Abstract
Hepatitis B and C viruses are major causative agents of liver fibrosis, cirrhosis, and liver cancer. Using comparative glycoproteomics, we identified a glycoprotein that is altered both in amount and in glycosylation as a function of liver fibrosis and cirrhosis. Specifically, this altered glycoprotein is an immunoglobulin G (IgG) molecule reactive to the heterophilic alpha-Gal epitope [Galα-1-3Galβ1-(3)4GlcNAc-R]. While similar changes in glycosylation have been observed in several autoimmune diseases, the specific immunoglobulins and their antigen recognition profiles were not determined. Thus, we provide the first report identifying the specific antigenic recognition profile of an immunoglobulin molecule containing altered glycosylation as a function of liver disease. This change in glycosylation allowed increased reactivity with several fucose binding lectins and permitted the development of a plate-based assay to measure this change. Increased lectin reactivity was observed in 100% of the more than 200 individuals with stage III or greater fibrosis and appeared to be correlated with the degree of fibrosis. The reason for the alteration in the glycosylation of anti-Gal IgG is currently unclear but may be related to the natural history of the disease and may be useful in the noninvasive detection of fibrosis and cirrhosis.
Worldwide, more than 500 million people have been chronically infected with hepatitis B or C virus (HBV or HCV) (1). Chronic infection with these viruses leads to liver damage, initially in the form of liver fibrosis (15). Without intervention, liver fibrosis can progress to cirrhosis and eventually lead to liver cancer (7).
For patients with chronic HBV and HCV infection, treatment decisions are based upon biochemical laboratory data, specifically, the circulating levels of hepatic transaminases and, more importantly, the degree of hepatic inflammation and fibrosis as determined by histological analysis (9). For example, in individuals with HCV or HBV infection, advanced fibrosis and cirrhosis are considered justifications to begin antiviral therapy (9, 18, 32). More importantly, the determination of hepatic fibrosis is critical to stage the severity of the liver disease in order to determine the prognosis and response to antiviral therapy (20). It is thus extremely important to be able to determine the presence of significant fibrosis and cirrhosis in a manner that allows routine clinical monitoring.
Using comparative glycoproteomics, we and others have observed changes in the N-linked glycans associated with serum glycoproteins upon the development of liver cirrhosis and liver cancer (3, 5). In this report, we show that the major serum glycoprotein containing altered glycosylation as a function of cirrhosis is not a liver-derived protein at all, but rather, is immunoglobulin G (IgG) that is specifically reactive to Galα-1-3Galβ1-(3)4GlcNAc-R (the alpha-Gal epitope). Anti-Gal antibodies are naturally occurring antibodies that in healthy subjects constitute ∼1% of total serum IgG. By definition, anti-Gal antibodies recognize a specific sugar linkage on glycolipids and glycoproteins that is present in nonhuman antigens. Briefly, this sugar linkage, referred to as the alpha-Gal epitope, is absent in humans but is abundantly synthesized by bacteria and nonprimate mammals. Although their function is not clearly known, it is hypothesized that anti-Gal antibodies control the level of Enterobacteriaceae, which are commonly found as a normal part of the human gut flora and express the alpha-Gal epitope (10).
Analysis of anti-Gal IgG molecules from healthy individuals or cirrhotic individuals revealed a shift in glycosylation from N links containing galactose residues to N links lacking galactose residues. This specific change in the glycosylation of immunoglobulins has been previously reported with various autoimmune diseases, such as rheumatoid arthritis and systemic lupus (26-28, 36), and recently with bacterial infection and chronic infection with human immunodeficiency virus (23). Our work is the first report that identifies a specific immunoglobulin with this modification.
This shift in glycosylation allowed the association with fucose binding lectins and permitted the development of a plate-based method to analyze this change. Using this assay, we have analyzed over 200 patients with various degrees of liver disease. Patients with mild fibrosis appeared to have less alteration in the glycosylation of their Galα1-3Galβ1-3GlcNAc (alpha-Gal) IgG, while patients with more severe fibrosis or cirrhosis had greater alterations in the glycosylation of their alpha-Gal IgG. This assay was capable of differentiating those patients with stage 1 or 2 fibrosis from patients without liver disease with a high level of sensitivity and specificity. In addition, the assay allowed the differentiation of severe fibrosis/cirrhosis (stages 3 to 6) from mild fibrosis (stages 1 and 2). The nature of this finding and the potential role in pathogenesis are discussed.
MATERIALS AND METHODS
Patients.
Patients for the current analysis were obtained from the University of Michigan under a study protocol that was approved by the University of Michigan Institutional Review Board. In addition, written informed consent was obtained from each subject. Demographic and clinical information was obtained, and a blood sample was collected from each subject. The blood sample from patients with chronic HCV infection was obtained at the time of liver biopsy and antiviral therapy. HCV was defined as the presence of HCV RNA with a lower limit of detection of <50 IU/ml at the University of Michigan Clinical Laboratory. All liver biopsy specimens were at least 30 mm long and 1.4 mm wide and were graded by three hepatic pathologists in a blinded fashion, and the amount of fibrosis was graded using the Ishak scoring system (16). Details on all patients are provided in Table 1. A group of individuals with no history of liver disease, alcohol consumption of less than 40 g a week, and no risk factors for viral hepatitis were enrolled from the general internal medicine clinics. All subjects in this control group were documented to have normal liver biochemistry and negative HCV antibodies. Patients with HCV-induced cirrhosis plus hepatocellular carcinoma (HCC) were enrolled from the liver clinic during this period. The diagnosis of HCC was made by histopathology (n = 87, including all T1 lesions) or, if histopathology was not available, by two imaging modalities (dynamic ultrasound, magnetic resonance imaging, or computed tomography). All patients with HCC were determined to have underlying cirrhosis based on histopathology (85%) and clinical parameters (15%). Each of the patients with a histological diagnosis of cirrhosis had a normal ultrasound and, if serum alpha-feto protein was elevated, magnetic resonance imaging of the liver within 3 months prior to enrollment and another 6 months after enrollment that showed no liver mass, in order to confirm that they had not developed HCC. The cirrhotic controls were followed for a median of 12 months (range, 7 to 18 months) after enrollment, and none developed HCC. The etiology of the liver disease for the patients without HCV infection was determined as previously described (21), and the definition of cirrhosis in these patients was also determined by histology.
TABLE 1.
Description of control subjects and patients with liver disease
| Variable | Value
|
|||||
|---|---|---|---|---|---|---|
| Controls | Patients at stage of disease (Ishak)a
|
|||||
| 1-2 | 3-5 | 6 (complete cirrhosis) | Non-HCV cirrhosisj | HCC + cirrhosis | ||
| Sample size | 113 | 24 | 19 | 57 | 34 | 87 |
| Ageb | 51 ± 11 | 50 ± 6 | 51 ± 4 | 53 ± 8 | 55 ± 8 | 56 ± 7 |
| % NHW/AA/H/Asianc | 90/10/0/0 | 98/1/1/0 | 96/2/1/1 | 85/11/2/2 | 98/0/1/0 | 88/4/2/6 |
| ALT (IU/ml)d | 28 ± 5 | 75 ± 7 | 71 ± 11 | 79 ± 19 | 85 ± 26 | 75 ± 12 |
| AST (IU/ml)e | 26 ± 3 | 73 ± 5 | 69 ± 8 | 93 ± 11 | 108 ± 24 | 101 ± 18 |
| Total bilirubinf (mg/dl) | 0.3 ± 0.1 | 0.3 ± 0.2 | 0.3 ± 0.4 | 1.2 ± 1.3 | 1.4 ± 0.7 | 1.6 ± 1.3 |
| % HCV genotype 1g | NAk | 78 | 72 | 76 | NA | 75 |
| HCV loadh (copies/ml) | 0 | 1.3 ± 0.8 | 1.6 ± 1 | 1.7 ± 1.5 | NA | 1.4 ± 1.3 |
| Serum gamma globulin (g/dl)i | 0.54 ± 0.1 | 0.85 ± 0.2 | 1.1 ± 0.4 | 1.3 ± 0.8 | 1.4 ± 0.8 | 1.1 ± 0.3 |
Fibrosis staging was based on the Ishak scoring system. For this study, stage 5 or greater was considered cirrhotic.
