Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV) envelope-associated glycoprotein B (gB) is involved in the initial steps of binding to host cells during KSHV infection. gB contains an RGD motif reported to bind the integrin α3β1 during virus entry. Although the ligand specificity of α3β1 has been controversial, current literature indicates that α3β1 ligand recognition is independent of RGD. We compared α3β1 to the RGD-binding integrin, αVβ3, for binding to envelope-associated gB and a gB(RGD) peptide. Adhesion assays demonstrated that β3-CHO cells overexpressing αVβ3 specifically bound gB(RGD), whereas α3-CHO cells overexpressing α3β1 did not. Function-blocking antibodies to αVβ3 inhibited the adhesion of HT1080 fibrosarcoma cells to gB(RGD), while antibodies to α3β1 did not. Using affinity-purified integrins and confocal microscopy, αVβ3 bound to gB(RGD) and KSHV virions, demonstrating direct receptor-ligand interactions. Specific αVβ3 antagonists, including cyclic and dicyclic RGD peptides and αVβ3 function-blocking antibodies, inhibited KSHV infection by 70 to 80%. Keratinocytes from α3-null mice lacking α3β1 were fully competent for infection by KSHV, and reconstitution of α3β1 function by transfection with α3 cDNA reduced KSHV infectivity from 74% to 55%. Additional inhibitory effects of α3β1 on the cell surface expression of αVβ3 and on αVβ3-mediated adhesion of α3-CHO cells overexpressing α3β1 were detected, consistent with previous reports of transdominant inhibition of αVβ3 function by α3β1. These observations may explain previous reports of an inhibition of KSHV infection by soluble α3β1. Our studies demonstrate that αVβ3 is a cellular receptor mediating both the cell adhesion and entry of KSHV into target cells through binding the virion-associated gB(RGD).
Kaposi's sarcoma-associated herpesvirus (KSHV)/human herpesvirus 8 was originally detected in KS lesions (12) and has been implicated in the etiology of KS and AIDS-associated pleural effusion lymphoma and multicentric Castleman's disease (52). KS is a multifocal lesion characterized by intense angiogenesis, proliferation, and infiltration by inflammatory cells (18). KSHV is detected in a number of cell types present in KS lesions, including the characteristic KS spindle cells, infiltrating B cells, and neovascular endothelial cells (4). KSHV infection is latent in these cells, with only a small percentage of cells undergoing active virus replication. In vitro, KSHV has a broad tropism and infects endothelial, epithelial, and mesenchymal cells (5, 47).
Similar to other herpesviruses, KSHV's infection of susceptible cells occurs through at least two separate binding events. The initial binding to heparan sulfate on cell-surface proteoglycans is mediated by the virion envelope-associated glycoproteins, K8.1 (63) and glycoprotein B (gB) (3). Although heparan-sulfate binding is reversible and not essential for virus entry, it is thought to localize virus on the cell surface, enhancing opportunities for direct interactions between virion glycoproteins and specific cellular entry receptors (3, 59). A number of entry receptor candidates have been reported for KSHV. The cystine transporter, xCT, was identified as a KSHV fusion-entry receptor by functional cDNA selection (27). DC-SIGN was shown to function as a receptor for KSHV on myeloid dendritic cells and macrophages (46). The α3β1 cellular integrin has also been identified as a potential entry receptor for KSHV (2), and integrin signaling has been implicated in the KSHV infection process and facilitates uptake into the host cell, intracellular trafficking of the viral capsid, and establishment of the latent infection (36, 39, 40, 55). These findings are of particular interest since the integrin receptor family is exploited by a number of other viruses as an entry pathway for infection.
While the KSHV virion proteins that interact with the xCT and DC-SIGN receptors have not yet been identified, the KSHV envelope-associated gB was reported to be the viral ligand recognized by the α3β1 integrin receptor (2). gB is highly conserved within members of the herpesvirus family and plays an important role in the infection of susceptible host cells by different herpesviruses (42). Sequence analysis of KSHV gB identified an N-terminal Arg-Gly-Asp (RGD) tripeptide motif, also found in extracellular matrix proteins that interact with members of the integrin receptor family (2, 49, 50). Integrin receptors consist of noncovalently associated αβ heterodimers, and ligand specificity is determined by different combinations of α and β monomers. The diverse integrin family is divided based on ligand specificity into subfamilies which include integrin receptors specific for RGD (group I), laminin (group II), collagen (group III), and leukocytes (group IV) (24). The presence of the RGD motif in the KSHV gB suggested that it might be a ligand of a group I RGD-binding integrin, such as αVβ3, αVβ5, or α5β1; however, gB was reported to interact with the laminin-binding integrin α3β1 (2). Although α3β1 was once regarded as a promiscuous receptor with weak affinity for RGD and other ligands, more-recent studies have shown that it binds with high affinity to laminin 5, laminin 10/11, and bacterial invasin in an RGD-independent manner (11, 14, 29, 30, 37, 41, 69). These findings raise questions regarding the direct involvement of α3β1 in RGD-mediated binding to KSHV gB and the role of α3β1 in RGD-mediated entry of KSHV into target cells.
We have used a variety of approaches to study the binding specificity of the RGD motif of KSHV gB and the role of RGD-binding integrins in KSHV infection. We have analyzed integrin binding to the native KSHV virion envelope-associated gB and to a peptide derived from the N-terminal RGD motif of KSHV gB [gB(RGD)] using cell adhesion and affinity binding studies. We have also used peptide antagonists and function-blocking antibodies to identify the integrin responsible for RGD-mediated binding and entry of KSHV into target cells. Our studies show that the group I integrin, αVβ3, specifically interacts with the RGD motif of KSHV gB and functions as a receptor in cell adhesion and KSHV entry during infection. We were unable to detect any specific interactions between the α3β1 integrin and gB or any of the other envelope-associated glycoproteins present on the KSHV virion. We observed an inhibition of KSHV infectivity and a downregulation of αVβ3 expression and function in cells overexpressing α3β1. These effects correlate with the transdominant inhibition of αVβ3 function by α3β1 and other integrins containing the β1 subunit seen by others (7, 22, 38) and may explain previous reports of inhibitory effects of soluble α3β1 on KSHV infectivity (2, 39, 55). Our results are consistent with the results of studies showing the importance of integrin-mediated entry and integrin-associated signaling during KSHV infection but suggest that the integrin receptor directly interacting with the RGD motif of KSHV gB is αVβ3 integrin.
MATERIALS AND METHODS
Cell lines.
The human HT1080 fibrosarcoma cell line was a gift of W. G. Carter. African green monkey Vero cells were from the American Type Culture Collection. The Chinese hamster ovary (CHO) parental cell line, pBJ, and human integrin-expressing transfected cell lines α3-CHO (60) and β3-CHO (61) which overexpress α3β1 and αVβ3 integrins, respectively, were a kind gift from Y. Takada. Cells were maintained in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum. The CHO cell medium also contained G418 (700 μg/ml). BCBL-1 cells latently infected with KSHV (48) were cultured in RPMI complete medium with 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 1.0 mM HEPES, and 0.01% 2-mercaptoethanol at 37°C. The mouse keratinocyte cell lines and culture medium have been described previously (16). The cell lines include MK-116 (MK+/+), derived from wild-type keratinocytes; MK-546 (MK−/−), derived from α3-null keratinocytes; and MK-546-hα3A (MK−/−,α3), derived from MK-546 cells stably transfected with human α3A integrin.
Immunological reagents.
The following integrin-specific mouse monoclonal antibodies (MAbs) were purchased from Chemicon: anti-α3 (clone ASC-6, clone P1B5, and clone 29A3 [for blotting]), anti-αVβ3 (clone LM609), anti-β3 (clone B3A), anti-α5β1 (clone JBS5), and anti-αVβ5 (clone P1F6). Other antibody reagents included mouse anti-K8.1A (clone 2A3) and rat anti-latency-associated nuclear antigen (LANA) (clone LN53) (Advanced Biotechnologies), mouse antibiotin (Molecular Probes), goat antibiotin-horseradish peroxidase (HRP) (Sigma), goat anti-mouse immunoglobulin G (IgG)-HRP, goat anti-mouse IgG-alkaline phosphatase (alk-phos), goat anti-rat IgG-HRP (BioSource), normal goat serum, nonimmune-mouse IgG, and mouse antibiotin-HRP (Jackson).
Peptides and purified integrin receptors.
Biotinylated peptides corresponding to the 12-amino-acid sequences of the RGD motif within the predicted N terminus of the mature KSHV gB and an RGE motif with a single-amino-acid mutation were synthesized by Res Gen (Huntsville, AL): gB(RGD), AHSRGDTFQTSSGCG, and gB(RGE), AHSRGETFQTSSGCG. The C-terminal GCG amino acids were added for coupling purposes. The αVβ3 antagonists and peptide controls were cyclo(Arg-Gly-Asp-D-Phe-Lys) (cRGD), dimeric H-Glu[cyclo(Arg-Gly-Asp-D-Phe-Lys)]2 (dcRGD), cyclo(Arg-Ala-Asp-D-Phe-Lys) (cRAD), and linear GRGDS and GRGES peptides (Peptides International). Affinity-purified human αVβ3 (cc1020) and α3β1 (cc1092) integrin receptors in an octyl-beta-d-glucopyranoside formulation were purchased from Chemicon.
Flow cytometry.
The expression levels of different integrins present on the CHO or HT1080 cells were determined by using fixed-cell suspensions incubated with anti-integrin antibodies and fluorescein isothiocyanate-labeled goat anti-mouse IgG and analyzed on a FACScan instrument. The data are given as the mean fluorescence intensities (MFIs) of the stained cell populations.
Adhesion assay.
