Abstract
A single blind, randomized, placebo-controlled, single-center phase I clinical trial of a CD8+ T-cell peptide epitope vaccine against infectious mononucleosis was conducted with 14 HLA B*0801-positive, Epstein-Barr virus (EBV)-seronegative adults. The vaccine comprised the HLA B*0801-restricted peptide epitope FLRGRAYGL and tetanus toxoid formulated in a water-in-oil adjuvant, Montanide ISA 720. FLRGRAYGL-specific responses were detected in 8/9 peptide-vaccine recipients and 0/4 placebo vaccine recipients by gamma interferon enzyme-linked immunospot assay and/or limiting-dilution analysis. The same T-cell receptor Vβ CDR3 sequence that is found in FLRGRAYGL-specific T cells from most EBV-seropositive individuals could also be detected in the peripheral blood of vaccine recipients. The vaccine was well tolerated, with the main side effect being mild to moderate injection site reactions. After a 2- to 12-year follow-up, 1/2 placebo vaccinees who acquired EBV developed infectious mononucleosis, whereas 4/4 vaccinees who acquired EBV after completing peptide vaccination seroconverted asymptomatically. Single-epitope vaccination did not predispose individuals to disease, nor did it significantly influence development of a normal repertoire of EBV-specific CD8+ T-cell responses following seroconversion.
Epstein-Barr Virus (EBV) is a human gammaherpesvirus that is usually transmitted orally during childhood and infects more than 90% of the world's population. The virus establishes a lifelong latent infection in B cells and is controlled in healthy seropositive individuals by CD8+ αβ T lymphocytes that recognize latent and lytic EBV antigens (38, 44). When infection with EBV is delayed until adolescence or adulthood, as is common in many Western countries, individuals have a 26 to 74% chance of developing glandular fever or infectious mononucleosis (IM) (19). IM is an acute, self-limiting disorder associated with fatigue, fever, sore throat, and generalized lymphadenopathy and is characterized by a pronounced blood monocytosis. The severity of symptoms varies from mild flu-like symptoms for a few weeks to prolonged and debilitating disease lasting several months (19), and in rare cases the disease can be fatal (59). IM caused by EBV has also been associated with increased risk of multiple sclerosis and Hodgkin's lymphoma (26, 41). In Western countries the incidence of IM has been estimated to be 45/100,000 per annum in the general community. However, the incidence is much higher among adolescents (15 to 19 years), at 320 to 370/100,000 per annum (18), and thus often affects young people at a critical time in their studies. The disease is less common in developing countries, where >90% of people generally seroconvert asymptomatically to EBV during childhood. In Western countries the number of children entering adolescence without being infected with EBV has been estimated at 10 to 20%. However, this figure may be increasing with improvements in living standards. In the Tokyo region this percentage has recently been estimated to have reached ≈50% by 2006 (57). Although ganciclovir has been used in severe cases and in immunocompromised patients (1) and corticosteroids are sometimes used (15), there are currently no treatments recommended for routine use in IM.
The ability of many individuals’ immune systems to control primary EBV infections without IM has prompted efforts toward the design of an IM vaccine (38, 39). Two approaches have been taken. The first involves a vaccine based on the major surface glycoprotein of EBV, gp350. This vaccine was originally conceived to induce neutralizing antibodies; however, antibody responses do not appear to correlate with protection, with some evidence suggesting that gp350-specific CD4 T-cell responses might mediate protection (40). The second approach seeks to generate EBV-specific CD8+ T cells that control the expansion of EBV-infected B cells after infection, thereby promoting asymptomatic seroconversion rather than preventing infection (38, 39). Such T cells are not only strongly implicated in controlling EBV in healthy individuals but have also been successfully used to treat EBV-associated posttransplant lymphoproliferative disease (PTLD) by adoptive transfer (29, 47, 49). Many EBV-specific CD8+ T cells recognize epitopes from the EBV nuclear antigens (EBNAs) (44). However, the association of these proteins with B-cell transformation precludes, and their large size complicates, their use in recombinant protein-based vaccines (54). A CD8+ T-cell epitope-based approach was thus pursued (39). Here we describe the results of the first phase I CD8+ T-cell epitope-based EBV vaccine trial. The vaccine comprised the HLA B*0801-restricted CD8+ T-cell epitope FLRGRAYGL (FLR) from the latent antigen EBNA3 (9, 10) and tetanus toxoid (TT) as a source of CD4+ T-cell help formulated in the water-in-oil adjuvant, Montanide ISA 720. This adjuvant has been successfully used to induce peptide-epitope-specific CD8+ T-cell responses in mice (20, 48) and has been used in several human vaccine trials (23, 36, 46, 62).
The primary aim of this trial was to establish that CD8+ T-cell epitope-based vaccination of EBV seronegative individuals was safe in the context of a primary EBV infection. IM is characterized by a high viral load and a pronounced lymphocytosis dominated by EBV-specific T cells (13, 14, 24). Asymptomatic seroconversion does not appear to be associated with a lymphocytosis, despite significant viral loads, suggesting that IM arises from an overreaction of T-cell responses (53). Whether a vaccine-induced EBV-specific memory T-cell response would subsequently expand following a primary infection and thereby promote lymphocytosis and IM remains an important question. In addition, since an FLR-based vaccine would induce CD8+ T-cell responses only to this single epitope, it also remains to be established whether immunodominance and/or immunodomination effects (17, 50) would narrow the immune response following a subsequent primary EBV infection to this epitope. Rapid recovery from IM symptoms has been correlated with broad T-cell reactivity to multiple CD8+ T-cell epitopes within lytic and latent antigens, whereas protracted illness was associated with a narrowly focused response (4). It has also been suggested that epitope-based vaccines might induce tolerance against the vaccine epitope and thus prevent a normal response to that epitope from developing (2, 61). This trial illustrates that single-epitope vaccination does not promote disease or predispose individuals to aberrant EBV-specific CD8+ T-cell response after seroconversion, indicating that an epitope-based approach for IM or PTLD vaccines is both feasible and safe.
MATERIALS AND METHODS
Study design.
The single-blind, randomized, placebo-controlled phase I (safety and immunogenicity) EBV vaccine trial was conducted at the Queensland Institute of Medical Research (QIMR), Australia and was approved by The Bancroft Centre Research Ethics Committee and conducted under the Australian Therapeutic Goods Administration CTN scheme. Volunteers were recruited from QIMR and the University of Queensland and were screened for HLA B*0801 status and EBV infection by serology. The initial HLA screening was performed by fluorescence-activated cell sorter (FACS) analysis using the HLA B*0801 monoclonal antibody (clone 59HA-1; One Lambda Inc.) and was later confirmed by full class I tissue typing (Princess Alexandra Hospital tissue typing facility, Brisbane, Australia). Serological testing for EBV capsid antibody (VCA) was initially done by immunofluorescence microscopy (37) and later confirmed by enzyme-linked immunosorbent assay specific for VCA immunoglobulin G (IgG) and IgM and EBNA IgG (Queensland Medical Laboratory, Brisbane, Queensland, Australia). The inclusion criteria for the trial were EBV-seronegative and HLA B*0801-positive status. To avoid adverse reactions to TT (27, 33), volunteers were excluded if they had anti-TT titers of above 5 IU/ml (Queensland Medical Laboratory) or a history of marked reaction to previous TT injections. Volunteers were also excluded if 2 weeks prior to vaccination (i) either full and differential blood counts, CD4/CD8 lymphocyte count, full blood biochemistry (including aspartate transaminase, alanine aminotransferase, gamma glutamyl transferase, urea, and creatinine), antinuclear factor, direct Coombs test, or urine protein was outside the normal range; (ii) females tested positive for serum or urine human chorionic gonadotropin; (iii) volunteers were seropositive for EBV, human immunodeficiency virus, or hepatitis B virus (tests performed by Queensland Health Pathology Services, Royal Brisbane Hospital); or (iv) the medical history or physical examination revealed clinical abnormalities. Separate information sessions and consent forms were provided for screening and enrollment into the trial.
Fourteen healthy, EBV-seronegative, HLA B*0801-positive, 18- to 50-year-old volunteers (seven females and seven males) were enrolled in the trial. They were randomly assigned by a computer-generated list into vaccine (n = 10, with 8 to be immunized with 5 μg and 2 with 50 μg peptide) and placebo (n = 4) groups (Table 1). Vaccine recipients were observed for 6 h following each immunization and contacted by telephone after 1 week. Clinical assessment was performed and blood was collected for immunological screening (including EBV serology and T-cell responses) before each injection and at 2, 4, 8, 10, and 12 weeks and 6, 12, and 24 months after each vaccination. Two vaccinations were offered, the first at day 0 and the second at week 8. Where possible, volunteers were also followed up after 8 to 12 years to test for EBV seroconversion and inquire whether they had suffered from IM since vaccination.
