Abstract
Classic studies on C57BL-derived mouse strains showed that they were resistant to mouse mammary tumor virus (MMTV) infection. Although one form of resistance mapped to the major histocompatibility complex (MHC) locus, at least one other, unknown gene was implicated in this resistance. We show here that B10.BR mice, which are derived from C57BL mice but have the same MHC locus (H-2k) as susceptible C3H/HeN mice, are resistant to MMTV, and show a lack of virus spread in their lymphoid compartments but not their mammary epithelial cells. Although in vivo virus superantigen (Sag)-mediated activation of T cells was similar in C3H/HeN and B10.BR mice, T cell-dependent B-cell and dendritic cell activation was diminished in the latter. Ex vivo, B10.BR T cells showed a diminished capacity to proliferate in response to the MMTV Sag. The genetic segregation of the resistance phenotype indicated that it maps to a single allele. These data highlight the role of Sag-dependent T-cell responses in MMTV infection and point to a novel mechanism for the resistance of mice to retroviral infection that could lead to a better understanding of the interplay between hosts and pathogens.
Many studies in human and animal populations have shown a genetic component to susceptibility to viruses. For example, there are a number of loci in mice, termed Fv, that confer resistance to infection by murine leukemia virus (14). Similarly, there are individuals who remain resistant to human immunodeficiency virus (HIV) type 1 in spite of multiple exposures; included in this group are individuals with germ line mutations in the gene encoding the chemokine receptor that functions as a coreceptor for HIV type 1 (27). It is clear that determining the genetic basis for resistance leads to elucidation of the infection pathway, as well as to the creation of novel treatment paradigms for viruses and other pathogens.
The mouse is particularly well suited for such genetic analysis because of the large number of genetically well-characterized inbred strains and the ability to generate transgenics and targeted germ line mutations. Mouse mammary tumor virus (MMTV), an endemic betaretrovirus found in many mouse strains, has been used extensively in a large number of genetic models to dissect its in vivo infection pathway (38). Genetic crosses performed early in the last century indicated that for some mouse strains, only females transmitted a trait of high breast cancer incidence. The classic studies of Bittner showed that this transmission was not genetic but due to a milk-borne agent acquired in the first week of life from females with high mammary tumor incidence (6).
It is now known that there are two mechanisms of MMTV acquisition, the milk-borne exogenous pathway and the inheritance of germ line copies of endogenous virus, termed Mtv loci. Like other retroviruses, the genome of MMTV includes gag, pol, and env genes, as well as a recently described rem gene involved in RNA export (29). In addition, the long terminal repeat (LTR) of both exogenous and endogenous MMTVs encodes a superantigen (Sag), a cell surface protein presented by major histocompatibility complex (MHC) class II proteins of antigen-presenting cells (APCs), such as B cells and dendritic cells (DCs), to CD4-positive (CD4+) T cells bearing specific T-cell receptor (TCR) Vβ chains. Sag presentation causes activation of specific Vβ-bearing T cells when it is recognized as foreign and deletion of such T cells when it is recognized as self (i.e., when expressed by endogenous proviruses or as a transgene) (37). Different proviruses cause the deletion or stimulation of different classes of Vβ-bearing T cells because they encode Sag proteins with different C-terminal amino acid sequences (termed the hypervariable region); this region of the Sag protein contacts the TCR Vβ molecule.
MMTV uses this Sag activity to amplify in lymphoid cells. MMTV first infects APCs in Peyer's patches, including dendritic and B cells (3, 7, 10, 28, 42). The infected APCs then present Sag to cognate CD4+ T cells, causing their stimulation and subsequent bystander B-cell activation that is dependent on CD40-CD40L interactions (9). This bystander activation sets up a reservoir of dividing, infection-competent cells; thus, Sag-dependent lymphocyte activation is critical for efficient virus spread (16). Virus infection spreads to other lymphoid organs, and B, T, and dendritic cells become MMTV infected (13, 28, 42). T and B cells, as well as DCs, are capable of producing infectious virus (10, 13), and infected lymphoid cells are required for virus spread within the mammary gland (18). Thus, MMTV represents a model system for the study of milk-borne retroviruses, such as HIV and human T-cell leukemia virus type 1, that initially infect lymphocytes in the gut mucosa (39, 40, 45).
Though MMTV is endemic in mice, mouse strains vary greatly in their susceptibilities to MMTV infection, and the level of infection ultimately affects both mammary tumor incidence and latency (2, 11). Several mechanisms of resistance have been identified. They include deletion of Sag-cognate T cells caused by Mtv loci; in this case, the retention of endogenous sag genes with the same Vβ specificity as those encoded by infectious virus greatly diminishes infection because the mice delete Sag-responsive T cells during the shaping of the immune repertoire (38). Similarly, C57BL/6 mice and related strains lack the appropriate MHC class II protein (I-E) required for Sag presentation, thereby abrogating the in vivo infection process at an early step (4, 23, 34). Other strains, such as I/LnJ mice and BALB/c congenic mice, lacking endogenous Mtv loci are also resistant to MMTV infection (8, 35).
Previous genetic studies mapped one major resistance gene to the MHC locus in C57BL mice and an additional resistance locus that could be genetically segregated from the MHC locus (11, 30). Here, we show that B10.BR mice, which are derived from C57BL mice but carry the same MHC class II allele (H-2k) as highly susceptible C3H/HeN mice, are resistant to MMTV infection. In vivo studies indicated that the block to MMTV infection was the result of decreased virus spread in the lymphoid compartment. Although Sag-induced T-cell stimulation was not diminished in B10.BR mice in vivo, subsequent Sag-dependent APC activation was dramatically reduced in B10.BR mice compared to C3H/HeN susceptible mice. Moreover, ex vivo B10.BR CD4+ T-cell proliferation was significantly diminished in response to MMTV Sag. These data suggest a defect in the CD4+ T-cell response to Sag that ultimately leads to diminished infection and mammary tumorigenesis in B10.BR mice.
MATERIALS AND METHODS
Mice.
C57BL/6, C3H/HeN MMTV-negative (MMTV−), and C3H/HeN MMTV+ mice were purchased from the National Cancer Institute, and B10.BR H2k H2-T18a/SgSnJ, C58J, and C57BR/cDJ mice were from The Jackson Laboratory. To examine milk-borne transmission in the mice, C3H/HeN MMTV+ females were used as foster mothers. All mice were housed according to the policies of the University of Pennsylvania.
Detection of integrated exogenous viral DNA by PCR.
To detect newly integrated copies of exogenous MMTV(C3H), splenic and thymic DNAs were amplified by semiquantitative PCR using LTR-specific primers, as previously described (18). These primers also amplify some endogenous MMTVs. To distinguish endogenous from exogenous MMTV sequences, each PCR amplification reaction mixture was incubated with MfeI restriction enzyme (New England Biolabs, Beverly, MA), as indicated in the figure legends, and the resulting products were analyzed on 1.5% agarose gels.
Detection of integrated exogenous viral DNA by RT-qPCR.