NeutrAvidin plates were coated with biotinylated gB(RGD) or gB(RGE) peptides at different concentrations. Maleimide plates (Pierce) were coated with laminin (20 μg/ml). The plates were blocked with phosphate-buffered saline-2% bovine serum albumin. HT1080 or CHO cell suspensions (2 × 104 cells/well) were incubated with immobilized peptides at 25°C for 1 h to allow cell adhesion. In some experiments, the cells were cooled to 4°C and pretreated for 1 h at 4°C with various function-blocking anti-integrin antibodies prior to being plated on peptide (10 μg/ml). Following cell adhesion at 25°C, the plates were inverted to remove nonadherent cells and then incubated at 37°C for 30 min to allow adherent cells to spread. The adherent cells were fixed, stained with 0.5% toluidine blue, and counted manually. The experiments were performed in triplicate or quadruplicate, and the means and standard errors of the cell numbers were determined.
Virus purification.
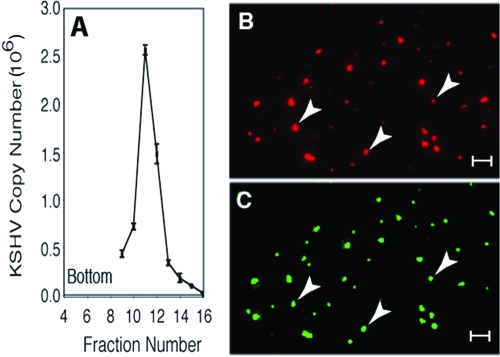
KSHV virions were purified from filtered culture supernatant (180 ml) of tetradecanoyl phorbol acetate (TPA)-treated BCBL-1 cells (20 ng/ml; 6 days) by centrifugation onto a cushion of 50% Opti-Prep (iodixanol) (19). An aliquot of the concentrated virus was labeled with 10 μg/ml NHS-PEO4-biotin (Pierce) and further purified by centrifugation on a 20%, 25%, 30%, and 40% Opti-Prep step gradient. Virus was quantitated by real-time quantitative PCR. A single DNA peak was obtained at the 20%-to-25% Opti-Prep interface (fractions 11 and 12; see Fig. 6A). The peak fractions were pooled and represent a 450-fold concentration of the original BCBL-1 supernatant. Virions were characterized by immobilization on poly-l-lysine-coated slides and staining with anti-K8.1 or antibiotin antibodies using goat anti-IgG-HRP and 488 and 594 tyramide signal amplification, respectively (Molecular Probes).
FIG. 6.
Gradient purification and characterization of KSHV virions for binding and infection studies. Infectious KSHV virions were purified from culture medium of TPA-induced BCBL cells by double-step gradient centrifugation in Opti-Prep. (A) Virus-containing fractions were identified by real-time quantitative PCR analysis for KSHV genomic DNA. (B, C) Biotinylated virions showed discrete staining and colocalization (arrows) of antibody reactivity to biotin (B) and KSHV envelope-associated glycoprotein K8.1 (C). Bar, 5 μm.
Rhesus rhadinovirus (RRV), a close homolog of KSHV (15), was used as a gB(RGD)-negative control virus. RRV, a gift of M. Axthelm and S. Wong, was propagated as described previously (9). RRV virions were concentrated, biotinylated, and gradient purified as described above for KSHV. The infectivity of the purified RRV was confirmed by using rhesus primary fetal fibroblasts (data not shown).
KSHV infection.
MK cell lines and Vero cells were seeded at 2.5 × 103 cells per well 24 h prior to infection. The cell cultures were infected with different dilutions of gradient-purified KSHV virions for 3 h at 37°C, washed, incubated for an additional 24 h, fixed, and stained for KSHV LANA using the LN53 anti-LANA antibody essentially as described previously (9). The nuclei were stained with TOPRO (Molecular Probes). The percentage of cells expressing nuclear LANA was determined manually using confocal images.
In some experiments, the ability of KSHV to infect HT1080 in the presence of specific αVβ3 antagonists was determined. HT1080 cells (3 × 104) were chilled on ice and pretreated with peptide (1 mM) or anti-αVβ3 antibody (LM609; 5 μg) for 1 h at 4°C. Gradient-purified KSHV titrated to yield an infection in 75% of the target cells (3 μl, approximately equivalent to 1 ml of BCBL-1 supernatant) was added and incubated for 1 h at 4°C. The cells were washed, cultured for 24 h, and fixed. The percentages of cells expressing LANA and the relative amounts of nuclear fluorescence were determined by using confocal imaging with the LN53 antibody. The histogram function within the Zeiss LSM software was used to measure the absolute frequency and intensity of fluorescent pixels within the nuclear LANA dots. The fluorescence intensities ranged from 0 (no fluorescence) to 255 (maximum fluorescence), and the lower threshold was set at 100 to eliminate nonspecific fluorescence.
Magnetic bead assays.
NeutrAvidin (Pierce) was coupled to Dynabeads with carboxylic acid using the EDC/NHS method (Dynal Biotech). The biotinylated gB(RGD) and gB(RGE) peptides (approximately 0.34 mg peptide/2.2 mg beads) or biotinylated gradient-purified KSHV or RRV virions were immobilized on the NeutrAvidin beads (50 μl virions/2 mg beads). To monitor coupling, the KSHV-coated beads were stained with anti-K8.1 antibody, while the RRV-coated beads and gB peptide-coated beads were stained with antibiotin antibody, and immunofluorescence was visualized by confocal microscopy.
(i) Affinity purification of integrins bound to immobilized peptide.
β3-CHO or α3-CHO cells (5 × 106) were solubilized with lysis buffer (100 mM octyl-beta-glucoside, 25 mM Tris HCl, 150 mM NaCl, 1.0 mM MgCl2, pH 7.4, containing 2 mM Pefabloc [Roche]) for 1 h at 4°C. The cell lysates were incubated with peptide-coated beads, and bound proteins were eluted with 20 mM EDTA, resolved on 10% NuPAGE gels (Invitrogen), and transferred to polyvinylidene difluoride membranes. The membranes were probed with either anti-β3 (MAb 2023) or anti-α3 (MAb 2290) antibody followed by alk-phos-labeled goat anti-IgG. Western blue alk-phos substrate (Promega) was used for the final enzymatic development.
(ii) Affinity purification of integrins bound to immobilized virions.
KSHV virion-coated beads or d-biotin-coated control beads were incubated with octylglucoside lysates of β3-CHO, α3-CHO, or HT1080 cells. The beads were immobilized on poly-l-lysine-coated slides, fixed, and blocked with 10% normal goat serum. Bound integrins were detected by confocal immunofluorescence microscopy using anti-β3 (MAb 2023) or anti-α3 (clone P1B5) antibody followed by goat anti-IgG-HRP with TSA 488 development. The Zeiss LSM software histogram function was used to determine the sum of the integrin fluorescence pixels (intensity range, 100 to 255) associated with individual beads. The mean sum ± standard deviation (SD) values for 10 beads per group were determined. The fluorescence pixels that were specific for integrin bound to KSHV were quantitated by subtracting the mean control bead values from the mean values for KSHV-bound integrin.
(iii) Binding of purified integrin receptor to immobilized peptides and purified virions.
Dynabeads coated with either gB(RGD) or gB(RGE) peptides or KSHV or RRV virions were incubated with 0.7 μg of affinity-purified αVβ3 or α3β1 integrin receptors in phosphate-buffered saline containing 1 mM MgCl2. The beads were washed and fixed, and bound integrins were detected by confocal immunofluorescence microscopy using the anti-β3 (clone B3A) or anti-α3 (clone P1B5) antibodies as described above.
RESULTS
The RGD motif in the KSHV envelope-associated gB has sequence similarity to extracellular matrix and viral ligands recognized by the αVβ3 integrin.
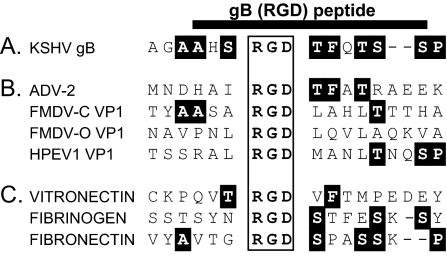
Sequence analysis of KSHV gB identified an Arg-Gly-Asp (RGD) motif near the N terminus of the predicted mature protein (49, 50). RGD motifs are recognized by a number of different integrin receptors, and the amino acids flanking RGD motifs contribute to receptor specificity (44). We compared the KSHV RGD motif and flanking region (Fig. 1A) to the RGD motifs and flanking regions of several integrin-binding extracellular matrix (Fig. 1C) and viral proteins (Fig. 1B) for clues to potential KSHV receptor specificity. Visual inspection of the sequences flanking the core RGD motif identified similarities in amino acid composition both upstream and downstream of the RGD motif (Fig. 1A). Alanine (A) residues upstream and a proline (P) residue downstream of the RGD motif were conserved in several RGD-containing ligands. An important conservation of the structurally similar serine (S) and threonine (T) residues flanking both sides of the RGD motif was noted between the KSHV sequence and other ligands. Similar to the sequences of vitronectin and the adenovirus 2 penton base, the KSHV gB contained a phenylalanine (F) residue 2 amino acids downstream of the RGD motif. Overall, the KSHV gB and adenovirus penton base proteins were the most similar, with 6 of 7 identical amino acids within the RGD motif and downstream flanking sequences. The RGD domain of the penton base protein has been shown to interact with the integrin heterodimers αVβ3 and αVβ5 (67), suggesting that the KSHV RGD motif might also bind to these or other integrins containing the αV integrin subunit.
FIG. 1.
