Abstract
Increased lymphocyte turnover is a hallmark of pathogenic lentiviral infection. To investigate perturbations in lymphocyte dynamics in natural hosts with nonpathogenic simian immunodeficiency virus (SIV) infection, the nucleoside analog bromodeoxyuridine (BrdU) was administered to six naturally SIV-infected and five SIV-negative sooty mangabeys. As a measure of lymphocyte turnover, we estimated the mean death rate by fitting a mathematical model to the fraction of BrdU-labeled cells during a 2-week labeling and a median 10-week delabeling period. Despite significantly lower total T- and B-lymphocyte counts in SIV-infected sooty mangabeys than in SIV-negative mangabeys, the turnover rate of B lymphocytes and CD4+ and CD8+ T lymphocytes was not increased in the SIV-infected animals. A small, rapidly proliferating CD45RA+ memory subset and a large, slower-proliferating CD45RA− central memory subset of CD4+ T lymphocytes identified in the peripheral blood of sooty mangabeys also did not show evidence of increased turnover in the context of SIV infection. Independently of SIV infection, the turnover of CD4+ T lymphocytes in sooty mangabeys was significantly higher (P < 0.01) than that of CD8+ T lymphocytes, a finding hitherto not reported in rhesus macaques or humans. The absence of aberrant T-lymphocyte turnover along with an inherently high rate of CD4+ T-lymphocyte turnover may help to preserve the pool of central memory CD4+ T lymphocytes in viremic SIV-infected sooty mangabeys and protect against progression to AIDS.
Perturbations in lymphocyte dynamics manifesting as increased lymphocyte turnover are a hallmark of chronic human immunodeficiency virus (HIV) infection in humans and simian immunodeficiency virus (SIV) infection in rhesus macaques and believed to be central to the pathogenesis of CD4+ T-lymphocyte depletion in AIDS (6, 11, 12, 14, 19, 20, 27, 28). Although the underlying mechanisms have been extensively debated, there is widespread consensus that increased T-cell activation is a critical factor driving the increased T-lymphocyte turnover in AIDS (10). A homeostatic response to CD4+ T-lymphocyte depletion is unlikely to be the sole or major driving force since it does not account for the increased turnover of other lymphocyte subsets such as CD8+ T lymphocytes, B lymphocytes, and NK cells (6). Furthermore, normalization of increased lymphocyte turnover in HIV-infected individuals on antiretroviral treatment is associated with suppression of viremia and immune activation and occurs prior to recovery from CD4+ T lymphocytopenia (10, 15, 20).
Sooty mangabeys are one of several nonhuman primate species of African origin that are natural hosts of SIV. Despite the presence of persistent viremia ranging between 103 and 107 SIV RNA copies/ml plasma, naturally SIV-infected sooty mangabeys typically do not develop AIDS (25, 29). Over the more than 20 years since SIV infection was first recognized in captive sooty mangabeys, there has been only one reported case of AIDS in this natural host (16). The mechanism of nonpathogenicity in this species is a subject of intense research. Unlike the case with pathogenic SIV infection, SIV-infected sooty mangabeys show little evidence of increased immune activation (29). Studies using the proliferation antigen marker Ki-67 have shown no difference in the fraction of Ki-67-positive CD8+ T lymphocytes (3, 29) between SIV-infected and SIV-negative sooty mangabeys, raising the possibility that lymphocyte turnover is not increased in nonpathogenic SIV infection in its natural host. However, the data on CD4+ T-lymphocyte proliferation in SIV-infected sooty mangabeys remain controversial. One study reported an increase in the fraction of Ki-67+ CD4+ T lymphocytes in the peripheral blood of SIV-infected sooty mangabeys (29), while another study found no difference in this fraction between SIV-negative and SIV-infected sooty mangabeys (3).
In this study, we have investigated lymphocyte dynamics in SIV-negative and SIV-infected sooty mangabeys by prolonged in vivo administration of the DNA nucleoside analog bromodeoxyuridine (BrdU). In contrast to Ki-67 antigen, which provides a snapshot view of cells proliferating at a given point in time, BrdU administration has the advantage of enabling dynamic follow-up of a population of dividing cells that incorporate BrdU during the labeling period. Additionally, Ki-67 positivity need not always denote cycling cells. Ki-67+ CD4+ T lymphocytes in HIV-infected individuals can show evidence of cell cycle arrest in the G1 phase (4). BrdU gets incorporated into the DNA of dividing cells via the nucleoside salvage pathway and can be detected by flow cytometry (27). Loss of BrdU label in a phenotypically defined population can signify cell death, decay of BrdU label due to repeated cell divisions, cell differentiation, or exit of a labeled cell out of the peripheral blood compartment being sampled. Modeling the rate of uptake and decay of BrdU label provides useful quantitative information on the dynamics of defined B- and T-lymphocyte populations in sooty mangabeys. Our results conclusively demonstrate an absence of increased turnover of multiple lymphocyte subsets including memory CD4+ T lymphocytes in viremic SIV-infected sooty mangabeys. We also report a significantly higher turnover rate of CD4+ T lymphocytes than of CD8+ T lymphocytes in sooty mangabeys, a finding hitherto not reported in rhesus macaques or humans. The absence of aberrant T-lymphocyte turnover combined with an inherently high turnover rate of CD4+ T lymphocytes may be an important factor protecting sooty mangabeys against SIV-induced CD4+ T-lymphocyte depletion and AIDS.
MATERIALS AND METHODS
Animals.
Sooty mangabeys were housed at the Yerkes National Primate Research Center and maintained in accordance with federal and institutional guidelines for animal care. Five SIV-negative sooty mangabeys ranging in age between 3.9 and 11 years (median, 6.6 years), and six naturally SIV-infected sooty mangabeys ranging in age between 7.3 and 14.1 years (median, 10.5 years) were enrolled in the study.
BrdU administration.
BrdU was administered daily for 14 days via the intraperitoneal route at a dose of 60 mg/kg of body weight/day. Blood was collected twice a week during the 2-week labeling period and the first 2 weeks of the decay phase. In all, BrdU was monitored for 4 to 13 weeks (median, 10 weeks) during the decay phase. Peripheral blood counts were monitored for anemia, leucopenia, and thrombocytopenia during the period of BrdU administration.
Flow cytometric detection of BrdU-labeled lymphocytes.