TABLE 1.
Vaccination summary for vaccine recipients, EBV seroconversion, and IM
| Vaccine | Vaccine recipient | EBV seroconversion (wk of test) | IM or asymptomatic | EBV serology
|
||
|---|---|---|---|---|---|---|
| VCA
|
EBNA IgG | |||||
| IgM | IgG | |||||
| Peptide | ||||||
| 5 μg | #01 | No (412) | Negative | Negative | Negative | |
| #02 | Yes (628) | Asymptomatic | Negative | Positive | Positive | |
| #04 | Yes (104) | Asymptomaticd | Low positive | Moderate | Negative | |
| #05 | No (542) | Negative | Negative | Negative | ||
| #06 | No (523) | Negative | Negative | Negative | ||
| #07a | No (520) | Negative | Negative | Negative | ||
| #08a | Yes (104) | Asymptomaticd | Negative | Moderate | Positive | |
| #09b | Yes (26) | Asymptomaticd | Negative | Moderate | Negative | |
| 50 μg | #13 | No (421) | Negative | Negative | Negative | |
| #14c | Yes (8) | ?d,e | Positive | Borderlinef | Negative | |
| Placebo | #03 | No (585) | Negative | Negative | Negative | |
| #10 | No (494) | Negative | Negative | Negative | ||
| #11a | Yes (438) | Asymptomatic | Negative | Borderlineg | Not tested | |
| #12 | Yes (392) | IM, treating doctor notified | ||||
Second immunization not given due to high anti-TT antibody titers.
Declined second immunization.
Second immunization not given due to EBV seroconversion.
Lymphocyte count within normal range at time of serology test.
Diagnosis unclear, possible mild IM or tonsillitis (see text).
Borderline. VCA IgM usually lasts for 1 to 2 months. Anti-EBNA IgG responses usually appear after 2 to 6 months.
FLR- and RAK-specific T-cell expansions seen by FACS (data not shown), confirming EBV-positive status.
The EBV vaccine.
The vaccine consisted of the synthetic peptide, FLR (9), mixed with TT in 5 mM phosphate buffer-isotonic saline and emulsified (3:7 [wt/wt]) with the water-in-oil adjuvant Montanide ISA 720 (Seppic, Paris, France) (20). The FLR peptide was chemically synthesized under GMP conditions (Auspep Ltd.). Two peptide doses were tested, 5 and 50 μg per vaccine dose, with the placebo vaccine containing no peptide. The peptide and placebo vaccines also contained 1.35 Lyme factor/ml (0.675 Lyme factor/dose) TT (CSL Ltd.) and thiomersal (0.01% [wt/vol]) (CSL Ltd.). Stoppered glass vials were filled with the vaccines under nitrogen by CSL Ltd. Volunteers were given 0.5 ml of vaccine per injection subcutaneously into the thigh.
Formal toxicology was undertaken by Pharmatox. Two 500-μl subcutaneous (day 1 and 15) injections of the vaccine (with 50 μg of peptide) given to five male and five female rats and guinea pigs failed to show clinical signs of toxicity over 29 days. Upon necroscopy, organ histology and blood hematology and biochemistry were not clinically different from those of animals injected with saline. The same dose also passed pyrogenicity testing in three rabbits. The vaccine showed no activity in the Ames test or sister chromatid exchange assay. The peptide was stable in the vaccine for 2 years at 4°C and was extracted from the vaccine by vigorous shaking with chloroform-0.1% trifluoroacetic acid in distilled water (1.5:1, vol/vol). The recovered peptide (in the aqueous phase) was analyzed by high-pressure liquid chromatography and was fully active in chromium release assays (9).
LCLs and PHA blasts.
EBV-transformed lymphoblastoid cell lines (LCLs) were established from each vaccinee's peripheral blood mononuclear cells (PBMC) by exogenous virus transformation of peripheral B cells using the QIMR Wil strain of virus (43). LCLs were maintained in medium comprising RPMI 1640 (Gibco), 2 mM glutamine (ICN Biomed. Aust. Pty Ltd., Seven Hills, Australia), 100 IU/ml penicillin and 100 mg/ml streptomycin (CSL Ltd., Melbourne, Australia), and 10% fetal calf serum (QIMR). Phytohemagglutinin (PHA) blasts were generated and maintained as described previously (31).
Ex vivo ELISPOT assay.
PBMC were separated by Ficoll density gradient centrifugation and frozen in 10% dimethyl sulfoxide and 90% fetal calf serum using the Cryo 1° freezing container (Nalgene). Gamma interferon (IFN-γ) enzyme-linked immunospot (ELISPOT) assays were performed as described before (5) using the FLR peptide. T-cell responses to the HLA B8-restricted epitopes QAKWRLQTL (QAK) and RAKFKQLL (RAK), and the HLA A2-restricted epitopes LLDFVRFMGV, CLGGLLTMV, YLLEMLWRL, YLQQNWWTL, GLCTLVAML, and ILIYNGWYA were also assessed for the indicated individuals. Peptides for in vitro assays were synthesized by Chiron Mimotopes (Melbourne, Australia).
LDA.
The limiting-dilution analysis (LDA) was performed as described previously (30). Briefly, PBMC from vaccinees were distributed in twofold dilutions from 6.25 × 103 to 5 × 104 cells per well in round-bottom microtiter plates and stimulated with irradiated (2,000 rads) FLR-sensitized autologous PBMC or irradiated (8,000 rads) autologous LCLs. On day 10, each of the 24 replicate microcultures for each dilution was split into two replicates and used as effectors in a standard 5-h 51Cr release assay against autologous PHA blasts sensitized with and without peptide epitope. Wells were scored as positive when the percent specific chromium release for peptide-sensitized target cells exceeded the mean release from control wells by 3 standard deviations. T-cell frequency was calculated by the method of maximum-likelihood estimation.
Bulk cultures.
Polyclonal T-cell effectors were generated from 2 × 106 PBMC stimulated (on day 0 and day 7) either with irradiated (8,000 rads) autologous LCLs (responder/stimulator ratio of 20:1) or with PBMC sensitized with synthetic peptides (10 μg/ml) (responder/stimulator ratio of 4:1). On day 10, these bulk effectors were used in a standard 51Cr release assay against peptide-sensitized autologous PHA blasts at a 20:1 effector-to-target ratio as described previously (8).
T-cell receptor (TCR) sequencing.
Total RNA was extracted from frozen PBMC or frozen bulk culture pellets containing 5 million cells by using TRIzol reagent (Invitrogen). The RNA pellets were resuspending in 20 μl of diethyl pyrocarbonate-treated double-distilled water (ICN) and were each added to a single-tube one-step reverse transcription-PCR (RT-PCR) system (Invitogen), with the addition of the TRBV7-8 family-specific forward primer (5′-TGAAGCTCAAACTAGACAAATCG-3′) and the FLR-specific reverse CDR3-2-7 primers (5′-CTGGTAGGCCTGBCCTAAGCTGCTGGC-3′ and 5′-AGTACTGCTCGTAGGCCTGBCC-3′). The RT-PCR cycling was performed at 50°C for 30 min and 94°C for 2 min. The PCR cycling was performed at 94°C for 15 s, 55°C for 30 s, and 68°C for 1 min for 40 cycles and at 68°C for 5 min for 1 cycle. The PCR products were cloned into the pGEM-T vector system (Promega) and transformed into DH5 cells (Invitrogen). White colonies (six to eight) were picked and placed into a M13 miniscreen PCR instrument. The PCR products were then sequenced with the ABI Prism Big Dye Terminator reaction kit (Applied Biosystems).
TCR RNA viability was evaluated in all samples with a total TCR constant-region RT-PCR with the addition of the TCR constant-region forward primer Cβ5′ (5′-CGTGTTCCCACCCGAGGTCGC-3′) and the TCR constant-region reverse primer CβB (5′-ATTCACCCACCAGCTCAGCTCCACG-3′) into the one-step RT-PCR system (Invitogen). The PCR cycling conditions were identical to those for the FLR CDR3-specific PCR described above.
Major histocompatibility complex (MHC)-peptide multimer analysis.