The RGD motif of KSHV gB has sequence similarity to ligands of the αVβ3 integrin. The RGD motif of KSHV gB (A) was aligned with the RGD motifs of viral (B) and extracellular matrix (C) ligands of cellular integrin receptors. The RGD motif is boxed, and conserved residues, including conservative (S)erine-(T)hreonine changes, are highlighted. The sequence from which the KSHV gB(RGD) peptide is derived is indicated. ADV-2, adenovirus 2 (penton base) (NCBI accession no. NP_040521); FMDV-C VP1, foot-and mouth-disease virus serotype C1 viral protein 1 (NCBI accession no. JC1329); FMDV-O VP1, (foot-and-mouth disease virus serotype O viral protein 1 (NCBI accession no. P03305); HPEV1 VP1, human parechovirus 1 viral protein 1 (NCBI accession no. Q66578). Vitronectin, NCBI accession no. P04004; fibrinogen, NCBI accession no. NP_000499; fibronectin, NCBI accession no. AAD00019.
The glycoprotein B RGD motif is a ligand for cell surface αVβ3 integrin.
Previously, the KSHV gB N-terminal domain was shown to promote RGD-dependent fibroblast adhesion; however, the receptor mediating gB binding was not identified (64). We therefore evaluated the ability of the α3β1 integrin, a proposed KSHV entry receptor (2), to mediate RGD-dependent cell adhesion to KSHV gB and compared it to that of αVβ3, one of the integrins predicted to bind the RGD motif of KSHV gB in our sequence alignment study. The α3-CHO cell line, which overexpresses human-hamster chimeric α3β1 integrin (60), was compared to the β3-CHO cell line, which overexpresses a chimeric αVβ3 integrin (61), for the ability to adhere to gB(RGD), a peptide derived from the N-terminal domain of the KSHV gB (see Fig. 1). Both chimeric integrins have been previously shown to be functional (60, 61). The pBJ-CHO cell line, which lacks an integrin insert, was used as a control. Initial analysis of the CHO cell lines by flow cytometry showed increased surface expression of the appropriate recombinant human integrins (Fig. 2A). To assess adhesion, tissue culture plates were coated with gB(RGD) peptide. Control experiments utilized a gB(RGE) peptide containing a single-amino-acid substitution within the RGD motif. The micrographs in Fig. 2 show that β3-CHO cells (Fig. 2C) adhered significantly more to gB(RGD) than did the α3-CHO cells (Fig. 2D) or the pBJ-CHO control cells (Fig. 2E). Adherent β3-CHO cells showed hallmarks of integrin activation and signaling with a spread morphology and the formation of vinculin-containing focal contacts (data not shown). The adhesion of the β3-CHO cells was specific since no adhesion to the gB(RGE) control peptide was detected (data not shown). Although the α3-CHO cells failed to show significant adhesion to gB(RGD), cell adhesion and spreading was observed when they were plated on a known α3β1 ligand, such as laminin (Fig. 2F).
FIG. 2.
The αVβ3 integrin mediates cell adhesion to KSHV gB(RGD). (A) Fluorescence-activated cell sorter analysis of integrin levels in the pBJ parental CHO cells compared to the levels in β3-CHO and α3-CHO recombinant cells expressing chimeric human-hamster αVβ3 and α3β1 integrins, respectively, using anti-αVβ3 (LM609) and anti-α3 (ASC-6) integrin antibodies. (B) Fluorescence-activated cell sorter analysis of αVβ3 levels in pBJ-CHO, β3-CHO, and α3-CHO cells. (C to F) Cell adhesion to immobilized gB(RGD) peptide (10 μg/ml) by β3-CHO (C), α3-CHO (D), and the parental pBJ-CHO (E) cells and to immobilized laminin by α3-CHO cells (F) is shown. Bar, 20 μm. (G) Cell adhesion to increasing concentrations of gB(RGD) peptide. The data represent the mean numbers ± standard errors of adherent cells in quadruplicate wells. Control experiments showed no adhesion to the gB(RGE) peptide with a single-amino-acid mutation in the RGD domain (data not shown).
Cell adhesion was dependent on the amount of immobilized gB(RGD) peptide. At 0.5 μg/ml, there were >500 adherent β3-CHO cells and no adherent α3-CHO or pBJ-CHO control cells (Fig. 2G). Maximal β3-CHO adhesion was observed at 1 μg/ml of gB(RGD). At the highest concentration of gB(RGD) tested, (2 μg/ml), there were a small number of adherent α3-CHO and pBJ-CHO cells (5% and 13% of the adherent β3-CHO cells, respectively). To determine whether the binding of the α3-CHO and pBJ-CHO cells could be due to endogenous hamster αVβ3, the level of αVβ3 expression for each CHO cell line was determined. As shown in Fig. 2B, the pBJ-CHO control cell line expressed low levels of hamster αVβ3 (MFI, 15) compared to the level in β3-CHO cells (MFI, 54). The α3-CHO cell line displayed even less αVβ3 integrin (MFI, 6). Thus, the levels of endogenous hamster αVβ3 expression in the different CHO cell lines correlate with their ability to adhere to gB(RGD) peptide. This suggests that the adhesion of the α3-CHO and pBJ-CHO cells seen at high concentrations of gB(RGD) peptide is mediated by the endogenous hamster αVβ3.
To examine the direct receptor-ligand interactions between αVβ3 and gB(RGD), gB(RGD) peptide-conjugated magnetic beads were used for affinity binding assays. Biotinylated gB(RGD) or gB(RGE) peptides bound to NeutrAvidin magnetic beads were incubated with octyl-beta-glucoside lysates of β3-CHO or α3-CHO cells in the presence of 1 mM MgCl2. The β3-CHO and α3-CHO cell lysates contained significant amounts of β3 (105 kDa) and α3 (160 kDa) integrins, respectively (Fig. 3, lanes 1 and 4, respectively). The beads were washed, and bound proteins were eluted by using EDTA. RGD interactions are stabilized by divalent cations, and dissociation with metal chelators, such as EDTA, is diagnostic of RGD-mediated binding (56-58). The eluted proteins were analyzed by immunoblotting using antibodies specific for either the α3 or β3 integrin. The αVβ3 integrin was detected in the EDTA eluates from the gB(RGD) beads (lane 2), whereas no α3β1 integrin was detected (lane 5). Neither integrin was detected in the eluates from the gB(RGE) control beads (Fig. 3, lanes 3 and 6). These studies demonstrate a specific interaction between αVβ3 and the RGD motif of KSHV gB.
FIG. 3.
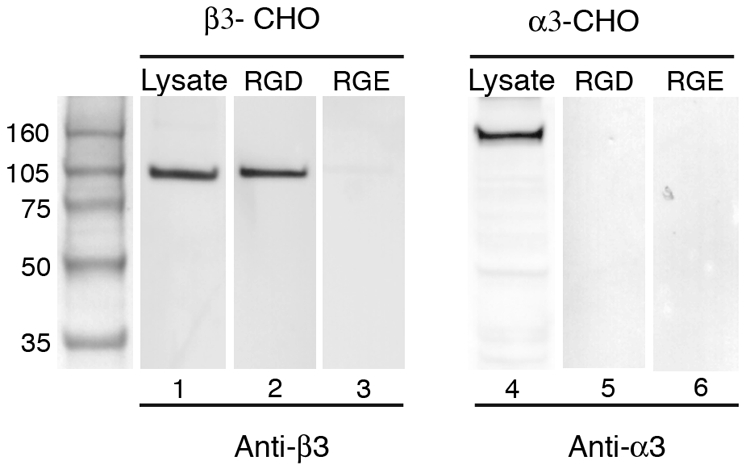
The RGD motif of KSHV gB specifically binds the αVβ3 integrin. Magnetic beads coated with the KSHV gB(RGD) peptide (lanes 2, 5) or gB(RGE) control peptide (lanes 3, 6) were incubated with lysates from either the β3-CHO (lanes 2, 3) or α3-CHO (lanes 5, 6) cells. Bound proteins were eluted with EDTA and characterized by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblot analysis using anti-β3 antibody (MAb 2023) to detect αVβ3 (lanes 1 to 3) and anti-α3 antibody (MAb 2290) to detect α3β1 (lanes 4 to 6). Integrin levels in the initial β3-CHO (lane 1) and α3-CHO (lane 4) cell lysates are shown.
KSHV gB(RGD)-mediated cell adhesion is blocked by αVβ3 antibodies.
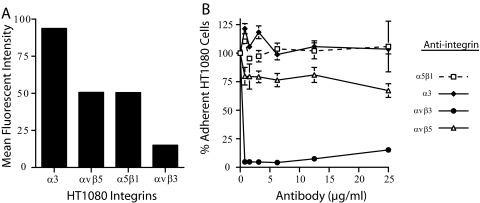
To show that the chimeric human/hamster integrin receptors overexpressed in CHO cells did not have an altered affinity for ligand, we also performed adhesion assays using the human fibrosarcoma cell line HT1080, which expresses human integrin receptors. In these studies, we evaluated the ability of integrin function-blocking antibodies to inhibit the adhesion of HT1080 cells to the gB(RGD) peptide. HT1080 cells were chosen due to their wide variety of expressed integrin receptors (65). The surface integrin expression on HT1080 cells was first determined by flow cytometry using a panel of antibodies to different α and β integrin subunits and receptor dimers. In all cases, single-peak histograms were observed, and the percentage of HT1080 cells staining with each antibody was high (87 to 99%) (Fig. 4A). Significant levels of the laminin-binding integrin α3β1 and the RGD-binding integrins αVβ3, αVβ5, and α5β1 were detected; however, the levels of αVβ3 were comparatively low, as has been previously reported (70).
FIG. 4.
αVβ3 function-blocking antibodies inhibit gB(RGD)-mediated HT1080 cell adhesion. (A) Flow cytometry analysis of cell surface integrin levels on HT1080 human fibrosarcoma cells using anti-α3 (ASC-6), anti-αVβ5 (P1F6), anti-α5β1 (JBS5), or anti-αVβ3 (LM609) antibodies. (B) HT1080 cell adhesion to immobilized gB(RGD) peptide (10 μg/ml) in the presence of different concentrations of the function-blocking integrin antibodies used for the experiments whose results are shown in panel A. Adherent cells were quantitated manually. The data represent the mean cell numbers ± standard errors of the results for triplicate wells. Standard errors smaller than the plot symbol are not shown.