Lymphocytes isolated from heparin blood shipped overnight to the New England Primate Research Center were processed for detection of BrdU-labeled lymphocyte subsets by five-color flow cytometry as previously described (27). At each time point, blood from an SIV-negative sooty mangabey that did not receive BrdU was also included in order to establish cutoff gates for a negative and positive BrdU signal. Briefly, blood collected in tubes containing the anticoagulant heparin was subjected to Ficoll-Hypaque density gradient centrifugation for isolation of peripheral blood mononuclear cells. After cells were washed with phosphate-buffered saline (PBS) containing 2% heat-inactivated fetal calf serum (wash medium), antibodies used for surface staining were added and the cells were incubated on ice for 30 min in the dark. After washing, the surface-stained cells were suspended in fresh 2% paraformaldehyde with 0.1% Tween 20 at 37°C for 30 min and then at room temperature for another 30 to 60 min. Cells were then washed and incubated for 30 min at 37°C with 50 Kunitz units of DNase I (Sigma-Aldrich, St. Louis, MO) in 0.15 M NaCl and 4.2 mM MgCl2, pH 5.0, to cleave DNA strands at random. Following another wash, cells were suspended in 0.5% Tween 20 in PBS along with anti-BrdU monoclonal antibody (clone B44) conjugated to fluorescein isothiocyanate (FITC) (BD Biosciences, San Jose, CA) and incubated at room temperature for 30 to 45 min. Cells were then washed twice with 0.1% Tween 20 in PBS and suspended in PBS with 2% paraformaldehyde until analysis. Samples were analyzed on a Becton Dickinson FACS Vantage flow cytometer equipped with a Coherent Enterprise laser simultaneously emitting 360 nm and 488 nm and a Coherent helium-neon laser emitting 630 nm. Twenty thousand lymphocyte events were collected and analyzed using Cellquest software (BD Biosciences) and Flow Jo software (Treestar Inc., San Carlos, CA).
Antibodies.
Monoclonal antibodies used included anti-BrdU monoclonal antibody (clone B44) conjugated to FITC, anti-CD3 (clone 6G12; kindly provided by J. Wong, Massachusetts General Hospital) conjugated to biotin or phycoerythrin (PE), anti-CD4 (clone SK3) conjugated to biotin or allophycocyanin (APC), anti-CD8 (clone 51.1; American Type Culture Collection) custom conjugated to Cascade Blue (Chromaprobe, Mountain View, CA), anti-CD20 (clone G44-26) conjugated to PE-Cy5, anti-CD45RA (clone L48) custom conjugated to APC, anti-CD44 (clone G44-26) conjugated to PE, anti-CD28 (clone 28.2) conjugated to PE or FITC, and anti-CD95 (clone DX2) conjugated to APC. Streptavidin Red613 (Gibco) was used as the secondary antibody. Antibodies were obtained from BD Biosciences unless stated otherwise.
Mathematical modeling of lymphocyte turnover.
The fraction of BrdU-labeled cells over time was fitted to a previously used model of BrdU labeling, and lymphocyte turnover was determined by estimating the mean death rate, d (per day), of defined cell populations as previously reported (6, 26). Briefly, we have a resting population that does not label, UR, but a fraction of which can expand into the dividing pool where labeling occurs at a rate a per cell. In addition to the expansion of the resting population into the dividing activated pool, the labeled fraction, LA, is also affected by the rates of proliferation and death of this population, pA and dA, respectively. During BrdU labeling, equations for the unlabeled and labeled (UA and LA, respectively) cells in the activated populations can be written (equation 1), where proliferation of an unlabeled cell results in two labeled daughter cells.
 |
(1) |
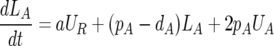 |
During the delabeling phase, activation from the resting pool results in unlabeled activated cells, and the equations for the activated population are
 |
(2) |
 |
From equations 1 and 2 we can deduce the fraction of labeled cells over time, before and after the labeling ends at time tE.
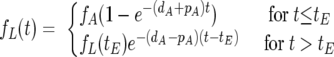 |
(3) |
where fA represents the fraction of activated cells, assumed constant and given by the quotient of activated cells over total cells.
Since the B-lymphocyte labeling profile showed an increase in the percentage of BrdU+ cells well past the end of BrdU intake, the labeling times, tE, in our fits of the B-lymphocyte kinetics were allowed to be free as described previously (6). The increase in BrdU+ B cells beyond the time of label administration was likely due to recirculating B lymphocytes.
From the curve fits, the fraction of dividing cells (fA) and the proliferation (pA) and death (dA) rates were estimated. The mean death rate d (per day) is defined as the weighted average of the death rates of the dividing and resting populations, i.e., dAfA + 0(1 − fA), because we assume that the resting population has a negligible death rate, at least within the time scales of this experiment. This has been shown to be a more robust parameter in this modeling approach and largely independent of the choice of the mathematical model (5).
With one exception, we were able to fit the data well and obtain estimates of the mean death rate of different lymphocyte subsets. The naïve CD4+ T-cell kinetics for one SIV-negative mangabey (mangabey FZN) did not give a reliable fit due to lack of convergence of the Levenberg-Marquardt procedure used to fit the data, and hence this animal was excluded from analysis of turnover of naïve CD4+ T lymphocytes.
Statistical analysis.
The nonparametric Mann-Whitney U test was used for unpaired comparisons and the nonparametric Wilcoxon signed-rank test was used for paired comparisons, while the Pearson test was used for correlation analysis. Statistical analysis was performed with Statview (Abacus Concepts, Berkeley, CA) and GraphPad Prism (GraphPad Software Inc., San Diego, CA).
RESULTS
Perturbations in peripheral T- and B-lymphocyte counts in SIV-infected sooty mangabeys.
Plasma SIV RNA levels ranged between 8.3 × 103 and 2.0 × 105 copies/ml (median, 7.8 × 104 copies/ml) in the six naturally SIV-infected sooty mangabeys. Based on the usual age of SIV seroconversion in captive sooty mangabeys (8), the SIV-infected mangabeys in this study would have been infected for at least 2 years (range, 2 to 9 years) at the time of BrdU administration. Peripheral T (CD3+)- and B (CD20+)-lymphocyte counts were significantly lower (P < 0.05) in the SIV-infected than in the SIV-negative sooty mangabeys (Fig. 1A). A trend for lower CD4+ T-lymphocyte counts in the SIV-infected mangabeys (median, 589/μl; range, 33 to 1,856/μl) than in the SIV-negative mangabeys (median, 1,233/μl; range, 892 to 1,684/μl) did not reach statistical significance (Fig. 1B). Since an age-related decline in CD4+ T-lymphocyte counts has been reported in sooty mangabeys (2), we investigated whether the differences in lymphocyte counts between SIV-negative and SIV-infected sooty mangabeys could be attributed to age. An inverse correlation between age and peripheral T- or B-cell counts was not observed (Fig. 1C). Thus, the B and T lymphopenia appears to be a direct consequence of SIV infection. These data indicate that SIV infection is not silent in its natural host.
