
- 15.Nishimura, Y., T. Igarashi, N. Haigwood, R. Sadjadpour, R. J. Plishka, A. Buckler-White, R. Shibata, and M. A. Martin. 2002. Determination of a statistically valid neutralization titer in plasma that confers protection against simian-human immunodeficiency virus challenge following passive transfer of high-titered neutralizing antibodies. J. Virol. 762123-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pal, R., V. S. Kalyanaraman, B. C. Nair, S. Whitney, T. Keen, L. Hocker, L. Hudacik, N. Rose, I. Mboudjeka, S. Shen, T. H. Wu-Chou, D. Montefiori, J. Mascola, P. Markham, and S. Lu. 2006. Immunization of rhesus macaques with a polyvalent DNA prime/protein boost human immunodeficiency virus type 1 vaccine elicits protective antibody response against simian human immunodeficiency virus of R5 phenotype. Virology 348341-353. [DOI] [PubMed] [Google Scholar]
- 17.Pantaleo, G., and R. A. Koup. 2004. Correlates of immune protection in HIV-1 infection: what we know, what we don't know, what we should know. Nat. Med. 10806-810. [DOI] [PubMed] [Google Scholar]
- 18.Parren, P. W., P. A. Marx, A. J. Hessell, A. Luckay, J. Harouse, C. Cheng-Mayer, J. P. Moore, and D. R. Burton. 2001. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 758340-8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poignard, P., R. Sabbe, G. R. Picchio, M. Wang, R. J. Gulizia, H. Katinger, P. W. Parren, D. E. Mosier, and D. R. Burton. 1999. Neutralizing antibodies have limited effects on the control of established HIV-1 infection in vivo. Immunity 10431-438. [DOI] [PubMed] [Google Scholar]
- 20.Pugach, P., A. J. Marozsan, T. J. Ketas, E. L. Landes, J. P. Moore, and S. E. Kuhmann. 2007. HIV-1 clones resistant to a small molecule CCR5 inhibitor use the inhibitor-bound form of CCR5 for entry. Virology 361212-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Purtscher, M., A. Trkola, G. Gruber, A. Buchacher, R. Predl, F. Steindl, C. Tauer, R. Berger, N. Barrett, A. Jungbauer, et al. 1994. A broadly neutralizing human monoclonal antibody against gp41 of human immunodeficiency virus type 1. AIDS Res. Hum Retrovir. 101651-1658. [DOI] [PubMed] [Google Scholar]
- 22.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27493-497. [Google Scholar]
- 23.Richman, D. D., T. Wrin, S. J. Little, and C. J. Petropoulos. 2003. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. USA 1004144-4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stiegler, G., C. Armbruster, B. Vcelar, H. Stoiber, R. Kunert, N. L. Michael, L. L. Jagodzinski, C. Ammann, W. Jager, J. Jacobson, N. Vetter, and H. Katinger. 2002. Antiviral activity of the neutralizing antibodies 2F5 and 2G12 in asymptomatic HIV-1-infected humans: a phase I evaluation. AIDS 162019-2025. [DOI] [PubMed] [Google Scholar]
- 25.Stiegler, G., R. Kunert, M. Purtscher, S. Wolbank, R. Voglauer, F. Steindl, and H. Katinger. 2001. A potent cross-clade neutralizing human monoclonal antibody against a novel epitope on gp41 of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 171757-1765. [DOI] [PubMed] [Google Scholar]
- 26.Trkola, A., H. Kuster, P. Rusert, B. Joos, M. Fischer, C. Leemann, A. Manrique, M. Huber, M. Rehr, A. Oxenius, R. Weber, G. Stiegler, B. Vcelar, H. Katinger, L. Aceto, and H. F. Gunthard. 2005. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat. Med. 11615-622. [DOI] [PubMed] [Google Scholar]
- 27.Trkola, A., M. Purtscher, T. Muster, C. Ballaun, A. Buchacher, N. Sullivan, K. Srinivasan, J. Sodroski, J. P. Moore, and H. Katinger. 1996. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J. Virol. 701100-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei, X., J. M. Decker, S. Wang, H. Hui, J. C. Kappes, X. Wu, J. F. Salazar-Gonzalez, M. G. Salazar, J. M. Kilby, M. S. Saag, N. L. Komarova, M. A. Nowak, B. H. Hahn, P. D. Kwong, and G. M. Shaw. 2003. Antibody neutralization and escape by HIV-1. Nature 422307-312. [DOI] [PubMed] [Google Scholar]
- 29.Yerly, S., H. F. Gunthard, C. Fagard, B. Joos, T. V. Perneger, B. Hirschel, and L. Perrin. 2004. Proviral HIV-DNA predicts viral rebound and viral setpoint after structured treatment interruptions. AIDS 181951-1953. [DOI] [PubMed] [Google Scholar]
- 30.Zwick, M. B., A. F. Labrijn, M. Wang, C. Spenlehauer, E. O. Saphire, J. M. Binley, J. P. Moore, G. Stiegler, H. Katinger, D. R. Burton, and P. W. Parren. 2001. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J. Virol. 7510892-10905. [DOI] [PMC free article] [PubMed] [Google Scholar]