PBMC or T-cell lines were incubated for 30 min at 4°C with an HLA B*0801-FLR or HLA B*0801-RAK phycoerythrin-labeled pentamer (ProImmune, Oxford, United Kingdom). Cells were then washed and labeled for 30 min at 4°C with Tri-color-labeled anti-human CD8 (Caltag, Burlingame, CA), allophycocyanin-labeled anti-human CD3 (BD Pharmingen, San Diego, CA), and one of fluorescein isothiocyanate-labeled anti-human-CD27 (Caltag, Burlingame, CA), anti-human CD28 (BD Pharmingen, San Diego, CA), anti-human CD45RA (Beckman-Coulter, Fullerton, CA), anti-human CD45RO (Beckman-Coulter, Fullerton, CA), or anti-human CD62L (Caltag, Burlingame, CA). Cells were then washed twice and analyzed on a four-color FACSCalibur using CellQuest software (Becton Dickinson, Mountain View, CA).
RESULTS
Volunteer selection.
A total of 1,075 resident students from the University of Queensland and staff members from QIMR were screened within a span of 5 years (1994 to 1999), and 25% were shown to be EBV seronegative. Of these, 72 (6.7%) were found to be EBV seronegative and HLA B*0801 positive during the initial screening. Ultimately 14 volunteers (1.5%) were enrolled in the trial. The main reasons for exclusion were anti-TT antibody titers of >5 IU/ml, EBV seroconversion prior to final enrollment, and subjects declining to continue.
Vaccination, adverse events, and seroconversion.
Fourteen volunteers were vaccinated, including eight with 5 μg peptide (volunteers designated #01, #02, #04, #05, #06, #07, #08, and #09), two with 50 μg peptide (#013 and #014), and four with placebo (#03, #010, #011, and #012). Three subjects (#07, #08, and #011) were not given the second vaccination because of an increase in anti-TT titers beyond 5 IU/ml. One subject (#014) was not given the second vaccination due to EBV seroconversion. One subject (#09) declined the second dose of vaccine because of the discomfort following the first dose (Table 1). The vaccine was well tolerated in most volunteers, and no serious adverse events were seen throughout the trial period. Side effects were restricted to self-limiting, mild to moderate injection site reactions, which are summarized in Table 2.
TABLE 2.
Injection site reactions and TT titers
| Vaccine | Vaccine recipient | Injection site reactiona after:
|
TT titer, IU/ml (wk after vaccinationb) | |
|---|---|---|---|---|
| First vaccination | Second vaccination | |||
| Peptide | ||||
| 5 μg | #01 | 11- by 6.5-cm red warm swelling at day 9, resolved at day 28 | 2- to 3-cm mild swelling | 0.25 (P), 0.91 (4), 1.1 (8), 1.2 (104) |
| #02 | 6-cm swelling at day 5-6, mild discomfort, resolving at day 14 | Slight thickening | 1.0 (P), 0.74 (4), 0.73 (8), 0.86 (12) | |
| #04 | Slight pain for 24 h, 5- by 4-cm induration at day 14, itching | No significant reaction | 1.6 (P), 1.5 (4), 1.2 (8), 1.3 (12), 1.1 (52) | |
| #05 | 10- by 8-cm firm warm raised swelling, resolved at day 7 | No significant reaction | 0.7 (P), 1.5 (4), 1.5 (8), 1.5 (10), 1.9 (12) | |
| #06 | No significant reaction | 9-cm swelling at day 3, slightly tender, resolved at day 5 | 2.6 (P), 1.5 (4), 2.40 (8), 3.0 (26) | |
| #07 | 11- by 8-cm raised erythema at day 2, pain, resolving at day 14 | NAc | 4.9 (P), 7.0 (4), 5.3 (8), 7.0 (14) | |
| #08 | 7-cm raised pink warm itchy area at day 3 | NA | 0.35 (P), 5.3 (4) | |
| #09 | 10-cm tender raised red swelling at day 2, 2 cm by day 4 | NA | 1.7 (P), 1.5 (4), 2.8 (26) | |
| 50 μg | #13 | No significant reaction | No significant reaction | 0.5 (P), 1.1 (4), 1.1 (8), 1.8 (10) |
| #14 | No significant reaction | NA | 3.0 (P), 3.9 (4), 3.9 (8) | |
| Placebo | #03 | 2- to 3-cm induration, slightly tender for 3 days | 5-cm red tender warm swelling at day 1, 3-cm induration at day 3, resolving at day 18 | 3.9 (P), 4.5 (4), 2.6 (8), 2.8 (10), 2.2 (12) |
| #10 | Slight tender swelling at day 5 | 6-cm warm swelling at day 5 | 0.55 (P), 3.3 (4), 3.6 (52) | |
| #11 | 2-cm induration, slightly tender at day 14 | NA | 1.1 (P), 5.9 (4) | |
| #12 | No significant reaction | 8-cm erythema, itchy, resolved after day 8 | 3.7 (P), 3.2 (4), 2.9 (8), 4.6 (156) | |
Measurements are diameters.
P, prevaccination. Boldface indicates TT values above the 5-IU/ml cutoff.
NA, not applicable because no second vaccination was given.
Two vaccinees (#04 and #08) were EBV seronegative at 52 weeks but had seroconverted by week 104, and one vaccinee (#09) was seronegative at 12 weeks but had seroconverted by week 26 (Table 1). These seroconversion events were asymptomatic, and no adverse events were experienced during these periods. A further vaccinee, #02, seroconverted between weeks 104 and 628 with no history of IM. One of the placebo-vaccinated volunteers (#12) was diagnosed with IM by their general practitioner using symptoms and serology at week 392 (Table 1).
Vaccinee #14 was found to be EBV seropositive at 8 weeks (Table 1) and at that time had significant responses against FLR (20/106 lymphocytes), RAK (480/106), and QAK 72/106) as measured by LDA. No responses were detected at weeks 2 and 4 (data not shown). The incubation period for EBV has been reported to be 38 days for one individual (56), which suggests that vaccinee 14 became infected within a few weeks of the first immunization. This vaccinee complained of a severe sore throat and fatigue, which lasted about 2 to 3 weeks and began at about week 7. No time off work was taken, biochemistry was normal, and no significant lymphocytosis was present at week 4 or 8. At week 4 the CD4 and CD8 counts were 1.64 × 109 and 0.45 × 109/liter, respectively, and this changed to 0.89 × 109 and 1.77 × 109/liter at week 8. This clinical picture may represent mild IM; however, this vaccinee had a history of repeated tonsillitis and was prescribed penicillin by her general practitioner. To try to determine whether vaccinee 14 had IM, interleukin-15 (IL-15) receptor status was determined, as this receptor has been reported to be permanently down regulated in T cells from individuals who have had IM (45). However, in contrast to the findings of Sauce et al. (45), we found no differences in IL-15 receptor status between several EBV-seronegative individuals, healthy EBV-seropositive individuals, and individuals recovered from IM (data not shown). Using monoclonal antibodies 151303 and 151307 (R&D Systems), 0.1 to 4% of CD4+ T cells and 3 to 15% of CD8+ T cells were IL-15 receptor positive in all groups and in vaccinee #14 (data not shown). The IL-15 receptor profile thus does not appear to be a reliable indicator of past IM.
Induction of FLR-specific T-cell responses postvaccination.
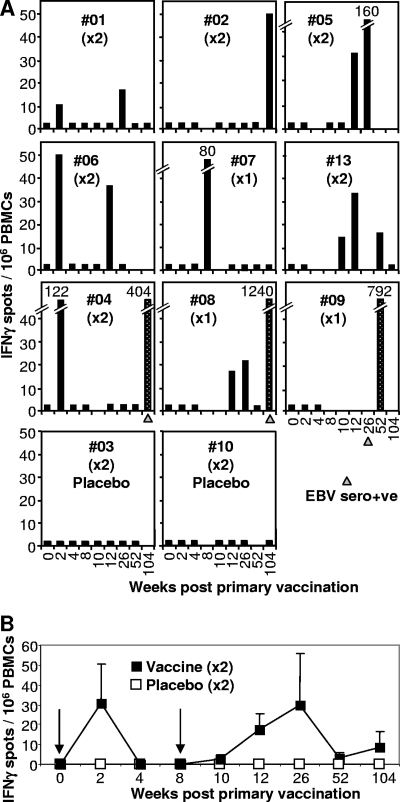
Ex vivo IFN-γ ELISPOT assays illustrated that the vaccine had generated detectable FLR-specific responses in 8/9 vaccine recipients. Responses were detected in 7/8 of the vaccinees (#01, #02, #04, #05, #06, #07, and #08) who received 5 μg of peptide and in one vaccinee (#13) who received 50 μg of peptide (Fig. 1A). T-cell data from vaccinee #14 are not presented (see above). Vaccinees #09 and #14 were the only vaccinees in whom FLR-specific responses could not be demonstrated postvaccination by ELISPOT assay. However, PBMC from #08, #10, and #12 weeks were not available for testing for vaccinee #09, and PBMC from only two time points (weeks 2 and 4) were available for vaccinee 14 before seroconversion. Appropriate Vβ TCR sequences were nevertheless identified in PBMC from vaccinee #09 at week 8 (see below). No responses were observed in any of the placebo vaccine recipients (#03 and #10 [Fig. 1] and #011 and #12 [data not shown]). When the mean responses for the six vaccinees who received two immunizations were plotted over time, clear postvaccination expansions of T-cell numbers followed by their expected contraction (22) could be seen (Fig. 1B).