Mean age in years. P = 0.01, HCC versus fibrosis stages 1 to 6.
NHW, non-Hispanic Caucasian; AA, African American; H, Hispanic. No significant differences among groups.
ALT, alanine aminotransferase. P < 0.0001, fibrosis stages 1 to 6 and HCC versus controls.
AST,aspartate aminotransferase. P < 0.0001, fibrosis stages 1 to 6 and HCC versus controls.
P < 0.0001 for fibrosis stages 1 through 5 and all samples with fibrosis.
No significant differences among groups.
No significant differences among groups.
P < 0.0001, fibrosis stages 1 to 6 and HCC versus controls.
Non-HCV cirrhosis: 5 hepatitis B, 6 autoimmune hepatitis, 14 cryptogenic cirrhosis, and 9 alcoholic.
NA, not applicable.
Glycan analysis of total serum.
Total-serum glycan analysis was performed on composite samples from 10 healthy patients, 10 patients with mild fibrosis, and 10 patients with cirrhosis to determine the glycan changes that occur with the development of liver cirrhosis. Briefly, 5 μl of serum was absorbed into a dehydrated 12% Tris-glycine gel plug. The gel plug was reduced and alkylated, and the proteins were fixed using 10% methanol and 7% acetic acid. The N-linked glycans were removed using N-Glycanase Plus (Prozyme, San Leandro, CA), as described previously (3, 5, 6), and labeled with 2-aminobenzoic acid (Ludger Ltd., Abingdon, United Kingdom) according to the manufacturer's directions (13, 31). Desialylation of labeled N-glycan was performed via incubation of dried glycan with 1 μnit/ml of Arthrobacter ureafaciens sialidase (Prozyme) according to the manufacturer's directions. Glycan structures were identified by the calculation of the glucose unit value through comparison to known standards and by sequential exoglycosidase digestion as described previously (12, 13).
Proteomic identification of fucosylated glycoproteins.
To determine the identities of those proteins that contained core fucosylated glycans, we utilized specific sugar (glycan) binding proteins (lectins) to enrich for fucosylated proteins prior to proteomic analysis via mass spectrometry (MS). Briefly, lectin extraction was performed using agarose-bound Aleuria aurantia lectin (AAL) (Vector Laboratories, Ventura, CA) and Affi Sep-AAL absorption buffer and elution buffers (GALAB, Germany). Fucosylated proteins from 10 healthy individuals and 10 cirrhotic individuals (sera were combined) were extracted, and the fucosylated proteins were dissolved in loading buffer and resolved by electrophoresis through 12% sodium dodecyl sulfate-polyacrylamide gels. Proteins of interest were excised from the gel and identified via liquid chromatography tandem MS (LC MS-MS). Samples were de-N-glycosylated prior to LC MS-MS analysis. Peptide identification was performed on an LCQ ion trap mass spectrometer (Thermo-Finnigan Corp., San Jose, CA) equipped with on-line microcapillary high-performance LC (HPLC) (Eldex, Napa, CA) and a microspray ionization source, as described previously (5). Peptide searches were performed using Sequest (Thermo-Finnigan Corp.) against the Swiss-Prot human database. Xcorr-versus-change-state parameters were set to 1.50, 2.0, and 2.50.
Lectin FLISA.
For the analysis of the glycan modification of total IgG and anti-Gal IgG, we utilized a lectin fluorophore-linked immunosorbent assay (FLISA)-based approach. Briefly, to remove the fucosylation of the capture antibody (mouse anti-human IgG; Bethyl Laboratories, Montgomery, TX), the antibody was incubated with 10 mM sodium periodate for 1 h at 4°C. An equal volume of ethylene glycol was added, and the oxidized antibody was brought to a concentration of 10 mg/ml with sodium carbonate buffer, pH 9.5. Antibody (5 μg/well), human serum albumin (HSA) attached to alpha-Gal (Dextra Laboratories), or HSA alone (Sigma-Aldrich) was added to the plate and, following incubation, washed with 0.1% Tween 20-phosphate-buffered saline (PBS), pH 7.4, and blocked overnight with 3% bovine serum albumin-PBS. For analysis, 3 μl of serum was diluted in 97 μl of 3% bovine serum albumin-PBS and added to the plates for 2 h, and the plates were washed five times in lectin incubation buffer (10 mM Tris, pH 8.0, 0.15 M NaCl, 0.1% Tween 20) before fucosylated IgG was detected with biotin-conjugated AAL (Vector Laboratories, Burlingame, CA). Bound lectin was detected using IRDye 800-conjugated streptavidin, and the signal intensity was measured using the Odyssey Infrared Imaging System (LI-COR Biotechnology, Lincoln, NE). In all cases, the sample intensity was compared to that of commercially purchased human serum (Sigma Inc., St. Louis, MO) (17, 18). All samples were run in triplicate, and intersample variation was less than 1%.
Purification and glycan analysis of anti-Gal IgG.
For analysis of anti-Gal IgG, the same samples described above were utilized. Briefly, synthetic alpha-Gal-HSA (Dextra Laboratories, Reading, United Kingdom) was coupled to a normal human serum-activated Sepharose 4 Fast Flow affinity column (GE Healthcare, Piscataway, NJ) according to the manufacturer's directions. Briefly, 50 μl of serum was incubated with the column, and the column was washed with 5 column volumes of Tris-buffered saline (TBS) with 0.1% Tween 20, followed by 1 column volume of TBS. The alpha-Gal-specific IgG was eluted using 0.1 M NaCl2-0.1 M glycine, pH 2.8, and immediately neutralized. For this study, an equal amount of anti-Gal IgG (1 μg) was reduced, alkylated, and separated on a 12% Tris-glycine acrylamide gel and stained using colloidal Coomassie. Anti-Gal IgG bands were excised and destained, and glycan analysis was performed as described previously (3, 5) and above.
Exoglycosidase treatment of purified IgG.
Briefly, IgG was purified from commercially purchased human serum (Sigma Chemicals) using the Melon Gel IgG purification kit (Pierce, Rockland, IL). The purified IgG was subsequently concentrated and the buffer exchanged for TBS. Half of the sample was incubated with 5 units/ml A. ureafaciens sialidase (Prozyme), 5 units/ml jack bean beta-galactosidase (Prozyme), and 5× exoglycosidase buffer [0.5 M citrate phosphate buffer, pH 4.5, 0.2 mM Zn(O2CCH3)2, 150 mM NaCl2] at 37°C overnight. The other half of the IgG sample was incubated with buffer alone at 37°C overnight. The next day, half of the sample was tested for lectin reactivity as described above and the other half was used for HPLC glycan analysis to confirm exoglycosidase digestion.
Statistical analysis.
Descriptive statistics for all patients were compared by scatter plots that included the outliers. All values were reported as mean values ± standard errors unless otherwise stated. As the data did not follow a typical Gaussian distribution, a nonparametrical test (a two-tailed, 95% confidence Mann-Whitney test) was used to determine statistical differences between groups. A two-tailed P value of 0.05 was used to determine statistical significance. All analyses were performed using GraphPad Prism (San Diego, CA).
RESULTS
Alterations in the human serum glycome and identification of anti-Gal IgG as the predominant altered glycoprotein in patients with cirrhosis.
As stated in the introduction, a change in glycosylation on serum-associated N-linked glycan from patients with a diagnosis of liver disease had been previously reported by us and by others (3-5). To extend those observations and to further analyze the proteins associated with the altered glycan in specific disease states, the profile of glycan derived from total proteins isolated from people with and without a diagnosis of cirrhosis was performed.