FIG. 1.
Comparison of lymphocyte counts and BrdU label in SIV-negative and SIV-infected sooty mangabeys. (A and B) Peripheral T (CD3+)- and B (CD20+)-lymphocyte counts (A) and CD4+ and CD8+ T-lymphocyte counts (B) in five SIV-negative and six SIV-infected sooty mangabeys. Lymphocyte counts prior to BrdU administration are shown. P values were determined by the Mann-Whitney U test. (C) Relation between age and lymphocyte counts in sooty mangabeys. Correlation coefficients (r) and P values were determined by the Pearson correlation test. (D to F) Percent BrdU-positive cells for CD4+ T lymphocytes (D), CD8+ T lymphocytes (E), and CD20+ B lymphocytes (F) during the label and decay phases.
Absence of increased T- and B-lymphocyte turnover in SIV-infected sooty mangabeys.
In order to investigate whether chronic SIV viremia also leads to perturbations in lymphocyte turnover in sooty mangabeys, the fraction of BrdU-labeled cells in the peripheral blood of six naturally SIV-infected and five SIV-negative sooty mangabeys given intraperitoneal BrdU was measured over time by five-color flow cytometry. Blood was sampled twice a week during the 2-week period of BrdU administration (label phase) and once or twice a week thereafter, for a median of 10 weeks (decay phase). BrdU label in the CD4+ T lymphocytes of one SIV-infected sooty mangabey with profound CD4+ T lymphocytopenia (Fig. 1B) could not be reliably assessed because of low cell numbers, and hence this animal was excluded from analysis of CD4+ T-lymphocyte turnover.
BrdU label accumulated and decayed exponentially in CD4+ and CD8+ T lymphocytes and in B lymphocytes (Fig. 1D to F). Despite the differences in lymphocyte counts between SIV-infected and SIV-negative sooty mangabeys, there was considerable overlap in the rate of uptake and decay of BrdU label in T and B lymphocytes between the two animal groups (Fig. 1D to F). At the end of the labeling period, B lymphocytes contained the highest proportion of BrdU-labeled cells (mean ± standard deviation [SD], 19.7% ± 5.9%), followed by CD4+ T lymphocytes (mean ± SD, 12.3% ± 5.5%) and CD8+ T lymphocytes (mean ± SD, 9.7% ± 3.5%).
The fraction of BrdU-labeled cells over time was fitted with a mathematical model and used to estimate the mean death rate d of different lymphocyte subsets as a measure of their turnover (Fig. 2A). Consistent with the considerable overlap in BrdU label uptake and decay between SIV-infected and SIV-negative sooty mangabeys, there was no difference in the mean death rates of CD4+ T lymphocytes, CD8+ T lymphocytes, and B lymphocytes between the two animal groups (Table 1), confirming the absence of increased lymphocyte turnover in sooty mangabeys naturally infected with SIV. Despite the presence of low CD4+ T-lymphocyte and B-lymphocyte counts in SIV-infected sooty mangabeys (Fig. 1A and B), there were no statistically significant correlations between CD4+ T or B lymphopenia and the mean death rate of the respective lymphocyte subsets (data not shown). The level of SIV viremia did not correlate with the turnover rate of any lymphocyte subset, and neither did it correlate with CD4+ T lymphocytopenia (data not shown).
FIG. 2.
Mean death rates of lymphocyte subsets in sooty mangabeys. (A) Representative curve-fit plots for CD4+ T lymphocytes, CD8+ T lymphocytes, and CD20+ B lymphocytes in one SIV-negative (FKU) and one SIV-infected (FDH) sooty mangabey. (B and C) Correlation between mean death rates of CD4+ and CD8+ T lymphocytes and B lymphocytes. Correlation coefficients (r) and P values were determined by the Pearson correlation test.
TABLE 1.
Lymphocyte turnover rates in SIV-negative and SIV-infected sooty mangabeys
| Lymphocyte type | Mean death rate d (per day)a
|
Pd | |
|---|---|---|---|
| SIV− (n = 5)b | SIV+ (n = 6)c | ||
| Total CD4+ T | 0.012 (0.009-0.016) | 0.011 (0.006-0.017) | 1.00 |
| Total CD8+ T | 0.008 (0.005-0.011) | 0.009 (0.006-0.013) | 0.33 |
| Total CD20+ B | 0.016 (0.013-0.020) | 0.020 (0.012-0.027) | 0.43 |
| Naïve CD4+ Te | 0.010 (0.002-0.018) | 0.006 (0.001-0.011) | 0.11 |
| CD45RA− memory CD4+ T | 0.012 (0.009-0.015) | 0.011 (0.006-0.016) | 1.00 |
| CD45RA+ memory CD4+ T | 0.018 (0.012-0.024) | 0.023 (0.012-0.034) | 0.42 |
| CD45RA− memory CD8+ T | 0.010 (0.006-0.015) | 0.008 (0.004-0.012) | 0.54 |
Numbers in parentheses are 95% confidence intervals of the means.
n = 4 for naïve CD4+ T lymphocytes because of exclusion of one animal with poor data fitting to curve.
n = 5 for CD4+ T lymphocytes due to exclusion of one SIV-infected sooty mangabey with severe CD4+ T lymphopenia.
Determined by Mann-Whitney U test.
CD45RA+ CD44lo/−.
There was a strong positive correlation between the turnover rates of total CD4+ and CD8+ T lymphocytes (Fig. 2B) and CD45RA− memory CD4+ and CD8+ T lymphocytes (r = +0.80; P = 0.005; data not shown). Thus, similar to rhesus macaques, the turnover of the two T-lymphocyte subsets is closely linked and likely regulated by the same factor(s). In contrast to that in rhesus macaques (6), B-lymphocyte turnover in the sooty mangabeys was inversely correlated with CD4+ T-lymphocyte turnover (Fig. 2C) and did not correlate with CD8+ T-lymphocyte turnover (data not shown).
High turnover rate of CD4+ T lymphocytes in sooty mangabeys.