Levels of integrated MMTV(LA) DNA in infected mouse tissues were determined by Sybr green real-time quantitative PCR (RT-qPCR) performed with primers specific to the MMTV(LA) LTR and to a single-copy mouse glyceraldehyde-phosphate-3-dehydrogenase (GAPDH) gene. Reactions were performed in triplicate using Sybr green 1 master mix and run on an ABI Prism model 7900HT, as previously described (32). Data are presented as relative levels of MMTV normalized to the single-copy GAPDH gene.
MMTV-XC cell injection.
Three- to 4-week-old female mice were injected with 107 XC cells expressing the MMTV hybrid provirus (HP) construct, a gift from Jaquelin Dudley, as described by Shackleford and Varmus (41). All injected females were bred, and RNA extracted from milk at their first pregnancy was subjected to RNase protection analysis.
RNase protection assay.
RNase T1 protection assays were performed as previously described using a probe specific for MMTV (C3H) viral transcripts (19). Forty micrograms of total RNA isolated from the lactating mammary glands and 5 μg of RNA isolated from the milk were used. Forty micrograms of Saccharomyces cerevisiae tRNA was used as a negative control.
Fluorescence-activated cell sorting (FACS).
The following monoclonal antibodies (conjugated with phycoerythrin, fluorescein isothiocyanate, or allophycocyanin; BD Bioscience, Inc.) were used: anti-CD71 (C2), anti-CD69 (H1.2F3), anti-B220 (RA3-6B2), anti-CD4 (RM4-5), anti-CD11c (HL3), anti-CD80 (16-10A1), anti-CD86 (MR1), anti-CD25 (PC61), and anti-CD40L (7D4). Cells were acquired on a FACS Calibur cytometer (Becton Dickinson) and analyzed using CellQuest software (Becton Dickinson Immunocytometry Systems).
Western blots.
Sera were obtained from infected and uninfected B10.BR and C3H/HeN mice, diluted 1:100, and used to probe Western blots of MMTV(LA) viral particles (1 μg/lane). Anti-mouse antibody conjugated to horseradish peroxidase (Amersham BioSciences) was used as the secondary antibody and was detected using ECL kits (Amersham BioSciences).
Virus isolation and injection.
Virus was purified from tumors, lactating mammary glands, or milk from MMTV(LA)- or MMTV(FM)-infected C3H/HeN mice, as previously described (19). MMTV(FM) or MMTV(LA) was diluted in sterile phosphate-buffered saline and injected into the right hind footpads of 1- to 2-month-old mice. Twenty-four and 96 hours later, the draining (right) and nondraining (left) popliteal lymph nodes were harvested, and the cells were analyzed by FACS. Dilutions of purified virus were tested for B-cell and Sag-mediated T-cell activation in C3H/HeN mice in vivo, and the highest dilution giving the maximum Sag-dependent stimulation (usually 1:200) was used for subsequent experiments. All virus preparations were also tested for lipopolysaccharide contamination, as previously described (7, 10, 36).
Mixed lymphocyte cultures.
Total lymphocytes were isolated from the lymph nodes of naïve B10.BR and C3H/HeN mice. CD4+ T cells were purified using a CD4+ T Cell Isolation Kit (Miltenyi Biotec, Inc.); the purity of the populations was determined by FACS analysis using anti-CD4 antibodies and was ≥96% (not shown). Unprimed B10.BR or C3H/HeN CD4+ T cells (1 × 106) were cultured in triplicate with 2 × 106 splenocytes isolated from HYB PRO transgenic mice (17) in 0.2 ml of RPMI 1640 complete medium (10% heat-inactivated fetal calf serum, 0.05 mM 2-mercaptoethanol), or 5 μg/ml concanavalin A (ConA) for the indicated times. T cells cultured alone or with autologous APCs served as controls. In some experiments, allogeneic splenocytes from C57BL/6 mice were also cocultured with lymphocytes from B10.BR and C3H/HeN mice. During the last 18 h of incubation, the cultures were pulsed with 1.0 μCi/well of [3H]thymidine (GE Healthcare, Inc.). The cells and supernatants were harvested, and thymidine incorporation was quantified.
Statistical analysis.
Statistical analysis was performed with a two-sample unequal-variance/two-tail distribution t test.
RESULTS
B10.BR mice are resistant to MMTV infection.
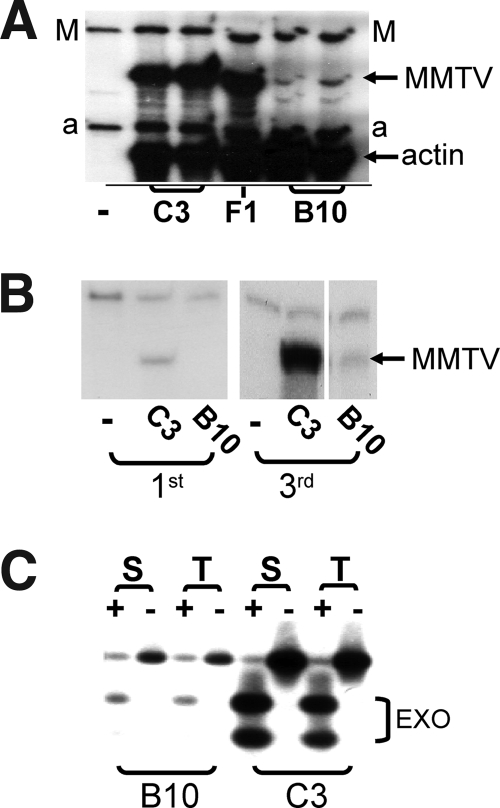
MMTV is naturally acquired through milk when neonates nurse on infected mothers. To determine whether B10.BR mice, which are H-2k and express the same MHC class II proteins as MMTV-susceptible C3H/HeN mice, were resistant to MMTV infection, B10.BR pups were foster nursed on C3H/HeN (MMTV+) mothers from 1 to 2 days after birth until they were weaned. C3H/HeN pups that had nursed on the same mothers served as controls. Female foster-nursed offspring of both strains were tested for infection when they reached adulthood. The mice were mated and sacrificed after the second pregnancy, and RNA isolated from lactating mammary glands and milk was subjected to RNase protection analysis to determine the virus load; we had previously shown that this is an accurate measure of the level of infection (21). B10.BR lactating mammary glands (Fig. 1A) and milk (Fig. 1B) had much lower levels of MMTV RNA than those of C3H/HeN mice. These data demonstrated that B10.BR mice have a block to infection that is MHC independent.
FIG. 1.
B10.BR mice show lower levels of virus infection in their mammary and lymphoid tissues and shed less virus in milk than C3H/HeN mice. (A and B) RNase protection analysis of RNA isolated from the lactating mammary glands at the second pregnancy (A) and milk at the first and third pregnancies (B). C3, C3H/HeN; B10, B10.BR; F1, C3H/HeN × B10.BR F1 females at their second pregnancies; M, MMTV-specific probe (17); a, mouse β-actin-specific probe. (C) PCR analysis of genomic DNAs from the spleens (S) and thymi (T) of milk-borne MMTV(C3H)-infected B10.BR and C3H/HeN mice to detect integrated exogenous MMTVs. The primers used amplified both endogenous and exogenous MMTVs. Following amplification, the amplicons were digested (+) with MunI, which restricts only the amplification products of exogenous MMTV (EXO) (13). The endogenous band after MunI digestion served as a control for DNA integrity.