FIG. 1.
Ex vivo IFN-γ ELISPOT analysis. (A) The vaccine was administered twice at week 0 and week 8 (×2), except for vaccinees #07, #08, and #09, who received only the first vaccination (×1). Vaccinees received a 5-μg dose of peptide, except #13, who received a 50-μg dose, and #03 and #10, who received no peptide (placebo). PBMC from vaccinees collected prior to vaccination (week 0) and at the indicated number of weeks after the first vaccination were analyzed by ex vivo IFN-γ ELISPOT assay using the FLR peptide and frozen PBMC. Three vaccinees seroconverted asymptomatically within 2 years (#04, #08, and #09), and the time at which serology first indicated EBV seroconversion is indicated with a gray triangle. Vaccine-induced responses are represented by black bars. Speckled bars illustrate responses after EBV seroconversion. Black bars at 2.5 IFN-γ spots/106 PBMC represent no significant response and are shown to indicate that an assay was performed on PBMC collected at that time point. (B) Mean numbers of IFN-γ spots (± standard errors) for vaccinees #01, #02, #04, #05, #06, and #13 (black squares), who received vaccinations at 0 and 8 weeks (arrows). The mean numbers of spots for all four placebo recipients are also shown (white squares). The two groups are significantly different (P = 0.041) by analysis of variance, which included a term for weeks.
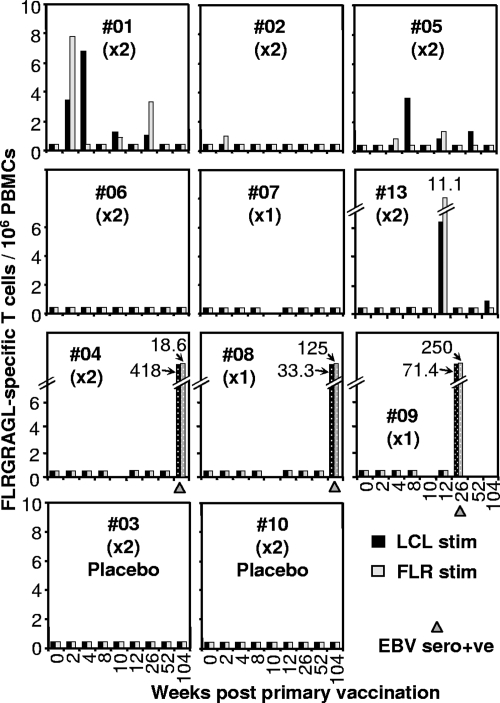
The LDA assays showed vaccine-induced FLR-specific responses in 3/9 vaccine recipients (#01, #05, and #13), with a marginal response in vaccinee 2 (Fig. 2). No responses were detected in the four placebo-vaccinated individuals (#03 and #10 [Fig. 2] and #11 and #12 [data not shown]).
FIG. 2.
LDA. Fresh PBMC from the same vaccines as described in the legend to Fig. 1 were analyzed by LDA. Cultures were stimulated with either autologous LCLs (black bars) or FLR peptide (10 μg/ml) (gray bars) and assessed in 51Cr release assays. Speckled bars illustrate responses after EBV seroconversion. The time when serology first indicated EBV seroconversion is indicated with a gray triangle. Bars at 0.4/106 PBMC represent no significant response and are shown to indicate that an assay was performed on PBMC collected at that time point.
PBMC from vaccinees 1, 6, and 13 were tested for long-term FLR-specific memory responses at weeks 412, 523, and 421, respectively, using the cultured ELISPOT method of Goonetilleke et al. (21). Responses of 0, 92.5, and 95 IFN-γ spots per 106 PBMC were obtained, respectively; however, the latter two did not reach significance over background (data not shown).
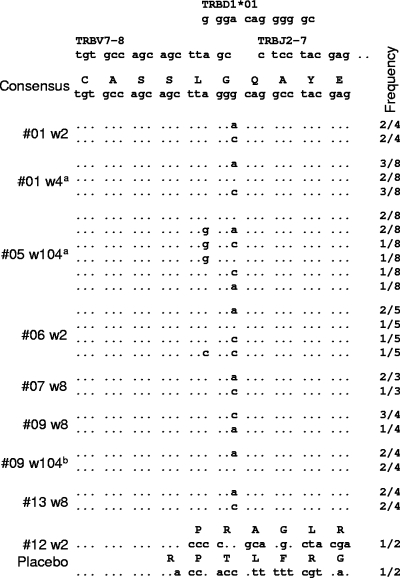
TCR sequence analysis.
FLR-specific CD8 T cells in nearly all HLA B8 EBV-seropositive individuals share the same Vβ TCR amino acid sequence (3, 52). RT-PCR and sequencing were therefore used to determine whether FLR vaccination induced CD8 T cells with this Vβ sequence. PBMC or bulk culture samples (containing 5 × 106 cells) from six vaccinees (#01, #05, #06, #07, #09, and #13) and three individuals receiving the placebo (#10, #11, and #12) taken at various times (see Fig. 3 legend) were analyzed by RT-PCR. All the samples from vaccinees produced PCR products that were detected by ethidium bromide staining (data not shown). In contrast, detectable PCR products were not generated from PBMC taken at week 0 from vaccinee #01 or from any of the six placebo PBMC samples, with the exception of PBMC from #12 taken at week 2 (data not shown). All samples produced bright, clear, and consistent PCR products when the TCR constant-region primers were used (data not shown). PCR products from no. 12 at week 2 and a selection of products from six vaccinees were cloned and sequenced. All clones providing TCR sequences from all six vaccinees showed the same Vβ amino acid sequence as that previously published for CD8 T cells recognizing FLR (3), although several different nucleotide sequences were seen (Fig. 3). The same nucleotide sequence was also found in #09 PBMC at 8 weeks and after EBV seroconversion at week 104. Two sequences were obtained from the PCR product from PBMC of the placebo recipient #12 at week 2 (Fig. 3, #12 w2 Placebo); however, neither showed any similarity to the consensus sequence. These data indicate that FLR peptide vaccination of EBV-seronegative individuals induced CD8 T cells with the same Vβ TCR amino acid sequence as that seen in the majority of HLA B8 individuals both during primary infection and after EBV seroconversion (52).
FIG. 3.
TCR Vβ sequences postvaccination. Sequences from cloned PCR products from PBMC or bulk cultures from vaccine recipients taken at the indicated times postvaccination (w, week) are shown. The frequency with which the indicated sequence was found over the total number of clones providing TCR sequence is indicated in the right column. a, from bulk cultures; b, vaccinee #09 had seroconverted at this time. The consensus sequence for LC13 (3) is shown at the top together with the germ line gene sequences that are used in the generation of this CDR3 region. Clear PCR products were obtained from #01 PBMC collected at weeks 2, 10, 26, 68, and 104; from #01 bulk cultures at week 4; from #05 bulk cultures at week 104; from #06 PBMC at weeks 2, 12, and 26; from #06 PBMC at weeks 2, 12, and 26; from #07 PBMC at week 8; from #09 PBMC at weeks 8, 10, 12, and 52; from placebo #12 PBMC at week 2; and from #13 PBMC at weeks 8 and 10. No detectable PCR products were obtained from #01 PBMC at week 0 (two samples), placebo #10 PBMC at weeks 2 and 4, placebo #11 PBMC at weeks 2 and 4, and placebo #12 PBMC at week 12 (data not shown).
FLR-specific T-cell responses post-EBV seroconversion.