HT1080 cell suspensions were pretreated at 4°C with function-blocking antibodies to different integrin receptors prior to being plated on immobilized gB(RGD), as described in Materials and Methods. Antibodies specific for αVβ3 blocked more than 95% of cell adhesion to gB(RGD) at concentrations as low as 0.4 μg/ml (Fig. 4B). These results confirmed the role of αVβ3 in cell adhesion to gB(RGD) seen with the transfected β3-CHO cells, described above. In contrast, function-blocking antibodies to the fibronectin receptor α5β1 and the laminin receptor α3β1 were unable to block the adhesion of HT1080 cells to gB(RGD). In fact, these antibody treatments increased cell adhesion to 110% and 125%, respectively, of that observed without antibody pretreatment. Antibodies to the RGD-binding integrin, αVβ5, inhibited 30% of adhesion at the highest antibody concentration. These results indicate that the RGD motif of KSHV gB has a strong specificity for integrins containing the αV integrin subunit, with the strongest reactivity to αVβ3, the vitronectin receptor.
Purified αVβ3 receptor binds directly to gB(RGD) and intact KSHV virions.
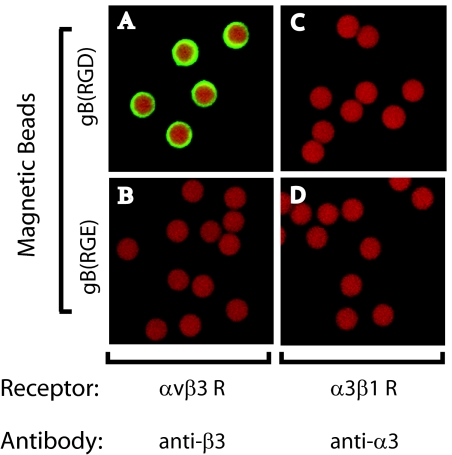
To demonstrate a direct interaction between the αVβ3 integrin and KSHV gB, we evaluated the binding of purified soluble αVβ3 integrin receptor to both gB(RGD) peptide and KSHV virion-coated beads. The results were compared to the binding of purified α3β1 integrin which has been previously reported to inhibit KSHV infection of fibroblasts (2). Initial experiments were performed to determine the binding specificities of the commercial preparations of affinity-purified αVβ3 and α3β1 receptors to gB(RGD) peptide-coated beads. NeutrAvidin beads coated with biotinylated gB(RGD) were incubated with either purified αVβ3 or α3β1 heterodimers. Bound receptors were fixed, and the beads were immobilized on poly-l-lysine-coated slides. Confocal immunofluorescence microscopy was used to detect bound receptors by reacting the beads with antibodies to either αVβ3 or α3β1. As shown in Fig. 5, αVβ3 receptor was detected on the surface of the gB(RGD)-coated beads (Fig. 5A), but not on the control gB(RGE)-coated beads (Fig. 5B). In contrast, the purified α3β1 receptor failed to bind to either the gB(RGD)- or gB(RGE)-coated beads (Fig. 5C, D). These results are consistent with the binding data obtained by using whole-cell lysates that are shown in Fig. 3.
FIG. 5.
Purified αVβ3 receptor specifically binds the KSHV gB(RGD) peptide. Biotinylated gB(RGD) and control gB(RGE) peptides were coupled to magnetic beads and incubated with commercial preparations of affinity-purified functional αVβ3 or α3β1 integrin receptors (R). Bound αVβ3 or α3β1 integrins were detected by using anti-β3 (A, B) or anti-α3 (α3β1) (C, D) antibodies and confocal microscopy (green fluorescence).
In order to examine the binding of purified integrins to native envelope-associated gB present on intact virions, KSHV virions were purified from TPA-induced BCBL-1 cell cultures by density gradient centrifugation in Opti-Prep (iodixanol) and biotinylated, as described in Materials and Methods. Real-time PCR analysis of KSHV DNA in the gradient fractions showed a single viral peak at the 25%-to-30% Opti-Prep interface (Fig. 6A). Biotinylated virions in the peak fractions were immobilized on poly-l-lysine-coated slides and characterized by confocal immunofluorescence microscopy using antibodies to biotin and to the KSHV envelope-associated K8.1 glycoprotein. Discrete particles were detected which showed a high degree of colocalization of biotin (Fig. 6B) and K8.1 (Fig. 6C) staining, consistent with the presence of intact, enveloped KSHV virions suitable for receptor binding studies.
The gradient-purified biotinylated KSHV virions were coupled to NeutrAvidin-coated beads and fixed. As a binding control, NeutrAvidin beads were coupled to gradient-purified preparations of biotinylated virions of RRV, a macaque herpesvirus that is closely related to KSHV (15). Sequence analysis of the RRV genome has identified a close homolog of the KSHV gB (53) which lacks a putative integrin-binding RGD motif. The relative amounts and distribution of virions coating the beads were determined by confocal immunofluorescence microscopy using the anti-K8.1A antibody for KSHV and the antibiotin antibody for RRV. Highly concentrated KSHV virions were detected at the bead surface, with some localized enhanced fluorescence concentrations possibly indicating areas of KSHV aggregation (Fig. 7A). The surface density of biotinylated RRV was similar to that of KSHV, but the uniform immunofluorescence pattern suggested fewer surface RRV aggregates (Fig. 7B). The KSHV and RRV virion-coupled beads were incubated with affinity-purified αVβ3 and α3β1 integrin receptors, and bound receptor was detected by confocal immunofluorescence using antibodies to β3 and α3 integrins. The antibodies to β3 reacted strongly with the KSHV-coated beads, demonstrating the presence of bound αVβ3 receptor (Fig. 7C). The pattern of fluorescence was similar to that seen with the anti-K8.1 antibody, indicating that the αVβ3 integrin receptor was bound to the KSHV virions. No binding of the affinity-purified α3β1 integrin to the KSHV-coated bead surface was detected (Fig. 7E). The binding of purified αVβ3 to the KSHV virion was specific since no binding was detected to the control macaque RRV virions which lack the gB RGD motif (Fig. 7D). The purified α3β1 integrin receptor was also unable to bind to the RRV virions (Fig. 7F).
FIG. 7.
Purified αVβ3 receptor specifically binds to KSHV virions. Gradient-purified KSHV and RRV virions which had been biotinylated and coupled to magnetic beads were reacted with anti-K8.1 (A) or antibiotin (B) antibodies, respectively, to monitor virus-bead coupling by confocal microscopy. −, no receptor. The virion-coated beads were incubated with commercial preparations of affinity-purified αVβ3 integrin (C, D) or α3β1 integrin (E, F), and bound integrin was detected by confocal microscopy using either anti-β3 (B3A) (C, D) or anti-α3 (ASC-6) (E, F) antibodies. Arrows show localized enhanced fluorescence concentrations possibly indicating areas of KSHV aggregation. Bound antibodies were detected as green fluorescence.
Soluble αVβ3 integrin in detergent-extracted cell lysates specifically binds KSHV virions.
To determine if αVβ3 associates with KSHV in the presence of other cellular proteins within whole-cell lysates, gradient-purified biotinylated KSHV virions were coupled to beads, and conjugated virus was detected by confocal immunofluorescence using antibody to the K8.1 envelope glycoprotein (Fig. 8E). The KSHV-coupled beads were incubated with octyl-beta-glucoside lysates of β3-CHO or α3-CHO cells. d-Biotin-conjugated beads were used as a control. Bound integrins were detected by immunofluorescence microscopy. High levels of the β3 integrin from the β3-CHO lysate bound to the KSHV beads (Fig. 8A). No binding to the control beads was detected (Fig. 8B). The α3 integrin from lysates of the α3-CHO cells was also detected on the KSHV beads, but at a lower level than in the β3 lysates (Fig. 8C). However, similar amounts of α3 also bound to the control beads containing no virus, suggesting that the binding was nonspecific. Quantitation of the bead-associated fluorescence revealed a significant and specific reaction between αVβ3 in cell lysates and the purified KSHV virions (Fig. 8F, G). This binding is similar to that seen with the purified αVβ3 receptor, described above. No significant association was detected between the KSHV virions and the α3β1 integrin even in the context of other cellular proteins in the cell lysate (Fig. 8F, G). These studies were repeated using lysates of human HT1080 cells which express significant levels of functional αVβ3 and α3β1 integrins with the same results (data not shown).
FIG. 8.
Soluble αVβ3 from cell lysates specifically binds to KSHV virions. Lysates from cells overexpressing either αVβ3 (β3-CHO) or α3β1 (α3-CHO) were incubated with gradient-purified KSHV virions coupled to magnetic beads (A, C) or control beads (B, D) with no virus. Bound αVβ3 and α3β1 were detected by using anti-β3 (B3A) (A, B) or anti-α3 (ASC-6) (C, D) antibodies, respectively. The presence of bead-bound KSHV was demonstrated by anti-K8.1 antibody immunofluorescence (E). Individual pixels of bead fluorescence (intensity range, 100 to 255) were quantitated, and the mean (n = 10) sum ± standard deviation of pixels was calculated (F). Specific binding to KSHV was determined by subtracting the control bead fluorescence (G). No significant binding of α3β1 was detected.
KSHV cell entry is inhibited by αVβ3 antagonists.