In order to ascertain whether the dynamics of normal lymphocyte turnover differed between sooty mangabeys and rhesus macaques, we investigated the relative turnover rates of different lymphocyte subsets in sooty mangabeys. Similar to findings in rhesus macaques (6), the turnover rate of B lymphocytes in sooty mangabeys was significantly higher than that of CD4+ and CD8+ T lymphocytes (Fig. 3A). The estimated mean half-life of total B lymphocytes was 41 days compared to 63 days for CD4+ T lymphocytes and 93 days for CD8+ T lymphocytes (Table 2). Interestingly, CD4+ T lymphocytes had a significantly higher mean death rate and shorter half-life than did CD8+ T lymphocytes (Fig. 3A and B; Table 3), an observation not previously reported in rhesus macaques or humans. The difference in turnover rate did not appear to be due to differences in the composition of memory cells within CD4+ and CD8+ T lymphocytes. CD45RA− memory cells were the dominant population and present at comparable frequencies within both CD4+ and CD8+ T lymphocytes (data not shown). Moreover, CD45RA− memory CD4+ T lymphocytes also had a significantly higher mean death rate than did CD45RA− memory CD8+ T lymphocytes (Table 3). Finally, the difference in turnover rate between CD4+ and CD8+ T lymphocytes was apparent even when the SIV-negative and SIV-infected mangabeys were analyzed separately (Fig. 3C), suggesting that it was independent of SIV infection and an inherent feature of lymphocyte turnover in sooty mangabeys.
FIG. 3.
Relative turnover rates of CD4+ T lymphocytes, CD8+ T lymphocytes, and B lymphocytes in sooty mangabeys. (A and B) Mean death rates (A) and half-lives (B) of CD4+ and CD8+ T lymphocytes and CD20+ B lymphocytes. (C) Comparison of mean death rates of CD4+ and CD8+ T lymphocytes in SIV-negative (n = 5) and SIV-infected (n = 5) sooty mangabeys. P values were determined by the Wilcoxon signed-rank test.
TABLE 2.
Half-lives of lymphocyte subsets in sooty mangabeys
| Cell type | Mean half-life (days) ± SD
|
||
|---|---|---|---|
| SIV− | SIV+ | All | |
| CD4+ T cells | |||
| Total | 59 ± 16 | 67 ± 23 | 63 ± 19 |
| Naïvea | 81 ± 35 | 138 ± 51 | 112 ± 51 |
| CD45RA− memoryb | 58 ± 9 | 70 ± 27 | 64 ± 20 |
| CD45RA+ memoryc | 41 ± 14 | 34 ± 15 | 38 ± 14 |
| CD8+ T cells | |||
| Total | 100 ± 35 | 86 ± 35 | 93 ± 34 |
| CD45RA− memoryb | 74 ± 21 | 100 ± 45 | 88 ± 37 |
| CD20+ B cells | |||
| Total | 43 ± 9 | 39 ± 12 | 41 ± 10 |
CD45RA+ CD44lo/−.
CD45RAlo/− CD44hi.
CD45RAhi CD44hi.
TABLE 3.
Comparison of CD4+ and CD8+ T-lymphocyte turnover in sooty mangabeys
| Cell type | Mean d (per day) ± SD for T-lymphocyte type:
|
Pa | |
|---|---|---|---|
| CD4+ | CD8+ | ||
| Total | 0.0119 ± 0.0035 | 0.0084 ± 0.0029 | 0.002 |
| CD45RA− memory | 0.0116 ± 0.0031 | 0.0091 ± 0.0035 | 0.004 |
Determined by Wilcoxon signed-rank test.
Turnover of naïve and memory CD4+ T lymphocytes in sooty mangabeys.
The absence of increased turnover of total CD4+ and CD8+ T lymphocytes in SIV-infected sooty mangabeys did not exclude the possibility of a subset of these cells, whether naïve or memory, being differentially affected by SIV infection. Since the five-color flow cytometry panel used for determining the fraction of BrdU-labeled cells included the phenotypic markers CD45RA and CD44, we first validated their use for defining naïve and memory CD4+ and CD8+ T-lymphocyte subpopulations in sooty mangabeys.
Dual staining with CD44 and CD45RA delineated three distinct subpopulations of CD4+ T lymphocytes; CD44lo/− CD45RA+ (designated as “naïve), CD44hi CD45RAlo/− (designated as “CD45RA− memory”), and CD44hi CD45RAhi (designated as “CD45RA+ memory”) (Fig. 4A). In separate four-color flow cytometry experiments performed on peripheral blood and tissue lymphocytes derived from SIV-negative sooty mangabeys, lymphocytes were stained for surface CD4, CD44, and CD45RA concurrent with CD95, a marker used to distinguish memory from naïve cells (24). CD4+ T lymphocytes designated as CD45RA− and CD45RA+ memory were larger in size (higher forward scatter) and expressed more CD95 than did cells designated as “naïve” (Fig. 4A). Consistent with a naïve phenotype, CD44lo/− CD45RA+ cells made up the majority of CD4+ T lymphocytes in a newborn (10-day-old) mangabey and declined with increasing age (Fig. 4B). Additional validation of the naïve and memory phenotypic designations was provided by their differential distribution at inductive and effector tissue sites. Tissue lymphocytes derived at necropsy from three SIV-negative sooty mangabeys that had died of natural causes were analyzed (Fig. 4C). Naïve CD4+ T lymphocytes were enriched at an inductive site represented by the lymph node but accounted for <15% of CD4+ T cells at effector sites represented by the jejunal mucosa and lung tissue (Fig. 4C). Conversely, CD45RA− memory cells accounted for >70% of CD4+ T lymphocytes at these two effector sites but were not the dominant population in the lymph nodes (Fig. 4C). Of note, the majority of CD45RA− memory CD4+ T lymphocytes in the peripheral blood were CD28+ (Fig. 4D), suggesting that they were central memory cells (24). In all, these data broadly support the naïve and memory designation of CD4+ T lymphocytes based on coexpression patterns of CD44 and CD45RA. Coexpression of CD44 and CD45RA on CD8+ T lymphocytes showed considerable variability in patterns of expression that could not be sufficiently validated by four-color flow cytometry (data not shown). Hence, turnover of only the CD45RA− memory subset of CD8+ T lymphocytes was analyzed.
FIG. 4.