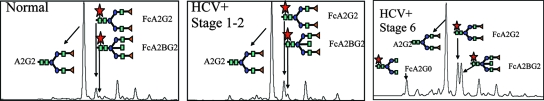
Figure 1 shows the desialylated N-linked glycan analysis of total sera from either control patients, HCV patients with stage 1 or 2 fibrosis, or patients with HCV and biopsy-confirmed cirrhosis as performed by normal-phase HPLC (13, 30). Each peak corresponds to a different glycan structure, and as Fig. 1A shows, consistent with previous work, significant changes in N-linked glycosylation can be seen in patient serum with the development of cirrhosis (4). Peaks that are significantly altered are indicated and correspond to an agalactosylated core fucosylated biantennary N-glycan (FcA2G0), a core fucosylated biantennary N-glycan (FcA2G2), and a bisected core fucosylated biantennary N-glycan (FcA2BG2).
FIG. 1.
Evidence that the glycosylation of the human serum glycome is altered with the development of cirrhosis and the identification of anti-Gal IgG as the glycoprotein with the altered glycan. Total serum desialylated glycan profiles from control samples (left), HCV samples with stage 1 and 2 fibrosis (middle), or samples from HCV patients with biopsy-confirmed cirrhosis (stage 5 and 6) are shown. The major peaks of interest are indicated and include a simple biantennary glycan (A2G2), a core fucosylated biantennary glycan (FcA2G2), and a core fucosylated bisected biantennary glycan (FcA2BG2). The cirrhotic samples were from patients with stage 6 fibrosis. The squares represent N-acetylglucosamine monosaccharides (GlcNAc), the circles represent mannose, the triangles represent galactose, and the stars represent fucose.
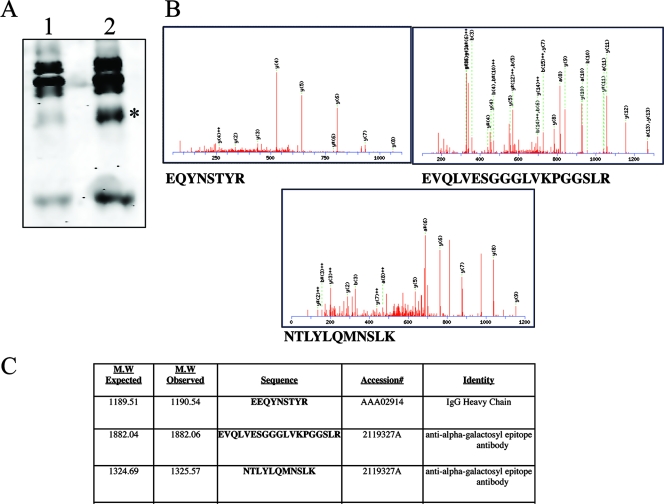
The identities of the major glycoproteins to which the fucosylated glycan was attached were determined by two approaches. The first was the observation that the difference in glycosylation, as shown in Fig. 1, was abolished if immunoglobulin was depleted from the serum prior to N-linked-glycan analysis (data not shown). Although the serum depletion approach provided circumstantial evidence that immunoglobulins were the source of elevated fucose in the serum, a second method was used to positively identify the fucosylated proteins. Briefly, undenatured proteins containing fucosylated N-linked glycans were extracted from serum by passing them over columns with the fucose binding lectin AAL. Polypeptides binding to the lectin represent the serum “fucome,” or “fucosylated proteome.” In this way, the fucosylated proteomes from healthy individuals or from patients with cirrhosis were analyzed by one-dimensional gel electrophoresis. As Fig. 2A shows, several differences can be seen between the fucosylated proteomes from healthy patients and those from patients with cirrhosis. A species at 50 kDa was highly elevated in patients with cirrhosis and was further examined by trypsination of the protein, followed by LC MS-MS analysis for protein identification (Fig. 2B). Three of the peptides identified are shown and were used to identify the 50-kDa species as IgG with reactivity to the sugar commonly referred to as the alpha-Gal epitope (Fig. 2B and C).
FIG. 2.
Identification of the major lectin-reactive protein(s) in the sera of patients with fibrosis/cirrhosis. (A) The major fucosylated proteins in the sera of cirrhotic patients were identified by extraction of fucosylated proteins from the sera using fucose-specific lectins and analyzed by one-dimensional gel electrophoresis. In lane 1 are the lectin-reactive proteins from healthy individuals; in lane 2 are the lectin-reactive proteins from cirrhotic individuals. The 50-kDa species, which is altered in the two patient groups, is indicated with an asterisk. (B) The 50-kDa species was digested with trypsin, and the protein was identified via LC MS-MS analysis. The MS-MS spectra for the three major peptides identified are shown. (C) The major identified fucosylated proteins observed in cirrhotic samples corresponded to IgG that was reactive to the alpha-Gal epitope.
Confirmation of alteration in the glycosylation of alpha-Gal-reactive IgG.
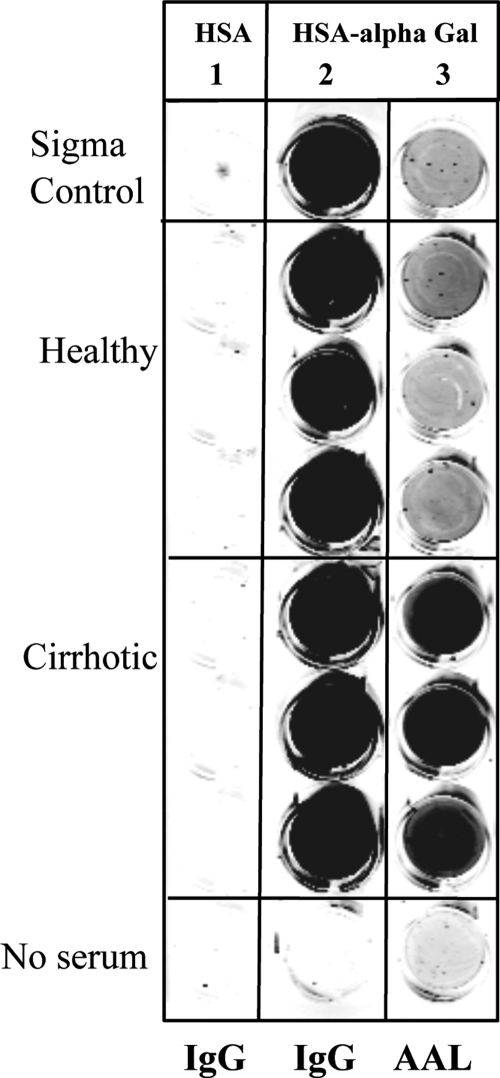
Confirmation of specificity for the alpha-Gal epitope was performed through a plate-based assay to measure the level of lectin-reactive IgG specific for the epitope. Briefly, as shown in Fig. 3, synthetic alpha-Gal linked to HSA was plated on 96-well plates and incubated with human sera from four healthy control individuals or three individuals with cirrhosis. Captured IgG was detected using anti-human IgG-conjugated secondary antibody or using the fucose-specific lectin AAL. As a control, unconjugated HSA was plated on adjacent wells to measure the specificity of binding. Columns 1 and 2 of Fig. 3 show the binding of human IgG to wells containing either HSA or HSA-alpha-Gal detected using an anti-human IgG secondary antibody. As the figure shows, and not surprisingly, while no human IgG molecules bound to the HSA (column 1), strong binding was observed to the HSA conjugated with the alpha-Gal sugar (column 2). The binding was present in all serum samples and is consistent with the report of anti-Gal antibodies being present in the sera of all patients (11). In column 3 is the lectin reactivity of the anti-Gal antibody. As column 3 shows, only the captured IgGs from patients with cirrhosis were reactive to the lectin, suggesting that the alpha-Gal antibody becomes lectin reactive with the development of severe fibrosis or cirrhosis. The relative increases in anti-Gal IgG, total IgG, and AAL-reactive anti-Gal IgG are shown in Table 2.