Since our data showed a strong reactivity between αVβ3 and KSHV virions, we performed infection-blocking studies to determine whether the αVβ3 integrin functions as a KSHV entry receptor. Cyclic RGD peptides are selective αVβ3 antagonists and block αVβ3 integrin binding at concentrations 80-fold lower than their linear counterparts (21, 43). Furthermore, changing the ligand valency by converting monomeric peptide to dimeric increases the affinity for αVβ3 (34). Therefore, we tested the ability of the cyclic RGD peptides to block KSHV infection mediated by αVβ3. cRGD and dcRGD were chosen since these peptides have high affinities for αVβ3 and low affinities for the αVβ5, αIIbβ3, and α5β1 integrins (25, 28). The inhibitory activities of the cyclic peptides were compared to the activity of the linear GRGDS peptide derived from the fibronectin sequence. The linear peptide GRGES and the cyclic peptide cRAD, which both contained single-amino-acid alterations in the RGD motif, were used as controls.
Adherent HT1080 cells were trypsinized, chilled (4°C) to prevent receptor internalization, and pretreated with peptide before infection with gradient-purified KSHV, as described in Materials and Methods. Latent infection was assayed at 24 h by confocal immunofluorescence detection of KSHV LANA. In the absence of inhibitor, approximately 75% of the HT1080 cells were infected with KSHV (Fig. 9A). Cells treated with the control peptides, cRAD and GRGES, showed only a small decrease in the number of LANA-expressing cells. In contrast, the αVβ3-specific cRGD peptide and the linear GRGDS peptide both inhibited approximately 78% of KSHV infection, while the dcRGD peptide inhibited approximately 64% (Fig. 9A).
FIG. 9.
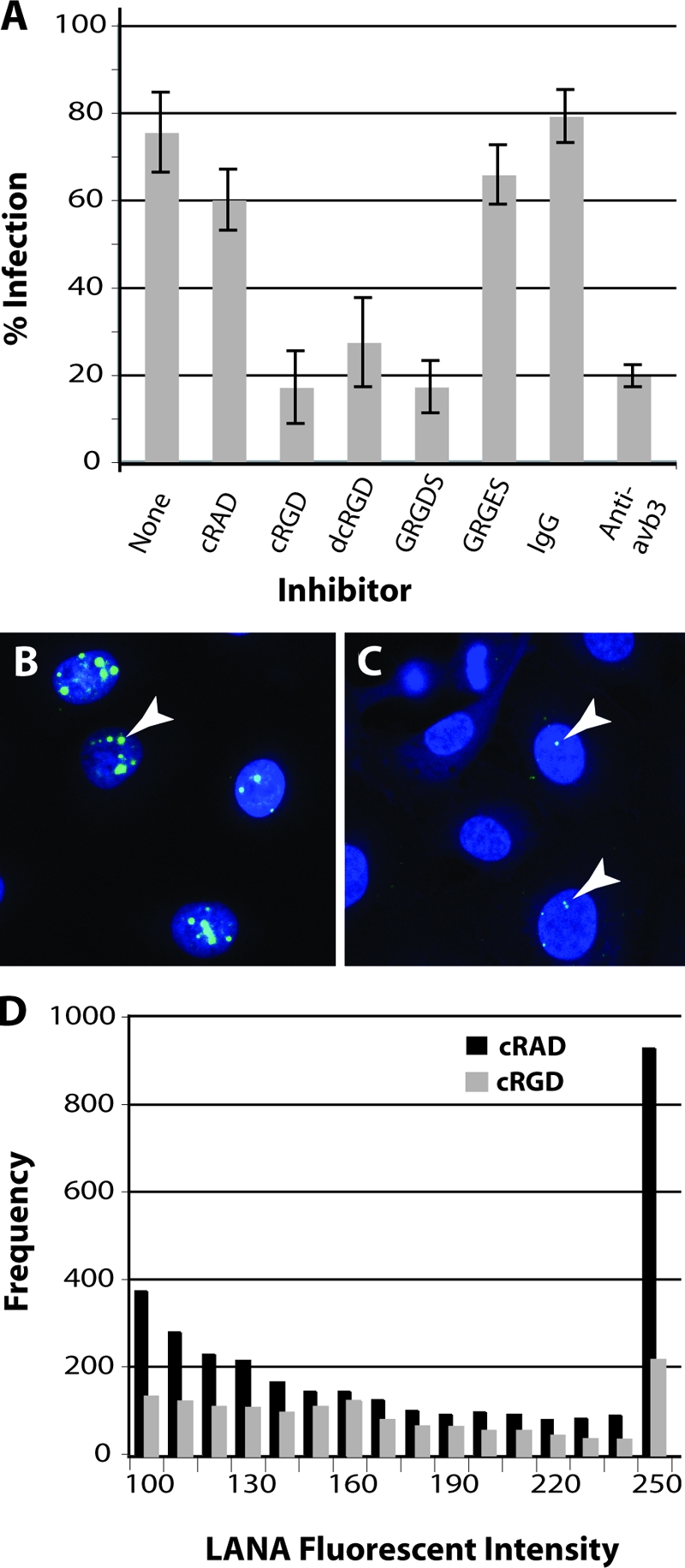
KSHV infection is inhibited by αVβ3-specific ligands and function-blocking antibodies. Infection-blocking studies were performed by pretreating HT1080 cells (4°C) with RGD-containing peptide ligands, including the linear GRGDS peptide and the αVβ3-specific cyclic (cRGD) and dicyclic (dcRGD) peptides or the αVβ3 function-blocking antibody (LM609). Controls included the linear GRGES and cyclic cRAD peptides and normal mouse IgG. Gradient-purified KSHV virions titrated to yield an infection in 75% of the cells were incubated with the cells for 1 h (4°C) in the presence of peptide or antibody. Unbound KSHV was removed by washing, and the percentage of cells expressing KSHV LANA was determined 24 h later in multiple fields by confocal microscopy (A). The standard deviation is shown. Representative micrographs showing nuclear LANA expression in control cRAD-treated (B) and cRGD-treated (C) HT1080 cells infected with KSHV are shown. Arrows indicate representative dots of LANA fluorescence. LANA fluorescence was quantitated, and the frequency and intensity (range, 100 to 255) of fluorescent pixels within LANA dots in individual nuclei (n = 30) were plotted (D).
Multiple large dots of LANA fluorescence were detected within the nuclei of cells infected in the absence of inhibitor (data not shown) and cells infected in the presence of the control cRAD peptide (Fig. 9B). A noticeable reduction in the number of LANA dots and the level of LANA expression in each dot was observed in the few cells infected in the presence of the αVβ3 cRGD antagonist (Fig. 9C). To quantitate changes in LANA expression, the fluorescence intensities of the LANA dots in 30 LANA-positive nuclei from the both cRAD- and cRGD-treated cells were compared. The frequency of fluorescent pixels and the intensity of each pixel (spanning a range of intensities from weakly fluorescent (100) to the brightest pixels (255) were determined for each dot. As shown in Fig. 9D, LANA expression was inhibited by treatment with the cRGD peptide across the intensity range. More importantly, there was an approximate fivefold reduction in the frequency of the brightest LANA pixels (intensity of 255).
To further substantiate the role of the αVβ3 integrin in KSHV entry, the function-blocking anti-αVβ3 MAb LM609 was tested for its ability to inhibit KSHV infection. As shown in Fig. 9A, the LM609 antibody was a potent inhibitor of KSHV infection and inhibited infection levels by 75%, equivalent to the results obtained with the cRGD and GRGDS peptides. No inhibition was detected with the normal IgG control antibodies. These studies and those with the αVβ3 peptide antagonists demonstrate that specific blocking of the RGD-mediated interaction with the αVβ3 receptor can inhibit KSHV infection and establishment of latency. The similar levels of inhibition seen with the cyclic and linear RGD peptides strongly implicate αVβ3 as a major KSHV entry receptor.
KSHV infection of mouse keratinocytes is independent of α3β1 integrin.
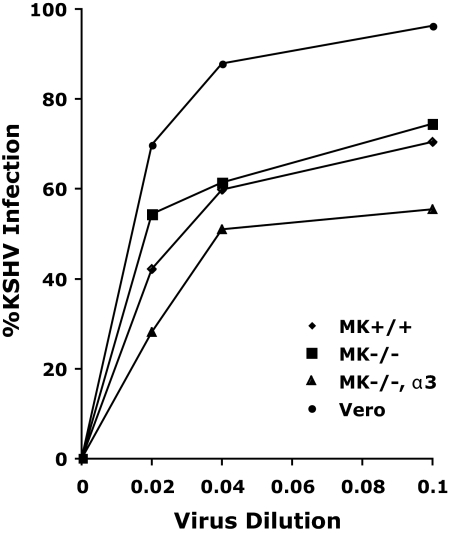
Although our studies failed to confirm the previously reported interaction between KSHV and the α3β1 integrin, the possibility remained that α3β1 could facilitate KSHV entry by synergizing with αVβ3 or other receptors, such as the xCT heavy chain which associates with integrins (35). We therefore compared the ability of KSHV to infect mouse keratinocytes derived from (i) wild-type mice (MK+/+), (ii) homozygous α3-null mice which lack functional cell surface α3β1 integrin receptors (MK−/−), or (iii) α3-null MK cells engineered to produce recombinant α3β1 (MK−/−,α3) (16). The α3-null and wild-type mouse keratinocytes express equivalent amounts of other integrins, including αV (16). The mouse keratinocytes were infected with different amounts of gradient-purified KSHV, and infection was quantitated 24 h later by immunofluorescence detection of nuclear LANA expression. The Vero cell line, which is highly infectible by KSHV, was used as a positive infection control. The virus infection curves for both the wild-type MK+/+ and the α3-null MK−/− cells were very similar, although at all points the infection of the α3-null MK−/− cells was higher than that of the wild-type cells, attaining 74% at the highest virus dose tested (Fig. 10). By comparison, this virus dose yielded >95% infection of the Vero cells. In contrast, MK−/−,α3 cells which overexpress the α3β1 integrin showed decreased infection levels, attaining only 55% infection of the cell culture at the highest virus dose tested. These results, which were corroborated in three independent experiments, indicate that KSHV infection of mouse keratinocytes is independent of α3β1. In fact, the reduced level of infection of the α3-null mouse keratinocytes transfected with recombinant α3A integrin cDNA suggested that the overexpression of α3β1 negatively affected KSHV entry and establishment of a latent infection.