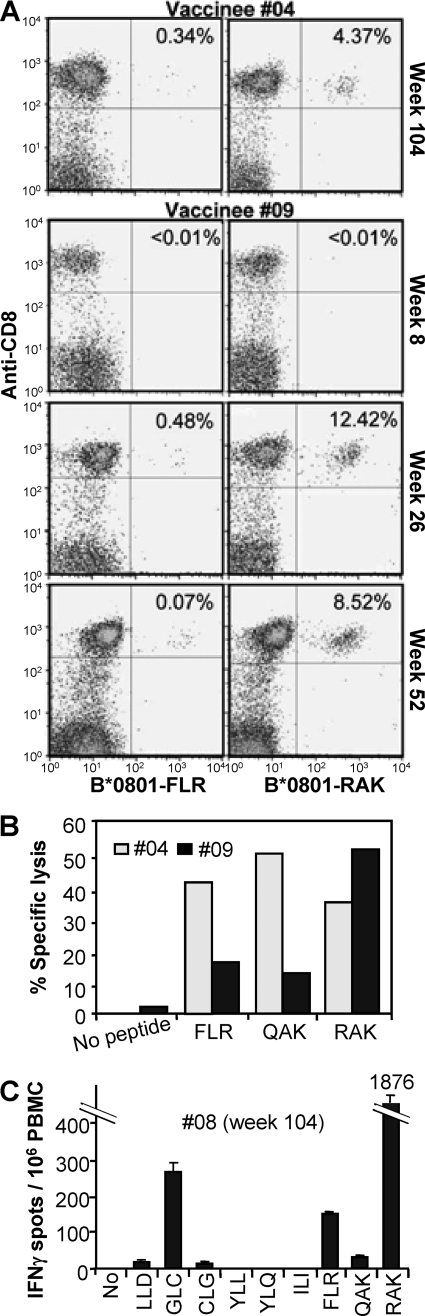
Three vaccinees, #04, #08, and #09, seroconverted asymptomatically within 2 years with no lymphocytosis evident at the time of positive EBV serology (Table 1). These vaccinees showed FLR-specific T-cell responses of 404, 1,240, and 792 IFN-γ spots per 106 PBMC after seroconversion (Fig. 1). These T-cell frequencies are comparable to those found in other studies using ex vivo ELISPOT analysis of FLR-specific T-cell responses in healthy EBV-seropositive HLA B8 individuals (32, 58). In our hands such individuals usually give FLR-specific responses in the range of 100 to 1,100 spots/106 PBMC in ex vivo IFN-γ ELISPOT assays (data not shown). The LDA frequencies following seroconversion (Fig. 2) are also comparable to those found in healthy EBV-seropositive individuals (11, 12). Analysis with MHC-peptide multimers of FLR-specific responses in vaccinees #04 and #09 (Fig. 4A, B*0801-FLR) also showed responses similar to those seen during IM and in healthy seropositive individuals (24). We were thus unable to find any evidence that vaccine-primed individuals were predisposed to abnormally high FLR-specific anemnestic responses after EBV seroconversion.
FIG. 4.
CD8 T-cell response of vaccinees after EBV seroconversion. (A) PBMC from vaccinees (no. 9 and 4) collected at the indicated time points postvaccination were assessed by FACS using FLR-specific (left panels, B*0801-FLR) and RAK-specific (right panels, B*0801-RAK) pentamers (x axis) and anti-CD8 monoclonal antibody (y axis). The percentage of CD8 T cells that are pentamer positive are indicated in the top right corner of each panel. (B) Standard chromium release assay using bulk cultures generated from PBMC from vaccinees #09 (week 52) and #04 (week 104) to asses lytic activity specific for FLR, QAK, and RAK. (C) Ex vivo IFN-γ ELISPOT assay using PBMC from vaccinee #08 (collected at week 104) to asses T-cell responses to the HLA A*0201-restricted epitopes LLDFVRFMGV (LLD), GLCTLVAML (GLC), CLGGLLTMV (CLG), ILIYNGWYA (ILI), YLLEMLWRL (YLL), and YLQQNWWTL (YLQ) and the HLA B*0801-restricted epitopes FLR, QAK, and RAK.
Examination of the phenotype of the B*0801-FLR multimer-positive cells for #04 (week 104) and #09 (week 26) showed the expected memory phenotype. However, significant proportions (25% and 57%, respectively) were CD45RA positive. For #09, this percentage dropped to 4% at week 52 (Table 3). In healthy seropositive individuals and during IM, latent antigen-specific CD8+ T cells usually remain CD45RA negative (16, 24).
TABLE 3.
Percentages of B*0801-FLR and -RAK tetramer-positive cells expressing CD27, CD28, CD45RA, CD45RO, and CD62L
| Vaccinee | Tetramer (wk) | %
|
|||||
|---|---|---|---|---|---|---|---|
| CD8 | CD27 | CD28 | CD45RA | CD45RO | CD62L | ||
| B*0801- | #04 (104) | 0.34a | 97 | 99 | 25 | 82 | 24 |
| FLR | #09 (26) | 0.48a | 97 | 99 | 57 | 91 | 58 |
| #09 (52) | 0.7a | 98 | 99 | 4 | 86 | 48 | |
| #01 (NA)b | 1.3 | 89 | 83 | 5 | 92 | 47 | |
| B*0801- | #04 (104) | 4.37a | 87 | 99 | 40 | 42 | 24 |
| RAK | #09 (26) | 12.42a | 79 | 99 | 26 | 47 | 28 |
| #09 (52) | 8.52a | 89 | 99 | 28 | 34 | 31 | |
| #01 (NA) | 3.41 | 90 | 97 | 38 | 61 | 40 | |
As shown in Fig. 3A.
Healthy seropositive donor. NA, not applicable.
Normal acquisition of CD8+ T-cell responses to other epitopes in vaccinees after EBV seroconversion.
To determine whether, after EBV seroconversion, vaccinees developed normal CD8+ T-cell responses to other epitopes or whether vaccination causes responses to be dominated by FLR-specific CD8+ T cells, PBMC from vaccinees #04 and #09 were analyzed with an MHC-peptide multimer for responses to the HLA B*0801-restricted epitope RAK from the lytic antigen BZLF1. Vaccinee #04 developed RAK responses (Fig. 4A, HLA B*0801-RAK) comparable to those seen in individuals recovering from IM or in individuals latently infected with EBV (24). Vaccinee 9 showed a clear expansion of RAK-specific responses at week 26, which fell at week 52 (Fig. 4A, HLA B*0801-RAK). Similar expansions and contractions of RAK responses have been well documented for IM patients as they recover from disease (24). The phenotypes of the HLA B*0801-RAK pentamer-positive cells were also similar to those seen in individuals recovering from IM (16, 25) (Table 3, HLA B*0801-RAK). Responses to FLR, the HLA B*0801-resticted epitope, QAK (from EBNA3), and RAK could also be detected in PBMC collected 26 and 104 weeks after immunization for vaccinees #04 and #09, respectively, using chromium release assays (Fig. 4B). In addition, ELISPOT assays using week 104 PBMC from vaccinee #08 showed HLA A2-resticted responses to GLCTLVAML (from BMLF1) as well as RAK responses similar to those found in healthy seropositive individuals (58) (Fig. 4C). Small responses to the HLA A2-restricted epitopes LLD (28) and CLG (34) were also detected (Fig. 4C). Thus, taken together, these data do not support the view that single-epitope immunization results in immunodominance or immunodomination effects (17) which would lead to the repression of responses to other epitopes following seroconversion.
DISCUSSION
Although dendritic cells pulsed with CD8 T-cell epitopes have been used to treat EBV-positive nasopharyngeal carcinoma patients (35), to our knowledge the current paper describes the first CD8+ T-cell-based EBV vaccine trial with EBV-seronegative individuals, and it illustrates that epitope-based vaccination is mostly well tolerated and immunogenic in most individuals. The same TCR Vβ sequences that are involved in recognizing FLR presented by EBV-infected cells in HLA B8 EBV-seropositive individuals (3) were also found in EBV-seronegative volunteers postvaccination. Importantly, vaccine-based priming of responses to the single FLR epitope did not predispose individuals to disease following a primary EBV infection, indicating that single-epitope immunization is safe in this context. In addition, neither FLR nor other EBV-specific CD8+ T-cell responses were demonstrably affected in vaccinees following primary EBV infection, suggesting that significant immunodominance or immunodomination effects did not occur (17). Insufficient numbers preclude any formal assessment of vaccine efficacy. Nevertheless, excluding vaccinee 14, who seroconverted near the time of vaccination, 4/4 of the peptide vaccine recipients who seroconverted failed to develop IM, whereas 1/2 of the placebo-vaccinated individuals who seroconverted developed IM. Published studies on the incidence of IM among young adults experiencing primary EBV seroconversion show a range from 25 to 74% (19).