FIG. 3.
Evidence that anti-Gal IgG molecules have altered glycosylation in patients with cirrhosis. Briefly, either HSA (column 1) or synthetic alpha-Gal-linked HSA (columns 2 and 3) was plated onto 96-well plates and incubated with human sera from four healthy control individuals or from three individuals with cirrhosis. The captured IgG was detected by using either anti-human IgG-conjugated secondary antibody (columns 1 and 2) or the fucose-specific lectin AAL (column 3).
TABLE 2.
Relative levels of total IgG, anti-Gal IgG, and AAL-reactive anti-Gal IgG in patients with limited fibrosis, severe fibrosis, or cirrhosis
| Patient groupa | Fold greater than healthy purchased controlb
|
||
|---|---|---|---|
| Total IgGc | Anti-Gal IgGd | AAL-reactive anti-Gal IgGe | |
| Experimental control samples | 1 ± 0.1 | 1 ± 0.1 | 1 ± 0.1 |
| F1-F2 | 1.8 ± 0.1 | 2 ± 0.2 | 3 ± 0.2 |
| F3-F5 | 2.1 ± 0.2 | 3.5 ± 0.1 | 9 ± 0.2 |
| Cirrhotic | 2.4 ± 0.1 | 3.7 ± 0.1 | 15 ± 0.2 |
Fibrosis staging was based on the Ishak scoring system. Samples were combined for analysis. Ten samples per group were used for analysis. F, fibrosis stage.
All values are provided as relative increases over commercially purchased sera ± standard deviations.
The relative increase in IgG compared to commercially purchased sera.
The relative increase in anti-Gal IgG compared to commercially purchased sera.
The relative increase in AAL-reactive anti-Gal IgG compared to commercially purchased sera.
The alpha-Gal antibodies become selectively reactive to the fucose binding lectin in people with fibrosis and cirrhosis.
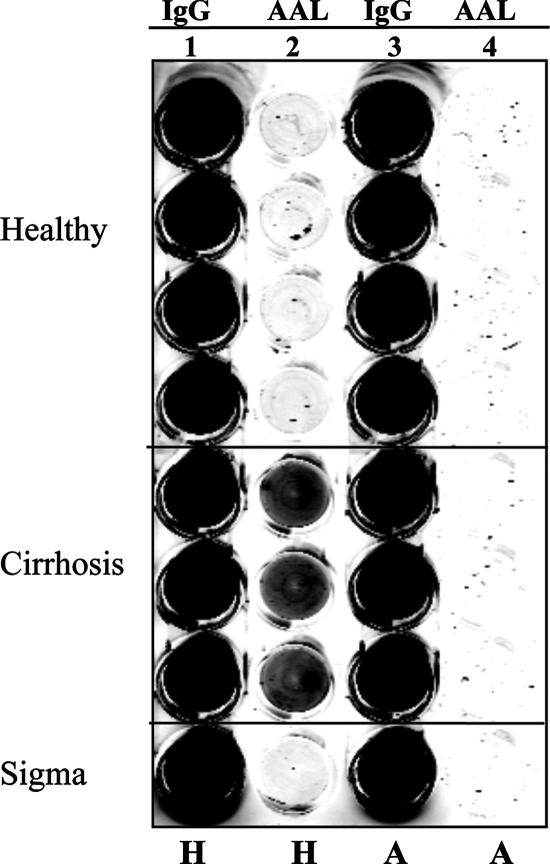
The increased binding of the AAL lectin to IgG from cirrhosis patients could be abolished if samples were precleared of anti-alpha-Gal antibodies prior to analysis. Figure 4 shows the results of a human IgG lectin FLISA using either sera from four healthy individuals or sera from three patients with cirrhosis. In this assay, a mouse anti-human IgG monoclonal antibody was used to capture all IgG molecules. Both serum samples were precleared with HSA or with alpha-Gal linked to HSA. Captured samples were probed either with a secondary mouse anti-human IgG antibody (column 1) or with the fucose binding lectin AAL (column 2). As columns 1 and 3 of Fig. 4 show, no significant change in IgG binding was observed in the healthy and cirrhotic patients as a function of blocking agents. Column 2 of Fig. 4 shows the lectin reactivities of the captured IgG molecules. As before, no signal was detected from the healthy samples regardless of pretreatment. In contrast, strong AAL binding of captured human IgG from patients with cirrhosis was observed when samples were precleared with HSA (column 2). However, when alpha-Gal-reactive antibodies were removed, even though large amounts of IgG were still captured on the plate (column 3), greatly reduced binding of the lectin was observed (column 4), indicating that alpha-Gal antibodies were specifically altered with the development of cirrhosis.
FIG. 4.
Evidence that only anti-Gal IgG is reactive with fucose binding lectins. Removal of heterophilic alpha-Gal antibodies prevents lectin reactivity of IgGs from cirrhotic patients. IgGs from either healthy individuals or individuals with cirrhosis were captured from sera by using a mouse anti-human IgG antibody. The amount of captured IgG (lane 1) or the level of AAL lectin reactivity (lane 2) was determined as for Fig. 3. Samples were precleared either with HSA (lanes 1 and 2) or with alpha-Gal-HSA (lanes 3 and 4) before analysis. H, precleared with HSA; A, precleared with alpha-Gal-HSA.
It was noted that when the IgG was denatured before analysis in an IgG lectin FLISA, all patient samples were reactive to the fucose-specific lectin, suggesting that the lectin reactivity of IgG is conformation dependent (data not shown).
The glycosylation of human IgG changes with the development of cirrhosis.
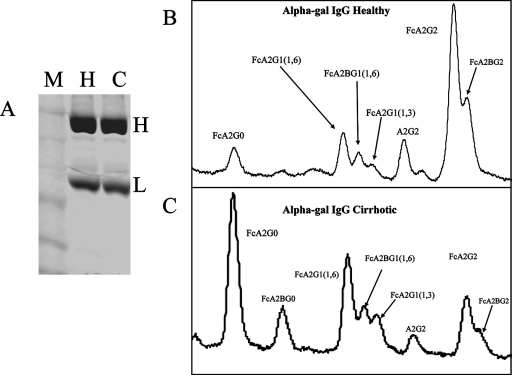
Based upon the results shown in Fig. 1 and 2, it appeared that anti-Gal IgGs from patients with cirrhosis had N-linked glycans with increased levels of fucose. Hence, we performed glycan analysis on anti-Gal IgG via N-linked glycan sequencing. Purified anti-Gal IgG was isolated from either control patients or patients with HCV-induced cirrhosis, and 1 μg was resolved via sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Fig. 5A). Subsequently, structural N-linked glycan analysis of the heavy chains was performed as shown in Fig. 5B and C. Significant differences could be seen in the glycans from patients with cirrhosis compared to those from the healthy patients (compare Fig. 5B with C). While healthy subjects contained primarily an FcA2G2 glycan structure, the heavy chains from patients with cirrhosis contained truncated structures with either a single galactose residue (FcA2G1) or no galactose residues (FcA2G0). The agalactosylated core fucosylated biantennary N-glycan (FcA2G0) was also observed in the glycomic analysis of total serum, as shown in Fig. 1A. N-linked glycans were not observed on light chains, either via lectin blotting or by glycan sequencing (data not shown). There thus appears to be a consistent structural difference between the IgGs isolated from healthy individuals and those from individuals with cirrhosis, and this change (agalactose) has been associated with a proinflammatory structure (17, 19).
FIG. 5.