FIG. 10.
KSHV infection of mouse keratinocytes is independent of α3β1 integrin. Wild-type mouse keratinocytes (MK+/+), keratinocytes derived from α3-null mice which lack α3β1 integrins (MK−/−), or α3-null cells transfected with human α3A integrin (MK−/−,α3) to reconstitute α3β1 expression were infected with different amounts of gradient-purified KSHV. Twenty-four hours postinfection, the percentage of infected cells was determined by quantitating cells expressing KSHV LANA using confocal microscopy. The KSHV-susceptible Vero cell line was used as a positive control. Cells analyzed: α3-null MK−/− (n = 353); MK+/+ (n = 532); MK−/−,α3 (n = 451); Vero (n = 293).
DISCUSSION
This study demonstrates that the αVβ3 integrin is a cellular receptor mediating both the cell adhesion and entry of KSHV into target cells through binding to the RGD motif of the virion-associated gB. This conclusion is supported by multiple lines of evidence. First, analysis of the RGD motif of the KSHV gB revealed strong sequence similarities to analogous motifs in known ligands of the αVβ3 integrin receptor. Second, the gB(RGD) peptide, derived from the KSHV gB sequence, promoted RGD-dependent cell adhesion that was specifically blocked by an αVβ3 function-blocking MAb. Function-blocking antibodies to α5β1or α3β1 integrins were unable to block adhesion. Antibodies to αVβ5 partially blocked adhesion, supporting the role of the αV integrin subunit in gB(RGD)-integrin interactions. Third, the overexpression of αVβ3 integrin receptors strongly increased the adherence of β3-CHO cells to gB(RGD) over that seen with the parental pBJ-CHO cells or α3-CHO cells overexpressing the α3β1 integrin. Fourth, immunoblot analysis revealed that the gB(RGD) peptide specifically bound to αVβ3 receptors in octyl-beta-glucoside lysates of β3-CHO cells in a divalent cation-dependent manner. Fifth, confocal microscopy demonstrated a specific binding between affinity-purified αVβ3 receptors and both gB(RGD) peptide and gradient-purified KSHV virions. Finally, KSHV infection of HT1080 fibrosarcoma cells was specifically blocked by αVβ3-specific cyclic RGD peptides and a specific αVβ3 function-blocking antibody.
The αVβ3 integrin is a cell surface receptor that is widely expressed in proliferating endothelial cells, arterial smooth muscle cells, and certain populations of leukocytes and tumor cells (23). Integrin αVβ3 mediates the adhesion of cells to a number of extracellular matrix proteins containing RGD motifs, including vitronectin and fibronectin, and is responsible for mediating cell-cell interactions through binding to cell-associated glycoproteins containing RGD motifs. The αVβ3 integrin has been implicated in cellular processes ranging from wound healing to tumor angiogenesis and tumor progression. In addition, αVβ3 has been coopted by a number of pathogens, including foot-and-mouth disease virus, coxsackievirus, hantavirus, and adenovirus (45), as a receptor for the entry of target cells during the infection process. Our finding that αVβ3 functions as an entry receptor for KSHV further expands the number of viruses that utilize this receptor for entry. In KS tumors, αVβ3 is highly expressed on KS tumor spindle cells and in neovascular endothelial cells and B cells that infiltrate KS tumors (8, 26, 51, 62). These cells are also consistently infected with KSHV in KS lesions, suggesting a central role for αVβ3 in KSHV infection and in the pathogenesis of KS.
The ability of the integrin αVβ3 to bind RGD-containing proteins has been exhaustively reported in the literature. Our finding that αVβ3 binds to the RGD motif of KSHV gB confirms this specificity. Ligand-binding studies have shown that αVβ3 and the closely related αVβ5 interact specifically with peptides containing the consensus sequence RGDXF, where X is S or T (33). Panning studies of phage libraries displaying cyclic peptides demonstrated that both the αVβ3 and αVβ5 integrins selectively bound the peptide sequence SRGDTF, which constitutes the core of the KSHV RGD motif (33). In contrast, the α5β1 and αIIbβ3 integrins, which also bind RGD motifs, did not select peptides with sequences similar to that of the KSHV RGD motif. These panning studies correlate with the results of our antibody-blocking studies which showed that antibodies to αVβ3 and αVβ5 could significantly block binding to the KSHV gB(RGD) peptide, whereas antibodies to α5β1 could not. These data strongly support the hypothesis that the RGD motif of KSHV gB specifically interacts with integrins containing the αV integrin subunit, especially αVβ3.
A previous study has shown that de novo infection of naïve cells with KSHV results in a Poisson distribution of viral episomes within the infected cells (1). Furthermore, the number and summed immunofluorescence of LANA dots in the infected cell nuclei were directly proportional to the amount of intracellular viral DNA. Our results showed that blocking KSHV infection with αVβ3 antagonists not only decreased the number of infected cells but also decreased the amount of LANA fluorescence in those cells that were infected. This suggests that blocking viral entry can reduce the number of virions that enter an infected cell and decrease the number of LANA dots and associated immunofluorescence. Studies are ongoing to confirm this.
A previous study reported that α3β1 interacts with KSHV gB and functions as a cellular entry receptor for KSHV (2). It was suggested that this interaction may be RGD mediated. However, recent studies have shown that α3β1 binding to its high-affinity ligands is RGD independent (11, 14, 20, 29, 30, 37, 41, 66, 69). Other studies using purified recombinant α3β1 detected no binding to RGD-containing proteins, such as fibronectin (17), while transfection studies demonstrated that the α3β1 integrin is not sufficient for cell attachment to matrices containing RGD motifs (14, 66). Furthermore, the characterized α3β1 recognition motifs NLR (37) and PPFLMLLKGSTR (31) are not present in the KSHV gB sequence. Our studies revealed no interaction between α3β1 and the RGD motif of KSHV gB, and α3β1 function-blocking antibodies failed to inhibit cell adhesion to the gB(RGD) peptide. Although α3-CHO cells overexpressing α3β1 showed enhanced adhesion to a laminin substrate, no adhesion to the gB(RGD) peptide greater that detected with the parental pBJ-CHO cells was observed. Affinity purification studies revealed no binding between the gB(RGD) peptide and solubilized α3β1 receptors from octyl-beta-glucoside lysates of α3-CHO cells. In addition, the gB(RGD) peptide and the native envelope-associated gB present on gradient-purified KSHV virions were unable to bind to commercial preparations of affinity-purified α3β1 receptors. Infection studies revealed that α3-null mouse keratinocytes lacking α3β1 integrin were competent for infection by KSHV, and the expression of α3β1 in either the parental mouse keratinocytes or α3-null mouse keratinocytes transfected with human α3 decreased infection levels.
While our study failed to detect a direct interaction between α3β1 and the RGD motif of KSHV gB, it did not directly address a potential secondary role for α3β1 in KSHV binding, entry, or downstream signaling. Studies have shown colocalization of αVβ3 and α3β1 integrin at cell surface focal adhesions due to shared interactions with other molecules, such as actin, focal adhesion kinase, vinculin, α-actinin, and paxillin which form the adhesion plaque (10, 13, 68). It has been suggested that the α3β1 integrin may mediate secondary responses after other integrins, such as αVβ3, bind their specific ligands (for a review, see reference 6). These secondary responses could include “integrin cross talk” and the modulation of downstream signaling or the recruitment of cytoskeleton and other integrins into membrane microdomains involved in KSHV entry. The data from our anti-α3 blocking studies and α3-CHO adhesion studies corroborate the results of others who have shown that α3β1 can modulate αVβ3 function (7, 22). Our infection studies revealed that α3-null mouse keratinocytes, which are completely lacking in α3β1, are competent for KSHV infection, bringing into question the importance of a secondary response, at least in mouse keratinocytes. However, it is possible that α3β1 plays a role in KSHV entry in other cell types, since our studies were based on different experimental approaches and target cells than used previously (2).
Several lines of evidence from our study confirm the transdominant inhibitory effect of α3β1 and other integrins containing the β1 subunit on the receptor function of αVβ3 that has been seen by others (7, 22, 32, 38). First, the antibody blocking of α3β1 function in our cell adhesion assays led to an increased level of αVβ3-mediated adhesion of HT1080 cells to the gB(RGD) peptide. Second, the overexpression of α3 integrin in the α3-CHO cells resulted in a 60% decrease in the surface expression of αVβ3 and a 62% decrease in αVβ3-dependent adhesion of the α3-CHO cells to the gB(RGD) peptide compared to those of the pBJ parental CHO cells. Third, the overexpression of α3 integrin in the rescued α3-null mouse keratinocytes resulted in decreased levels of KSHV infection compared to the levels in both α3-null mouse keratinocytes and wild-type mouse keratinocytes. The inhibitory effect of α3β1 on αVβ3 function may explain the ability of soluble α3β1 to inhibit KSHV infection, as reported previously (2, 39, 55).
Several previous studies have implicated integrin binding and the downstream activation of integrin signaling pathways in the KSHV infection process (36, 40, 54, 64). Our data showing that αVβ3 functions as a major integrin receptor mediating RGD-dependent interactions with the KSHV gB during viral entry are compatible with the results of these studies.
Acknowledgments
We acknowledge William Carter for his gift of anti-integrin antibodies and his advice on integrins; Yoshikazu Takada for his gift of the integrin-expressing CHO cell lines; Michael Axthelm and Scott Wong for their gift of RRV; and Lynn Rose, Kellie Burnside, Jonathan Ryan and Jeannette Staheli for invaluable editing of the manuscript.
This work was supported in part by grants to T. M. Rose from the M. J. Murdock Charitable Trust exceptional opportunity grants, the National Cancer Institute (CA91760), and the National Institute of Allergy and Infectious Disease (K02-AI49275).