Characterization of naïve and memory CD4+ T lymphocytes in sooty mangabeys. (A) Representative plots showing surface expression of CD44, CD45RA, and CD95 and size (forward scatter; FSC) for naïve and memory subsets of CD4+ T lymphocytes. (B) Changes in naïve and memory CD4+ T lymphocytes with age. Data on four SIV-negative sooty mangabeys are shown. (C) Distribution of naïve and memory CD4+ T lymphocytes at different tissue sites. Peripheral blood mononuclear cells (PBMC) were compared with lymph node (LN), jejunum, and lung. Means and standard errors of the means of data from three SIV-negative sooty mangabeys are shown. Tissues were obtained at necropsy. (D) CD28 expression on CD45RA− memory and CD45RA+ memory CD4+ T lymphocytes. Data on peripheral blood lymphocytes in 10 sooty mangabeys are shown. (E) Comparison of half-lives of naïve and memory CD4+ T lymphocytes. P values were determined by the Wilcoxon signed-rank test.
Mathematical modeling showed a good fit of the BrdU label data for the CD45RA− memory CD4+ and CD8+ T lymphocytes (Fig. 5A). The fit quality was not as good for the naïve and CD45RA+ memory CD4+ T lymphocytes, possibly due to the smaller size of these pools leading to greater variation in the data (Fig. 5A). Nevertheless, we found that the mean death rate estimated for the whole CD4+ T-lymphocyte population and the weighted mean death rate, considering the fractions and death rates of each subpopulation, differed by an average of only 6%.
FIG. 5.
Turnover of naïve and memory T lymphocyte subsets in sooty mangabeys. (A) Representative curve fits of BrdU-labeled naïve and memory T lymphocytes in one SIV-negative mangabey (FKU; top) and one SIV-positive sooty mangabey (FDH; bottom). (B) Comparison of peripheral naive and memory CD4+ and CD8+ T-lymphocyte counts in SIV-infected and SIV-negative sooty mangabeys.
SIV-infected sooty mangabeys had lower absolute numbers of naïve and memory CD4+ T lymphocytes than did SIV-negative sooty mangabeys, although the differences did not reach statistical significance (Fig. 5B). Similar to the total CD4+ and CD8+ T-lymphocyte population, there was no difference in the mean death rate of the naïve and memory CD4+ and memory CD8+ T-lymphocyte subpopulations between SIV-infected and SIV-negative sooty mangabeys (Table 1), suggesting that the absence of increased turnover in viremic SIV-infected sooty mangabeys applies to both naïve and memory T-lymphocyte populations.
Consistent with their naïve and memory designations, naïve CD4+ T lymphocytes had a longer half-life than did the two memory CD4+ T-lymphocyte populations (Fig. 4E; Table 2). The CD45RA+ memory cells, which accounted for <10% (range, 3.1 to 8.0%) of CD4+ T lymphocytes, were composed of rapidly proliferating cells with a significantly shorter half-life (mean, 38 days) than that of the naïve subset and the dominant CD45RA− memory subset of CD4+ T lymphocytes (Fig. 4E; Table 2). However, the difference in turnover rate of naïve and CD45RA− memory CD4+ T lymphocytes failed to reach statistical significance (Fig. 4E). An overlap between the naïve and the rapidly proliferating CD45RA+ memory CD4+ T lymphocytes (Fig. 4A) might have led to an overestimation of the turnover rate of the naïve CD4+ T lymphocytes.
The turnover of total CD4+ T lymphocytes was strongly correlated with the dominant CD45RA− memory population (r = +0.70, P = 0.02) and less so with the minor CD45RA+ memory population (r = +0.54, P = 0.10). There was no correlation between the turnover of total and naïve CD4+ T lymphocytes (r = +0.007, P = 0.98) or between CD45RA− memory and naïve CD4+ T lymphocytes (r = +0.06, P = 0.88), suggesting that the homeostatic mechanisms for maintaining naïve and memory CD4+ T lymphocytes in the peripheral blood are independently regulated.
DISCUSSION
By modeling the rate of uptake and decay of BrdU label in vivo in sooty mangabeys, this study provides the first dynamic estimates of lymphocyte turnover in a natural host of SIV. Our study confirms the absence of increased turnover of CD8+ T lymphocytes in viremic naturally SIV-infected sooty mangabeys and, additionally, extends this observation to multiple other lymphocyte subsets, namely, total, naïve, and memory CD4+ T lymphocytes and total B lymphocytes. Other notable findings include the identification of a rapidly proliferating CD44hi CD45RAhi memory subset of CD4+ T lymphocytes in sooty mangabeys and demonstration of a faster turnover of CD4+ T lymphocytes than of CD8+ T lymphocytes in this species.
Similar to pathogenic lentiviral infection, SIV-infected sooty mangabeys had significantly lower total T- and B-lymphocyte counts and a trend toward lower numbers of naïve and memory CD4+ T lymphocytes compared to SIV-negative sooty mangabeys. The lymphopenia was not related to aging and was likely a direct result of SIV infection. Despite these perturbations, lymphocyte turnover was not increased and there was no correlation between lymphocyte turnover and viral load or the extent of CD4+ T lymphocytopenia in SIV-infected sooty mangabeys. These findings contrast with the twofold-or-greater increase in turnover of multiple lymphocyte subsets (CD4+ and CD8+ T lymphocytes, B lymphocytes, and NK cells) directly related to the level of viremia and extent of CD4+ T lymphocytopenia observed in SIV-infected rhesus macaques (6, 19, 27). The presence of T and B lymphopenia but not increased lymphocyte turnover in naturally SIV-infected sooty mangabeys suggests that homeostatic mechanisms do not contribute in any significant manner to the occurrence of aberrant lymphocyte turnover in AIDS. Instead, these data provide further support for chronic immune activation being the principal driving force behind the increased lymphocyte turnover of pathogenic lentiviral infection. Why chronic viremia is not associated with aberrant immune activation in natural hosts of SIV is still not known. We have previously shown that the magnitude and quality of the SIV-specific T-cell response do not differ between naturally SIV-infected sooty mangabeys and rhesus macaques with chronic pathogenic SIV infection (31). Hence, it is unlikely that the aberrant immune activation in AIDS reflects immunopathology due to an inappropriate or excessive antiviral T-cell immune response. The presence of an intact adaptive T-cell response to SIV in sooty mangabeys suggests that proliferation of SIV-specific T lymphocytes has little or no contribution to the increased T-cell turnover of pathogenic chronic lentiviral infection.