The most common side effect in our study was inflammation and discomfort at the site of injection (Table 2), an observation also made in previous trials using this adjuvant (36, 62). The side effects were similar in the peptide vaccine and placebo groups (Table 2), indicating that they were unrelated to the peptide component of the formulation. Following a pretrial vaccination of an HLA B8 EBV-seropositive individual with the 5-μg FLR vaccine, TT titers rose from 8.3 to >50,000 IU/ml. This was associated with a severe injection site reaction, consisting of a 15-cm-diameter erythema and induration, with suppuration lasting ≈2 months (data not shown). TT is thus the likely cause of injection site reactions (27, 33), despite the fact that only 1/10th the amount of TT in the conventional TT-alum vaccine was delivered. The 5-IU/ml cutoff adopted for this trial was implemented to minimize the risk of such inflammatory responses. However, this meant that ≈45% of volunteers could not enter the trial, and 3/14 vaccinees did not receive a second dose of vaccine (Table 2). TT thus does not appear to be a good choice for providing CD4+ T-cell help in such vaccines, although adequate help for CD8 induction was likely provided by the TT, since all vaccinees increased their TT titer and/or showed a pronounced reaction to the vaccine. Such responses/reactions are likely due to activation of TT-specific CD4 T cells (7), which should provide the necessary help for CD8 T-cell induction (6, 48). However, alternative proteins or peptides that have not previously been seen by the patient's immune system might provide sufficient help without the associated anamnestic inflammatory response (42). gp350 from EBV is clearly an obvious candidate (40).
The presence of CD45RA-positive latent-antigen-specific CD8+ T cells during acute asymptomatic seroconversions in vaccinees #04 and #09 (Table 3) contrasts with the observation that such cells are usually CD45RA negative during IM and in healthy seropositive individuals (16, 24). Such CD45RA, CD45R0 double-positive memory cells have been reported previously after primary human cytomegalovirus infection (63). It is unclear whether this is a general feature of acute asymptomatic seroconversions or due to vaccination. The expansion of RAK-specific CD8+ T cells in #09 at week 26 (Fig. 4A) in the absence of a lymphocytosis was also unexpected, since a previous study has demonstrated no major perturbations in the blood TCR repertoire in three out of four individuals undergoing asymptomatic seroconversion (53). The TCR repertoire recognizing the dominant RAK epitope can be diverse (51), so conceivably TCR repertoire analysis may not detect such expansions. This RAK expansion is unlikely to be a result of FLR vaccination and therefore indicates that lytic antigen-specific expansions can occur during asymptomatic seroconversions.
Due to the HLA diversity across human populations, we have estimated that about 25 CD8+ T-cell epitopes would be needed to cover >90% of individuals in Western countries. Although complex peptide mixtures can be effectively codelivered in water-in-oil adjuvants (20), manufacture of a vaccine with so many individual components is difficult. EBV peptide-based vaccines with fewer epitopes might be envisaged for PTLD patients, where the HLA is often known, or for IM if specific populations are targeted or where reduced population coverage is acceptable. Combining large numbers of epitopes for an EBV vaccine might be achieved using the polyepitope or polytope approach (55, 60), which has recently shown some success in human trials (21). The data presented here suggest that an epitope-based approach for EBV vaccines is safe and does not appear to predispose individuals to immunological problems after EBV infection.
Acknowledgments
S. Elliott, A. Suhrbier, and J. Miles contributed equally to this work and should be considered joint first authors.
This work was funded by the National Cancer Institute (grant CA 57952), the NH&MRC (Australia), and the Co-operative Research Centre for Vaccine Technology.
We thank the staff at QIMR and students at the University of Queensland for participating in the trial. We also acknowledge Richard Kemp (Royal Brisbane Hospital) and Bill Woods and David Ryan from CSL Ltd. for their unstinting support of the study. We thank the Queensland Medical Laboratory for doing the serology and the Royal Brisbane Hospital Pathology and the Princess Alexandra Hospital tissue typing facilities for volunteering their services for the trial.
Footnotes
Published ahead of print on 21 November 2007.
REFERENCES
- 1.Adams, L. A., B. Deboer, G. Jeffrey, R. Marley, and G. Garas. 2006. Ganciclovir and the treatment of Epstein-Barr virus hepatitis. J. Gastroenterol. Hepatol. 211758-1760. [DOI] [PubMed] [Google Scholar]
- 2.Aichele, P., K. Brduscha-Riem, S. Oehen, B. Odermatt, R. M. Zinkernagel, H. Hengartner, and H. Pircher. 1997. Peptide antigen treatment of naive and virus-immune mice: antigen-specific tolerance versus immunopathology. Immunity 6519-529. [DOI] [PubMed] [Google Scholar]
- 3.Argaet, V. P., C. W. Schmidt, S. R. Burrows, S. L. Silins, M. G. Kurilla, D. L. Doolan, A. Suhrbier, D. J. Moss, E. Kieff, T. B. Sculley, and I. S. Misko. 1994. Dominant selection of an invariant T cell antigen receptor in response to persistent infection by Epstein-Barr virus. J. Exp. Med. 1802335-2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bharadwaj, M., S. R. Burrows, J. M. Burrows, D. J. Moss, M. Catalina, and R. Khanna. 2001. Longitudinal dynamics of antigen-specific CD8+ cytotoxic T lymphocytes following primary Epstein-Barr virus infection. Blood 982588-2589. [DOI] [PubMed] [Google Scholar]
- 5.Bharadwaj, M., M. Sherritt, R. Khanna, and D. J. Moss. 2001. Contrasting Epstein-Barr virus-specific cytotoxic T cell responses to HLA A2-restricted epitopes in humans and HLA transgenic mice: implications for vaccine design. Vaccine 193769-3777. [DOI] [PubMed] [Google Scholar]
- 6.Black, C. A. 1999. Delayed type hypersensitivity: current theories with an historic perspective. Dermatol. Online J. 57. [PubMed] [Google Scholar]
- 7.Brown, A. E., L. Markowitz, S. Nitayaphan, P. Morgan, S. Sukwit, S. Chinaworapong, S. Leelasupasri, and D. Birx. 2000. DTH responsiveness of HIV-infected Thai adults. J. Med. Assoc. Thai. 83633-639. [PubMed] [Google Scholar]
- 8.Burrows, S. R., J. Gardner, R. Khanna, T. Steward, D. J. Moss, S. Rodda, and A. Suhrbier. 1994. Five new cytotoxic T cell epitopes identified within Epstein-Barr virus nuclear antigen 3. J. Gen. Virol. 752489-2493. [DOI] [PubMed] [Google Scholar]
- 9.Burrows, S. R., S. J. Rodda, A. Suhrbier, H. M. Geysen, and D. J. Moss. 1992. The specificity of recognition of a cytotoxic T lymphocyte epitope. Eur. J. Immunol. 22191-195. [DOI] [PubMed] [Google Scholar]
- 10.Burrows, S. R., T. B. Sculley, I. S. Misko, C. Schmidt, and D. J. Moss. 1990. An Epstein-Barr virus-specific cytotoxic T cell epitope in EBV nuclear antigen 3 (EBNA 3). J. Exp. Med. 171345-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burrows, S. R., S. L. Silins, S. M. Cross, C. A. Peh, M. Rischmueller, J. M. Burrows, S. L. Elliott, and J. McCluskey. 1997. Human leukocyte antigen phenotype imposes complex constraints on the antigen-specific cytotoxic T lymphocyte repertoire. Eur. J. Immunol. 27178-182. [DOI] [PubMed] [Google Scholar]