Footnotes
Published ahead of print on 28 November 2007.
REFERENCES
- 1.Adang, L. A., C. H. Parsons, and D. H. Kedes. 2006. Asynchronous progression through the lytic cascade and variations in intracellular viral loads revealed by high-throughput single-cell analysis of Kaposi's sarcoma-associated herpesvirus infection. J. Virol. 8010073-10082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2002. Integrin alpha3beta1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108407-419. [DOI] [PubMed] [Google Scholar]
- 3.Akula, S. M., F. Z. Wang, J. Vieira, and B. Chandran. 2001. Human herpesvirus 8 interaction with target cells involves heparan sulfate. Virology 282245-255. [DOI] [PubMed] [Google Scholar]
- 4.Antman, K., and Y. Chang. 2000. Kaposi's sarcoma. N. Engl. J. Med. 3421027-1038. [DOI] [PubMed] [Google Scholar]
- 5.Bechtel, J. T., Y. Liang, J. Hvidding, and D. Ganem. 2003. Host range of Kaposi's sarcoma-associated herpesvirus in cultured cells. J. Virol. 776474-6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belkin, A. M., and M. A. Stepp. 2000. Integrins as receptors for laminins. Microsc. Res. Tech. 51280-301. [DOI] [PubMed] [Google Scholar]
- 7.Borza, C. M., A. Pozzi, D. B. Borza, V. Pedchenko, T. Hellmark, B. G. Hudson, and R. Zent. 2006. Integrin alpha3beta1, a novel receptor for alpha3(IV) noncollagenous domain and a trans-dominant inhibitor for integrin alphavbeta3. J. Biol. Chem. 28120932-20939. [DOI] [PubMed] [Google Scholar]
- 8.Brooks, P. C., R. A. Clark, and D. A. Cheresh. 1994. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science 264569-571. [DOI] [PubMed] [Google Scholar]
- 9.Burnside, K. L., J. T. Ryan, H. Bielefeldt-Ohmann, A. Gregory Bruce, M. E. Thouless, C. C. Tsai, and T. M. Rose. 2006. RFHVMn ORF73 is structurally related to the KSHV ORF73 latency-associated nuclear antigen (LANA) and is expressed in retroperitoneal fibromatosis (RF) tumor cells. Virology 354103-115. [DOI] [PubMed] [Google Scholar]
- 10.Burridge, K., K. Fath, T. Kelly, G. Nuckolls, and C. Turner. 1988. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu. Rev. Cell Biol. 4487-525. [DOI] [PubMed] [Google Scholar]
- 11.Carter, W. G., M. C. Ryan, and P. J. Gahr. 1991. Epiligrin, a new cell adhesion ligand for integrin alpha 3 beta 1 in epithelial basement membranes. Cell 65599-610. [DOI] [PubMed] [Google Scholar]
- 12.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 2661865-1869. [DOI] [PubMed] [Google Scholar]
- 13.Critchley, D. R., M. R. Holt, S. T. Barry, H. Priddle, L. Hemmings, and J. Norman. 1999. Integrin-mediated cell adhesion: the cytoskeletal connection. Biochem. Soc. Symp. 6579-99. [PubMed] [Google Scholar]
- 14.Delwel, G. O., A. A. de Melker, F. Hogervorst, L. H. Jaspars, D. L. Fles, I. Kuikman, A. Lindblom, M. Paulsson, R. Timpl, and A. Sonnenberg. 1994. Distinct and overlapping ligand specificities of the alpha 3A beta 1 and alpha 6A beta 1 integrins: recognition of laminin isoforms. Mol. Biol. Cell 5203-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desrosiers, R. C., V. G. Sasseville, S. C. Czajak, X. Zhang, K. G. Mansfield, A. Kaur, R. P. Johnson, A. A. Lackner, and J. U. Jung. 1997. A herpesvirus of rhesus monkeys related to the human Kaposi's sarcoma-associated herpesvirus. J. Virol. 719764-9769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DiPersio, C. M., M. Shao, L. Di Costanzo, J. A. Kreidberg, and R. O. Hynes. 2000. Mouse keratinocytes immortalized with large T antigen acquire alpha3beta1 integrin-dependent secretion of MMP-9/gelatinase B. J. Cell Sci. 1132909-2921. [DOI] [PubMed] [Google Scholar]
- 17.Eble, J. A., K. W. Wucherpfennig, L. Gauthier, P. Dersch, E. Krukonis, R. R. Isberg, and M. E. Hemler. 1998. Recombinant soluble human alpha 3 beta 1 integrin: purification, processing, regulation, and specific binding to laminin-5 and invasin in a mutually exclusive manner. Biochemistry 3710945-10955. [DOI] [PubMed] [Google Scholar]
- 18.Ensoli, B., C. Sgadari, G. Barillari, M. C. Sirianni, M. Sturzl, and P. Monini. 2001. Biology of Kaposi's sarcoma. Eur. J. Cancer. 371251-1269. [DOI] [PubMed] [Google Scholar]
- 19.Ford, T., J. Graham, and D. Rickwood. 1994. Iodixanol: a nonionic iso-osmotic centrifugation medium for the formation of self-generated gradients. Anal. Biochem. 220360-366. [DOI] [PubMed] [Google Scholar]
- 20.Gresham, H. D., I. L. Graham, G. L. Griffin, J. C. Hsieh, L. J. Dong, A. E. Chung, and R. M. Senior. 1996. Domain-specific interactions between entactin and neutrophil integrins. G2 domain ligation of integrin alpha3beta1 and E domain ligation of the leukocyte response integrin signal for different responses. J. Biol. Chem. 27130587-30594. [DOI] [PubMed] [Google Scholar]
- 21.Haubner, R., H. J. Wester, U. Reuning, R. Senekowitsch-Schmidtke, B. Diefenbach, H. Kessler, G. Stocklin, and M. Schwaiger. 1999. Radiolabeled alpha(v) beta3 integrin antagonists: a new class of tracers for tumor targeting. J. Nucl. Med. 401061-1071. [PubMed] [Google Scholar]
- 22.Hodivala-Dilke, K. M., C. M. DiPersio, J. A. Kreidberg, and R. O. Hynes. 1998. Novel roles for alpha3beta1 integrin as a regulator of cytoskeletal assembly and as a trans-dominant inhibitor of integrin receptor function in mouse keratinocytes. J. Cell Biol. 1421357-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horton, M. A. 1997. The alpha v beta 3 integrin “vitronectin receptor.” Int. J. Biochem. Cell Biol. 29721-725. [DOI] [PubMed] [Google Scholar]
- 24.Hynes, R. O. 2002. Integrins: bidirectional, allosteric signaling machines. Cell 110673-687. [DOI] [PubMed] [Google Scholar]
- 25.Janssen, M. L., W. J. Oyen, I. Dijkgraaf, L. F. Massuger, C. Frielink, D. S. Edwards, M. Rajopadhye, H. Boonstra, F. H. Corstens, and O. C. Boerman. 2002. Tumor targeting with radiolabeled alpha(v) beta(3) integrin binding peptides in a nude mouse model. Cancer Res. 626146-6151. [PubMed] [Google Scholar]
- 26.Kaaya, E. E., E. Castanos-Velez, H. Amir, L. Lema, J. Luande, J. Kitinya, M. Patarroyo, and P. Biberfeld. 1996. Expression of adhesion molecules in endemic and epidemic Kaposi's sarcoma. Histopathology 29337-346. [DOI] [PubMed] [Google Scholar]
- 27.Kaleeba, J. A., and E. A. Berger. 2006. Kaposi's sarcoma-associated herpesvirus fusion-entry receptor: cystine transporter xCT. Science 3111921-1924. [DOI] [PubMed] [Google Scholar]
- 28.Kantlehner, M., P. Schaffner, D. Finsinger, J. Meyer, A. Jonczyk, B. Diefenbach, B. Nies, G. Holzemann, S. L. Goodman, and H. Kessler. 2000. Surface coating with cyclic RGD peptides stimulates osteoblast adhesion and proliferation as well as bone formation. Chembiochem 1107-114. [DOI] [PubMed] [Google Scholar]
- 29.Kikkawa, Y., N. Sanzen, and K. Sekiguchi. 1998. Isolation and characterization of laminin-10/11 secreted by human lung carcinoma cells. Laminin-10/11 mediates cell adhesion through integrin alpha3 beta1. J. Biol. Chem. 27315854-15859. [DOI] [PubMed] [Google Scholar]
- 30.Kikkawa, Y., M. Umeda, and K. Miyazaki. 1994. Marked stimulation of cell adhesion and motility by ladsin, a laminin-like scatter factor. J. Biochem. (Tokyo) 116862-869. [DOI] [PubMed] [Google Scholar]
- 31.Kim, J. M., W. H. Park, and B. M. Min. 2005. The PPFLMLLKGSTR motif in globular domain 3 of the human laminin-5 alpha3 chain is crucial for integrin alpha3beta1 binding and cell adhesion. Exp. Cell Res. 304317-327. [DOI] [PubMed] [Google Scholar]
- 32.Kim, J. P., K. Zhang, R. H. Kramer, T. J. Schall, and D. T. Woodley. 1992. Integrin receptors and RGD sequences in human keratinocyte migration: unique anti-migratory function of alpha 3 beta 1 epiligrin receptor. J. Investig. Dermatol. 98764-770. [DOI] [PubMed] [Google Scholar]
- 33.Koivunen, E., B. Wang, and E. Ruoslahti. 1995. Phage libraries displaying cyclic peptides with different ring sizes: ligand specificities of the RGD-directed integrins. Biotechnology (N. Y.) 13265-270. [DOI] [PubMed] [Google Scholar]
- 34.Kok, R. J., A. J. Schraa, E. J. Bos, H. E. Moorlag, S. A. Asgeirsdottir, M. Everts, D. K. Meijer, and G. Molema. 2002. Preparation and functional evaluation of RGD-modified proteins as alpha(v)beta(3) integrin directed therapeutics. Bioconjug. Chem. 13128-135. [DOI] [PubMed] [Google Scholar]