This study has provided important insights into the biology of normal lymphocyte turnover in sooty mangabeys and points to the intriguing possibility of species-specific differences between hosts with pathogenic and nonpathogenic lentiviral infection. With the exception of Ki-67 expression (3), there are no comparative data on differences or similarities in the biology of lymphocyte turnover between sooty mangabeys and rhesus macaques. In published studies that have used in vivo BrdU labeling to compute lymphocyte turnover in rhesus macaques, BrdU was either administered orally for 3 weeks (6, 19) or administered for 2 weeks via the intraperitoneal and oral routes (27). Since the measured death rate of lymphocytes using in vivo DNA labeling techniques is subject to variation with the length of the labeling period, dosage, and route of administration and the mathematical model used for calculation of turnover rates (1, 5), comparison of actual turnover rates between different studies is difficult. However, interspecies comparisons based on relative turnover rates of different lymphocyte subsets may still be meaningful. Thus, BrdU labeling studies have highlighted certain similarities in lymphocyte turnover between sooty mangabeys and rhesus macaques (6, 19, 27). In both species, B lymphocytes turn over at a significantly higher rate than do T lymphocytes. Additionally, the strong direct correlation between CD4+ and CD8+ T-lymphocyte turnover suggests that the turnover of the two major T-lymphocyte subsets is closely linked and likely influenced by similar factors in the two nonhuman primate species.
Two possible differences from rhesus macaques were observed. The turnover rate of CD4+ T lymphocytes in sooty mangabeys was significantly higher than that of CD8+ T lymphocytes irrespective of the presence or absence of SIV infection. Such a difference has not to our knowledge been reported for rhesus macaques. In one BrdU labeling study which used the same mathematical model used in the current study, the measured mean death rates of CD4+ and CD8+ T lymphocytes in rhesus macaques did not appear to be different from each other. In contrast, CD8+ T-lymphocyte turnover in SIV-negative rhesus macaques appeared to be equal to or higher than CD4+ T-lymphocyte turnover (6). The calculated mean daily replacement rates for CD3+ CD4+ and CD3+ CD4− T lymphocytes in SIV-negative rhesus macaques were 0.9% and 1.1%, respectively (6, 19). In our study, the corresponding mean daily replacement rates in sooty mangabeys were 1.2% for CD4+ T lymphocytes and 0.8% for CD8+ T lymphocytes. It should be noted that because of different study designs, including duration and route of BrdU administration, the interstudy comparison does not allow us to ascertain whether the turnover rate of CD4+ T lymphocytes in sooty mangabeys is significantly different from that in rhesus macaques. Although our study revealed a roughly 30% higher turnover rate of CD4+ T lymphocytes than of CD8+ T lymphocytes in sooty mangabeys, studies based on Ki-67 expression have not reported such a difference (3, 29). This discrepancy is likely related to differences in the methodology of measuring lymphocyte turnover. While Ki-67 antigen provides a snapshot view of proliferating cells, in vivo BrdU administration results in cumulative information on labeled cycling cells which can be followed over time. A time series analysis of labeling and delabeling in lymphocytes provides a more powerful and sensitive measure of T-lymphocyte turnover.
Another difference from rhesus macaques was the lack of any correlation between B-lymphocyte and CD8+ T-lymphocyte turnover and an inverse correlation between the turnover of B lymphocytes and CD4+ T lymphocytes in sooty mangabeys. In rhesus macaques, B-lymphocyte turnover was shown to be directly correlated with both CD4+ and CD8+ T-lymphocyte turnover (6). Since this analysis mainly included SIV-infected macaques (6), it is possible that the direct correlation between B-lymphocyte turnover and turnover of T-lymphocyte subsets reflected increased turnover of all lymphocyte subsets in SIV infection rather than a true difference in lymphocyte turnover biology between rhesus macaques and sooty mangabeys.
The finding of a higher turnover rate of CD4+ T lymphocytes relative to CD8+ T lymphocytes in sooty mangabeys was unexpected. A higher turnover rate of CD4+ T lymphocytes is contrary to what would be expected from the comparative proliferative capacity of CD4+ and CD8+ T lymphocytes in response to antigen exposure. CD4+ T lymphocytes proliferate less than CD8+ T lymphocytes in response to in vitro antigenic stimulation (7). Compared to CD8+ T lymphocytes, CD4+ T lymphocytes undergo limited division and clonal expansion in vivo in response to viral infections (13, 21). In human subjects with bone marrow transplant or chemotherapy-induced myelosuppression, regeneration of CD8+ T lymphocytes occurs significantly faster than does CD4+ T-lymphocyte regeneration (18). However, factors responsible for antigen-driven proliferation may be quite different from those driving normal turnover of memory T lymphocytes. In the murine system, it has been shown that normal turnover of memory CD8+ T lymphocytes is T-cell receptor independent and mediated by interleukin 15 (32). Interestingly, proliferation of different memory subsets of CD4+ T lymphocytes in mice can be as high as or higher than that of CD8+ T lymphocytes (30, 32). Recently, a higher turnover of CD4+ T cells than of CD8+ T cells has also been observed in HIV-negative volunteers from ex vivo BrdU labeling studies of peripheral blood mononuclear cells (Marta Catalfamo, personal communication). Hence, the finding of higher turnover rates of CD4+ T lymphocytes may not be unique to sooty mangabeys. It remains to be determined whether sooty mangabeys have a greater homeostatic proliferative capacity of CD4+ T lymphocytes than do rhesus macaques. If true, it raises the intriguing possibility that an inherently higher rate of CD4+ T-lymphocyte turnover helps protect sooty mangabeys against developing irreversible CD4+ T-lymphocyte depletion.
This paper is also the first to report on the cycling properties of naïve and memory CD4+ T-lymphocyte subsets in sooty mangabeys. Based on coexpression of CD44 and CD45RA, a long-lived CD45RA− memory population and a short-lived CD45RA+ memory population of peripheral blood CD4+ T lymphocytes were identified. The rapidly cycling CD45RA+ memory CD4+ T lymphocytes constituted <10% of peripheral CD4+ T lymphocytes and are likely effector memory cells (12, 17). The slower-proliferating CD45RA− memory subset, which made up most of the memory CD4+ T lymphocytes, largely consists of CD28+ cells in the peripheral blood and thus is likely to consist predominantly of central memory CD4+ T lymphocytes. There was no evidence of increased turnover of either the short-lived effector or the long-lived central memory CD4+ T lymphocytes in SIV-infected mangabeys, consistent with immune activation being the principal driver of increased proliferation in AIDS. This observation is particularly striking in light of the recent observation that acute SIV infection induces significant depletion of CD4+ T lymphocytes at effector sites even in its natural host (9, 23). Even though it is likely that depletion of CD4+ T lymphocytes at effector sites continues into the chronic phase of SIV infection in sooty mangabeys, the absence of increased turnover of memory CD4+ T lymphocytes suggests that its extent is limited in SIV-infected sooty mangabeys. Thus, constraints on central memory CD4+ T-lymphocyte homeostasis imposed by continuing loss of effector memory CD4+ T lymphocytes that are observed in SIV-infected rhesus macaques progressing to AIDS (22) appear to be restricted or absent in SIV-infected sooty mangabeys.