B10.BR mammary tissue is susceptible to infection.
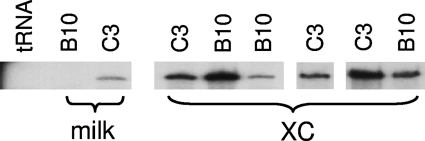
As described above, milk-borne MMTV infection initiates in lymphoid cells, at least in part through the action of its Sag, and then spreads to the mammary epithelia during puberty and pregnancy. To determine whether the block to infection in B10.BR mice was due to a defect in mammary epithelial cell infection, we injected 3-week-old B10.BR and C3H/HeN females with rat XC cells expressing high levels of a molecular clone of MMTV, HYB PRO, that carries the MMTV(C3H) sag; this mode of infection is Sag independent (43). Following infection, the mice were bred, and RNA isolated from their milk after the first pregnancy was subjected to RNase protection analysis for viral sequences. The B10.BR and C3H/HeN mice injected with the MMTV-producing XC cells shed similar levels of virus in milk (Fig. 2), indicating that there was no block to infection of mammary epithelial cells. This was in contrast to mice that were infected by milk-borne transmission (Fig. 1 and 2). Moreover, examination of virus production after the second and third pregnancies revealed no differences in infection (not shown). Thus, the mammary epithelial cells of B10.BR mice showed no block to direct infection.
FIG. 2.
B10.BR mammary glands are susceptible to infection. Virus RNA was isolated from the milk of B10.BR and C3H/HeN mice that received mammary gland injections of MMTV-producing XC cells at 3 weeks of age at the first pregnancy and subjected to RNase protection analysis using a probe specific for exogenous MMTV (XC). Shown for comparison is RNase protection analysis of RNA isolated from the milk from mammary glands of age- and pregnancy-matched C3H/HeN and B10.BR mice that nursed on MMTV-infected C3H/HeN mothers (milk).
B10.BR lymphocytes show lower levels of MMTV infection.
We also examined lymphocyte infection via milk-borne infection. First, we examined Sag-mediated deletion of Vβ14 cognate T cells (21) in B10.BR and C3H/HeN mice that were foster nursed on C3H/HeN MMTV+ mothers. Deletion of these T cells was slightly delayed in B10.BR mice relative to C3H/HeN mice, although they still showed substantial loss of this T-cell population, indicating that Sag presentation did occur (not shown). Next, we directly examined infection of lymphoid tissues from these mice. DNA was isolated from the spleens and thymi of age-matched B10.BR and C3H/HeN mice nursed on the same C3H/HeN MMTV+ mothers and was subjected to PCR analysis for integrated exogenous viral DNA, as previously described (13). Shown in Fig. 1C is a representative PCR from one set of mice. In all cases, the B10.BR lymphoid tissue showed much lower levels of virus infection than did the C3H/HeN tissue. These data indicated that the block to infection in B10.BR mice was due to decreased virus spread in the lymphoid compartment.
B10.BR mice show diminished Sag-dependent APC activation in vivo.
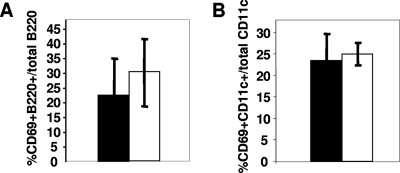
It is well established that efficient infection of lymphocytes by MMTV requires Sag-dependent T-cell activation (37). We next investigated whether lymphoid cell responses were affected in B10.BR mice. MMTV has two phases of lymphocyte activation. At early times after infection, virus binds to and activates APCs, at least in part through interaction with toll-like receptor 4 (TLR4) (7, 10, 36). To determine if initial APC activation occurred in B10.BR mice, we performed subcutaneous injection of either MMTV(LA) or MMTV(FM) into adult mice and determined whether the CD69 activation marker was up-regulated on CD11c+ DCs and B220+ B cells in the draining lymph node. B-cell and DC activation in resistant B10.BR mice at 18 h after injection was similar to that seen in susceptible C3H/HeN mice, indicating that the TLR4-mediated activation by MMTV was not altered in B10.BR mice (Fig. 3A and B). In support of this, we also found that the responses to the TLR4 ligand lipopolysaccharide were equivalent in B10.BR and C3H/HeN mice (not shown).
FIG. 3.
Early activation of B cells and DCs is similar in B10.BR and C3H/HeN mice. B10.BR (filled bars) and C3H/HeN (open bars) mice received subcutaneous injections of MMTV(FM) in their footpads, and at 18 h, the lymphocytes from their draining lymph nodes were analyzed by FACS for CD69 on B220+ B cells (A) and CD69 on CD11c+ cells (B). The data presented are the averages of three mice and are representative of at least 10 independent experiments. The error bars indicate standard deviations.
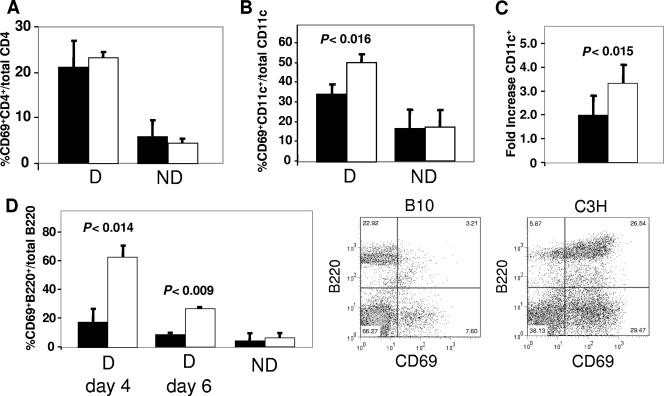
After their initial activation, infected APCs present the viral Sag to cognate CD4+ T cells. These T cells in turn provide costimulation to the APCs, causing their activation and migration into the lymph node; activation peaks at days 3 and 4 after inoculation and declines thereafter. To determine whether Sag-mediated T-cell or subsequent APC activation was affected in resistant B10.BR mice, we again injected MMTV(LA) or MMTV(FM), both of which encode Sags that mediate a robust T-cell response, and examined a number of activation markers on DCs and B and T cells at 4 and 6 days postinoculation. Sag-mediated activations of T cells were similar in B10.BR and C3H/HeN mice, using CD69 (Fig. 4A) or CD40L and CD25 (Table 1) as markers. Moreover, the characteristic MMTV Sag-mediated increases in Vβ2-, Vβ6-, and Vβ14-bearing [MMTV(LA)] or Vβ8.1-bearing [MMTV(FM)] T cells were similar in the draining lymph nodes of C3H/HeN and B10.BR mice (not shown). In contrast, activation of B220+ B cells was significantly reduced in B10.BR draining lymph nodes at both 4 and 6 days postinoculation, using CD69 (Fig. 4D) or CD80 and CD86 (Table 1) as the markers. CD69 up-regulation on CD11c+ DCs was also reduced in response to MMTV(LA) (Fig. 4B), as was the recruitment of CD11c+ DCs into the lymph node (Fig. 4C); similar results were obtained when MMTV(FM) was injected into C3H/HeN and B10.BR mice (not shown). These results indicated that Sag-dependent activation of APCs was diminished in B10.BR mice and that this diminution was independent of the particular class of Vβ-bearing T cells activated by the Sag.