- 12.Burrows, S. R., S. L. Silins, D. J. Moss, R. Khanna, I. S. Misko, and V. P. Argaet. 1995. T cell receptor repertoire for a viral epitope in humans is diversified by tolerance to a background major histocompatibility complex antigen. J. Exp. Med. 1821703-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Callan, M. F., N. Steven, P. Krausa, J. D. Wilson, P. A. Moss, G. M. Gillespie, J. I. Bell, A. B. Rickinson, and A. J. McMichael. 1996. Large clonal expansions of CD8+ T cells in acute infectious mononucleosis. Nat. Med. 2906-911. [DOI] [PubMed] [Google Scholar]
- 14.Callan, M. F., L. Tan, N. Annels, G. S. Ogg, J. D. Wilson, C. A. O'Callaghan, N. Steven, A. J. McMichael, and A. B. Rickinson. 1998. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein-Barr virus in vivo. J. Exp. Med. 1871395-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Candy, B., and M. Hotopf. 2006. Steroids for symptom control in infectious mononucleosis. Cochrane Database Syst. Rev. 3CD004402. [DOI] [PubMed] [Google Scholar]
- 16.Catalina, M. D., J. L. Sullivan, R. M. Brody, and K. Luzuriaga. 2002. Phenotypic and functional heterogeneity of EBV epitope-specific CD8+ T cells. J. Immunol. 1684184-4191. [DOI] [PubMed] [Google Scholar]
- 17.Chen, W., and J. McCluskey. 2006. Immunodominance and immunodomination: critical factors in developing effective CD8+ T-cell-based cancer vaccines. Adv. Cancer Res. 95203-247. [DOI] [PubMed] [Google Scholar]
- 18.Chetham, M. M., and K. B. Roberts. 1991. Infectious mononucleosis in adolescents. Pediatr. Ann. 20206-213. [DOI] [PubMed] [Google Scholar]
- 19.Crawford, D. H., K. F. Macsween, C. D. Higgins, R. Thomas, K. McAulay, H. Williams, N. Harrison, S. Reid, M. Conacher, J. Douglas, and A. J. Swerdlow. 2006. A cohort study among university students: identification of risk factors for Epstein-Barr virus seroconversion and infectious mononucleosis. Clin. Infect. Dis. 43276-282. [DOI] [PubMed] [Google Scholar]
- 20.Elliott, S. L., S. Pye, T. Le, L. Mateo, J. Cox, L. Macdonald, A. A. Scalzo, C. A. Forbes, and A. Suhrbier. 1999. Peptide based cytotoxic T-cell vaccines; delivery of multiple epitopes, help, memory and problems. Vaccine 172009-2019. [DOI] [PubMed] [Google Scholar]
- 21.Goonetilleke, N., S. Moore, L. Dally, N. Winstone, I. Cebere, A. Mahmoud, S. Pinheiro, G. Gillespie, D. Brown, V. Loach, J. Roberts, A. Guimaraes-Walker, P. Hayes, K. Loughran, C. Smith, J. De Bont, C. Verlinde, D. Vooijs, C. Schmidt, M. Boaz, J. Gilmour, P. Fast, L. Dorrell, T. Hanke, and A. J. McMichael. 2006. Induction of multifunctional human immunodeficiency virus type 1 (HIV-1)-specific T cells capable of proliferation in healthy subjects by using a prime-boost regimen of DNA- and modified vaccinia virus Ankara-vectored vaccines expressing HIV-1 Gag coupled to CD8+ T-cell epitopes. J. Virol. 804717-4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haring, J. S., V. P. Badovinac, and J. T. Harty. 2006. Inflaming the CD8+ T cell response. Immunity 2519-29. [DOI] [PubMed] [Google Scholar]
- 23.Hersey, P., S. W. Menzies, B. Coventry, T. Nguyen, M. Farrelly, S. Collins, D. Hirst, and H. Johnson. 2005. Phase I/II study of immunotherapy with T-cell peptide epitopes in patients with stage IV melanoma. Cancer Immunol. Immunother. 54208-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hislop, A. D., N. E. Annels, N. H. Gudgeon, A. M. Leese, and A. B. Rickinson. 2002. Epitope-specific evolution of human CD8(+) T cell responses from primary to persistent phases of Epstein-Barr virus infection. J. Exp. Med. 195893-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hislop, A. D., N. H. Gudgeon, M. F. Callan, C. Fazou, H. Hasegawa, M. Salmon, and A. B. Rickinson. 2001. EBV-specific CD8+ T cell memory: relationships between epitope specificity, cell phenotype, and immediate effector function. J. Immunol. 1672019-2029. [DOI] [PubMed] [Google Scholar]
- 26.Hjalgrim, H., K. E. Smedby, K. Rostgaard, D. Molin, S. Hamilton-Dutoit, E. T. Chang, E. Ralfkiaer, C. Sundstrom, H. O. Adami, B. Glimelius, and M. Melbye. 2007. Infectious mononucleosis, childhood social environment, and risk of Hodgkin lymphoma. Cancer Res. 672382-2388. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs, R. L., R. S. Lowe, and B. Q. Lanier. 1982. Adverse reactions to tetanus toxoid. JAMA 24740-42. [PubMed] [Google Scholar]
- 28.Kerr, B. M., N. Kienzle, J. M. Burrows, S. Cross, S. L. Silins, M. Buck, E. M. Benson, B. Coupar, D. J. Moss, and T. B. Sculley. 1996. Identification of type B-specific and cross-reactive cytotoxic T-lymphocyte responses to Epstein-Barr virus. J. Virol. 708858-8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khanna, R., S. Bell, M. Sherritt, A. Galbraith, S. R. Burrows, L. Rafter, B. Clarke, R. Slaughter, M. C. Falk, J. Douglass, T. Williams, S. L. Elliott, and D. J. Moss. 1999. Activation and adoptive transfer of Epstein-Barr virus-specific cytotoxic T cells in solid organ transplant patients with posttransplant lymphoproliferative disease. Proc. Natl. Acad. Sci. USA 9610391-10396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khanna, R., S. R. Burrows, J. Nicholls, and L. M. Poulsen. 1998. Identification of cytotoxic T cell epitopes within Epstein-Barr virus (EBV) oncogene latent membrane protein 1 (LMP1): evidence for HLA A2 supertype-restricted immune recognition of EBV-infected cells by LMP1-specific cytotoxic T lymphocytes. Eur. J. Immunol. 28451-458. [DOI] [PubMed] [Google Scholar]
- 31.Khanna, R., S. R. Burrows, A. Suhrbier, C. A. Jacob, H. Griffin, I. S. Misko, T. B. Sculley, M. Rowe, A. B. Rickinson, and D. J. Moss. 1993. EBV peptide epitope sensitization restores human cytotoxic T cell recognition of Burkitt's lymphoma cells. Evidence for a critical role for ICAM-2. J. Immunol. 1505154-5162. [PubMed] [Google Scholar]
- 32.Klatt, T., Q. Ouyang, T. Flad, I. Koetter, H. J. Buhring, H. Kalbacher, G. Pawelec, and C. A. Muller. 2005. Expansion of peripheral CD8+ CD28− T cells in response to Epstein-Barr virus in patients with rheumatoid arthritis. J. Rheumatol. 32239-251. [PubMed] [Google Scholar]
- 33.Kuhlwein, A., and A. Bleyl. 1985. Tetanus antitoxin levels and reactions following tetanus vaccination. Hautarzt 36462-464. [PubMed] [Google Scholar]
- 34.Lee, S. P., W. A. Thomas, R. J. Murray, F. Khanim, S. Kaur, L. S. Young, M. Rowe, M. Kurilla, and A. B. Rickinson. 1993. HLA A2.1-restricted cytotoxic T cells recognizing a range of Epstein-Barr virus isolates through a defined epitope in latent membrane protein LMP2. J. Virol. 677428-7435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin, C. L., W. F. Lo, T. H. Lee, Y. Ren, S. L. Hwang, Y. F. Cheng, C. L. Chen, Y. S. Chang, S. P. Lee, A. B. Rickinson, and P. K. Tam. 2002. Immunization with Epstein-Barr virus (EBV) peptide-pulsed dendritic cells induces functional CD8+ T-cell immunity and may lead to tumor regression in patients with EBV-positive nasopharyngeal carcinoma. Cancer Res. 626952-6958. [PubMed] [Google Scholar]
- 36.Lopez, J. A., C. Weilenman, R. Audran, M. A. Roggero, A. Bonelo, J. M. Tiercy, F. Spertini, and G. Corradin. 2001. A synthetic malaria vaccine elicits a potent CD8(+) and CD4(+) T lymphocyte immune response in humans. Implications for vaccination strategies. Eur. J. Immunol. 311989-1998. [DOI] [PubMed] [Google Scholar]
- 37.Moss, D. J., S. R. Burrows, D. J. Castelino, R. G. Kane, J. H. Pope, A. B. Rickinson, M. P. Alpers, and P. F. Heywood. 1983. A comparison of Epstein-Barr virus-specific T-cell immunity in malaria-endemic and -nonendemic regions of Papua New Guinea. Int. J. Cancer 31727-732. [DOI] [PubMed] [Google Scholar]