In summary, this study conclusively demonstrates the absence of increased turnover of multiple lymphocyte subsets during the chronic phase of infection in a natural host of SIV. Our data suggest that normal T-cell turnover, an intrinsically high rate of CD4+ T-lymphocyte turnover compared to CD8+ T-lymphocyte turnover, and an intact central memory CD4+ T-lymphocyte pool may all be contributing factors that help maintain adequate CD4+ T-cell homeostasis for a prolonged duration in SIV-infected sooty mangabeys.
Acknowledgments
This work was supported by Public Health Service grants RR 00168 (A.K. and R.P.J.) and A149809 (A.K.), AI24833 and RR06555 (A.S.P.), and P20-RR18754 (R.M.R.). Portions of this work were done under the auspices of the U.S. Department of Energy under contract DE-AC52-06NA25396.
We thank Kristen Toohey for assistance with figures, Carolyn O'Toole for help with manuscript preparation, and the staff of the Yerkes National Primate Research Center for ably conducting the animal studies.
Footnotes
Published ahead of print on 21 November 2007.
REFERENCES
- 1.Asquith, B., C. Debacq, D. C. Macallan, L. Willems, and C. R. Bangham. 2002. Lymphocyte kinetics: the interpretation of labelling data. Trends Immunol. 23596-601. [DOI] [PubMed] [Google Scholar]
- 2.Chakrabarti, L. A., S. R. Lewin, L. Zhang, A. Gettie, A. Luckay, L. N. Martin, E. Skulsky, D. D. Ho, C. Cheng-Mayer, and P. A. Marx. 2000. Age-dependent changes in T cell homeostasis and SIV load in sooty mangabeys. J. Med. Primatol. 29158-165. [DOI] [PubMed] [Google Scholar]
- 3.Chakrabarti, L. A., S. R. Lewin, L. Zhang, A. Gettie, A. Luckay, L. N. Martin, E. Skulsky, D. D. Ho, C. Cheng-Mayer, and P. A. Marx. 2000. Normal T-cell turnover in sooty mangabeys harboring active simian immunodeficiency virus infection. J. Virol. 741209-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Combadere, B., C. Blanc, T. Li, G. Carcelain, C. Delaugerre, V. Calvez, R. Tubiana, P. Debre, C. Katlama, and B. Autran. 2000. CD4+Ki67+ lymphocytes in HIV-infected patients are effector T cells accumulated in the G1 phase of the cell cycle. Eur. J. Immunol. 303598-3603. [DOI] [PubMed] [Google Scholar]
- 5.De Boer, R. J., H. Mohri, D. D. Ho, and A. S. Perelson. 2003. Estimating average cellular turnover from 5-bromo-2′-deoxyuridine (BrdU) measurements. Proc. R. Soc. Lond. B 270849-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Boer, R. J., H. Mohri, D. D. Ho, and A. S. Perelson. 2003. Turnover rates of B cells, T cells, and NK cells in simian immunodeficiency virus-infected and uninfected rhesus macaques. J. Immunol. 1702479-2487. [DOI] [PubMed] [Google Scholar]
- 7.Foulds, K. E., L. A. Zenewicz, D. J. Shedlock, J. Jiang, A. E. Troy, and H. Shen. 2002. Cutting edge: CD4 and CD8 T cells are intrinsically different in their proliferative responses. J. Immunol. 1681528-1532. [DOI] [PubMed] [Google Scholar]
- 8.Fultz, P. N., T. P. Gordon, D. C. Anderson, and H. M. McClure. 1990. Prevalence of natural infection with simian immunodeficiency virus and simian T-cell leukemia virus type I in a breeding colony of sooty mangabey monkeys. AIDS 4619-625. [DOI] [PubMed] [Google Scholar]
- 9.Gordon, S. N., N. R. Klatt, S. E. Bosinger, J. M. Brenchley, J. M. Milush, J. C. Engram, R. M. Dunham, M. Paiardini, S. Klucking, A. Danesh, E. A. Strobert, C. Apetrei, I. V. Pandrea, D. Kelvin, D. C. Douek, S. I. Staprans, D. L. Sodora, and G. Silvestri. 2007. Severe depletion of mucosal CD4+ T cells in AIDS-free simian immunodeficiency virus-infected sooty mangabeys. J. Immunol. 1793026-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hazenberg, M. D., J. W. Stuart, S. A. Otto, J. C. Borleffs, C. A. Boucher, R. J. de Boer, F. Miedema, and D. Hamann. 2000. T-cell division in human immunodeficiency virus (HIV)-1 infection is mainly due to immune activation: a longitudinal analysis in patients before and during highly active antiretroviral therapy (HAART). Blood 95249-255. [PubMed] [Google Scholar]
- 11.Hellerstein, M., M. B. Hanley, D. Cesar, S. Siler, C. Papageorgopoulos, E. Wieder, D. Schmidt, R. Hoh, R. Neese, D. Macallan, S. Deeks, and J. M. McCune. 1999. Directly measured kinetics of circulating T lymphocytes in normal and HIV-1-infected humans. Nat. Med. 583-89. [DOI] [PubMed] [Google Scholar]
- 12.Hellerstein, M. K., R. A. Hoh, M. B. Hanley, D. Cesar, D. Lee, R. A. Neese, and J. M. McCune. 2003. Subpopulations of long-lived and short-lived T cells in advanced HIV-1 infection. J. Clin. Investig. 112956-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaur, A., C. L. Hale, S. Ramanujan, R. K. Jain, and R. P. Johnson. 2000. Differential dynamics of CD4+ and CD8+ T-lymphocyte proliferation and activation in acute simian immunodeficiency virus infection. J. Virol. 