FIG. 4.
Sag-dependent B-cell and DC activation is impaired in B10.BR mice. B10.BR (filled bars) and C3H/HeN (open bars) mice received subcutaneous injections of MMTV(LA) in their footpads, and after 4 days (A to D) or 6 days (D), the lymphocytes from their draining lymph nodes were analyzed by FACS for CD69 on CD4+ T cells (A), CD69 on CD11c+ DCs (B), the increase in the percentage of CD11c+ cells in the draining compared to the nondraining contralateral lymph node (C), and CD69 on B220+ B cells (D). A representative FACS plot of cells from the draining lymph nodes of B10.BR and C3H/HeN mice stained with anti-CD69 and -B220 is also shown. D, draining lymph node; ND, contralateral nondraining lymph node. The data presented are the averages of three mice and are representative of at least 10 independent experiments with MMTV(LA) or MMTV(FM). The error bars indicate standard deviations.
TABLE 1.
Activation marker expression on CD4+ T and B220+ B cells in response to MMTV Saga
| Cell type | Marker | Expressionb
|
|||
|---|---|---|---|---|---|
| C3H/HeN
|
B10.BR
|
||||
| D | ND | D | ND | ||
| B220+ | CD86 | 29.8 ± 2.9 | 9.0 | 16.7 ± 2.2 | 9.8 |
| B220+ | CD80 | 15.6 ± 2.9 | 10.8 | 9.2 ± 0.7 | 8.0 |
| CD4+ | CD25 | 10.0 ± 1.8 | 6.2 | 9.3 ± 0.6 | 8.0 |
| CD4+ | CD40L | 7.1 ± 0.6 | 0.9 | 7.64 ± 0.6 | 1.8 |
Mice were injected with MMTV(FM), and 4 days later, lymphocytes in the draining (D) and contralateral nondraining (ND) lymph nodes were examined by FACS for the different cell surface markers.
Shown are the percentages of total B220+ B cells or CD4+ T cells that expressed each marker; n = 3 mice per group. Nondraining lymph nodes from the three mice were pooled for analysis.
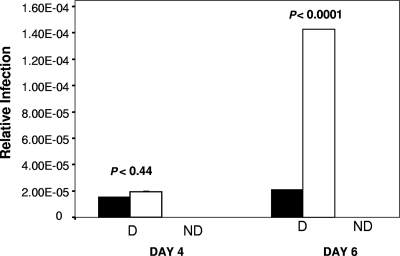
To ensure that the diminished lymphocyte activation in B10.BR mice reflected the level of infection, we also performed RT-qPCR on lymphocytes isolated from mice that received subcutaneous injections of MMTV(LA), using primers that specifically detect the exogenous viral sequences. At 4 days postinoculation, little viral DNA was detected in the draining lymph nodes of either C3H/HeN or B10.BR mice (Fig. 5). By 6 days postinoculation, the level of infection in C3H/HeN mice had increased dramatically, while no increase was seen in B10.BR mice. Thus, the diminished Sag-dependent APC activation in B10.BR mice was paralleled by a lack of virus spread.
FIG. 5.
In vivo infection of lymphocytes from B10.BR mice is lower than in those from C3H/HeN mice. B10.BR (filled bars) and C3H/HeN (open bars) mice received subcutaneous footpad injections of MMTV(LA), and at 4 and 6 days postinoculation, the lymphocytes from their draining lymph nodes were analyzed for MMTV(LA) sequences by RT-qPCR. MMTV signals were normalized to GAPDH. D, draining lymph node; ND, contralateral nondraining lymph node.
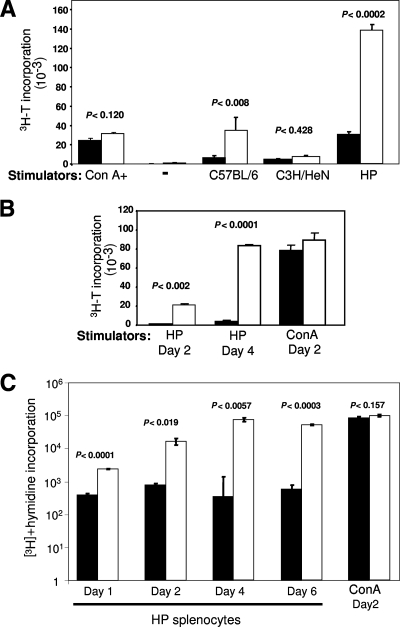
B10.BR T cells showed diminished T-cell responses ex vivo.
Sag-mediated CD4+ T-cell activation occurs when APCs present this virus protein. The activated T cells then provide help and interact in turn with additional APCs. The diminished activation of B10.BR APCs in response to T cells could be due to cell-intrinsic differences in the ability to respond to signals from the Sag-activated T cells or to differences in the ability of B10.BR T cells to provide these signals after Sag activation. We therefore tested in an ex vivo mixed lymphocyte culture assay whether CD4+ T cells from B10.BR mice responded to Sag to the same extent as those isolated from C3H/HeN mice. We used splenocytes from HP transgenic mice expressing the Sag from MMTV(C3H) as APCs, which we had previously demonstrated were able to activate Sag-responsive T cells (17). Sag-mediated induction of the CD69 activation marker on T cells was similar for both B10.BR and C3H responder cells (Table 2), as was seen in vivo (Fig. 4A). However, Sag-mediated T-cell proliferation was dramatically reduced with responders isolated from B10.BR mice in comparison to those from C3H mice, even after 4 days of coculture (Fig. 6A); similar results were obtained when purified T cells were used as responders (Fig. 6B). Additionally, B10.BR T cells showed a diminished proliferative response to allogeneic APCs (Fig. 6A). This was not due to a general defect in B10.BR T cells, since their response to the T-cell mitogen ConA was similar to that seen with C3H T cells (Fig. 6A, B, and C). While C3H/HeN CD4+ T-cell proliferation in response to the MMTV Sag increased during up to 6 days of coculture with HP APCs, there was no change in B10.BR T-cell responses (Fig. 6C).
TABLE 2.
Ex vivo T-cell activation by MMTV Saga
| Responder cells | % CD69+ CD4+ T cells for activatorb:
|
|||
|---|---|---|---|---|
| Nothing | ConA | C3H/HeN | HP | |
| B10.BR | 7.78 ± 0.48 | 55.36 ± 3.23 | 8.22 ± 1.49 | 69.81 ± 3.19 |
| C3H/HeN | 7.63 ± 0.51 | 65.27 ± 1.19 | 7.64 ± 0.36 | 76.37 ± 4.20 |
Four days after coculture of lymph node lymphocytes with the indicated cells or treatments, the cells were stained for the CD69 activation marker and CD4.