Glycosylation analysis of anti-Gal IgG sfrom healthy or cirrhotic individuals via HPLC-based glycan sequencing. (A) Coomassie staining of anti-Gal IgGs purified from either healthy individuals (H) or cirrhotic individuals (C). The marker lane is also indicated (M). The heavy (H) and light (L) chains are indicated. (B and C) Glycan analysis of the desialylated N-linked glycans associated with the heavy chain from healthy individuals (B) or a cirrhotic individual (C). FcA2G0, core fucosylated (1,6) agalactosylated biantennary glycan; FcA2BG0, core fucosylated (1,6) agalactosylated biantennary glycan with a bisecting GlcNac; FcA2G1(1,6) core fucosylated (1,6) biantennary glycan with a single galatose residue on the 1,6 arm; FcA2BG1(1,6), core fucosylated (1,6) biantennary glycan with a single galatose residue on the 1,6 arm and a bisecting GlcNac; FcA2G2G1(1,3), core fucosylated (1,6) biantennary glycan with a single galatose residue on the 1,3 arm; FcA2G2, core fucosylated biantennary N-glycan (FcA2G2); FcA2BG2, bisected core fucosylated biantennary N-glycan.
The change in glycosylation of human IgG allows interaction with fucose binding lectins.
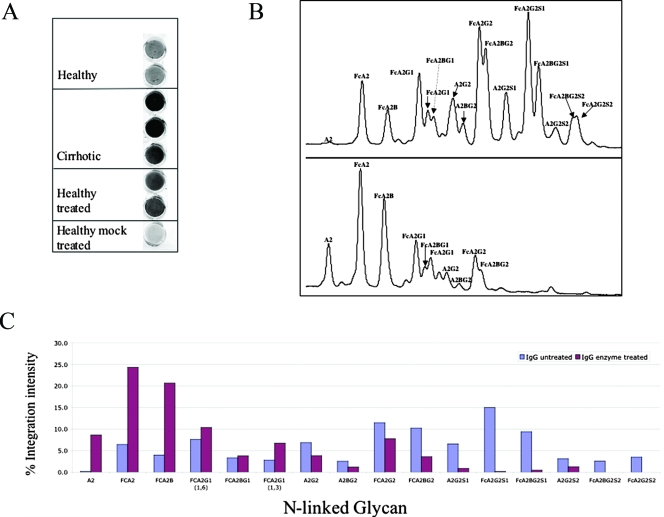
The relationship between the change in glycosylation observed in cirrhosis (Fig. 5B and C) and the increased reactivity with fucose binding lectins (Fig. 1 to 4) was tested by altering the glycan structure on IgGs from healthy individuals to mimic that observed in patients with fibrosis/cirrhosis. This was done by treating purified IgG from healthy individuals, which does not interact with the fucose binding lectin AAL, with sialidase (A. ureafaciens) and beta-galactosidase (jack bean), to create the degalactosylated N-linked glycan normally found on IgG patients with cirrhosis (24). The reactivities of these samples with the fucose binding lectins were tested using a lectin FLISA for captured IgG. Consistent with the results shown in Fig. 2 to 5, while IgG from control serum was not reactive with fucose binding lectins, IgG from cirrhotic patients was highly reactive (Fig. 6A). Reactivity with fucose binding lectin was obtained when IgG was degalactosylated enzymatically, suggesting that the increased reactivity of IgG to the fucose binding lectins was partially associated with the degalactosylated glycan structure (Fig. 6A and B). Treatment of samples with sialidase alone had no effect on binding, while treatment with beta-galactosidase or beta-galactosidase alone had only a minor effect (data not shown). Glycan sequencing on treated IgG was performed to confirm glycan modification (Fig. 6C). Thus, the increased reactivity of the IgGs from cirrhotic individuals with fucose binding lectins is partially related to the truncated glycosylation of the IgG molecule.
FIG. 6.
The reactivity of human IgG to the fucose binding lectin AAL is dependent on the type of N-linked glycan attached. (A) IgG from healthy individuals (purchased from Sigma Chemicals) was digested overnight with sialidase (A. ureafaciens) and beta(1-4)-galactosidase (jack bean) to create IgG molecules with the degalactosylated glycans observed in patients with cirrhosis. As a control, a mock sample, treated identically but without enzyme, was used. While IgG purified from healthy individuals had low reactivity with the fucose binding lectin, IgG purified either from cirrhotic individuals or from healthy serum that has been treated with the sialidase and beta-galactosidase had much greater reactivity, indicating that the lectin reactivity is directly associated with the FcA2G0 glycan structure. (B) Glycan analysis of IgG from either mock-treated samples (top) or enzyme-treated samples (bottom) to confirm enzymatic digestion. The major peaks are indicated. For the abbreviations, see the legend to Fig. 5B and C. Additional structures not presented in Fig. 5: A2G2S1, monosialylated biantennary glycan; FCA2G2S1, monosialylated core fucosylated biantennary N-glycan; FCA2BG2S1, monosialylated core fucosylated biantennary glycan with a bisecting GlcNac; A2G2S2, disialylated biantennary glycan; FCA2BG2S2, disialylated core fucosylated biantennary glycan; FcA2BG2S2, disialylated core fucosylated biantennary glycan with a bisecting GlcNac. (C) Quantification of results shown in panel B. The x axis is the glycan structure as detailed in panel B; the y axis is the relative contribution of each glycan structure to the total N-linked glycan profile. Structures are as in panel B.
Increased reactivity of a fucose-specific lectin with human IgG appears to be correlated with the development of cirrhosis.
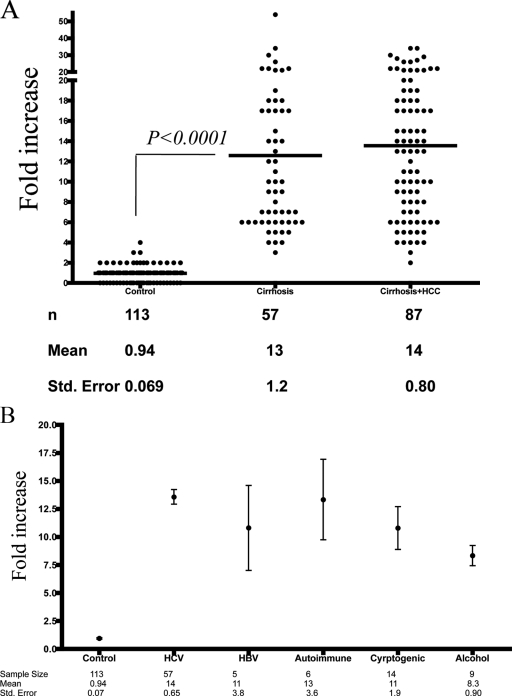
Using the same lectin FLISA shown in Fig. 2 to 4, the relative amounts of lectin-reactive IgG were determined in 257 coded serum samples from either control subjects, those with HCV-induced cirrhosis, or those with HCV-induced cirrhosis plus HCC. The results are shown in Fig. 7A and are expressed as the increase over commercially purchased human serum.
FIG. 7.
Analysis of lectin-reactive IgG in patients with HCV-induced fibrosis and cirrhosis. (A) Scatter plot of control individuals, individuals with HCV-induced cirrhosis, and individuals with HCV-induced cirrhosis plus HCC. The n value, mean, and standard error are provided for each group below the graph. The x axis represents the patient group; the y axis is the increase in lectin-reactive IgG compared to commercially purchased sera. The statistical difference between the HCV-induced cirrhosis group and control subjects was obtained (P < 0.0001; Mann-Whitney test). (B) The levels of lectin-reactive anti-Gal IgG were measured in patients with autoimmune disease-, HBV-, alcohol-, or cryptogenically induced cirrhosis. The n value, mean, and standard error are provided for each group below the graph. The x axis represents the patient group; the y axis is the increase in fucosylated IgG compared to commercially purchased sera. The values for each group are provided as the mean and standard error of the mean. The statistical differences were obtained for all cirrhosis groups and control subjects (P < 0.0001; Mann-Whitney test), but not between individual cirrhotic groups.