748413-8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kovacs, J. A., R. A. Lempicki, I. A. Sidorov, J. W. Adelsberger, B. Herpin, J. A. Metcalf, I. Sereti, M. A. Polis, R. T. Davey, J. Tavel, J. Falloon, R. Stevens, L. Lambert, R. Dewar, D. J. Schwartzentruber, M. R. Anver, M. W. Baseler, H. Masur, D. S. Dimitrov, and H. C. Lane. 2001. Identification of dynamically distinct subpopulations of T lymphocytes that are differentially affected by HIV. J. Exp. Med. 1941731-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lempicki, R. A., J. A. Kovacs, M. W. Baseler, J. W. Adelsberger, R. L. Dewar, V. Natarajan, M. C. Bosche, J. A. Metcalf, R. A. Stevens, L. A. Lambert, W. G. Alvord, M. A. Polis, R. T. Davey, D. S. Dimitrov, and H. C. Lane. 2000. Impact of HIV-1 infection and highly active antiretroviral therapy on the kinetics of CD4+ and CD8+ T cell turnover in HIV-infected patients. Proc. Natl. Acad. Sci. USA 9713778-13783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ling, B., C. Apetrei, I. Pandrea, R. S. Veazey, A. A. Lackner, B. Gormus, and P. A. Marx. 2004. Classic AIDS in a sooty mangabey after an 18-year natural infection. J. Virol. 788902-8908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macallan, D. C., D. Wallace, Y. Zhang, C. De Lara, A. T. Worth, H. Ghattas, G. E. Griffin, P. C. Beverley, and D. F. Tough. 2004. Rapid turnover of effector-memory CD4+ T cells in healthy humans. J. Exp. Med. 200255-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackall, C. L., T. A. Fleisher, M. R. Brown, M. P. Andrich, C. C. Chen, I. M. Feuerstein, I. T. Magrath, L. H. Wexler, D. S. Dimitrov, and R. E. Gress. 1997. Distinctions between CD8+ and CD4+ T-cell regenerative pathways result in prolonged T-cell subset imbalance after intensive chemotherapy. Blood 893700-3707. [PubMed] [Google Scholar]
- 19.Mohri, H., S. Bonhoeffer, S. Monard, A. S. Perelson, and D. D. Ho. 1998. Rapid turnover of T lymphocytes in SIV-infected rhesus macaques. Science 2791223-1227. [DOI] [PubMed] [Google Scholar]
- 20.Mohri, H., A. S. Perelson, K. Tung, R. M. Ribeiro, B. Ramratnam, M. Markowitz, R. Kost, A. Hurley, L. Weinberger, D. Cesar, M. K. Hellerstein, and D. D. Ho. 2001. Increased turnover of T lymphocytes in HIV-1 infection and its reduction by antiretroviral therapy. J. Exp. Med. 1941277-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monceaux, V., R. H. T. Fang, M. C. Cumont, B. Hurtrel, and J. Estaquier. 2003. Distinct cycling CD4+- and CD8+-T-cell profiles during the asymptomatic phase of simian immunodeficiency virus SIVmac251 infection in rhesus macaques. J. Virol. 7710047-10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okoye, A., M. Meier-Schellersheim, J. M. Brenchley, S. I. Hagen, J. M. Walker, M. Rohankhedkar, R. Lum, J. B. Edgar, S. L. Planer, A. Legasse, A. W. Sylwester, M. Piatak, Jr., J. D. Lifson, V. C. Maino, D. L. Sodora, D. C. Douek, M. K. Axthelm, Z. Grossman, and L. J. Picker. 2007. Progressive CD4+ central memory T cell decline results in CD4+ effector memory insufficiency and overt disease in chronic SIV infection. J. Exp. Med. 2042171-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pandrea, I. V., R. Gautam, R. M. Ribeiro, J. M. Brenchley, I. F. Butler, M. Pattison, T. Rasmussen, P. A. Marx, G. Silvestri, A. A. Lackner, A. S. Perelson, D. C. Douek, R. S. Veazey, and C. Apetrei. 2007. Acute loss of intestinal CD4+ T cells is not predictive of simian immunodeficiency virus virulence. J. Immunol. 1793035-3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pitcher, C. J., S. I. Hagen, J. M. Walker, R. Lum, B. L. Mitchell, V. C. Maino, M. K. Axthelm, and L. J. Picker. 2002. Development and homeostasis of T cell memory in rhesus macaque. J. Immunol. 16829-43. [DOI] [PubMed] [Google Scholar]
- 25.Rey-Cuille, M. A., J. L. Berthier, M. C. Bomsel-Demontoy, Y. Chaduc, L. Montagnier, A. G. Hovanessian, and L. A. Chakrabarti. 1998. Simian immunodeficiency virus replicates to high levels in sooty mangabeys without inducing disease. J. Virol. 723872-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ribeiro, R. M., H. Mohri, D. D. Ho, and A. S. Perelson. 2002. In vivo dynamics of T cell activation, proliferation, and death in HIV-1 infection: why are CD4+ but not CD8+ T cells depleted? Proc. Natl. Acad. Sci. USA 9915572-15577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenzweig, M., M. A. DeMaria, D. M. Harper, S. Friedrich, R. K. Jain, and R. P. Johnson. 1998. Increased rates of CD4+ and CD8+ T lymphocyte turnover in simian immunodeficiency virus-infected macaques. Proc. Natl. Acad. Sci. USA 956388-6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sachsenberg, N., A. S. Perelson, S. Yerly, G. A. Schockmel, D. Leduc, B. Hirschel, and L. Perrin. 1998. Turnover of CD4+ and CD8+ T lymphocytes in HIV-1 infection as measured by Ki-67 antigen. J. Exp. Med. 1871295-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silvestri, G., D. L. Sodora, R. A. Koup, M. Paiardini, S. P. O'Neil, H. M. McClure, S. I. Staprans, and M. B. Feinberg. 2003. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity 18441-452. [DOI] [PubMed] [Google Scholar]
- 30.Tough, D. F., and J. Sprent. 1994. Turnover of naive- and memory-phenotype T cells. J. Exp. Med. 1791127-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang, Z., B. Metcalf, R. M. Ribeiro, H. McClure, and A. Kaur. 2006. Th-1-type cytotoxic CD8+ T-lymphocyte responses to simian immunodeficiency virus (SIV) are a consistent feature of natural SIV infection in sooty mangabeys. J. Virol. 802771-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang, X., S. Sun, I. Hwang, D. F. Tough, and J. Sprent. 1998. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity 8591-599. [DOI] [PubMed] [Google Scholar]