Shown are the percentages of CD69+ CD4+ T cells of the total population of CD4+ T cells. The cultures were done in triplicate.
FIG. 6.
B10.BR T cells show lower proliferation than C3H/HeN T cells in response to MMTV Sag. (A) Responder cells from the lymph nodes of B10.BR (filled bars) or C3H/HeN (open bars) mice were cocultured for 4 days alone (−), with mitomycin-treated splenocytes from MMTV transgenic mice (HP) or C3H/HeN or C57BL/6 mice, or in the presence of ConA. (B) T cells purified from B10.BR or C3H/HeN mice were cocultured as in panel A for the indicated times. (C) Responder cells from the lymph nodes of B10.BR (filled bars) or C3H/HeN (open bars) mice were cocultured with splenocytes from mitomycin-treated MMTV transgenic mice (HP) for the indicated times or with ConA for 2 days. During the last 18 h of culture, the cells were pulsed with 1.0 μCi/well of [3H]thymidine at the indicated times (days). The error bars indicate standard deviations.
B10.BR mice do not make increased protective humoral antibody responses.
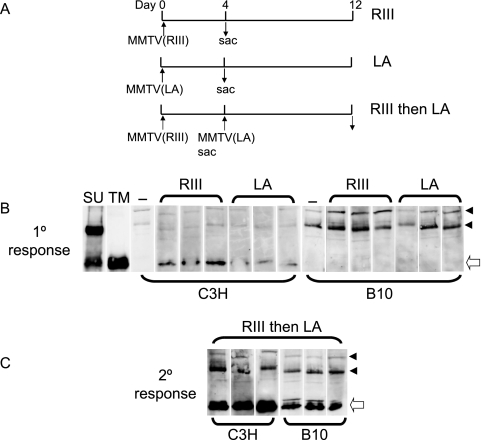
Previously it was demonstrated that adult mice immunized with one MMTV strain are resistant to challenge with a second strain because they make a protective humoral immune response (26). Moreover, at least one mouse strain, I/LnJ, is resistant to MMTV because infected mice make a hyper-humoral immune response to virus after milk-borne transmission (35). To test if B10.BR mice made a stronger initial or challenge humoral immune response to MMTV than C3H/HeN mice, which protected them from infection, we performed subcutaneous injection of MMTV(RIII) or MMTV(LA) into naive B10.BR and C3H/HeN adult mice. Four days after injection, the mice were sacrificed. A second set of mice received an initial inoculation with MMTV(RIII) and at day 4 were challenged with MMTV(LA). At day 12 after the primary infection, the mice were sacrificed (Fig. 7A).
FIG. 7.
B10.BR mice do not make a more robust humoral immune response to MMTV. (A) Scheme of virus injections. (B) C3H/HeN and B10.BR mice (three each) received subcutaneous injections of either MMTV(LA) or MMTV(RIII). Four days after injection, the mice were bled and their sera were tested for anti-MMTV antibodies by Western blot analysis of virion proteins. The blots incubated with C3H/HeN sera were exposed for <5 s; the blots incubated with B10.BR sera were exposed for 30 s. As controls, the blots were stripped and incubated with mouse monoclonal antibodies against the MMTV SU or TM protein; −, naïve mice. (C) C3H/HeN or B10.BR mice were inoculated with MMTV(RIII). Four days after injection, the mice were inoculated with MMTV(LA). Eight days later, the mice were bled and their sera were tested for anti-MMTV antibodies. The arrowheads point to background bands used to align the blots. The open arrow indicates the TM protein.
The sera from these mice were then used to probe Western blots of MMTV virions to determine if antiviral antibodies were produced. C3H/HeN, but not B10.BR, mice that received a single inoculation of either MMTV(LA) or MMTV(RIII) made anti-TM antibodies (Fig. 7B). When the mice were challenged with a second injection of virus, both strains made anti-TM antibodies, although C3H/HeN mice appeared to make a stronger response (Fig. 7C). These results indicate that resistance to MMTV infection by B10.BR mice is not the result of greater humoral immune response. Indeed, these data support previous observations that the humoral immune response to MMTV relies on Sag-mediated T-cell activation (25).
Genetic analysis of B10.BR resistance.
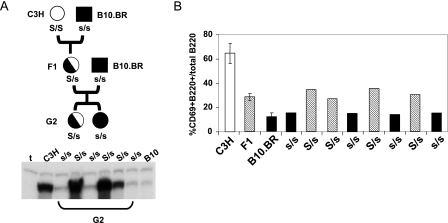
We next analyzed the segregation patterns of resistance to MMTV infection in F1 and G2 backcrosses. B10.BR males were crossed with C3H/HeN (MMTV+) females, and F1 females were generated. The MMTV(C3H)+ F1 females were then crossed with B10.BR males, and mammary gland infections in their female offspring were determined by RNase protection analysis of virus in milk. Figure 8A shows a representative analysis of 6 mice out of 151 mice analyzed; 68 G2 females showed low levels of infection similar to those in B10.BR females, while the remainder showed high levels, similar to those in C3H/HeN mice. F1 females showed high levels of mammary gland infection, similar to that seen with C3H/HeN susceptible mice (Fig. 1A). These data indicated that resistance to MMTV infection segregated as a recessive trait and were consistent with its mapping to a single genetic locus.
FIG. 8.
The B10.BR resistance cell phenotype segregates as a single gene. (A) Milk RNAs isolated from G2 backcross, C3H/HeN, and B10.BR mice that had nursed on MMTV+ mothers were subjected to RNase protection analysis for exogenous MMTV. Shown above the G2 lanes are the presumed genotypes of the mice (S/s, susceptible; s/s, resistant). Shown are the data for six representative mice. (B) B-cell activation in B10.BR (filled bars), C3H (open bars), F1 (hatched bars), and eight G2 backcross mice. The mice received subcutaneous injections of MMTV, and after 4 days, the lymphocytes from their draining lymph nodes were analyzed by FACS for CD69 on B220+ B cells. The data are the averages of three mice each for the C3H/HeN, F1, and B10.BR samples. The error bars indicate standard deviations. Shown below the line are the presumed genotypes of the G2 mice.
To test whether the Sag-dependent activation of APCs also showed similar segregation, we generated a cohort of 85 uninfected G2 backcrossed mice. B-cell and DC activation levels for C3H/HeN, B10.BR, F1, and G2 mice were compared 4 days after subcutaneous virus inoculation. We found that F1 mice routinely showed virus-dependent B-cell activation that was intermediate between those seen in susceptible C3H/HeN and resistant B10.BR mice (Fig. 8B), indicating that the C3H allele was semidominant, using lymphocyte activation as the phenotype; similar segregation was also seen using DC activation as the phenotype readout (not shown). When this assay was performed with the G2 backcross mice, the mice showed B-cell and DC (not shown) stimulation similar to that seen with either the F1 or B10.BR mice (shown for eight G2 offspring in Fig. 8B). Of the 85 G2 mice analyzed, 41 showed the low B10.BR B-cell/DC activation phenotype (not shown). This suggests that the lymphocyte activation phenotype also maps to a single locus.