- 35.Kolesnikova, T. V., B. A. Mannion, F. Berditchevski, and M. E. Hemler. 2001. Beta1 integrins show specific association with CD98 protein in low density membranes. BMC Biochem. 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krishnan, H. H., N. Sharma-Walia, D. N. Streblow, P. P. Naranatt, and B. Chandran. 2006. Focal adhesion kinase is critical for entry of Kaposi's sarcoma-associated herpesvirus into target cells. J. Virol. 801167-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krutzsch, H. C., B. J. Choe, J. M. Sipes, N. Guo, and D. D. Roberts. 1999. Identification of an alpha(3)beta(1) integrin recognition sequence in thrombospondin-1. J. Biol. Chem. 27424080-24086. [DOI] [PubMed] [Google Scholar]
- 38.Ly, D. P., K. M. Zazzali, and S. A. Corbett. 2003. De novo expression of the integrin alpha5beta1 regulates alphavbeta3-mediated adhesion and migration on fibrinogen. J. Biol. Chem. 27821878-21885. [DOI] [PubMed] [Google Scholar]
- 39.Naranatt, P. P., S. M. Akula, C. A. Zien, H. H. Krishnan, and B. Chandran. 2003. Kaposi's sarcoma-associated herpesvirus induces the phosphatidylinositol 3-kinase-PKC-ζ-MEK-ERK signaling pathway in target cells early during infection: implications for infectivity. J. Virol. 771524-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naranatt, P. P., H. H. Krishnan, M. S. Smith, and B. Chandran. 2005. Kaposi's sarcoma-associated herpesvirus modulates microtubule dynamics via RhoA-GTP-diaphanous 2 signaling and utilizes the dynein motors to deliver its DNA to the nucleus. J. Virol. 791191-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishiuchi, R., O. Murayama, H. Fujiwara, J. Gu, T. Kawakami, S. Aimoto, Y. Wada, and K. Sekiguchi. 2003. Characterization of the ligand-binding specificities of integrin alpha3beta1 and alpha6beta1 using a panel of purified laminin isoforms containing distinct alpha chains. J. Biochem. (Tokyo) 134497-504. [DOI] [PubMed] [Google Scholar]
- 42.Pereira, L. 1994. Function of glycoprotein B homologues of the family herpesviridae. Infect. Agents Dis. 39-28. [PubMed] [Google Scholar]
- 43.Pfaff, M., K. Tangemann, B. Muller, M. Gurrath, G. Muller, H. Kessler, R. Timpl, and J. Engel. 1994. Selective recognition of cyclic RGD peptides of NMR defined conformation by alpha IIb beta 3, alpha V beta 3, and alpha 5 beta 1 integrins. J. Biol. Chem. 26920233-20238. [PubMed] [Google Scholar]
- 44.Pierschbacher, M. D., and E. Ruoslahti. 1987. Influence of stereochemistry of the sequence Arg-Gly-Asp-Xaa on binding specificity in cell adhesion. J. Biol. Chem. 26217294-17298. [PubMed] [Google Scholar]
- 45.Plow, E. F., T. A. Haas, L. Zhang, J. Loftus, and J. W. Smith. 2000. Ligand binding to integrins. J. Biol. Chem. 27521785-21788. [DOI] [PubMed] [Google Scholar]
- 46.Rappocciolo, G., F. J. Jenkins, H. R. Hensler, P. Piazza, M. Jais, L. Borowski, S. C. Watkins, and C. R. Rinaldo, Jr. 2006. DC-SIGN is a receptor for human herpesvirus 8 on dendritic cells and macrophages. J. Immunol. 1761741-1749. [DOI] [PubMed] [Google Scholar]
- 47.Renne, R., D. Blackbourn, D. Whitby, J. Levy, and D. Ganem. 1998. Limited transmission of Kaposi's sarcoma-associated herpesvirus in cultured cells. J. Virol. 725182-5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Renne, R., W. Zhong, B. Herndier, M. McGrath, N. Abbey, D. Kedes, and D. Ganem. 1996. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat. Med. 2342-346. [DOI] [PubMed] [Google Scholar]
- 49.Rose, T. M., K. B. Strand, and M. L. Bosch. January 1999. Glycoprotein B of the RFHV/KSHV subfamily of herpesviruses. U.S. patent 6,015,565.
- 50.Russo, J. J., R. A. Bohenzky, M. C. Chien, J. Chen, M. Yan, D. Maddalena, J. P. Parry, D. Peruzzi, I. S. Edelman, Y. Chang, and P. S. Moore. 1996. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. USA 9314862-14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salcedo, R., and M. Patarroyo. 1995. Constitutive alpha V beta 3 integrin-mediated adhesion of human lymphoid B cells to vitronectin substrate. Cell. Immunol. 160165-172. [DOI] [PubMed] [Google Scholar]
- 52.Schulz, T. F. 1998. Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8). J. Gen. Virol. 791573-1591. [DOI] [PubMed] [Google Scholar]
- 53.Searles, R. P., E. P. Bergquam, M. K. Axthelm, and S. W. Wong. 1999. Sequence and genomic analysis of a Rhesus macaque rhadinovirus with similarity to Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8. J. Virol. 733040-3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharma-Walia, N., H. H. Krishnan, P. P. Naranatt, L. Zeng, M. S. Smith, and B. Chandran. 2005. ERK1/2 and MEK1/2 induced by Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) early during infection of target cells are essential for expression of viral genes and for establishment of infection. J. Virol. 7910308-10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharma-Walia, N., P. P. Naranatt, H. H. Krishnan, L. Zeng, and B. Chandran. 2004. Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 envelope glycoprotein gB induces the integrin-dependent focal adhesion kinase-Src-phosphatidylinositol 3-kinase-Rho GTPase signal pathways and cytoskeletal rearrangements. J. Virol. 784207-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith, J. W., and D. A. Cheresh. 1988. The Arg-Gly-Asp binding domain of the vitronectin receptor. Photoaffinity cross-linking implicates amino acid residues 61-203 of the beta subunit. J. Biol. Chem. 26318726-18731. [PubMed] [Google Scholar]
- 57.Smith, J. W., and D. A. Cheresh. 1990. Integrin (alpha v beta 3)-ligand interaction. Identification of a heterodimeric RGD binding site on the vitronectin receptor. J. Biol. Chem. 2652168-2172. [PubMed] [Google Scholar]
- 58.Smith, J. W., R. S. Piotrowicz, and D. Mathis. 1994. A mechanism for divalent cation regulation of beta 3-integrins. J. Biol. Chem. 269960-967. [PubMed] [Google Scholar]
- 59.Spear, P. G., and R. Longnecker. 2003. Herpesvirus entry: an update. J. Virol. 7710179-10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Symington, B. E., Y. Takada, and W. G. Carter. 1993. Interaction of integrins alpha 3 beta 1 and alpha 2 beta 1: potential role in keratinocyte intercellular adhesion. J. Cell Biol. 120523-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takagi, J., T. Kamata, J. Meredith, W. Puzon-McLaughlin, and Y. Takada. 1997. Changing ligand specificities of alphavbeta1 and alphavbeta3 integrins by swapping a short diverse sequence of the beta subunit. J. Biol. Chem. 27219794-19800. [DOI] [PubMed] [Google Scholar]
- 62.Uccini, S., L. P. Ruco, F. Monardo, A. Stoppacciaro, E. Dejana, I. L. La Parola, D. Cerimele, and C. D. Baroni. 1994. Co-expression of endothelial cell and macrophage antigens in Kaposi's sarcoma cells. J. Pathol. 17323-31. [DOI] [PubMed] [Google Scholar]
- 63.Wang, F. Z., S. M. Akula, N. P. Pramod, L. Zeng, and B. Chandran. 2001. Human herpesvirus 8 envelope glycoprotein K8.1A interaction with the target cells involves heparan sulfate. J. Virol. 757517-7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang, F. Z., S. M. Akula, N. Sharma-Walia, L. Zeng, and B. Chandran. 2003. Human herpesvirus 8 envelope glycoprotein B mediates cell adhesion via its RGD sequence. J. Virol. 773131-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wayner, E. A., and W. G. Carter. 1987. Identification of multiple cell adhesion receptors for collagen and fibronectin in human fibrosarcoma cells possessing unique alpha and common beta subunits. J. Cell Biol. 1051873-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weitzman, J. B., R. Pasqualini, Y. Takada, and M. E. Hemler. 1993. The function and distinctive regulation of the integrin VLA-3 in cell adhesion, spreading, and homotypic cell aggregation. J. Biol. Chem. 2688651-8657. [PubMed] [Google Scholar]
- 67.Wickham, T. J., P. Mathias, D. A. Cheresh, and G. R. Nemerow. 1993. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell 73309-319. [DOI] [PubMed] [Google Scholar]
- 68.Wozniak, M. A., K. Modzelewska, L. Kwong, and P. J. Keely. 2004. Focal adhesion regulation of cell behavior. Biochim. Biophys. Acta 1692103-119. [DOI] [PubMed] [Google Scholar]
- 69.Wu, C., A. E. Chung, and J. A. McDonald. 1995. A novel role for alpha 3 beta 1 integrins in extracellular matrix assembly. J. Cell Sci. 1082511-2523. [DOI] [PubMed] [Google Scholar]
- 70.Xue, W., I. Mizukami, R. F. Todd III, and H. R. Petty. 1997. Urokinase-type plasminogen activator receptors associate with beta1 and beta3 integrins of fibrosarcoma cells: dependence on extracellular matrix components. Cancer Res. 571682-1689. [PubMed] [Google Scholar]