The signal detected in the samples from the 113 control subjects was similar to that observed in commercially purchased serum, with a mean relative value of 0.94 (Fig. 7A). In contrast, 57 patients with HCV-induced cirrhosis had values greater than 3-fold above commercially purchased serum, with a mean increase of 13-fold. This difference was statistically significant (P < 0.0001; two-tailed Mann-Whitney test), confirming our results in Fig. 2 to 6.
As the fucosylation of many proteins has been shown to be increased in HCC (5, 8), it was of interest to see if greater lectin reactivity would be observed in patients with cirrhosis plus HCC. Therefore, the levels of lectin-reactive IgGs were measured in 87 patients with HCV-induced cirrhosis plus HCC. As Fig. 7A shows, patients with cirrhosis plus HCC had a mean 14-fold increase in lectin-reactive IgG, which was not statistically different from the increase observed in patients with cirrhosis alone. Thus, no further increases in lectin binding could be observed with the development of HCC.
Increased lectin reactivity to anti-Gal IgG is observed in patients with cirrhosis from multiple etiologies.
To determine if the lectin reactivity with IgG was a phenomenon that was correlated with the development of cirrhosis independently of the etiology, we examined the levels of lectin-reactive IgG in patients with cirrhosis induced by excessive alcohol consumption, autoimmune disease, or HBV infection or from patients with an unknown underlying disease. As Fig. 7B shows, although the sample size was limited, the trend was very clear: IgGs from patients with cirrhosis, regardless of the underlying etiology, had greater reactivity to fucose-specific lectins than IgGs from healthy controls. Similar to the results shown in Fig. 3 to 5, all patients with cirrhosis had >3-fold elevations in lectin-reactive IgG. Statistical differences were observed for all individual cirrhotic groups (from non-HCV-induced cirrhosis) compared to control subjects (P < 0.0001; Mann-Whitney test). However, no statistical difference was observed between individual cirrhotic groups (one-way analysis of variance; Kruskal-Wallis test).
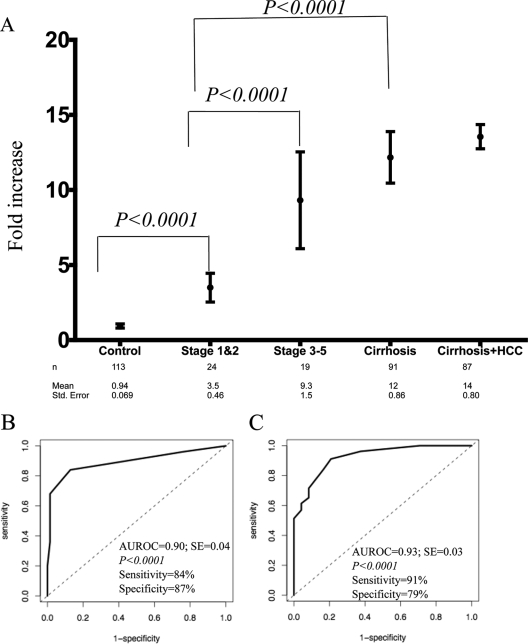
Increased lectin reactivity of anti-Gal IgG is observed in patients with fibrosis.
As the level of lectin-reactive anti-Gal IgG was increased in patients with cirrhosis, it was of interest to determine if similar changes could be observed in patients with fibrosis. Thus, we examined the levels of lectin-reactive anti-Gal IgG in patients with either limited fibrosis (stages 1 and 2) or more severe fibrosis (stages 3 and 4). These data are shown in Fig. 8A, along with the data from patients from the control or cirrhosis group. As the figure shows, sera from stage 1 and 2 fibrotic patients had a mean increase of 3.5-fold over commercially purchased sera, compared to only a 0.94-fold increase in the control patients. Even with the small sample size (n = 24), this increase was statistically different from the control group (P < 0.0001; two-tailed Mann-Whitney test), implying that the change in IgG starts with the development of liver fibrosis.
FIG. 8.
Increase in lectin-reactive anti-Gal IgG with the development of liver fibrosis. (A) Levels of lectin-reactive anti-Gal IgG in the control group and people with stage 1 or 2 fibrosis, stage 3 or 5 fibrosis, and cirrhosis. The mean value for each group is plotted, along with the 95% confidence interval for the mean. The x axis represents the patient group; the y axis is the increase in lectin-reactive IgG compared to commercially purchased serum. The n value, mean, and standard error are provided for each group below the graph. All patients with cirrhosis (from Fig. 5A and B) are included. (B) ROC analysis comparing people with stage 1 and 2 fibrosis with the control group. Using an optimal cutoff of threefold above commercially purchased sera, fucosylated immunoglobulin had a sensitivity of 84% and a specificity of 87%. (C) ROC analysis comparing stage 1 and 2 fibrosis with stage 3 and 6 fibrosis/cirrhosis. Using an optimal cutoff of fivefold above commercially purchased sera, fucosylated immunoglobulin had a sensitivity of 91% and a specificity of 79%. AUROC, area under the ROC curve.
Sera from patients with bridging fibrosis and incomplete cirrhosis (stages 3 to 5) had an even greater level of lectin-reactive IgG, with a mean increase of 9.3-fold over commercially purchased sera. Although the sample size was limited (n = 19), a significant statistical difference (P < 0.0001; two-tailed Mann-Whitney test) was observed between those patients with limited fibrosis (stages 1 and 2) and those with more severe fibrosis (stages 3 to 5). No statistical difference was observed between patients with bridging fibrosis (stages 3 to 5) and those patients with cirrhosis (P > 0.5).
Receiver operator characteristic (ROC) curves were plotted to help define the optimal cutoff values for differentiating the different stages of liver fibrosis and cirrhosis. ROC analysis was performed between stage 1 and 2 fibrosis and control subjects and between stage 1 and 2 fibrosis and stage 3 to 6 fibrosis/cirrhosis. Figure 8B shows the area under the ROC curve for the analysis of control subjects versus stage 1 and 2 fibrosis was 0.90, with a significant statistical difference between the observed curve and the no-discrimination line (P < 0.0001). Using a cutoff of threefold above commercially purchased sera, lectin-reactive anti-Gal IgG had a sensitivity of 84%, a specificity of 87%, a positive predictive value of 70%, and a negative predictive value of 94%. The ROC analysis for the differentiation of those patients with limited fibrosis (stages 1 and 2) and those with stage 3 or greater fibrosis is shown in Fig. 8C. In such an analysis, the area under the ROC curve for fucosylated IgG was 0.93, with a sensitivity of 91% and a specificity of 79%, using an optimal cutoff of 5 relative units.
DISCUSSION
This is the first report describing changes in the glycosylation of IgG with the development of liver disease. In addition, while changes in the glycosylation of IgG molecules have been reported for several other diseases, this is the first report identifying a specific immunoglobulin that contains the altered glycosylation. This alteration in anti-Gal IgG molecules allowed interaction with fucose binding lectins and permitted the development of a plate-based test to quantify these changes. Quite surprisingly, this change was observed in 100% of patients with biopsy-confirmed fibrosis and cirrhosis. Furthermore, a possible correlation between the amount of lectin-reactive anti-Gal IgG and the degree of fibrosis has been shown.
Recently, it has become evident that alterations in bacterial flora occur with the development of cirrhosis and that they may play a role in liver pathology (reviewed in reference 29). However, much of this evidence has been gathered from animal models that may not truly represent the human condition. As stated above, the alpha-Gal antibodies are believed to be responsive to the Enterobacteriaceae, and thus, our results add strong evidence that alterations in bacterial flora are occurring in humans. What role this response plays in disease pathology remains unclear, but a direct association is not ruled out. Recent reports have indicated that liver-associated B cells may play a significant role in the development of liver fibrosis (25). Thus, it is possible that the alpha-Gal antibodies observed in the sera of patients with fibrosis and cirrhosis are derived from these liver-resident B cells and may contribute in some way to liver pathogenesis. For example, the production of proinflammatory antibodies to the Enterobacteriaceae could lead to increased levels of bacterial products, such as lipopolysaccharide, that could contribute to liver fibrosis or cirrhosis (35).