Finally, to determine whether the defect in lymphocyte activation was unique to B10.BR mice or was shared by other strains derived from a C57 background, we tested MMTV-mediated B-cell and T-cell activation in C57BR/cdJ and C58/J mice, both of which are also H-2k. We also tested lymphocyte activation in B10.BR × C57BL/6 F1 mice; C57BL/6 mice, which are H-2b, lack the MHC class II I-E molecule required for efficient presentation of most MMTV Sags (34). All the C57-derived strains showed lower levels of B-cell (Table 3) activation than did C3H/HeN mice; in contrast, T-cell activation was similar to that in C3H/HeN mice, except for C58/J mice, in which it was lower (not shown). C57BL/6 mice showed no lymphocyte activation, as previously described (4, 23, 34). Interestingly, the B10.BR × C57BL/6 F1 mice showed low-level B-cell activation similar to that seen in B10.BR mice, indicating that the two strains might contain the same allele responsible for this phenotype.
TABLE 3.
Lack of B-cell response in B10.BR × C57BL/6 F1 micea
| Strain | % CD69+ B220+b |
|---|---|
| C3H/HeN | 63.5 ± 6.2 |
| B10.BR | 37.8 ± 3.8 |
| C57BL/6 | 6.2 ± 0.9 |
| C57BL/6 × B10.BR F1 | 30.9 ± 3.9 |
| C58J | 44.7 |
| C57BR/cDJ | 20.5 |
Mice were injected with MMTV(LA), and 4 days later, lymphocytes in the draining lymph nodes were examined by FACS for CD69 and B220 cell surface markers.
Shown are the percentages of CD69+ B220+ B cells out of the total population of B220+ B cells; n = 3 mice for the B10.BR, C3H/HeN, and C57BL/6 mice; n = 4 for the F1 mice; n = 2 for the C58J and C57BR/cDJ mice.
DISCUSSION
The genetics of susceptibility to MMTV infection has been a subject of investigation since the 1930s, when Bittner described a milk-borne transmissible agent that causes breast cancer in mice (6). Much has been learned about the genes that confer resistance or susceptibility to infection during the 70 years following his initial description. For example, early studies had shown that C57BL and derivative strains were resistant to tumor induction by most MMTV strains (11). With the discovery of the virus-borne sag, it was recognized that C57BL and H-2b strains, which genetically lack the MHC class II I-E gene, had poor presentation of this antigen to cognate CD4+ T cells, leading to resistance to infection (4, 34). Similarly, mice containing germ line MMTVs with the same Sag as exogenous MMTV, either as a transgene or as an endogenous provirus, delete Sag-cognate T cells during the shaping of the immune repertoire, rendering them resistant to infection by exogenous MMTVs carrying sag genes with the same T-cell specificity, and Vβ8.2 TCR transgenic mice cannot be infected by exogenous MMTVs whose Sag proteins do not bind Vβ8.2-bearing T cells (16, 22).
Other mechanisms of resistance to MMTV infection also occur. For example, I/LnJ mice show wild-type levels of lymphocyte infection but little or no transfer of virus to mammary tissue or to subsequent generations (15). These mice develop high-titer anti-MMTV antibodies as they age, which coat the virions and thereby block mammary gland infection and milk-borne transmission to the next generation (35). It has also recently been shown that BALB/c congenic mice lacking endogenous Mtv loci are resistant to infection; the mechanism of this resistance is not yet known (5). These and other genetic studies have led to an understanding of the pathway of virus infection and mammary tumor induction in vivo.
Early genetic studies also indicated that C57BL-derived mice might have resistance alleles in addition to the MHC class II restriction. The block to infection in this mouse strain appeared to occur prior to mammary gland infection, since C57BL mammary tissue transplanted into the cleared fat pads of susceptible hosts developed into tumors with the same kinetics and frequency as tissue transplanted from susceptible mice (12, 31). Indeed, we show here that in B10.BR mice, which have the same MHC allele as susceptible C3H/HeN mice, resistance to MMTV infection resides at a step prior to mammary gland infection. By systematically examining the steps of the in vivo infection pathway, we were able to demonstrate that B10.BR CD4+ T cells were activated by the MMTV Sag, indicating that initial infection of APCs by MMTV was not affected. Instead, although B10.BR mice have APCs with the appropriate MHC class II molecules and have T cells bearing TCRs capable of interacting with MMTV Sags, the subsequent Sag-dependent T-cell help to B cells and DCs was greatly diminished. This was seen at the level of B-cell and DC activation and in the ability of B10.BR mice to make anti-MMTV antibodies; it had been previously established that the anti-MMTV humoral immune response requires Sag-mediated T-cell help (25, 26).
Neither Sag-mediated T-cell deletion after milk-borne infection nor activation of T cells after virus inoculation was significantly diminished in B10.BR mice. This may be due to the lack of sensitivity of Sag-mediated effects as a readout assay; we have previously shown that very low levels of Sag expression on APCs can produce maximum T-cell stimulation (17). Although their in vivo activation was not reduced, ex vivo proliferation of B10.BR T cells was defective compared to that of C3H/HeN T cells, suggesting that it is the T cells themselves, rather than the B cells and DCs, that are unable to respond appropriately to the MMTV Sag. Indeed, we also found that the B10.BR T cells showed a decreased ex vivo allogeneic response compared to those from C3H/HeN mice. Taken together, these data suggest that defective Sag-mediated T-cell stimulation in B10.BR mice accounts for the lack of efficient virus spread in the lymphocyte compartment and thus results in lower levels of lymphocyte infection after both experimental inoculation and milk-borne MMTV infection. However, we cannot rule out the possibility that B10.BR B cells and DCs are also deficient in the ability to respond to T-cell help.
Sag proteins typically stimulate up to 20% of all CD4+ T cells in a given mouse by directly binding to both MHC class II and particular TCR Vβ chains. In this study, we used four different MMTV strains, MMTV(C3H) for milk-borne transmission and MMTV(FM), MMTV(RIII), and MMTV(LA) for experimental inoculation. The different MMTVs encode Sag proteins with different Vβ specificities: MMTV(C3H) interacts with Vβ14-bearing, MMTV(FM) with Vβ8.1-bearing, MMTV(RIII) with Vβ2-bearing, and MMTV(LA) (a mixture of three viruses) with Vβ2-, Vβ14-, and Vβ6-bearing T cells (20, 24, 32, 44). We showed that B10.BR mice were resistant to milk-borne infection by MMTV(C3H) and that this resistance occurred in the lymphoid compartment. We have also found that milk-borne infection with MMTV(LA) is reduced in B10.BR mice (not shown). Because the MMTV(C3H) Sag is weaker than those encoded by other MMTVs, we could not use an acute-infection assay to functionally dissect the block to infection in lymphocytes. Instead, using either MMTV(LA) or MMTV(FM), we showed that B10.BR mice had altered Sag-dependent B-cell and DC stimulation in vivo. That several different viruses encoding different Sags showed similar phenotypes in B10.BR mice argues that the lack of response and subsequent amplification of MMTV infection in lymphocytes is not specific to a particular strain of MMTV but represents a generalized resistance to infection by the virus. This is in contrast to recent work demonstrating that in some cases, MMTVs encoding “strong” Sag proteins can infect mice in the absence of a robust Sag-mediated T-cell response (33).