- 38.Moss, D. J., C. Schmidt, S. Elliott, A. Suhrbier, S. Burrows, and R. Khanna. 1996. Strategies involved in developing an effective vaccine for EBV-associated diseases. Adv. Cancer Res. 69213-245. [DOI] [PubMed] [Google Scholar]
- 39.Moss, D. J., A. Suhrbier, and S. L. Elliott. 1998. Candidate vaccines for Epstein-Barr virus. BMJ 317423-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moutschen, M., P. Leonard, E. M. Sokal, F. Smets, M. Haumont, P. Mazzu, A. Bollen, F. Denamur, P. Peeters, G. Dubin, and M. Denis. 2007. Phase I/II studies to evaluate safety and immunogenicity of a recombinant gp350 Epstein-Barr virus vaccine in healthy adults. Vaccine 254697-4705. [DOI] [PubMed] [Google Scholar]
- 41.Nielsen, T. R., K. Rostgaard, N. M. Nielsen, N. Koch-Henriksen, S. Haahr, P. S. Sorensen, and H. Hjalgrim. 2007. Multiple sclerosis after infectious mononucleosis. Arch. Neurol. 6472-75. [DOI] [PubMed] [Google Scholar]
- 42.Parra-Lopez, C., J. M. Calvo-Calle, T. O. Cameron, L. E. Vargas, L. M. Salazar, M. E. Patarroyo, E. Nardin, and L. J. Stern. 2006. Major histocompatibility complex and T cell interactions of a universal T cell epitope from Plasmodium falciparum circumsporozoite protein. J. Biol. Chem. 28114907-14917. [DOI] [PubMed] [Google Scholar]
- 43.Pope, J. H. 1968. Establishment of cell lines from Australian leukaemic patients: presence of a herpes-like virus. Aust. J. Exp. Biol. Med. Sci. 46643-645. [DOI] [PubMed] [Google Scholar]
- 44.Rickinson, A. B., and D. J. Moss. 1997. Human cytotoxic T lymphocyte responses to Epstein-Barr virus infection. Annu. Rev. Immunol. 15405-431. [DOI] [PubMed] [Google Scholar]
- 45.Sauce, D., M. Larsen, S. J. Curnow, A. M. Leese, P. A. Moss, A. D. Hislop, M. Salmon, and A. B. Rickinson. 2006. EBV-associated mononucleosis leads to long-term global deficit in T-cell responsiveness to IL-15. Blood 10811-18. [DOI] [PubMed] [Google Scholar]
- 46.Saul, A., G. Lawrence, A. Smillie, C. M. Rzepczyk, C. Reed, D. Taylor, K. Anderson, A. Stowers, R. Kemp, A. Allworth, R. F. Anders, G. V. Brown, D. Pye, P. Schoofs, D. O. Irving, S. L. Dyer, G. C. Woodrow, W. R. Briggs, R. Reber, and D. Sturchler. 1999. Human phase I vaccine trials of 3 recombinant asexual stage malaria antigens with Montanide ISA720 adjuvant. Vaccine 173145-3159. [DOI] [PubMed] [Google Scholar]
- 47.Savoldo, B., J. A. Goss, M. M. Hammer, L. Zhang, T. Lopez, A. P. Gee, Y. F. Lin, R. E. Quiros-Tejeira, P. Reinke, S. Schubert, S. Gottschalk, M. J. Finegold, M. K. Brenner, C. M. Rooney, and H. E. Heslop. 2006. Treatment of solid organ transplant recipients with autologous Epstein Barr virus-specific cytotoxic T lymphocytes (CTLs). Blood 1082942-2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scalzo, A. A., S. L. Elliott, J. Cox, J. Gardner, D. J. Moss, and A. Suhrbier. 1995. Induction of protective cytotoxic T cells to murine cytomegalovirus by using a nonapeptide and a human-compatible adjuvant (Montanide ISA 720). J. Virol. 691306-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sherritt, M. A., M. Bharadwaj, J. M. Burrows, L. E. Morrison, S. L. Elliott, J. E. Davis, L. M. Kear, R. E. Slaughter, S. C. Bell, A. J. Galbraith, R. Khanna, and D. J. Moss. 2003. Reconstitution of the latent T-lymphocyte response to Epstein-Barr virus is coincident with long-term recovery from posttransplant lymphoma after adoptive immunotherapy. Transplantation 751556-1560. [DOI] [PubMed] [Google Scholar]
- 50.Sherritt, M. A., J. Gardner, S. L. Elliott, C. Schmidt, D. Purdie, G. Deliyannis, W. R. Heath, and A. Suhrbier. 2000. Effect of pre-existing cytotoxic T lymphocytes on therapeutic vaccines. Eur. J. Immunol. 30671-677. [DOI] [PubMed] [Google Scholar]
- 51.Silins, S. L., S. M. Cross, S. L. Elliott, S. J. Pye, J. M. Burrows, D. J. Moss, and I. S. Misko. 1997. Selection of a diverse TCR repertoire in response to an Epstein-Barr virus-encoded transactivator protein BZLF1 by CD8+ cytotoxic T lymphocytes during primary and persistent infection. Int. Immunol. 91745-1755. [DOI] [PubMed] [Google Scholar]
- 52.Silins, S. L., S. M. Cross, S. L. Elliott, S. J. Pye, S. R. Burrows, J. M. Burrows, D. J. Moss, V. P. Argaet, and I. S. Misko. 1996. Development of Epstein-Barr virus-specific memory T cell receptor clonotypes in acute infectious mononucleosis. J. Exp. Med. 1841815-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Silins, S. L., M. A. Sherritt, J. M. Silleri, S. M. Cross, S. L. Elliott, M. Bharadwaj, T. T. Le, L. E. Morrison, R. Khanna, D. J. Moss, A. Suhrbier, and I. S. Misko. 2001. Asymptomatic primary Epstein-Barr virus infection occurs in the absence of blood T-cell repertoire perturbations despite high levels of systemic viral load. Blood 983739-3744. [DOI] [PubMed] [Google Scholar]
- 54.Suhrbier, A. 1997. Multi-epitope DNA vaccines. Immunol. Cell Biol. 75402-408. [DOI] [PubMed] [Google Scholar]
- 55.Suhrbier, A. 2002. Polytope vaccines for the codelivery of multiple CD8 T-cell epitopes. Expert Rev. Vaccines 1207-213. [DOI] [PubMed] [Google Scholar]
- 56.Svedmyr, E., I. Ernberg, J. Seeley, O. Weiland, G. Masucci, K. Tsukuda, R. Szigeti, M. G. Masucci, H. Blomogren, and W. Berthold. 1984. Virologic, immunologic, and clinical observations on a patient during the incubation, acute, and convalescent phases of infectious mononucleosis. Clin. Immunol. Immunopathol. 30437-450. [DOI] [PubMed] [Google Scholar]
- 57.Takeuchi, K., K. Tanaka-Taya, Y. Kazuyama, Y. M. Ito, S. Hashimoto, M. Fukayama, and S. Mori. 2006. Prevalence of Epstein-Barr virus in Japan: trends and future prediction. Pathol. Int. 56112-116. [DOI] [PubMed] [Google Scholar]
- 58.Tan, L. C., N. Gudgeon, N. E. Annels, P. Hansasuta, C. A. O'Callaghan, S. Rowland-Jones, A. J. McMichael, A. B. Rickinson, and M. F. Callan. 1999. A re-evaluation of the frequency of CD8+ T cells specific for EBV in healthy virus carriers. J. Immunol. 1621827-1835. [PubMed] [Google Scholar]
- 59.Tattevin, P., Y. Le Tulzo, S. Minjolle, A. Person, J. M. Chapplain, C. Arvieux, R. Thomas, and C. Michelet. 2006. Increasing incidence of severe Epstein-Barr virus-related infectious mononucleosis: surveillance study. J. Clin. Microbiol. 441873-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thomson, S. A., R. Khanna, J. Gardner, S. R. Burrows, B. Coupar, D. J. Moss, and A. Suhrbier. 1995. Minimal epitopes expressed in a recombinant polyepitope protein are processed and presented to CD8+ cytotoxic T cells: implications for vaccine design. Proc. Natl. Acad. Sci. USA 925845-5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Toes, R. E., R. Offringa, R. J. Blom, C. J. Melief, and W. M. Kast. 1996. Peptide vaccination can lead to enhanced tumor growth through specific T-cell tolerance induction. Proc. Natl. Acad. Sci. USA 937855-7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Toledo, H., A. Baly, O. Castro, S. Resik, J. Laferte, F. Rolo, L. Navea, L. Lobaina, O. Cruz, J. Miguez, T. Serrano, B. Sierra, L. Perez, M. E. Ricardo, M. Dubed, A. L. Lubian, M. Blanco, J. C. Millan, A. Ortega, E. Iglesias, E. Penton, Z. Martin, J. Perez, M. Diaz, and C. A. Duarte. 2001. A phase I clinical trial of a multiepitope polypeptide TAB9 combined with Montanide ISA 720 adjuvant in non-HIV-1 infected human volunteers. Vaccine 194328-4336. [DOI] [PubMed] [Google Scholar]
- 63.Wills, M. R., A. J. Carmichael, M. P. Weekes, K. Mynard, G. Okecha, R. Hicks, and J. G. Sissons. 1999. Human virus-specific CD8+ CTL clones revert from CD45ROhigh to CD45RAhigh in vivo: CD45RAhighCD8+ T cells comprise both naive and memory cells. J. Immunol. 1627080-7087. [PubMed] [Google Scholar]