It was noted that changes in glycosylation were not observed on non-anti-Gal IgG reactive to Escherichia coli, Klebsiella, or Helicobacter pylori. Additionally, analysis of our samples for reactivity to other antibodies, such as the Paul-Bunnell antigen test (MonoSpot test), showed no reactivity (data not shown). However, it is unclear if reactivity to the Hanganutziu and Deicher antigen is present (14).
A trivial explanation for the results we observed is that we were only measuring an increase in the total level of IgG related to liver failure. That is, with advanced fibrosis and cirrhosis, there is a shunting of blood flow away from sinusoids. As many glycoproteins (including antibodies) are cleared by Kupffer cells in the sinusoids, one could expect to see an increase in IgG (and other glycoproteins) as a result of portal-hepatic shunting. Indeed, as Table 2 shows, the levels of total IgG increased approximately two- to threefold with the development of fibrosis and cirrhosis, and this no doubt contributed to the glycan profile observed using total serum by us and by others (22, 34, 37). However, there was a specific alteration in the glycosylation of anti-Gal IgG that was independent of this phenomenon. That is, while 2- to 3-fold changes could been seen in the level of total IgG, there was over a 13-fold (mean) increase in the level of alpha-Gal IgG with the development of severe fibrosis and cirrhosis (as shown in Fig. 4A). Therefore, our results cannot be explained as only the result of an increase in immunoglobulin levels. In addition, while human IgG is present at a concentration of 10 to 20 mg/ml, our plate-based approach has a capacity of only 100 to 500 ng/ml. Thus, what we observed was a “snapshot” analysis of a specific antibody in any given patient.
A more exciting explanation for our results is that they were due to immune stimulation. That is, it has been shown that a decrease in galactosylation of IgG is associated with repeated immunization and response to a specific antigen. Surprisingly, this is the same alteration that we observed, and it strongly suggests that there may be clonal expansion of a specific set of B cells, presumably those that secrete alpha-Gal antibodies. It was noted that the glycosylation of IgG was shown to be directly associated with immune function (2). That is, it has recently been reported that IgG mediates pro- and anti-inflammatory activities through the engagement of its Fc domain with distinct Fc receptors. One type of interaction generates a proinflammatory effect, while other interactions generate anti-inflammatory effects. The type of interaction is dependent upon the presence of terminal sialic acid residues on the N-linked glycan present on the IgG molecule (17). IgG molecules containing terminal sialic acid molecules lead to anti-inflammatory responses, while IgG molecules lacking terminal sialic acid lead to a proinflammatory response. In the results presented here, the shift to N-linked glycan lacking terminal galactose residues, which prevents the further addition of sialic acid molecules, would lead to an inflammatory condition, which itself could be associated with pathology.
It was noted that while IgG from healthy individuals was heavily fucosylated, in the native form it was not reactive to the lectin AAL, presumably as a result of limited access to the core fucose residue on the Fc domain. In contrast, native IgGs from patients with fibrosis and cirrhosis were more reactive to the lectin AAL. This could be due to either a conformational change on IgG or increased lectin reactivity with the altered glycan structures found on IgGs from patients with fibrosis and cirrhosis (degalactosylated core fucosylated structures). The two conserved oligosaccharides in the Fc region of normal serum IgG are buried between the two CH2 domains (2). Reports have previously indicated that these glycans had limited association with several lectins and became reactive only after heat denaturation, indicating that alteration of IgG tertiary structure by denaturation led the carbohydrate moiety to become accessible to the lectins (33). It thus could be assumed that AAL-reactive IgG from the fibrotic/cirrhotic patients, but not from healthy individuals, has altered tertiary structure with an exposed carbohydrate moiety.
The work described here may be applicable as a screening tool in patient populations that are at an elevated risk of liver disease, such as those infected with HBV or HCV. Such a test could be utilized to select patients with significant fibrosis that would warrant further evaluation by other, more invasive methods, such as biopsy. Alternatively, such an assay could be utilized in the monitoring of patients postbiopsy to measure progression (or regression) of liver disease. Additionally, such a simple assay would potentially have great benefit in the developing world. Although larger, more diverse studies are needed and a direct comparison to other noninvasive tests for cirrhosis is required (5, 35-37), this work lays the groundwork by suggesting that the determination of the amount of lectin-reactive anti-Gal IgG will have value in the management of people with liver disease. In addition, the finding that alpha-Gal antibodies have increased reactivity to fucose binding lectins will allow the development of lectin FLISA-based methods for the analysis of glycoproteins that become hyperfucosylated with the development of HCC.
Acknowledgments
This work was supported by grant UO1 CA084951-06 from the National Cancer Institute (NCI) Early Detection Research Network (EDRN); grant R01 CA120206-01, also from the NCI; the Hepatitis B Foundation; and an appropriation from The Commonwealth of Pennsylvania.
Bevin Gangadharan, Brent Kopenhaver, and Andrew H. Talal are thanked for their careful reading of the manuscript.
Footnotes
Published ahead of print on 28 November 2007.
REFERENCES
- 1.Alter, M. J. 1997. Epidemiology of hepatitis C. Hepatology 2662S-65S. [DOI] [PubMed] [Google Scholar]
- 2.Arnold, J. N., M. R. Wormald, R. B. Sim, P. M. Rudd, and R. A. Dwek. 2006. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu. Rev. Immunol. 2521-50. [DOI] [PubMed] [Google Scholar]
- 3.Block, T. M., M. A. Comunale, M. Lowman, L. F. Steel, P. R. Romano, C. Fimmel, B. C. Tennant, W. T. London, A. A. Evans, B. S. Blumberg, R. A. Dwek, T. S. Mattu, and A. S. Mehta. 2005. Use of targeted glycoproteomics to identify serum glycoproteins that correlate with liver cancer in woodchucks and humans. Proc. Natl. Acad. Sci. USA 102779-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Callewaert, N., H. Van Vlierberghe, A. Van Hecke, W. Laroy, J. Delanghe, and R. Contreras. 2004. Noninvasive diagnosis of liver cirrhosis using DNA sequencer based total serum protein glycomics. Nat. Med. 10429-434. [DOI] [PubMed] [Google Scholar]
- 5.Comunale, M. A., M. Lowman, R. E. Long, J. Krakover, R. Philip, S. Seeholzer, A. A. Evans, H. W. L. Hann, T. M. Block, and A. S. Mehta. 2006. Proteomic analysis of serum associated fucosylated glycoproteins in the development of primary hepatocellular carcinoma. J. Proteome Res. 6308-315. [DOI] [PubMed] [Google Scholar]