Although we have yet to determine the precise molecular process leading to resistance to MMTV in B10.BR mice, we do have evidence suggesting that resistance to MMTV infection in this mouse strain segregates as a recessive autosomal allele in crosses with C3H/HeN mice. Interestingly, when we used mammary gland infection as the readout, F1 mice were fully susceptible to infection (Fig. 1A). In contrast, when lymphocyte activation was used as the assay, the F1 phenotype was intermediate between susceptible C3H/HeN and B10.BR mice (Fig. 8B), indicating that the gene determining the Sag-mediated lymphocyte response to MMTV in the former is semidominant. These data indicate that even though F1 mice have lower levels of lymphocyte infection than do C3H/HeN mice, this level of lymphocyte infection was sufficient to achieve the maximum mammary tissue infection, at least at the time point we studied (2 to 3 months of age). It is also possible that additional alleles that restrict or enhance virus replication in vivo beyond the initial steps of lymphocyte activation determine the level of mammary gland infection in F1 mice. Further studies are in progress to determine the exact mechanism of resistance to MMTV observed in the B10.BR mouse strain and to map the allele that affects Sag-mediated lymphocyte activation.
It is likely that B10.BR mice will show differential susceptibilities to other pathogens, in addition to MMTV. Indeed, B10.BR mice are known to be resistant to staphylococcal enterotoxin B (SEB)-induced lethal shock, while C3H/HeJ mice, genetically similar to the C3H/HeN mice used here, are susceptible (1). Although T cells were required for disease induction in the SEB model, a non-T-cell compartment was implicated in the B10.BR resistance. Whether the resistance to MMTV is also the result of a non-T-cell compartment and whether the MMTV and SEB-induced disease resistance phenotypes map to the same genetic locus will be the subjects of future experiments. Importantly, B10.BR mice were not bred for any particular mutation but represent a “normal” genetic variant. Identification of the gene(s) involved in resistance to the viral and bacterial pathogens in different inbred mice, like B10.BR, is likely to lead to greater understanding of the role genetics plays in the response to infectious disease and to help develop new treatment paradigms.
Acknowledgments
We thank Tatyana Golovkina and Wayne Clemmons for the early work that led to these studies and Jennifer Meyers for technical assistance. The HP-transfected XC cells were a gift from Jaquelin Dudley, and the anti-MMTV SU and TM hybridomas were a gift from Tatyana Golovkina.
This work was supported by NIH RO1CA45954 (to S.R.R.); C.M.O. was supported by training grant NIH/NCI T32-CA9140.
Footnotes
Published ahead of print on 14 November 2007.
REFERENCES
- 1.Anderson, M. B., and M. Tary-Lehmann. 2001. Staphylococcal enterotoxin-B-induced lethal shock in mice is T-cell-dependent, but disease susceptibility is defined by the non-T-cell compartment. Clin. Immunol. 9885-94. [DOI] [PubMed] [Google Scholar]
- 2.Bentvelzen, P., J. Brinkhof, and J. J. Haaijman. 1978. Genetic control of endogenous murine mammary tumour viruses reinvestigated. Eur. J. Cancer 141137-1147. [DOI] [PubMed] [Google Scholar]
- 3.Beutner, U., E. Draus, D. Kitamura, K. Rajewsky, and B. T. Huber. 1994. B cells are essential for murine mammary tumor virus transmission, but not for presentation of endogenous superantigens. J. Exp. Med. 1791457-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beutner, U., B. McLellan, E. Draus, and B. T. Huber. 1996. Lack of MMTV superantigen presentation in MHC class II-deficient mice. Cell Immunol. 168141-147. [DOI] [PubMed] [Google Scholar]
- 5.Bhadra, S., M. M. Lozano, S. M. Payne, and J. P. Dudley. 2006. Endogenous MMTV proviruses induce susceptibility to both viral and bacterial pathogens. PLoS Pathog. 2e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bittner, J. J. 1936. Some possible effects of nursing on the mammary gland tumor incidence in mice. Science 84162. [DOI] [PubMed] [Google Scholar]
- 7.Burzyn, D., J. C. Rassa, D. Kim, I. Nepomnaschy, S. R. Ross, and I. Piazzon. 2004. Toll-like receptor 4-dependent activation of dendritic cells by a retrovirus. J. Virol. 78576-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Case, L. K., A. Purdy, and T. V. Golovkina. 2005. Molecular and cellular basis of the retrovirus resistance in I/LnJ mice. J. Immunol. 1757543-7549. [DOI] [PubMed] [Google Scholar]
- 9.Chervonsky, A. V., J. Xu, A. K. Barlow, M. Khery, R. A. Flavell, and C. A. Janeway, Jr. 1995. Direct physical interaction involving CD40 ligand on T cells and CD40 on B cells is required to propagate MMTV. Immunity 3139-146. [DOI] [PubMed] [Google Scholar]
- 10.Courreges, M. C., D. Burzyn, I. Nepomnaschy, I. Piazzon, and S. R. Ross. 2007. Critical role of dendritic cells in mouse mammary tumor virus in vivo infection. J. Virol. 813769-3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dux, A. 1972. Genetic aspects in the genesis of mammary cancer, p. 301-308. In P. Emmelot and P. Bentvelzen (ed.), RNA viruses and host genome in oncogenesis. North-Holland Publishers, Amsterdam, The Netherlands.
- 12.Dux, A., and P. Demant. 1987. MHC-controlled susceptibility to C3H-MTV-induced mouse mammary tumors is predominantly systemic rather than local. Int. J. Cancer 40372-377. [DOI] [PubMed] [Google Scholar]
- 13.Dzuris, J. L., T. V. Golovkina, and S. R. Ross. 1997. Both T and B cells shed infectious MMTV. J. Virol. 716044-6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goff, S. P. 2004. Genetic control of retrovirus susceptibility in mammalian cells. Annu. Rev. Genet. 3861-85. [DOI] [PubMed] [Google Scholar]
- 15.Golovkina, T. V. 2000. A novel mechanism of resistance to mouse mammary tumor virus infection. J. Virol. 742752-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Golovkina, T. V., A. Chervonsky, J. P. Dudley, and S. R. Ross. 1992. Transgenic mouse mammary tumor virus superantigen expression prevents viral infection. Cell 69637-645. [DOI] [PubMed] [Google Scholar]
- 17.Golovkina, T. V., A. Chervonsky, J. A. Prescott, C. A. Janeway, and S. R. Ross. 1994. The mouse mammary tumor virus envelope gene product is required for superantigen presentation to T cells. J. Exp. Med. 179439-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golovkina, T. V., J. P. Dudley, and S. R. Ross. 1998. Superantigen activity is need for mouse mammary tumor virus spread within the mammary gland. J. Immunol. 1612375-2382. [PubMed] [Google Scholar]
- 19.Golovkina, T. V., A. B. Jaffe, and S. R. Ross. 1994. Coexpression of exogenous and endogenous mouse mammary tumor virus RNA in vivo results in viral recombination and broadens the virus host range. J. Virol. 685019-5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golovkina, T. V., I. Piazzon, I. Nepomnaschy, V. Buggiano, M. de Olano Vela, and S. R. Ross. 1997. Generation of a tumorigenic milkborne mouse mammary tumor virus by recombination between endogenous and exogenous viruses. J. Virol. 713895-3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golovkina, T. V., J. A. Prescott, and S. R. Ross. 1993. Mouse mammary tumor virus-induced tumorigenesis in sag transgenic mice: a laboratory model of natural selection. J. Virol. 677690-7694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Held, W., A. N. Shaknow, S. Izui, G. A. Waanders, L. Scarpellino, H. R. MacDonald, and H. Acha-Orbea. 1993. Superantigen-reactive CD4+ T cells are required to stimulate B cells after infection with mouse mammary tumor virus. J. Exp. Med. 177359-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Held, W., G. A. Waanders, H. R. MacDonald, and H. Acha-Orbea. 1994. MHC class II hierarchy of superantigen presentation predicts efficiency of infection with mouse mammary tumor virus. Int. Immunol. 61403-1407. [DOI] [PubMed] [Google Scholar]
- 24.Ignatowicz, L., J. Kappler, and P. Marrack. 1992. The effects of chronic infection with a superantigen-producing virus. J. Exp. Med. 175917-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luther, S. A., A. Gulbranson-Judge, H. Acha-Orbea, and I. C. M. MacLennan. 1996. Viral superantigen drives extrafollicular and follicular B cell differentiation leading to virus-specific antibody production. J. Exp. Med. 185551-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luther, S. A., I. Maillard, F. Luthi, L. Scarpellino, H. Diggelmann, and H. Acha-Orbea. 1997. Early neutralizing antibody response against mouse mammary tumor virus; critical role of viral infection and superantigen-reactive T cells. J. Immunol. 1592807-2814. [PubMed] [Google Scholar]
- 27.Marmor, M., K. Hertzmark, S. M. Thomas, P. N. Halkitis, and M. Vogler. 2006. Resistance to HIV infection. J. Urban Health 835-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin, P., S. R. Ruiz, G. Martinez del Hoyo, F. Anjuere, H. H. Vargas, M. Lopez-Bravo, and C. Ardavin. 2002. Dramatic increase in lymph node dendritic cell numbers during infection by the mouse mammary tumor virus occurs by a CD62L-dependent blood-borne DC recruitment. Blood 991282-1288. [DOI] [PubMed] [Google Scholar]
- 29.Mertz, J. A., M. S. Simper, M. M. Lozano, S. M. Payne, and J. P. Dudley. 2005. Mouse mammary tumor virus encodes a self-regulatory RNA export protein and is a complex retrovirus. J. Virol. 7914737-14747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muhlbock, O., and A. Dux. 1972. MMTV-variants and histocompatibility, p. 11-20. In J. Mouriquand (ed.), Fundamental research on mammary tumours. INSERM, Paris, France.
- 31.Nandi, S., M. Handin, A. Robinson, D. R. Pitelka, and L. E. Webber. 1966. Susceptibility of mammary tissues of “genetically resistant” strains of mice to mammary tumor virus. J. Natl. Cancer Inst. 36783-801. [DOI] [PubMed] [Google Scholar]
- 32.Okeoma, C. M., N. Lovsin, B. M. Peterlin, and S. R. Ross. 2007. APOBEC3 inhibits mouse mammary tumor virus replication in vivo. Nature 445927-930. [DOI] [PubMed] [Google Scholar]
- 33.Pobezinskaya, Y., A. V. Chervonsky, and T. V. Golovkina. 2004. Initial stages of mammary tumor virus infection are superantigen independent. J. Immunol. 1725582-5587. [DOI] [PubMed] [Google Scholar]
- 34.Pucillo, C., R. Cepeda, and R. J. Hodes. 1993. Expression of a MHC class II transgene determines superantigenicity and susceptibility to mouse mammary tumor virus infection. J. Exp. Med. 1781441-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Purdy, A., L. Case, M. Duvall, M. Overstrom-Coleman, N. Monnier, A. Chervonsky, and T. Golovkina. 2003. Unique resistance of I/LnJ mice to a retrovirus is due to sustained IFN-gamma dependent production of virus-neutralizing antibodies. J. Exp. Med. 197233-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rassa, J. C., J. L. Meyers, Y. Zhang, R. Kudaravalli, and S. R. Ross. 2002. Murine retroviruses activate B cells via interaction with Toll-like receptor 4. Proc. Natl. Acad. Sci. USA 992281-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ross, S. R. 1997. MMTV and the immune system. Adv. Pharmacol. 3921-46. [DOI] [PubMed] [Google Scholar]
- 38.Ross, S. R. 2000. Using genetics to probe host-virus interactions: the mouse mammary tumor virus model. Microbes Infect. 21215-1223. [DOI] [PubMed] [Google Scholar]
- 39.Saji, F., K. Ohashi, Y. Tokugawa, S. Kamiura, C. Azuma, and O. Tanizawa. 1990. Perinatal infection of human T-lymphotropic virus type I, the etiologic virus of adult T-cell leukemia/lymphoma. Cancer 661933-1937. [DOI] [PubMed] [Google Scholar]
- 40.Seltzer, V., and F. Benjamin. 1990. Breast-feeding and the potential for human immunodeficiency virus transmission. Obstet. Gynecol. 75713-715. [PubMed] [Google Scholar]
- 41.Shackleford, G. M., and H. E. Varmus. 1988. Construction of a clonable, infectious, and tumorigenic mouse mammary tumor virus provirus and a derivative genetic vector. Proc. Natl. Acad. Sci. USA 859655-9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vacheron, S., S. J. Luther, and H. Acha-Orbea. 2002. Preferential infection of immature dendritic cells and B cells by mouse mammary tumor virus. J. Immunol. 1683470-3476. [DOI] [PubMed] [Google Scholar]
- 43.Wrona, T. J., M. Lozano, A. A. Binhazim, and J. P. Dudley. 1998. Mutational and functional analysis of the C-terminal region of the C3H mouse mammary tumor virus superantigen. J. Virol. 724746-4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshimoto, T., H. Nagase, H. Nakano, A. Matsuzawa, and H. Nariuchi. 1994. A V β8.2-specific superantigen from exogenous mouse mammary tumor virus carried by FM mice. Eur. J. Immunol. 241612-1619. [DOI] [PubMed] [Google Scholar]
- 45.Ziegler, R. B., R. O. Johnson, D. A. Cooper, and J. Gold. 1985. Postnatal transmission of AIDS-associated retrovirus from mother to infant. Lancet i896-898. [DOI] [PubMed] [Google Scholar]