- 6.Comunale, M. A., T. S. Mattu, M. A. Lowman, A. A. Evans, W. T. London, O. J. Semmes, M. Ward, R. Drake, P. R. Romano, L. F. Steel, T. M. Block, and A. Mehta. 2004. Comparative proteomic analysis of de-N-glycosylated serum from hepatitis B carriers reveals polypeptides that correlate with disease status. Proteomics 4826-838. [DOI] [PubMed] [Google Scholar]
- 7.Di Bisceglie, A. M. 1997. Hepatitis C and hepatocellular carcinoma. Hepatology 2634S-38S. [DOI] [PubMed] [Google Scholar]
- 8.Drake, R. R., E. E. Schwegler, G. Malik, J. Diaz, T. Block, A. Mehta, and O. J. Semmes. 2006. Lectin capture strategies combined with mass spectrometry for the discovery of serum glycoprotein biomarkers. Mol. Cell Proteomics 51957-1967. [DOI] [PubMed] [Google Scholar]
- 9.Fung, S. K., and A. S. Lok. 2004. Management of hepatitis B patients with antiviral resistance. Antivir. Ther. 91013-1026. [PubMed] [Google Scholar]
- 10.Galili, U. 2005. The alpha-Gal epitope and the anti-Gal antibody in xenotransplantation and in cancer immunotherapy. Immunol. Cell Biol. 83674-686. [DOI] [PubMed] [Google Scholar]
- 11.Galili, U., F. Anaraki, A. Thall, C. Hill-Black, and M. Radic. 1993. One percent of human circulating B lymphocytes are capable of producing the natural anti-Gal antibody. Blood 822485-2493. [PubMed] [Google Scholar]
- 12.Guile, G. R., D. J. Harvey, N. O'Donnell, A. K. Powell, A. P. Hunter, S. Zamze, D. L. Fernandes, R. A. Dwek, and D. R. Wing. 1998. Identification of highly fucosylated N-linked oligosaccharides from the human parotid gland. Eur. J. Biochem. 258623-656. [DOI] [PubMed] [Google Scholar]
- 13.Guile, G. R., P. M. Rudd, D. R. Wing, S. B. Prime, and R. A. Dwek. 1996. A rapid high-resolution high-performance liquid chromatographic method for separating glycan mixtures and analyzing oligosaccharide profiles. Anal. Biochem. 240210-226. [DOI] [PubMed] [Google Scholar]
- 14.Higashi, H., M. Naiki, S. Matuo, and K. Okouchi. 1977. Antigen of “serum sickness” type of heterophile antibodies in human sera: indentification as gangliosides with N-glycolylneuraminic acid. Biochem. Biophys. Res. Commun. 79388-395. [DOI] [PubMed] [Google Scholar]
- 15.Hoofnagle, J. H. 1997. Hepatitis C: the clinical spectrum of disease. Hepatology 2615S-20S. [DOI] [PubMed] [Google Scholar]
- 16.Ishak, K., A. Baptista, L. Bianchi, F. Callea, J. De Groote, F. Gudat, H. Denk, V. Desmet, G. Korb, R. N. MacSween, et al. 1995. Histological grading and staging of chronic hepatitis. J. Hepatol. 22696-699. [DOI] [PubMed] [Google Scholar]
- 17.Kaneko, Y., F. Nimmerjahn, and J. V. Ravetch. 2006. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 313670-673. [DOI] [PubMed] [Google Scholar]
- 18.Kweon, Y. O., Z. D. Goodman, J. L. Dienstag, E. R. Schiff, N. A. Brown, E. Burchardt, R. Schoonhoven, D. A. Brenner, and M. W. Fried. 2001. Decreasing fibrogenesis: an immunohistochemical study of paired liver biopsies following lamivudine therapy for chronic hepatitis B. J. Hepatol. 35749-755. [DOI] [PubMed] [Google Scholar]
- 19.Lastra, G. C., S. J. Thompson, A. S. Lemonidis, and C. J. Elson. 1998. Changes in the galactose content of IgG during humoral immune responses. Autoimmunity 2825-30. [DOI] [PubMed] [Google Scholar]
- 20.Liaw, Y. F. 2003. Results of lamivudine trials in Asia. J. Hepatol. 39(Suppl. 1)S111-S115. [DOI] [PubMed] [Google Scholar]
- 21.Marrero, J. A., R. J. Fontana, G. L. Su, H. S. Conjeevaram, D. M. Emick, and A. S. Lok. 2002. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology 361349-1354. [DOI] [PubMed] [Google Scholar]
- 22.Martin, D., D. Vroon, and S. Nasrallah. 1984. Value of serum immunoglobulins in the diagnosis of liver disease. Liver 4214-218. [DOI] [PubMed] [Google Scholar]
- 23.Moore, J. S., X. Wu, R. Kulhavy, M. Tomana, J. Novak, Z. Moldoveanu, R. Brown, P. A. Goepfert, and J. Mestecky. 2005. Increased levels of galactose-deficient IgG in sera of HIV-1-infected individuals. AIDS 19381-389. [DOI] [PubMed] [Google Scholar]
- 24.Nimmerjahn, F., R. M. Anthony, and J. V. Ravetch. 2007. Agalactosylated IgG antibodies depend on cellular Fc receptors for in vivo activity. Proc. Natl. Acad. Sci. USA 1048433-8437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Novobrantseva, T. I., G. R. Majeau, A. Amatucci, S. Kogan, I. Brenner, S. Casola, M. J. Shlomchik, V. Koteliansky, P. S. Hochman, and A. Ibraghimov. 2005. Attenuated liver fibrosis in the absence of B cells. J. Clin. Investig. 1153072-3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parekh, R. B., R. A. Dwek, and T. W. Rademacher. 1988. Rheumatoid arthritis as a glycosylation disorder. Br. J. Rheumatol 27(Suppl. 2)162-169. [DOI] [PubMed] [Google Scholar]
- 27.Parekh, R. B., R. A. Dwek, B. J. Sutton, D. L. Fernandes, A. Leung, D. Stanworth, T. W. Rademacher, T. Mizuochi, T. Taniguchi, K. Matsuta, et al. 1985. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature 316452-457. [DOI] [PubMed] [Google Scholar]
- 28.Parekh, R. B., I. M. Roitt, D. A. Isenberg, R. A. Dwek, B. M. Ansell, and T. W. Rademacher. 1988. Galactosylation of IgG associated oligosaccharides: reduction in patients with adult and juvenile onset rheumatoid arthritis and relation to disease activity. Lancet i966-969. [DOI] [PubMed] [Google Scholar]
- 29.Riordan, S. M., and R. Williams. 2006. The intestinal flora and bacterial infection in cirrhosis. J. Hepatol. 45744-757. [DOI] [PubMed] [Google Scholar]
- 30.Rudd, P. M., and R. A. Dwek. 1997. Rapid, sensitive sequencing of oligosaccharides from glycoproteins. Curr. Opin. Biotechnol. 8488-497. [DOI] [PubMed] [Google Scholar]
- 31.Rudd, P. M., T. S. Mattu, N. Zitzmann, A. Mehta, C. Colominas, E. Hart, G. Opdenakker, and R. A. Dwek. 1999. Glycoproteins: rapid sequencing technology for N-linked and GPI anchor glycans. Biotechnol. Genet. Eng. Rev. 161-21. [DOI] [PubMed] [Google Scholar]
- 32.Sherman, M. 2005. Hepatocellular carcinoma: epidemiology, risk factors, and screening. Semin. Liver Dis. 25143-154. [DOI] [PubMed] [Google Scholar]
- 33.Terai, I., K. Kobayashi, J. P. Vaerman, and N. Mafune. 2006. Degalactosylated and/or denatured IgA, but not native IgA in any form, bind to mannose-binding lectin. J. Immunol. 1771737-1745. [DOI] [PubMed] [Google Scholar]
- 34.Tsianos, E. V., A. M. Di Bisceglie, N. M. Papadopoulos, R. Costello, and J. H. Hoofnagle. 1990. Oligoclonal immunoglobulin bands in serum in association with chronic viral hepatitis. Am. J. Gastroenterol. 851005-1008. [PubMed] [Google Scholar]
- 35.Versalovic, J. 2007. Probiotics: intestinal gatekeeping, immunomodulation, and hepatic injury. Hepatology 46618-621. [DOI] [PubMed] [Google Scholar]
- 36.Watson, M., P. M. Rudd, M. Bland, R. A. Dwek, and J. S. Axford. 1999. Sugar printing rheumatic diseases: a potential method for disease differentiation using immunoglobulin G oligosaccharides. Arthritis Rheum. 421682-1690. [DOI] [PubMed] [Google Scholar]
- 37.Watt, K., J. Uhanova, Y. Gong, K. Kaita, K. Doucette, N. Pettigrew, and G. Y. Minuk. 2004. Serum immunoglobulins predict the extent of hepatic fibrosis in patients with chronic hepatitis C virus infection. J. Viral Hepat. 11251-256. [DOI] [PubMed] [Google Scholar]